ABSTRACT
Introduction: Current medical therapy for glaucoma consists of topical agents that lower intraocular pressure (IOP). Prostaglandin F2α analogues, the most commonly used class of IOP-lowering drugs, bind to prostaglandin FP receptors in tissues of the uveoscleral pathway. This binding increases the expression of matrix metalloproteinases, which degrade the extracellular matrix of the ciliary body, creating inter-muscular spaces allowing aqueous humor to exit the eye. Drawbacks to prostaglandin F2α analogues include cosmetic side effects, especially prostaglandin-associated periorbitopathy (PAP) syndrome.
Areas covered: This review describes the novel prostaglandin E2 receptor antagonist, omidenepag isopropyl, which reduces IOP by improving drainage of uveoscleral and trabecular outflow, increasing the facility of outflow. In contrast to prostaglandin F2α analogues, omidenepag does not inhibit adipogenesis or promote eyelash growth. This review describes preclinical studies of omidenepag, published results of phase I–III clinical trials, and preliminary results of phase III trials currently in progress.
Expert opinion: Omidenepag appears to provide IOP reductions comparable to those of prostaglandin F2α analogues, but without the cosmetic side effects common to prostaglandin F2α analogues, especially PAP syndrome. The lack of association between omidenepag and PAP suggests that long-term use of this agent may have advantages in patients with glaucoma.
1. Introduction
Glaucoma is a collective term for a group of ocular conditions characterized by progressive degeneration of the optic nerve with loss of retinal ganglion cells and their axons. This results in loss of the peripheral visual field, which can, if left untreated, lead to visual dysfunction and blindness [Citation1]. The most common form of glaucoma is primary open-angle glaucoma (POAG), the pathogenesis of which is complex and multifactorial, with a strong genetic basis [Citation2]. The most important known risk factor for POAG is elevated intraocular pressure (IOP). Other conditions that can raise IOP and cause secondary glaucoma include ocular conditions (e.g. pigment dispersion syndrome, trauma, pseudo-exfoliation syndrome, and uveitis) and use of medications (e.g. corticosteroids). IOP is considered a causal factor, in that the reduction of elevated IOP can delay or prevent the development of glaucoma in at-risk eyes with ocular hypertension (OHT) [Citation3] and reduce the risk of progression in eyes afflicted with glaucoma [Citation4,Citation5]. The prevalence of POAG in the United States is estimated at 1.9%, with approximately 3.3 million Americans affected in 2020 [Citation6]. Globally, the prevalence of glaucoma in subjects aged 40 to 80 years is estimated at 3.54%, with 76 million people in this age group affected in 2020 [Citation7].
2. Current medical therapy for glaucoma
Therapy for glaucoma consists of lowering IOP to prevent disease progression to symptomatic visual dysfunction and blindness. This can be accomplished through a variety of medications, although laser techniques and surgical procedures have also been utilized. Historically and currently, topical medical therapy remains the preferred first-line approach to IOP reduction.
Medical therapy for glaucoma consists of one or more daily applications of topical IOP-lowering eye drop medications. Drug classes that effectively lower IOP include prostaglandin F2α analogues, beta-adrenergic antagonists, carbonic anhydrase inhibitors, selective alpha-2 adrenergic agonists, rho kinase inhibitors, and parasympathomimetics. These drugs lower IOP by reducing the production of aqueous humor or by improving the drainage outflow of aqueous humor from the eye via the trabecular, uveoscleral, and/or episcleral venous outflow pathways (). Among these various drug classes, the prostaglandin F2α analogues are most commonly used as first-line medical therapy due to their superior efficacy and safety compared with other options, and once-daily dosing convenience; with the availability of generic formulations, they are affordable as well.
Table 1. Commonly used topical medications for intraocular pressure reduction in eyes having or suspected of having glaucoma
Advantages of medical therapy include robust characterization of efficacy and safety profiles from high-quality phase 3 regulatory trials and noninvasiveness. Medical treatments have drawbacks, however, the most important being non-adherence to treatment, in that many patients do not take their drops as prescribed [Citation20–23]. This non-adherence significantly increases the likelihood for disease progression over time [Citation24]. Safety is another limitation of medical therapy. Although many side effects, such as hypersensitivity to the medication or conjunctival hyperemia, are acute and easily detectable, others are chronic and more insidious. The latter include adverse events such as the lethargy or depression associated with beta-blockers, fatigue associated with alpha-2 agonists, and periorbitopathy associated with prostaglandin F2α analogues. Tolerability is also a limiting factor and can contribute to non-adherence. For example, topical glaucoma medications have been found to cause and aggravate symptoms of ocular surface diseases [Citation25], which are experienced by 30–70% of glaucoma patients [Citation26–32].
3. Unmet needs and current advances in glaucoma therapy
The array of therapeutic options for IOP reduction in glaucoma has never been broader, but several key gaps still remain. Although the prostaglandin F2α analogues are effective and safe, there are limits to their use. For example, conjunctival hyperemia, eyelash changes, and iris and skin hyperpigmentation are cosmetic side effects that are poorly tolerated by or socially unacceptable to many patients, particularly younger patients still active in their professional lives. Prostaglandin-associated periorbitopathy (PAP) syndrome, characterized by loss of orbital fat and resulting enophthalmos, has also been described; this latter issue is insidious and was only recognized as being associated with prostaglandin F2α analogues after 10+ years of their widespread use [Citation33–35]. PAP syndrome, which can occur intraoperatively or postoperatively, has cosmetic effects on patients, including skin and iris hyperpigmentation and abnormal eyelash growth [Citation36]. Moreover, because it is frequently accompanied by enophthalmos, PAP may increase the difficulties and risks associated with cataract surgery, such as deepening of the upper eyelid sulcus [Citation36]. When these medications are dosed unilaterally, it can aggravate the cosmetic impact of these side effects. A once-daily topical medication with comparable efficacy and superior safety relative to prostaglandin F2α analogues would be of value to physicians and patients.
4. Omidenepag: a novel prostanoid EP2 receptor agonist
Prostaglandins are lipid-derived autacoids that regulate multiple bodily systems, including the cardiovascular, endocrine, immune, and central nervous systems. There are at least nine distinct prostaglandin receptors, the activation of which promote a variety of effects, some of which overlap among receptors and others of which are distinct to one or more receptor [Citation37].
The prostaglandin F2α analogues lower IOP through agonist binding to the prostanoid FP receptor, a G-protein-coupled receptor present in the tissues of the uveoscleral outflow pathway (iris, ciliary body, and sclera) [Citation38]. This binding upregulates the expression of various matrix metalloproteinases (MMPs), which degrade the extracellular matrix (ECM) of the ciliary body, creating inter-muscular spaces through which aqueous humor flows to exit the eye [Citation39,Citation40].
Prostanoid EP2 receptor agonists promote similar effects, upregulating MMPs [Citation41] and remodeling the ECM to produce inter-muscular spaces in the ciliary body for aqueous egress [Citation40], reducing IOP by improving drainage of uveoscleral outflow and increasing the facility of outflow, primarily through the trabecular meshwork [Citation42,Citation43]. In contrast to FP agonists, EP2 agonists do not promote eyelash growth [Citation44] and do not inhibit adipogenesis [Citation45]. Another key difference is that EP2 agonists increase outflow facility by improving the drainage of both trabecular and uveoscleral outflow [Citation46]. Thus, a selective EP2 agonist has the potential to lower IOP with than current prostaglandin F2α analogues, with PAP being unlikely with the former.
Omidenepag () is a non-prostaglandin selective agonist of the prostanoid EP2 receptor [Citation47]. It is formulated as the prodrug omidenepag isopropyl and hydrolyzed to its active form by corneal esterases during ocular penetration. Omidenepag, which is in free acid form, binds EP2 receptors with high affinity (Ki = 3.6 nM) and has high agonist activity at the receptor (EC50 = 8.3 nM); its isopropyl prodrug has no effects at any prostaglandin receptors. In preclinical animal models, omidenepag lowered IOP in normotensive rabbits, dogs and monkeys, and in monkeys with laser-induced ocular hypertension [Citation46,Citation47]. This IOP reduction was achieved by improving the drainage of both uveoscleral and trabecular outflow [Citation46]. Notably, omidenepag has no measurable effect at the FP receptor [Citation47], differentiating it from the prostaglandin F2α analogues currently in clinical use for IOP reduction and eliminating the risk of FP-mediated adverse events such as lash growth and PAP [Citation44,Citation45,Citation48].
In a phase 1 safety evaluation, seven healthy Japanese and seven healthy Caucasian subjects were dosed once daily with omidenepag isopropyl 0.0025% in an ophthalmic solution in both eyes for 7 days [Citation49]. Mean IOP reduction of 4–5 mmHg was achieved by day 3 and was maintained through day 7. Omidenepag was rapidly absorbed into the systemic circulation, with maximum plasma concentration occurring within 10 minutes of instillation; it was cleared rapidly as well, with a serum half-life of approximately 30 minutes and no systemic accumulation after 7 days of dosing. Adverse events included mild conjunctival hyperemia in three subjects (21.4%) and mild photophobia in two (14.3%). There were no serious adverse events or discontinuations, and no systemic safety signals were observed.
4.1. Omidenepag isopropyl – global clinical development program and real-world data
Multiple phase 2 studies of omidenepag have been conducted. Three identically-designed phase 2 dose-finding studies were conducted in a total of 338 patients with POAG or OHT in the United States and Japan [Citation50]. Subjects were randomized to once daily therapy in both eyes with one of seven concentrations of omidenepag isopropyl, ranging from 0.0003% to 0.003%, with each study assessing different concentrations within this range, or, as controls, to latanoprost or placebo. The studies ranged in duration from 1–3 months. Pharmacodynamic analysis revealed a dose-response relationship between IOP reduction and conjunctival hyperemia; the 0.002% and 0.0025% concentrations produced IOP reductions comparable to those achieved with latanoprost (~ 5–8 mmHg), with 0.002% omidenepag isopropyl having higher response rates than 0.0025% omidenepag. These two concentrations of omidenepag isopropyl had conjunctival hyperemia rates of 14.3–-27.2%. In addition to conjunctival hyperemia, 10.0–14.3% of eyes treated with 0.002% and 0.0025% omidenepag isopropyl experienced photophobia and 3.3–14.3% experienced eye pain. Based on these findings, the 0.002% concentration was selected for further clinical development.
Pooled results have shown that conjunctival hyperemia is the most frequently observed ocular adverse event in Japanese patients treated with omidenepag isopropyl [Citation51]. The second most frequent ocular adverse event in these patients was macular edema, including cystoid macular edema, which was especially frequent in pseudophakic eyes [Citation51,Citation52]. The Japanese PMDA therefore listed pseudophakia as a contraindication for omidenepag isopropyl [Citation53]. In comparison, approximately 2–5% of high-risk pseudophakic and aphakic eyes treated with latanoprost developed clinically symptomatic and angiographically documented CME [Citation54,Citation55]. In contrast, a recent nested case-control study in 508 patients and 5080 controls found that postoperative use of the prostaglandin analogues bimatoprost and travoprost/travoprost-z was significantly associated with the incidence of pseudophakic CME, whereas the association between latanoprost treatment and pseudophakic CME was not statistically significant [Citation56].
An additional phase 2 study in the United States, (SPECTRUM 6) was performed to compare 0.002% omidenepag isopropyl dosed once and twice daily in 98 patients with POAG or OHT [Citation57]. In this double-masked trial, treatment once daily for 6 weeks lowered IOP significantly, from 24.6 ± 1.9 mmHg at baseline to 18.37 ± 0.41 mmHg at 6 weeks. Similarly, twice daily treatment significantly lowered IOP, from 25.4 ± 2.9 mmHg at baseline to 17.77 ± 0.43 mmHg at 6 weeks. Twice daily dosing did not significantly increase the IOP-lowering efficacy of once-daily dosing but did increase the rate of side effects. Omidenepag isopropyl 0.002% dosed once daily was confirmed as being the optimal dose frequency and was therefore selected for phase 3 clinical development.
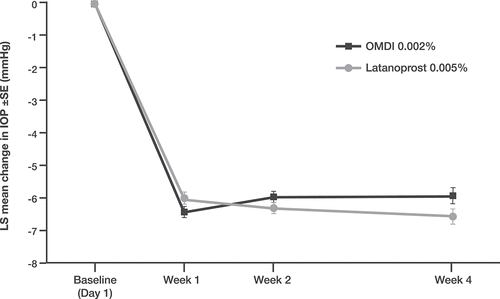
The phase 3 AYAME study randomized 190 Japanese subjects with POAG or OHT 1:1 to omidenepag isopropyl 0.002% or latanoprost 0.005%, each dosed once daily for 4 weeks [Citation58]. In this non-inferiority trial, both omidenepag isopropyl and latanoprost lowered IOP significantly from pretreatment baseline (~6 mmHg), with a small, statistically significant difference of 0.63 mmHg (p = 0.0477) favoring latanoprost (). Conjunctival hyperemia (24.5% versus 10.4%) and corneal thickening (11.7% versus 1.0%) were more common, whereas punctuate keratitis was less common (0% versus 7.3%), in eyes treated with omidenepag isopropyl. The higher rates of conjunctival hyperemia and corneal thickening observed in patients treated with omidenepag isopropyl were not trivial and may be of clinical importance.
The FUJI study was a phase 3 open-label evaluation of omidenepag isopropyl 0.002% once daily in eyes with previous poor response to latanoprost [Citation59]. Japanese patients with POAG or OHT underwent an 8-week run-in with latanoprost, and those with IOP reductions ≤15% were crossed over to omidenepag isopropyl treatment for 4 weeks. Mean diurnal IOP on latanoprost for 8 weeks was 23.1 mmHg, and it further decreased by 3 mmHg to 20.2 mmHg after 4 weeks of omidenepag isopropyl treatment (p < 0.0001).
The phase 3 RENGE study was an open-label, single-arm evaluation of long-term IOP reduction with omidenepag in Japanese patients with POAG or OHT and high (22 mmHg to ≤34 mmHg) or low (16 mmHg to ≤22 mmHg) baseline IOP [Citation52]. After 6 months of daily treatment with omidenepag isopropyl 0.002%, mean IOP was reduced 2.4 mmHg in the low baseline IOP group (n = 48) and 4.9 mmHg in the high baseline IOP group (n = 37). Conjunctival hyperemia was seen in 18.8% of the combined groups and macular edema in 5.9%, all of the latter in pseudophakic eyes. Other adverse events included cystoid macular edema (5.9%), central corneal thickening (2.4%), iritis (2.4%), and eye pain (2.4%). There were no reports of cosmetic AEs, such as increased pigmentation of the iris, eyelid, and eyelashes, in either group.
In these combined trials, the EP2 receptor agonist omidenepag isopropyl was associated with conjunctival hyperemia, a common side effect of FP receptor agonists. Hyperemia, however, was both mild and reversible. Moreover, none of the eyes treated with omidenepag isopropyl experienced abnormal eyelash growth or PAP. A recent Japanese case series described 11 eyes with prostaglandin F2α analogue-associated PAP that were prospectively switched to omidenepag isopropyl 0.002% once daily and underwent external ocular photography at baseline and 3 and 6 months later [Citation48]. At 6 months, deepening of the upper eyelid sulcus improved in three patients, flattening of the lower eyelid bags improved in two, ciliary hypertrichosis improved in two, and periorbital skin hyperpigmentation improved in eight.
An extension of this study, in 12 patients with PAP syndrome who were switched to omidenepag isopropyl 0.002%, found that, after 12 months, deepening of the upper eyelid sulcus improved in six patients, flattening of the lower eyelid bags improved in three, upper eyelid ptosis improved in two, and eyelid hyperpigmentation improved in three [Citation60]. All six patients with improved deepening of the upper eyelid sulcus had been previously receiving bimatoprost or travoprost.
A retrospective trial evaluated the efficacy and safety of omidenepag isopropyl 0.002% in patients with normal-tension glaucoma [Citation61]. The 54 patients (54 eyes) included 22 men and 32 women of mean age 55.0 ± 14.1 years who were treated with omidenepag isopropyl 0.002% once daily for 4 months. IOP was significantly lower after 1–2 months (13.5 ± 2.3 mmHg) and after 3–4 months (13.6 ± 2.4 mmHg) than at baseline (15.7 ± 2.6 mmHg)(p < 0.0001). Eleven patients were non-responders, defined as having a < 10% reduction in baseline IOP. Four patients experienced adverse effects, including three with conjunctival hyperemia and one with eye pain.
Another retrospective study analyzed treatment discontinuation rates in 233 patients treated with omidenepag isopropyl 0.002%, including those switched from other drugs, and 95 treated with latanoprost 0.005% [Citation62]. Rates of treatment failure, defined as drug discontinuation due to any adverse event or change in therapy, were 20% in patients treated with omidenepag isopropyl, and 15% in patients treated with latanoprost. The median persistence times in patients treated with omidenepag isopropyl and latanoprost were 165 and 188 days, respectively, and the median times to discontinuation were 45 and 102 days, respectively.
summarizes the results of clinical trials with omidenepag isopropyl.
Table 2. Clinical studies of omidenepag isopropyl (OMDI) in patients with glaucoma or ocular hypertension
5. Summary and conclusion
Omidenepag isopropyl 0.002% is an EP2 agonist that lowers IOP by facilitating uveoscleral and trabecular outflow [Citation46]. It is comparable in IOP-lowering efficacy to latanoprost, with once-daily dosing. Like prostaglandin F2α analogues, omidenepag isopropyl 0.002% treatment is associated with conjunctival hyperemia; but unlike prostaglandin F2α analogues, it does not seem to cause lash changes or periorbital fat loss (at least in the short term). Omidenepag isopropyl 0.002% solution once daily has been approved in Japan for the reduction of IOP in eyes with glaucoma and or OHT and in Korea, Taiwan, Thailand and Singapore for reduction of IOP in eyes open-angle glaucoma and OHT. In the United States, omidepenag isopropyl is in late-stage clinical development, with phase 3 studies currently ongoing. It has the potential to provide safe and effective IOP reduction in eyes with POAG and OHT without the cosmetic side effects common to prostaglandin F2α analogues, but with somewhat higher rates of other side effects, like conjunctival hyperemia, than the prostaglandin F2α analogues.
6. Expert opinion
The introduction of prostaglandin F2α analogues for IOP reduction in the 1990s revolutionized medical therapy for glaucoma. Prostaglandin F2α analogues offered unparalleled efficacy and safety, and quickly supplanted beta-blockers as preferred first-line medical therapy for glaucoma. Beta-blockers were themselves a paradigm-changer when introduced in the 1970s. While they were not free of limitations, which included systemic side effects and tachyphylaxis over time, beta-blockers were far superior drugs when compared with the miotics and epinephrine compounds available at the time.
Like timolol in 1978, latanoprost in 1996 was far superior to what was currently available, yet not without problems of its own. Now, after 25 years of experience with prostaglandin F2α analogues as preferred first-line therapy, we have become more familiar with the consequences of chronic exposure. Most of the side effects of prostaglandin analogues are cosmetic in nature, including conjunctival hyperemia, eyelash growth, iris and periorbital skin pigmentation, and fine vellus hair growth on lower lids. While not typically a reason to avoid or discontinue therapy in most patients, the occurrence of these side effects can limit the use of prostaglandin F2α analogues for unilateral therapy and in younger patients who are more concerned about cosmetic appearances. While still associated with conjunctival hyperemia, the EP2 receptor agonist omidenepag isopropyl appears to be free of most of these more bothersome cosmetic side effects and may be more acceptable for some image-conscious patients, as well as for those requiring unilateral therapy. Conjunctival hyperemia may reflect a shared physiologic effect of multiple prostaglandin receptors and is generally a less bothersome side effect for many patients, as well as being an infrequent reason for discontinuation of therapy.
PAP is a more troubling consequence of long-term prostaglandin F2α analogue use. Its onset is insidious and is frequently overlooked. PAP can be symptomatic in some patients, even producing an audible clicking sound upon blinking [Citation63,Citation64]. Asymptomatic cases can become problematic at the time of ocular surgery, in that enophthalmos associated with PAP can increase the complexity of intraocular surgery, as well as having implications for patients undergoing oculoplastic surgery. In addition, one of the signs of PAP, deepening of upper eye lid sulcus, has been reported to be a risk factor for surgical failure in patients undergoing trabeculectomy [Citation36]. Studies to date suggest that omidenepag isopropyl may not cause PAP, as expected of a drug that does not inhibit adipogenesis. Long-term and well-controlled studies will be necessary to more fully characterize any relationship between chronic omidenepag isopropyl use and the development of PAP. If omidenepag isopropyl and/or other prostanoid EP2 receptor agonists are found to not cause PAP, these agents will have advantages over prostaglandin F2α analogues in many patients.
There is an unmet need for medical therapy that is more effective and/or safer and/or more long-lasting than prostaglandin F2α analogues. Omidenepag isopropyl appears to provide IOP reductions comparable to prostaglandin F2α analogues with a somewhat different safety profile and to lower IOP in non- and low responders to latanoprost. Future research will be necessary to characterize the long-term efficacy and safety profile of this drug and also to evaluate its additivity to other classes of IOP-lowering therapy. Clinical trials have demonstrated that most patients will require more than one medication for long-term glaucoma management. Most classes of drugs show additive effects to prostaglandin F2α analogues, although the relatively weak additivity of beta-blockers to prostaglandin F2α analogues has prevented the commercialization of fixed combinations in the United States (but not in other global markets). It will be particularly interesting to see if the additivity of beta-blockers to omidenepag isopropyl exceeds that of the prostaglandin F2α analogues and if prostaglandin-beta-blocker combinations become commercially available in the US.
Finally, the landscape for therapy of glaucoma is rapidly changing. In recognition of the numerous limitations of topical medical therapy – including poor adherence, chronic inflammation contributing to ocular surface disease, and the general negative impact that daily self-administration of eye-drop therapy has on quality of life – there is a rapid expansion of clinical investigations into sustained-release drug delivery platforms to overcome these issues. One, the bimatoprost anterior segment implant, has already been approved for use in the US. Others, such as punctal plugs, fornix-based systems, intraocular implants, and other technologies, are in late-stage clinical development. Selective laser trabeculoplasty and minimally invasive glaucoma surgery offer additional alternatives for primary glaucoma care. Paradigm changes seem inevitable over the next 5–10 years, although with so many new and emerging therapeutic options, including omidenepag isopropyl, it is unclear what form the new paradigm may take.
Declaration of interest
M Aihara has received financial support from Santen Pharmaceutical Co. Ltd., Senju Pharmaceutical Co. Ltd., Alcon Japan, Novartis, Pfizer, Kowa Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Wakamoto Pharmaceutical Co. Ltd., Johnson & Johnson, Glaukos, TOMEY, Ono Pharmaceutical Co. Ltd., CREWT medical systems, Sato Pharmaceutical Co. Ltd.; been a consultant for Santen Pharmaceutical Co. Ltd., Senju Pharmaceutical Co. Ltd., Alcon Japan, Pfizer Pharmaceutical Co. Ltd., Kowa Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Wakamoto Pharmaceutical Co. Ltd., HOYA, Glaukos, IRIDEX, CREWT medical systems, Astellas Pharmaceutical Co. Ltd; received lecture fees, travel fees, drugs from Santen Pharmaceutical Co. Ltd., Senju Pharmaceutical Co. Ltd., Alcon Japan, Novartis, Pfizer, Kowa Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Johnson & Johnson, HOYA, Glaukos, TOMEY, IRIDEX, CREWT medical systems, CANON, Carl Zeiss Meditec, Sato Pharmaceutical Co. Ltd. T Aung has received consultancy fees, research support and lecture honoraria from Santen Inc., Allergan, Novartis, Sun Pharma, Alcon, and Belkin Laser. J Bacharach, has been an investigator and consultant for Santen Pharmaceuticals. L Cantor has been a consultant for Carl Zeiss, Inc and Santen, Inc, and is a stockholder for Mati, Inc. T Nakazawa has been a consultant and received honoraria from Santen. KH Park has been a consultant for Santen, Allergan, Novartis and Sensimed. DW Lu has been a consultant for Santen and Allergan.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Weinreb RN, Friedman DS, Fechtner RD, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138(3):458–467.
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911.
- Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713.
- Heijl A, Leske MC, Bengtsson B, et al. Early manifest glaucoma trial group. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279.
- Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295–1304.
- Friedman DS, Wolfs RCW, O’Colmain BJ, et al. Eye diseases prevalence research group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538.
- Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090.
- Pfizer, Inc. Xalatan® (Latanoprost Ophthalmic Solution) 0.005%. Prescribing Information. New York City, NY: Pfizer, Inc.; 2014.
- Allergan, Inc. Lumigan® (Bimatoprost Ophthalmic Solution) 0.01%. Prescribing Information. Irvine, CA: Allergan, Inc.; 2017.
- Alcon Laboratories, Inc. Travatan Z® (Travoprost Ophthalmic Solution) 0.004%. Prescribing Information. Fort Worth, TX: Alcon Laboratories, Inc.; 2017.
- Akorn, Inc. Zioptan® (Tafluprost Ophthalmic Solution) 0.0015%. Prescribing Information. Lake Forest, IL: Akorn, Inc; 2014.
- Bausch & Lomb, Inc. Timoptic 0.25% and 5% (Timolol Maleate Ophthalmic Solution). Prescribing Information. Bridgewater, NJ: Bausch & Lomb, Inc.; 2016.
- Sonntag JR, Brindley GO, Shields MB. Effect of timolol therapy on outflow facility. Invest Ophthalmol Vis Sci. 1978;17(3):293–296.
- Merck & Co., Inc. Trusopt® (Dorzolamide Hydrochloride Ophthalmic Solution) 2%. Prescribing Information. Whitehouse Station, NJ: Merck & Co., Inc.; 2014.
- Alcon Laboratories, Inc. Azopt® (Brinzolamide Ophthalmic Suspension) 1%. Prescribing Information. Fort Worth, TX: Alcon Laboratories, Inc.; 2015.
- Allergan, Inc. Alphagan® P (Brimonidine Tartrate Ophthalmic Solution) 0.1% and 0.15%. Prescribing Information. Irvine, CA: Allergan, Inc; 2013.
- Aerie Pharmaceuticals, Inc. Rhopressa® (Netarsudil Ophthalmic Solution) 0.02%. Prescribing Information. Irvine, CA: Aerie Pharmaceuticals, Inc.; 2017.
- Tanihara H, Inoue T, Yamamoto T, et al. K-115 Clinical Study Group. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156(4):731–736.
- Somerset Therapeutics. Pilocarpine prescribing information. Somerset, NJ 2019.
- Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116(11 Suppl):S30–6.
- Newman-Casey PA, Dayno M, Robin AL. Systematic review of educational interventions to improve glaucoma medication adherence: an update in 2015. Expert Rev Ophthalmol. 2016;11(1):5–20.
- Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316.
- Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Curr Opin Ophthalmol. 2006;17(2):190–195.
- Newman-Casey PA, Niziol LM, Gillespie BW, et al. The association between medication adherence and visual field progression in the collaborative initial glaucoma treatment study. Ophthalmology. 2020;127(4):477–483.
- Baudouin C, Labbe A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334.
- Labbe A, Terry O, Brasnu E, et al. Tear film osmolarity in patients treated for glaucoma or ocular hypertension. Cornea. 2012;31(9):994–999.
- Ghosh S, O’Hare F, Lamoureux E, et al. Prevalence of signs and symptoms of ocular surface disease in individuals treated and not treated with glaucoma medication. Clin Exp Ophthalmol. 2012;40(7):675–681.
- Valente C, Iester M, Corsi E, et al. Symptoms and signs of tear film dysfunction in glaucomatous patients. J Ocul Pharmacol Ther. 2011;27(3):281–285.
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355.
- Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621.
- Rossi GC, Pasinetti GM, Scudeller L, et al. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur J Ophthalmol. 2013;23(3):296–302.
- Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1–9.
- Peplinski LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81(8):574–577.
- Tappeiner C, Perren B, Iliev ME, et al. [Orbital fat atrophy in glaucoma patients treated with topical bimatoprost–can bimatoprost cause enophthalmos?]. Klin Monbl Augenheilkd. 2008;225(4):443–445.
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24(4):302–307.
- Miki T, Naito T, Fujiwara M, et al. Effects of presurgical administration of prostaglandin analogs on the outcome of trabeculectomy. PLoS One. 2017;12(7):e0181550.
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103(2):147–166.
- Crowston JG, Lindsey JD, Aihara M, et al. Effect of latanoprost on intraocular pressure in mice lacking the prostaglandin FP receptor. Invest Ophthalmol Vis Sci. 2004;45(10):3555–3559.
- Oh DJ, Martin JL, Williams AJ, et al. Analysis of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human ciliary body after latanoprost. Invest Ophthalmol Vis Sci. 2006;47(3):953–963.
- Richter M, Krauss AHP, Woodward DF, et al. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest Ophthalmol Vis Sci. 2003;44(10):4419–4426.
- Yen JH, Kocieda VP, Jing H, et al. E2 induces matrix metalloproteinase 9 expression in dendritic cells through two independent signaling pathways leading to activator protein 1 (AP-1) activation. J Biol Chem. 2011;286(45):38913–38923.
- Saeki T, Ota T, Aihara M, et al. Effects of prostanoid EP agonists on mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2009;50(5):2201–2208.
- Nilsson SFE, Drecoll E, Lütjen-Drecoll E, et al., The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 2006;47(9): 4042–4049.
- Esaki Y, Katsuta O, Kamio H, et al. The antiglaucoma agent and EP2 receptor agonist omidenepag does not affect eyelash growth in mice. J Ocul Pharmacol Ther. 2020;36(7):529–533.
- Yamamoto Y, Taniguchi T, Inazumi T, et al. Effects of the selective EP2 receptor agonist omidenepag on adipocyte differentiation in 3T3-L1 cells. J Ocul Pharmacol Ther. 2020;36(3):162–169.
- Fuwa M, Toris CB, Fan S, et al., Effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, on aqueous humor dynamics in laser-induced ocular hypertensive monkeys. J Ocul Pharmacol Ther. 2018;34(7): 531–537.
- Kirihara T, Taniguchi T, Yamamura K, et al., Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Invest Ophthalmol Vis Sci. 2018;59(1): 145–153.
- Nakakura S, Terao E, Fujisawa Y, et al. Changes in prostaglandin-associated periorbital syndrome after switch from cnventional prostaglandin F2α treatment to omidenepag isopropyl in 11 consecutive patients. J Glaucoma. 2020;29(4):326–328.
- Aihara M, Lu F, Kawata H, et al. Pharmacokinetics, safety, and intraocular pressure-lowering profile of omidenepag isopropyl, a selective, nonprostaglandin, prostanoid EP2 receptor agonist, in healthy Japanese and Caucasian volunteers (phase I study). J Ocul Pharmacol Ther. 2019;35(10):542–550.
- Aihara M, Lu F, Kawata H, et al., Phase 2, randomized, dose-finding studies of omidenepag isopropyl, a selective EP2 agonist, in patients with primary open-angle glaucoma or ocular hypertension. J Glaucoma. 2019;28(5): 375–385.
- Duggan S. Omidenepag isopropyl ophthalmic solution 0.002%: first global approval. Drugs. 2018;78(18):1925–1929.
- Aihara M, Lu F, Kawata A, et al. Six-month efficacy and safety outcomes of a novel selective EP2 agonist omidenepag isopropyl: the Renge study (phase 3). Invest Ophthalmol Vis Sci. 2018;59(9):1229.
- Santen Pharmaceutical Co., Inc. EYBELIS ophthalmic solution 0.002%. Prescribing information [Japanese]. Osaka, Japan: Santen Pharmaceutical Co.; 2018. Available from: http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/300237_13197C0Q1028_1_02
- Lima MC, Jr PA, Salim S, et al. Visually significant cystoid macular edema in pseudophakic and aphakic patients with glaucoma receiving latanoprost. J Glaucoma. 2000;9(4):317–321.
- Wand M, Gaudio AR, Shields MB. Latanoprost and cystoid macular edema in high-risk aphakic or pseudophakic eyes. J Cataract Refract Surg. 2001;27(9):1397–1401.
- Wendel C, Zakrzewski H, Carleton B, et al. Association of postoperative topical prostaglandin analog or beta-blocker use and incidence of pseudophakic cystoid macular edema. J Glaucoma. 2018;27(5):402–406.
- Olander K, Sato MA, Abrams MA, et al. A randomized phase 2 trial assessing the safety and efficacy of omidenepag isopropyl 0.002% once and twice daily in subjects with primary open-angle glaucoma or ocular hypertension (Spectrum 6). Invest Ophthalmol Vis Sci. 2020;61(7):4258.
- Aihara M, Lu F, Kawata H, et al. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the Phase 3 AYAME study. Am J Ophthalmol. 2020;220:53–63.
- Aihara M, Ropo A, Lu F, et al., Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol. 2020;64(4): 398–406.
- Oogi S, Nakakura S, Terao E, et al. One-year follow-up study of changes in prostaglandin-associated periorbital syndrome after switch from conventional prostaglandin F2α to omidenepag isopropyl. Cureus. 2020;12(8):e10064.
- Inoue K, Inoue J, Kunimatsu-Sanuki S, et al. Short-term efficacy and safety of omidenepag isopropyl in patients with normal-tension glaucoma. Clin Ophthalmol. 2020;14:2943–2949.
- Nakakura S, Kanamori A, Fukuma Y, et al. Evaluation of early medication persistence with omidenepag isopropyl, a topical selective prostaglandin EP2 agonist, in patients with glaucoma: a retrospective two-institute study. BMJ Open. 2021;11(1):e040301.
- Skorin L, Dailey KH. Clicking eyelids: a new finding of prostaglandin-associated periorbitopathy. Optom Vis Sci. 2016;93(7):779–781.
- Abedi F, Chappell A, Craig JE. Audible clicking on blinking: an adverse effect of topical prostaglandin analogue medication. Clin Exp Ophthalmol. 2017;56(3):304–306.