ABSTRACT
Introduction
Infantile nystagmus syndrome (INS) is the most common nystagmus type in children, and can be idiopathic or associated with ocular, neurologic, and systemic disease. Current treatment options for INS aim to optimize vision by reducing the intensity of nystagmus and correcting anomalous head postures and associated strabismus. A PubMed search of all articles published from January 1980 to July 2021 on the management of INS was performed.
Areas covered
This review will address the principles of nystagmus management, including optical, pharmacological and surgical options. Pharmacological treatments, such as gabapentin and memantine, are now being implemented in clinical practice. In patients with obvious torticollis, eye muscle surgery aims to shift the nystagmus null zone into the primary position.
Expert opinion
Successful management of INS depends on accurate diagnosis and identification of patient needs and concerns. Recent advances in pediatric retinal imaging and the identification of new genes have refined our understanding of INS subtypes, facilitate early diagnosis, and allow for individualized management. Due to the lack of randomized controlled trials, there is still no consensus on a clinical pathway for INS; however, there are several therapeutic options, which can result in improved vision and amelioration of anomalous head postures.
1. Introduction
Infantile nystagmus syndrome (INS) is an involuntary rhythmic oscillation of the eyes, which usually develops within the first 3–6 months of life [Citation1]. The prevalence of INS in the general population has been estimated to be 0.14% [Citation2], and visual acuity (VA) is typically reduced due to the excessive motion of images on the retina [Citation3]. INS can be idiopathic (infantile idiopathic nystagmus, IIN), or associated with other ocular diseases such as albinism, retinal dystrophies, or visual deprivation early in life. Around 50% of cases of INS have a sensory visual deficit [Citation4]. Other types of nystagmus in infancy include manifest latent nystagmus (MLN) or fusion maldevelopment nystagmus syndrome (FMNS) and spasmus nutans.
Occasionally, infantile nystagmus can be a manifestation of neurological disease, and in these cases, it is usually associated with other red flags pointing at the underlying etiology [Citation5]. Immediate neuroimaging investigation for chiasmal and optic nerve tumors is required in cases of asymmetrical (dissociated) or unilateral nystagmus, vertical or torsional nystagmus (in the absence of retinal disease), acquired nystagmus, and in the presence of accompanying neurological and ophthalmological findings, such as RAPD, optic atrophy, or papilledema [Citation6,Citation7]. For example, monocular vertical nystagmus can be associated with chiasmal or hypothalamic gliomas [Citation8,Citation9]. In addition, patients with see-saw nystagmus should be investigated for suprasellar lesions that compress the mesodiencephalic region [Citation10]. See-saw nystagmus is a type of pendular nystagmus in which there is simultaneous intorsion and elevation of one eye and extorsion and depression of the fellow eye [Citation10]. Patients with achiasma may also present with see-saw nystagmus [Citation11]. Nystagmus should be also differentiated form saccadic intrusions and oscillations, which are abnormal fast eye movements, disrupt visual fixation, and occur in association with cerebellar, brainstem, or cerebral pathology [Citation12]. They are clearly non-rhythmic and are initiated by saccades rather than by the slow (smooth) eye movements, which initiate nystagmus. These are uncommon in children and include square-wave jerks, opsoclonus, ocular flutter and ocular bobbing. In these cases neuroimaging is indicated due to the possible association with systemic abnormalities, i.e. opsoclonus and neuroblastoma [Citation12].
In some nystagmus forms, such as in IIN and albinism, patients have often a ‘null point,’ where the nystagmus is less intense. If the null zone does not coincide with the primary gaze position, patients adopt anomalous head postures (AHP), in order to move the null zone in the primary gaze and improve acuity and binocularity [Citation13]. It has been estimated that up to 60% of patients with infantile nystagmus have a null zone on eccentric gaze and 70% adopt an AHP [Citation14,Citation15]. The recent advances in pediatric retinal imaging, by means of handheld optical coherence tomography (HH-OCT), and the identification of new genes, have greatly enhanced our understanding of INS and facilitated early diagnosis and management [Citation16,Citation17].
Nystagmus is a lifelong condition with a negative impact on quality of life, as patients are confronted with poor self-esteem, increased dependency, and social relationship issues [Citation18–20]. Hence, establishing the correct diagnosis and introducing the appropriate treatment is key in ameliorating the visual function and social life of affected individuals. Treatment of nystagmus aims to optimize vision by increasing foveation duration, to reduce the intensity of nystagmus and correct AHP and associated strabismus. Current interventions include optical, pharmacological, and surgical options. Recent discoveries in the field of genetics have also paved the way for novel gene therapies by introducing adeno-associated virus (AAV)-based gene interventions for albinism in animal models [Citation21].
A PubMed search of all articles published from January 1980 to July 2021 on the management of INS was performed. Searches included a combination of the following terms: ‘nystagmus therapy,’ ‘nystagmus treatment,’ ‘nystagmus management,’ ‘nystagmus surgery,’ ‘anomalous head posture,’ ‘head tilt,’ ‘head turn,’ ‘congenital nystagmus,’ ‘infantile nystagmus,’ ‘manifest latent nystagmus.’ The resulting references were then reviewed for pertinent articles. Selected key papers of historical importance published before 1980 were also included.
2. Optical treatment
2.1. Refractive correction
The vast majority of patients with INS have high refractive errors as a result of impaired emmetropization [Citation22]. Significant refractive errors have been found in 57% of patients with nystagmus [Citation23]. Assessment of refraction under cycloplegia must be meticulous, because correcting even small amounts of refractive errors in INS can cause subjective amelioration in VA [Citation4,Citation24,Citation25].
High myopia is observed in congenital stationary night blindness (CSNB). Astigmatism and hyperopia are also more common in children with INS, especially in the presence of albinism [Citation22,Citation26,Citation27]. Healey et al. reported that the trend of refractive errors in INS is predominantly hyperopic (67% of the sample were albinos) [Citation28], albeit Sampath et al. claim the opposite, that the trend is more myopic (especially in INS) [Citation29]. In this study, the authors also demonstrated that individuals with INS have high astigmatism, which is typically with-the-rule (WTR) [Citation29,Citation30]. Similar results have been obtained by Wang et al., who have shown that WTR astigmatism was common in children with INS. They also demonstrated that the magnitude of astigmatism was increasing with age during the first 8 years of life [Citation31]. Moreover, children with INS most commonly remained hyperopic and the mean spherical equivalent was found to be larger than in the reference normal group [Citation31]. WTR astigmatism is also common among children with albinism and it often increases within the first 10 years of life [Citation32]. These findings highlight the importance of regular cycloplegic refractions in children with INS, especially in the first decade of life. Most authors generally agree on prescribing the full cycloplegic and anisometropic correction in children with INS and the highest correction tolerated by adults [Citation4,Citation30]. It has been suggested that by giving the full cycloplegic refraction, both visual acuity and fusional convergence can be maximized to synergistically improve infantile nystagmus [Citation24].
Furthermore, the coexistence of strabismus and amblyopia in these patients is common, so appropriate management with full cycloplegic correction and/or occlusion is mandatory [Citation4,Citation14,Citation23,Citation33]. In particular, there is a higher risk of developing strabismus in children with secondary nystagmus due to albinism rather than those with IIN [Citation33,Citation34]. It is estimated that approximately 50% of patients with albinism have strabismus [Citation35].
2.2. Contact lenses
The fact that many patients adopt AHPs and have an eccentric ‘null zone’ might reduce the efficacy of spectacles due to prismatic effects and spherical aberrations in the periphery of the lens, especially in high refractive errors () [Citation30,Citation36]. The above optical phenomena may degrade image resolution, enhance aniseikonia and prevent fusion and binocularity [Citation36]. On the other hand, contact lenses (CLs) increase the proportion of time a patient with nystagmus is looking along the visual axis of the correcting lens, as CLs do not have the problems of decentration and obstruction of the visual axis by spectacle frames [Citation1,Citation37,Citation38]. The improvement in visual acuity with the use of CLs may be attributed to reduced optical aberrations and prismatic effects, increased peripheral visual field, and a possible proprioceptive dampening effect mediated via the trigeminal nerve, which can be very helpful for patients with high refractive errors and a significant AHP [Citation39–41].
Figure 1. This girl with idiopathic infantile nystagmus (IIN) has a left head turn and slight left head tilt (a). The efficacy of her spectacles is possibly reduced because the visual axis is partially obstructed by the spectacle frame (b)

However, the relative benefits of CLs in comparison to spectacles in INS remain unclear. The use of CLs in infantile nystagmus was first reported in 1962 by Hale [Citation42]. Both rigid gas-permeable (RGP) and soft contact lenses (SCL) have been used in patients with INS, and to date, there are only two published randomized trials investigating CL use in INS [Citation43,Citation44]. Jayaramachandran et al. performed a randomized, controlled cross-over trial comparing the use of spectacles, SCL, and RGP. The trial included 24 patients (12 idiopathic, 12 with albinism). They concluded that there was no significant difference in nystagmus indices with either CL type compared with baseline spectacle wear [Citation43]. Moreover, patients wearing SCL had worst best-corrected VA (BCVA), reading acuity, and critical print size compared with patients wearing RGP and spectacles (P < 0.05), although mean differences were less than 1 logarithm of the minimum angle of resolution (logMAR) line () [Citation43]. It was hypothesized that the deterioration of vision with SCL wear in this study was probably due to rotation of the toric SCL out of position by approximately 30° with no realignment of the lens occurring via blinks or other means [Citation43]. It was hypothesized that the misalignment of the SCL is caused by the torsional forces of the quick phase of horizontal nystagmus in combination with the asymmetric distribution of mass vertically in the contact lens [Citation43]. Theodorou et al. conducted a pilot randomized controlled trial comparing fully corrective SCL with plano SCL (with corrective spectacles if required) in a group of 38 adults with idiopathic INS [Citation44]. Although there was a small effect size, they noticed an amelioration in BCVA in both groups. Moreover, there was an improvement in some of the nystagmus waveform parameters [Citation44]. Further small case series and case reports have also suggested an improvement in VA or/and nystagmus intensity by using CLs [Citation36,Citation38–41,Citation45–49].
Figure 2. Mean best-corrected visual acuity (BCVA) (error bars = standard error of mean) under soft contact lenses (SCL), rigid gas-permeable lenses (RGPL) and baseline spectacle wear (average of 2 measurements) for albinism (open symbols) and idiopathic (filled symbols) participants for near (0.4 m, circles and solid lines) and distance (4 m, squares and dashed lines) viewing under binocular viewing conditions. SCLs lead to a small but significant deterioration in VA compared with both RGPL and spectacle wear, although, overall, effect sizes were not clinically relevant. logMAR = logarithm of the minimum angle of resolution. Reproduced with permission from Jayaramachandran et al. [Citation43]
![Figure 2. Mean best-corrected visual acuity (BCVA) (error bars = standard error of mean) under soft contact lenses (SCL), rigid gas-permeable lenses (RGPL) and baseline spectacle wear (average of 2 measurements) for albinism (open symbols) and idiopathic (filled symbols) participants for near (0.4 m, circles and solid lines) and distance (4 m, squares and dashed lines) viewing under binocular viewing conditions. SCLs lead to a small but significant deterioration in VA compared with both RGPL and spectacle wear, although, overall, effect sizes were not clinically relevant. logMAR = logarithm of the minimum angle of resolution. Reproduced with permission from Jayaramachandran et al. [Citation43]](/cms/asset/844cd92f-598b-4159-960c-769bb0528220/ierl_a_1970533_f0002_b.gif)
There are several theories about the mechanism that induces nystagmus dampening by using CLs. Dell’Osso et al. first hypothesized that CLs reduce nystagmus intensity via afferent stimulation of the proprioceptive pathways mediated by the ophthalmic division of the trigeminal nerve [Citation37]. It is suggested that CLs employ afferent feedback to damp INS slow phases over a wider range of gaze angles, and broaden the eXpanded Nystagmus Aquity Function (NAFX) [Citation37]. The NAFX objectively estimates the nystagmus foveation quality and also predicts the BCVA for subjects without afferent visual system defects [Citation50]. Taibbi et al. confirmed this hypothesis by demonstrating 133% broadening of the NAFX curve [Citation41]. This theory was also supported by the fact that instillation of topical anesthetic reverses the beneficial effects of CLs wear. The same effect was present when plano CLs were tested [Citation46]. Another potential mechanism suggested by Abadi is that CLs produce additional vergence and accommodative effort, which might reduce nystagmus amplitude and frequency [Citation38]. Hence, CL wear in patients with INS possibly permits a wider range of high foveation quality field and improves foveation at each gaze angle in addition to any improvement in primary-position visual acuity they may provide.
The conflicting results between studies for CL wear in INS are possibly related to differences in study design, such as trial duration, study population, age range, and type of CLs (RGP vs. SCL). Additionally, many of these studies are case reports or small case series. CLs should be considered in patients with INS, but larger randomized controlled trials are necessary, to investigate their safety and efficacy in different nystagmus forms and across a wide age range. An interesting field is the use of CLs in young children with INS, who have greater plasticity in the visual cortex and would possibly benefit more from this treatment [Citation51]. However, the potential benefit of CL wear should be also viewed in the light of some risks, such as infections. In general, literature findings regarding the use of CLs in INS are contradictory, and currently, there is paucity of well-designed clinical trials.
2.3. Prisms
The dampening of nystagmus in convergence broadens the range of gaze angles with higher foveation quality, which means that the null region is broadened [Citation52], and also reduces nystagmus indices in patients with INS, especially IIN [Citation53,Citation54]. Hence, the primary use of a convergence null is to either optically or surgically induce convergence innervation when viewing at distance.
Prisms can be used to damp nystagmus, where the amplitude of nystagmus is smaller in convergence, and the patient has binocular vision and strong fusion [Citation23]. Prisms (5Δ–7Δ base out) create an artificial divergence that the patient is required to overcome by converging the eyes even when looking at distance [Citation55,Citation56]. Gauthier et al. suggest the use of base-out prisms up to 9Δ, where is afforded [Citation57]. Prisms are not indicated in patients who lack binocularity, because they damp nystagmus by means of fusional convergence [Citation23].
In cases of an eccentric null zone with AHP, yoked prisms (apex pointed bilaterally in the direction of the patient’s gaze) have been used to move fixation to that null zone and alleviate the AHP when the angle is small. However, if the null zone is too eccentric, this method is difficult to apply, because it requires high-power prisms which are heavy, cosmetically unacceptable and decrease VA [Citation1]. Thus, Anderson recommends a surgical procedure initially, and then correction of postoperative residual small AHPs with prisms [Citation58]. It is worth to mention that Dell’Osso reported that some patients use both types of nulls. In these cases, he suggested the use of two unequal base-out prisms in order to promote both gaze-angle null and convergence null, accompanied with −1.00 sph. in both spectacle lenses [Citation59].
The prisms in INS might be used in the long term to fix AHP and improve VA or preoperatively for short term [Citation57]. Dell’Osso and Gauthier et al. reported that prisms induce significant improvement of VA [Citation57,Citation59]. However, other authors showed that prisms do not decrease nystagmus and their only usage is for cosmetic reasons [Citation60,Citation61].
Press-on prisms have been also described by Tang et al. in order to correct residual AHP in 28 children with INS who underwent nystagmus surgery [Citation62]. The use of press-on prisms did not have a significant effect on VA, but they reduced visual distortion and dizziness of patients while wearing spectacles. Additionally, 67% of children almost had disappearance of residual compensatory head turn [Citation62].
To conclude, it seems that prisms are an effective method to reduce small AHPs in patients with INS. The use of base-out prisms of approximately 7Δ with −1.00 sph. to induce convergence is a simple and effective treatment and according to studies it broadens the range of gaze-angles. On the other hand, the use of prisms to move the images to the gaze-angle null is seldom useful due to the large amount of prisms required. In these cases, it might be better to perform surgery to correct the AHP and then exploit the use of prisms to fix any residual AHP.
3. Pharmacological treatment
3.1. Topical
A preliminary report on a subject with INS suggested that topical brinzolamide resulted in improved-foveation waveforms over a broadened range of gaze angles and that its therapeutic effects were equivalent to systemic carbonic anhydrase inhibitors (CAIs) [Citation63]. The beneficial effect of topical brinzolamide on measures of nystagmus and visual acuity was later confirmed by a randomized controlled trial in five adult patients with INS, suggesting that topical CAIs may emerge as a viable addition to optical, surgical, behavioral, and systemic drug therapies for INS [Citation64]. The mechanism of action of topical brinzolamide is possibly related to the contribution of carbonic anhydrase in the response of sensory stretch receptors of the trigeminal nerve and its nerve endings, as a functioning carbonic anhydrase system may be involved in facilitating enthesial neuronal feedback to central ocular motor areas [Citation65]. A more recent observational study of 23 adult patients with IIN and albinism-associated nystagmus who received topical brinzolamide has also shown reduction of nystagmus in 5 patients, increase in VA in 9 patients and reduced AHP in 4 patients [Citation66]. However, the observed improvement in VA was not statistically significant. Clearly, larger randomized controlled studies with pediatric patients and longer follow-up period are needed in order to investigate the efficacy of topical agents in INS.
3.2. Oral agents
Pharmacological treatment for INS has been explored recently, although drugs, such as baclofen, gabapentin, cannabis, memantine, and aminopyridines have been administered for a long time in acquired nystagmus [Citation67–69]. The GABA-agonist baclofen has been shown to be effective in some patients with congenital periodic alternating nystagmus (PAN) usually at doses between 15 and 60 mg/day [Citation70,Citation71].
The use of gabapentin and memantine, drugs which may have an antiglutaminergic action, was associated with reduction of nystagmus in INS patients in a retrospective case series [Citation72]. Gabapentin binds a subunit of voltage-dependent calcium channels. Memantine selectively blocks excess glutamatergic activity. The mechanism behind how these interventions improve nystagmus is still unclear. In the only available randomized controlled trial, McLean et al. reported improved vision and eye movement recordings in adults with INS treated with slowly increasing doses of gabapentin (up to 2400 mg/day in divided doses) and memantine (up to 40 mg/day in divided doses) versus placebo () [Citation73]. The tolerability of the drugs was good and only mild side effects, such as dizziness and tiredness, were noted. The trial showed that BCVA improved significantly in patients with IIN. On the contrary, in those with secondary nystagmus, i.e. albinism, there was no significant difference; however, nystagmus intensity and foveation improved after medication in all subjects [Citation73]. The reduced nystagmus amplitude with pharmacological treatment can also lead to improved well-being and cosmesis, which is often very important to patients with nystagmus. On subjective questioning, significantly more patients taking memantine or gabapentin reported that their vision improved more compared to those taking placebo [Citation73]. However, all patient groups showed significantly improved scores in the Visual Function 14 questionnaire (VF-14) and the Social Functioning Questionnaire (SFQ) [Citation73]. This dissociation between subjective perception of vision and functional consequences of impaired vision highlights the importance of further vision-related quality of life measures beyond BCVA.
Figure 3. Change in horizontal eye movement before and after treatment for memantine, gabapentin and placebo patients in infantile idiopathic nystagmus (IIN) and secondary nystagmus (SN). Decrease in amplitude of nystagmus for memantine and gabapentin and no change in nystagmus for placebo. Reproduced with permission from McLean et al. [Citation73]
![Figure 3. Change in horizontal eye movement before and after treatment for memantine, gabapentin and placebo patients in infantile idiopathic nystagmus (IIN) and secondary nystagmus (SN). Decrease in amplitude of nystagmus for memantine and gabapentin and no change in nystagmus for placebo. Reproduced with permission from McLean et al. [Citation73]](/cms/asset/34a34dab-9a68-4ff5-a91f-181373a9479f/ierl_a_1970533_f0003_b.gif)
Interestingly, oral acetazolamide has been studied in an adult with INS and resulted in a broadened range of gaze angles and improved foveation waveforms. Hence, the authors suggested that hereditary INS is associated with an inherited channelopathy, and other agents with known effects on ion channels may represent potential therapeutic targets for INS [Citation74].
In conclusion, there is clinical evidence for the efficacy of systemic drugs in INS. They have been used in patients over 16 years of age, and they are still not included in routine clinical practice, due to the lack of long-term safety studies especially for younger patients. Additionally, the off-label use of these drugs is a potential reason for their limited use. However, evidence from randomized controlled trials suggests that pharmacological options should be offered to patients with nystagmus after detailed explanation of their mode of action and possible side effects.
4. Surgical treatment
Surgery for nystagmus has been first introduced in the 1950s by Kestenbaum and Anderson for surgical alleviation of AHP by shifting the null zone to primary position [Citation58,Citation75]. The aim of surgery for nystagmus is to correct AHP or to improve visual acuity by dampening nystagmus. Surgeries used to treat nystagmus and AHP associated with IIN are also used to treat albinism and other nystagmus forms. The most common surgeries performed involve muscle recession, resection, tenotomy, or a combination of these.
Surgery for correction of AHPs is performed in cases of an eccentric gaze null point, in order to shift the null zone of nystagmus into primary position, resolve the head torticollis, and allow patients to use their maximal visual acuity () [Citation76]. Usually, null zones are either in the primary position of gaze or along the horizontal plane (head turns), less commonly in the vertical plane (chin-up or chin-down AHP) and least commonly they are torsional (head tilts) [Citation1]. In some patients with horizontal nystagmus, there are vertical AHPs or head tilts, or a combination of AHPs in different directions [Citation1]. Furthermore, patients with vertical IIN can also present with horizontal AHPs [Citation1]. The most important reason to correct the AHP in patients who wear glasses, is to enable them to view through their glasses centrally and achieve better optical correction [Citation77]. If the AHP is preventing children with low vision to look through their glasses, they are at risk of developing amblyopia () [Citation78]. Additionally, the AHP is corrected for cosmetic reasons and also to alleviate neck problems and headaches that can arise [Citation77]. Another benefit of nystagmus surgery is a possible enlargement of the null zone [Citation79,Citation80]. If there is associated manifest strabismus, surgery for AHP should be performed on the fixing eye and the deviation resulting from AHP surgery and preexisting strabismus should be addressed accordingly in the non-fixing eye [Citation1]. The principle underlying nystagmus surgery is to rotate the eyes in the direction of the head turn.
Figure 4. This child with albinism-associated nystagmus and a left head turn (a) underwent a Kestenbaum procedure for correction of his head posture. No head turn is present after the operation (b). Reproduced with permission from Papageorgiou et al. [Citation77]
![Figure 4. This child with albinism-associated nystagmus and a left head turn (a) underwent a Kestenbaum procedure for correction of his head posture. No head turn is present after the operation (b). Reproduced with permission from Papageorgiou et al. [Citation77]](/cms/asset/85c62b6b-5c70-42cc-82f8-1f79e572b88b/ierl_a_1970533_f0004_oc.jpg)
4.1. Kestenbaum–Anderson procedure
Anderson suggested recession of the two muscles that rotate the eyes toward the preferred horizontal gaze position, and Kestenbaum proposed combined recessions with resections in both eyes [Citation58,Citation75]. The Kestenbaum–Anderson procedure is the standard surgical approach to correct AHP occurring secondary to an eccentric null zone in INS (). The classical procedure by Kestenbaum included 5 mm surgery on each muscle and was subsequently modified by Parks to the 5, 6, 7, 8 mm procedure [Citation81], and by Pratt–Johnson to 10 mm surgery on all four muscles [Citation82].
Multiple surgical protocols have been described in the literature depending on the severity of head turn and the presence of strabismus. The Kestenbaum procedure on all four horizontal recti has been recommended for patients with large horizontal head turns. For patients with smaller horizontal head turns many authors favor the Anderson procedure or its modifications, such as the augmented Anderson procedure, which was described by DeDecker and includes large recessions of two horizontal recti [Citation24,Citation56,Citation83]. Generally, most authors suggest augmentation of the classical surgical amounts by 40% for AHPs up to 30° and 60% for AHPs exceeding 45° [Citation84–89]. It has been reported that 72–100% of patients achieve an AHP of 15° or less postoperatively [Citation84,Citation87,Citation89–93]. However, up to 40% of patients may require additional surgery after a mean interval of 6 years for under-corrections [Citation94]. Overcorrection and limitation of ductions following large recessions have been occasionally reported [Citation95–97].
4.2. Vertical AHPs
Vertical shifting of the nystagmus null zone to correct vertical AHP was first proposed by Pierse, who weakened the inferior recti and superior oblique in both eyes to correct chin-up head postures [Citation98]. In general, smaller surgical amounts are required to correct a vertical AHP [Citation44]. Chin-down AHPs have been managed with bilateral superior rectus recession, inferior oblique myectomy and a horizontal rectus recession or tenotomy. On the other hand, chin-up AHPs have been treated with bilateral superior oblique tenectomy, inferior rectus recession and a horizontal rectus recession or tenotomy. Surgery on the oblique muscles is performed to avoid cyclotorsion [Citation99]. For correction of chin-down and chin-up AHPs, other authors have used recessions or tucking of the vertical recti between 6 and 7 mm, in combination with surgery on the superior oblique muscles (6–8 mm tucking in the case of chin-down, or recession in the case of chin-up AHP) [Citation100]. Bilateral superior oblique posterior 7/8th tenectomy in conjunction with bilateral inferior rectus recession has been also used to improve chin-up AHPs [Citation101].
Simultaneous recessions and resections of the vertical recti of both eyes (4–6 mm) are commonly used to treat chin-up and chin-down AHPs while sparing the oblique muscles [Citation56,Citation102]. Recessions of the inferior recti for chin-up AHPs or the superior recti for chin-down AHPs have been also described [Citation51]. Transposition of the vertical recti horizontally is sometimes used in larger recessions or resections, to prevent alphabetic patterns [Citation56]. For example, bilateral inferior rectus recessions may cause A-pattern deviation because of weakened adduction in downgaze. The inferior rectus may be transposed nasally to avoid creating an A-pattern [Citation51].
4.3. Head tilts
Head tilts are less common and can be corrected by rotatory or oblique Kestenbaum procedures, as described by Conrad and de Decker [Citation103]. This type of surgery includes recession and resection of all oblique muscles in order to rotate both eyes around the sagittal axis [Citation103]. The intortor on the side of the tilt, i.e. the superior oblique, should be weakened and the extortor on the other side, i.e. the inferior oblique, strengthened [Citation102].
A similar technique has been described by Lueder et al. and involves anterior tenectomy of the superior oblique tendon combined with contralateral recession of the inferior oblique muscle () [Citation104].
Figure 5. A, Inferior oblique muscle recession, left eye, surgeon’s view. The inferior oblique muscle has been cut near its insertion. Its anterior edge is reattached 6 mm posterior to the lateral border of the inferior rectus muscle insertion. The posterior fibers are reattached at a 45° angle posteriorly and laterally from the anterior insertion. B, Superior oblique tenectomy, right eye, surgeon’s view. The fibers of the superior oblique tendon are disinserted from the anterior border of the insertion to 50% of the width of the insertion. The anterior 50% of the tendon is then removed between the insertion and the lateral border of the superior rectus muscle. IO, inferior oblique muscle; IR, inferior rectus muscle; SO, superior oblique tendon; SR, superior rectus muscle. Reproduced with permission from [Citation104]
![Figure 5. A, Inferior oblique muscle recession, left eye, surgeon’s view. The inferior oblique muscle has been cut near its insertion. Its anterior edge is reattached 6 mm posterior to the lateral border of the inferior rectus muscle insertion. The posterior fibers are reattached at a 45° angle posteriorly and laterally from the anterior insertion. B, Superior oblique tenectomy, right eye, surgeon’s view. The fibers of the superior oblique tendon are disinserted from the anterior border of the insertion to 50% of the width of the insertion. The anterior 50% of the tendon is then removed between the insertion and the lateral border of the superior rectus muscle. IO, inferior oblique muscle; IR, inferior rectus muscle; SO, superior oblique tendon; SR, superior rectus muscle. Reproduced with permission from [Citation104]](/cms/asset/890d3771-2947-4dde-b81c-22cee79893ee/ierl_a_1970533_f0005_b.gif)
Another approach to correct head tilts is to induce torsion by moving the insertion of the rectus muscles, as suggested by von Noorden [Citation1,Citation105]. For example, the horizontal recti could be shifted up and down and the vertical recti nasally and temporally, in order to induce cycloduction. For example, de Castro-Abeger et al. have described transposition of the vertical rectus muscles of each eye to induce cyclotorsion in the direction of the head tilt () [Citation106].
Figure 6. To correct a right head tilt: A nasal transposition of the right superior rectus and left inferior rectus and/or temporal transposition of the right inferior rectus and the left superior rectus leads to excyclotorsion of the right eye and incyclotorsion of the left eye respectively. To correct a left head tilt: A nasal transposition of the right inferior rectus and left superior rectus and/or temporal transposition of the right superior rectus and left inferior rectus leads to incyclotorsion of the right eye and excyclotorsion of the left eye respectively. Reproduced with permission from De Castro-abeger et al. [Citation106]
![Figure 6. To correct a right head tilt: A nasal transposition of the right superior rectus and left inferior rectus and/or temporal transposition of the right inferior rectus and the left superior rectus leads to excyclotorsion of the right eye and incyclotorsion of the left eye respectively. To correct a left head tilt: A nasal transposition of the right inferior rectus and left superior rectus and/or temporal transposition of the right superior rectus and left inferior rectus leads to incyclotorsion of the right eye and excyclotorsion of the left eye respectively. Reproduced with permission from De Castro-abeger et al. [Citation106]](/cms/asset/f73dedfd-2bc5-463a-ac30-b0469179ac79/ierl_a_1970533_f0006_oc.jpg)
The various surgical approaches for correction of head tilts are summarized in [Citation93,Citation107].
Figure 7. Right head tilt with left cycloversion. An opposing right cycloversion may be obtained by creating excyclotorsion of the right eye and incyclotorsion of the left eye. This may be obtained by (1) recess-resect of the oblique muscles [Citation103], (2) surgery on the four cyclovertical mucles: recession of the right SO and adjustable suture recession of the right SR (both incyclotortors), and recession of the left iO and adjustable suture recession of the left iR (both excyclotortors) [Citation107], (3) slanting of the four rectus muscles: an oblique recession of 7 mm of the superior fibers of the MR, the nasal fibers of the iR, the inferior fibers of the LR, and the temporal fibers of the SR creates incyclotorsion. Conjugate slanting of the rectus muscles in the other eye creates excyclotorsion [Citation93], (4) horizontal transposition of the vertical rectus muscle tendon. This transposition may be extended to any rectus muscle, depicted here as also involving horizontal rectus muscles [Citation105]. SO, superior oblique muscle; SR, superior rectus muscle; iO, inferior oblique muscle; iR, inferior rectus muscle; MR, medial rectus muscle; LR, lateral rectus muscle. Reproduced with permission from Spielmann [Citation93]
![Figure 7. Right head tilt with left cycloversion. An opposing right cycloversion may be obtained by creating excyclotorsion of the right eye and incyclotorsion of the left eye. This may be obtained by (1) recess-resect of the oblique muscles [Citation103], (2) surgery on the four cyclovertical mucles: recession of the right SO and adjustable suture recession of the right SR (both incyclotortors), and recession of the left iO and adjustable suture recession of the left iR (both excyclotortors) [Citation107], (3) slanting of the four rectus muscles: an oblique recession of 7 mm of the superior fibers of the MR, the nasal fibers of the iR, the inferior fibers of the LR, and the temporal fibers of the SR creates incyclotorsion. Conjugate slanting of the rectus muscles in the other eye creates excyclotorsion [Citation93], (4) horizontal transposition of the vertical rectus muscle tendon. This transposition may be extended to any rectus muscle, depicted here as also involving horizontal rectus muscles [Citation105]. SO, superior oblique muscle; SR, superior rectus muscle; iO, inferior oblique muscle; iR, inferior rectus muscle; MR, medial rectus muscle; LR, lateral rectus muscle. Reproduced with permission from Spielmann [Citation93]](/cms/asset/aa910c30-f2b7-47b7-ab2a-648f67481dc2/ierl_a_1970533_f0007_b.gif)
4.4. Large horizontal recessions of all four horizontal muscles
Another variation of the recession/resection procedure is the large symmetric recession (11–13 mm) of all four horizontal recti muscles with new insertion posterior to the equator [Citation108,Citation109]. Initial reports showed moderate improvement in visual acuity, decrease in nystagmus amplitude, and improvement in AHP without significantly limiting ocular motility [Citation108–110]. It has been hypothesized that the cutting of the extraocular muscles at its enthesis contributes in large part toward the improved nystagmus waveform and visual function [Citation111]. Large four-muscle recessions are useful for patients with PAN and AHPs in both directions, to correct the AHP. Some authors do not recommend large recessions of the four horizontal recti for INS patients, especially those who have binocularity, suggesting that they are at risk to develop diplopia and undesirable exotropia [Citation112].
4.5. Tenotomy
The four-muscle tenotomy is another procedure used in the surgical treatment of nystagmus and involves detaching and reattaching the rectus muscles at their insertion [Citation111]. Tenotomy includes the removal of the tendon organs responsible for proprioception, and hence the nystagmus is reduced and the null region is broadened [Citation113]. This procedure can be applied on both horizontal and vertical muscles and can be combined with strabismus correction. Hertle et al. reported a series of 75 patients with congenital nystagmus, who underwent either recess/resect surgery or tenotomy [Citation114]. There was a postoperative improvement of 0.1 logMar in VA in 71% of patients and a significant improvement in null zone width. Other studies have also described improvement in foveation characteristics and small VA gains, but only a few individual patients had VA improvement that exceeded test–retest variability [Citation115–117]. Hence, the efficacy of four-muscle tenotomy has yet to be determined in larger-scale studies.
4.6. Myectomy of the extraocular muscles
In 2002, it was suggested that total extirpation and disposal of the anterior portions of the four horizontal recti for horizontal nystagmus without a null point was effective in improving horizontal eye movements and visual acuity [Citation118]. A modification of this procedure, myectomy without reattachment, which is a measured myectomy at posterior Tenon’s fascia, was later proposed in order to reduce the risks of limitation of versions, intraoperative orbital bleeding, and postoperative strabismus [Citation119]. However, subsequent studies have failed to prove the efficacy of this procedure, which was associated with postoperative symptomatic complications, such as diplopia, oscillopsia, AHP along with vestibular and saccadic dysfunction, hypo-accommodation, and lack of convergence [Citation120].
4.7. Artificial divergence surgery
Another less common procedure is the Cüppers artificial divergence surgery, which creates a divergent resting position (exophoria) that must be overcome by fusional convergence, thus decreasing the nystagmus intensity [Citation121]. This method is indicated in patients whose nystagmus damps with convergence and have good binocular function [Citation1]. The aim of the surgery is to increase convergence and to trigger a convergence impulse even at distance by creating a divergent resting position (exophoria) [Citation1,Citation102]. Preoperatively, it is necessary to measure the fusion range and also test for at least 1–2 weeks the optimal strength of base out prisms in order to ensure adequate potential for the surgery and surgical dose [Citation1].
In some patients, a convergence null can interact with a gaze null, if present. In these cases, combined surgery, consisting of the Anderson-Kestenbaum procedure and the Cüppers artificial divergence procedure gave better vision improvement than the Anderson-Kestenbaum operation for the treatment of infantile nystagmus with head turn [Citation122].
4.8. Preoperative considerations and timing of surgery
In order to choose the appropriate surgical approach, it is of paramount importance to identify the existence of PAN, MLN or strabismus during the preoperative examination. Hence, candidates for nystagmus surgery should be observed for at least 5–7 min to exclude PAN [Citation51]. The evaluation of AHP should be performed by testing both with and without glasses at both distance and near, monocularly and binocularly, with documentation of the direction and degree of AHP during maximal visual effort [Citation51]. In INS patients, dynamic targets, such as videos, are also used to elicit AHPs that may not be evident on testing of static visual acuity [Citation51].
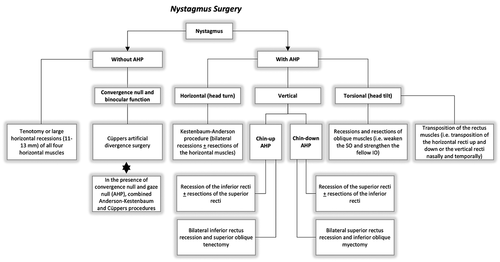
Surgical intervention for nystagmus can be performed at any age [Citation1]. In most cases, surgery is typically deferred until 6–8 years of age to be sure that the AHP has stabilized and the child is cooperative and easier to examine [Citation1,Citation102]. AHP may become more obvious and frequent at school age due to the increasing visual demands associated with schoolwork. However, surgery should be performed earlier in children with significant torticollis, who are not able to wear glasses due to large AHPs and have amblyopia, in order to optimize visual function during the sensitive period of visual development [Citation123]. The surgical techniques and indications for INS patients are summarized in .
4.9. Manifest latent nystagmus or fusion maldevelopment nystagmus syndrome (FMNS)
Manifest latent nystagmus (MLN) or fusion maldevelopment nystagmus syndrome (FMNS) is almost always associated with the congenital squint syndrome and represents the most common form of non-INS nystagmus in childhood [Citation51]. MLN is characterized by horizontal nystagmus, which beats in the direction of the fixing eye and reverses with alternate occlusion. In MLN, binocular VA is typically better than VA during monocular testing, because the nystagmus increases on occlusion of either eye. In many patients with MLN, the nystagmus damps in adduction of the fixing/open eye; therefore, it can induce a face turn to the side of the fixing eye. Patients with MLN typically present with strabismus and abnormal binocular functions and often have amblyopia [Citation124]. Interestingly, INS with a latent component can have a similar clinical presentation and the distinction between those two entities is possible by means of eye movement recordings. In the presence of MLN, eye muscle surgery in the fixing eye should aim to correct both the esotropia and the head turn [Citation122]. Patients with MLN and strabismus may substantially benefit from strabismus correction (surgical or optical) because their nystagmus can decrease, and their vision can improve significantly if the squint angle is smaller. If exotropia and abnormal head position are present, the surgery is complex as recessions of lateral rectus muscles (to reduce the squint) and medial rectus muscles (to reduce the AHP) may be necessary. Patching therapy for amblyopia is also effective and can also reduce the nystagmus intensity in MLN [Citation125,Citation126]. Simonsz has suggested that occlusion of the better eye in children with MLN and amblyopia should be prescribed only in days per week, not in hours per day [Citation126].
5. Laser refractive surgery
In 1998, Siganos et al. introduced the use of photorefractive keratectomy (PRK) in infantile nystagmus and suggested that patients with infantile nystagmus and myopia are eligible candidates for PRK [Citation127]. Laser in situ keratomileusis (LASIK) and intraepithelial LASIK (intraLASIK) were performed with success in adult patients with INS [Citation128–131]. In order to manage the continuous oscillations and stabilize the eye, an active eye-tracking system or manual fixation of the globe (forceps or fixation ring) has been used [Citation130]. Most studies generally agree that patients with INS, who are older than 18 years and have stable myopia for at least 1 year, are good candidates for successful refractive surgery. Furthermore, in most patients, postoperative uncorrected VA is equal or better to the preoperative spectacle-corrected VA. In some of these patients, postoperative VA can be further improved with spectacles. Hence, refractive surgery in INS patients leads to significant improvement in VA, contrast sensitivity and nystagmus symptoms and has a positive impact in their quality of life [Citation132,Citation133]. Mahler et al. reported the case of a patient who became eligible for a driver’s license after surgery [Citation130].
Refractive surgeries share some common advantages with CLs in patients with INS due to abolition of prismatic and peripheral spectacle aberrations, especially when there is an AHP. However, refractive surgery spares the chronic side effects of CLs wear (i.e infection, dry eye disease, irritation or abrasions of the cornea or eyelids, hypoxia, giant papillary conjunctivitis) and is preferred for patients who meet the criteria for these surgeries.
6. Botulinum toxin injections
Botulinum toxin A (Botox) injections into the extraocular muscles or retrobulbar space in order to weaken the extraocular muscles, is another approach used in the treatment of nystagmus. Botulinum toxin A is produced by the bacterium Clostridium botulinum. It is a neurotoxic protein that induces a persistent but reversible paralysis of muscles with cholinergic innervation [Citation134]. Botulinum toxin A is a potent therapeutic tool in the world of ophthalmology. Its current use in the field of neuromuscular diseases such as blepharospasm, hemifacial spasm, corneal exposure in lagophthalmos, strabismus, and nystagmus made it valuable for many patients [Citation135].
6.1. Botox in infantile nystagmus
There are few studies and case series in patients with INS. The preferred technique is to inject approximately 25 units of botulinum toxin A (Botox) direct to the horizontal rectus muscles of both eyes. The results were generally good with improvement of nystagmus amplitude, AHP and VA [Citation136–138]. Oleszczynska reported amelioration of both distant and near VA in 32 children with infantile nystagmus [Citation137]. However, improvement was transient and repetition of injection every 2–4 months was necessary. Carruthers et al. reported that three of four patients achieved a significant enough change in acuity to receive daytime-restricted driver’s licenses, while no complications were caused by the procedure [Citation136]. Hernández-García et al. reported effectiveness of botulinum toxin in an infant with albinism-associated nystagmus. He also suggested that the reduction of nystagmus amplitude permits early development of binocular vision [Citation138]. However, Botox use in INS is still very limited due to the limited period of action and the need for reinjection every 2–4 months, anesthesia in children, and side effects, such as bruising, red eye, recurrent filamentary keratitis, presence of oscillopsia during head movements due to loss of the vestibulo-ocular reflex ptosis, diplopia and strabismus, as the neurotoxic effect is unlikely to be identical in all injected muscles.
6.2. Botox in acquired nystagmus
The use of botulinum toxin A in acquired nystagmus has been investigated more extensively than in INS, in an attempt to reduce severe oscillopsia. In acquired nystagmus, the preferred method is to inject botulinum toxin A (Botox, Oculinum) into the retrobulbar space [Citation139–144] rather than directly into the horizontal rectus muscles [Citation139,Citation145]. Hence, this technique carries the risks of retrobulbar injections, which has restricted its use in clinical routine practice.
7. Acupuncture
A preliminary study of Blekher et al. examined the effects of acupuncture at the sternocleidomastoid muscles on foveation characteristics in six patients with infantile nystagmus [Citation146]. Four of the six patients showed improved foveation at the commencement of treatment; three maintained this response throughout the treatment period and after the needles were removed. Hence, the authors suggested that that projections from the neck and face to the reticular formation and vestibular nucleus may alter the behavior of the pathophysiological mechanism underlying infantile nystagmus [Citation146]. A more recent study with three patients with IIN and three patients with acquired nystagmus found that acupuncture on the body and ears led to improvement in the visual function (BCVA and contrast sensitivity) and thus, in their quality of life [Citation147]. Due to the limited number of participants, the efficacy of acupuncture in INS and the pathogenetic mechanism are still unclear and larger studies are needed to prove its value.
8. Biofeedback
Microperimetric biofeedback training (BFT) is a visual rehabilitation strategy. The aim is to stabilize patient fixation by reinforcing or creating a new preferential fixation locus [Citation148]. This method ‘reeducates’ the visual pathway by promoting retina-brain transmission and improving cortical plasticity [Citation148]. In practice, patients are requested to follow a training locus, which is located more eccentric in the retina. When the patient gaze is close to the selected locus target, an auditory signal increases its frequency. This method has been recently applied in patients with loss of foveal function due to different maculopathies, in order to ameliorate fixation [Citation149]. Historically, the first application of eye movement auditory biofeedback in the control of nystagmus was introduced in 1982 by Ciuffreda et al., and demonstrated promising results in improvement of vision and cosmesis [Citation150]. Grenga et al. perfomed BFT in a patient with oculocutaneous albinism, showing significant fixation improvement but without significant VA increase [Citation151]. In a recent BFT retrospective study with 12 pediatric patients with INS, Caputo et al. found significant improvement in fixation stability, but not for BCVA [Citation152]. A pilot BFT study with 10 pediatric patients with IIN showed improvements in fixation stability, BCVA, contrast sensitivity, stereopsis, and reading speed [Citation153]. However, the use of microperimetric BFT is still limited due to lack of large randomized controlled trials, the small study samples and inconsistent standards of practice. It is speculated that BFT in the pediatric population with INS may not improve VA as much as that achieved in adults with macular disease [Citation152]. There is also uncertainty regarding the cooperation of children with low vision during the cumbersome microperimetry examination, where deep attention over a long period of time is required [Citation152].
9. Gene therapies
Gene therapy has already shown promising results in patients with inherited retinal diseases, such as retinitis pigmentosa, congenital achromatopsia, and Leber’s congenital amaurosis [Citation154]. INS is a genetically heterogenous disorder associated with mutations of genes expressed in the retina and brain. Recently, Thomas et al. developed a next-generation sequencing (NGS) panel for nystagmus patients, in order to facilitate early diagnosis and enable accurate genetic counseling [Citation155]. This information is a first step toward individualized diagnosis for patients with atypical manifestations of INS and personalized genetic treatment ().
Figure 9. Diagnostic workflow for patients with nystagmus. The current pathway suggests a number of different investigations in order to identify the underlying etiology: optical coherence tomography, eye movement recordings, electrodiagnostic tests, MRI brain and genetic testing. The new pathway that involves using NGS as a frontline diagnostic tool would help establish a genetic diagnosis and thus guide further investigations and targeted treatment. Reproduced from Thomas et al. [Citation155] under Creative Commons
![Figure 9. Diagnostic workflow for patients with nystagmus. The current pathway suggests a number of different investigations in order to identify the underlying etiology: optical coherence tomography, eye movement recordings, electrodiagnostic tests, MRI brain and genetic testing. The new pathway that involves using NGS as a frontline diagnostic tool would help establish a genetic diagnosis and thus guide further investigations and targeted treatment. Reproduced from Thomas et al. [Citation155] under Creative Commons](/cms/asset/24c398d3-c079-4510-bea2-3b601bdedff3/ierl_a_1970533_f0009_b.gif)
In the field of nystagmus, gene therapy has been introduced in albino animal models. Surace et al. first reported an adeno-associated virus (AAV)-mediated gene transfer therapy for ocular albinism type 1 gene (OA1) [Citation21,Citation156,Citation157]. AAV is a single-stranded DNA virus and AAV-based vectors infect the retina via subretinal or intravitreal injection without causing any toxicity in both animals and humans [Citation158]. OA1 gene encapsulated by AAV-vectors was delivered subretinally to the adult Oa1−/- mice. After 4 weeks, ERG demonstrated significantly higher a- and b-wave amplitudes under scotopic conditions in treated mice and melanosome density in the RPE of treated mice was higher than controls.
Gargiulo et al. used an AAV-based vector expressing the human tyrosine (TYR) gene in mice [Citation156]. After tyrosinase gene complementation by AAV gene transfer, there was melanosome biogenesis and restoration of retinal functions in the ERG [Citation156].
Song et al. used single-stranded DNA (ssDNA) and CRISP/Cas9-mediated homology-directed repair (HDR), which is able to exchange and insert single nucleotide polymorphisms (SNP) in a specific position accurately in animals [Citation159]. New Zealand white rabbits’ embryos with albinism from TYR mutations were injected with ssDNA, where nucleotide C instead of A was positioned at 1118 in codon 373 of TYR gene [Citation159]. The mutant newborn rabbits increased melanin synthesis in both hair follicles and irises, thus providing functional validation of the albinism-associated T373K SNP at the animal level [Citation159].
Although all the above studies did not report on nystagmus, these data demonstrate the potential benefit of gene interventions for albinism by achieving both structural and functional improvement in albino animal models. On the other hand, the generalizability of those results to human albinism should be investigated more thoroughly. For example, using the ERG as on outcome measure in gene studies should take into account the differences in the electrophysiological findings between the Oa1 (-/-) mouse retina and the human albino retina. The Oa1 (-/-) mouse displays retinal electrophysiological abnormalities, including significant decrease in a- and b-wave amplitude and delayed recovery of b-wave amplitude from photoreceptor desensitization following bright light exposure [Citation21]. On the other hand, the ERG in patients with albinism is usually recorded as normal or accentuated [Citation160,Citation161]. Hence, the current evidence from animal studies points the need for clinical trials in order to establish the safety and efficacy of these treatments in humans.
The treatment approaches for INS are summarized in .
Table 1. Overview of treatment approaches for infantile nystagmus syndrome
10. Conclusions
Nystagmus has a profound effect on vision and psychosocial aspects of life and has long been considered an untreatable condition with limited options for pharmacological or surgical intervention. Currently, a variety of therapeutic methods is available and should be considered on an individualized basis after detailed investigation of the underlying nystagmus etiology. Refractive correction by spectacles or contact lenses, possibly with the inclusion of prisms, and surgery are the most effective methods to improve vision and correct AHPs and strabismus. Recently, the efficacy of novel pharmacological agents, such as gabapentine and memantine, has been proven in randomized clinical trials and can now be offered to selected patients as an additional option. Finally, the advent of OCT and next-generation sequencing enable early and accurate diagnosis of nystagmus and pave the way for future individualized genetic approaches. Interestingly, there are currently no consensus recommendations on nystagmus treatment; hence, future studies should address the need for more randomized clinical trials.
11. Expert opinion
Children with nystagmus are common in pediatric ophthalmic practice. Due to the variability in clinical presentation, complexity of pathophysiologic mechanisms and possibility of serious underlying disorders, clinicians often face diagnostic and therapeutic challenges when dealing with affected children. The first step in choosing the appropriate treatment for a child with nystagmus is the correct diagnosis. Hence, the identification of INS in the clinical practice signals the beginning of a diagnostic pathway, in order to accurately characterize the underlying cause. Recent advances in pediatric retinal imaging and genetic testing in combination with eye movement recordings allow the early and exact diagnosis of nystagmus. Brain neuroimaging and electrodiagnostic testing are also necessary in some children to determine the underlying etiology. The recent development of next-generation sequencing panels will enable the identification of a genetic cause and the characterization of phenotype-genotype correlations in order to guide further individualized treatment [Citation155]. However, the limited availability of these genetic panels in the clinical setting across different countries is a potential difficulty that has to be addressed, as gene replacement treatments are arising.
Treatment of nystagmus in children begins with established generic approaches, such as refractive correction, by means of spectacles or contact lenses and therapy of amblyopia, in order to achieve optimal development of the visual system during the sensitive period [Citation162]. Children with nystagmus should be monitored regularly and refracted under cycloplegia from young ages, because they have impaired emmetropization and a higher prevalence of refractive errors compared to normal children. Additionally, both RGP and SCL are an option for patients with nystagmus, especially those with high refractive errors. Caution should be taken when using toric CLs in patients with significant amounts of astigmatism due to their possible misalignment and degradation of VA. Furthermore, adding yoked prisms to the spectacles of patients with AHPs, can be useful in alleviating small AHPs. Bilateral base-out prisms may also be helpful for patients with fusion whose nystagmus damps in convergence. Memantine and gabapentine are both oral agents that have been tested for their efficacy and safety in patients with nystagmus and can be offered as additional treatment option at least in adults.
The benefit of surgery for correcting AHPs in nystagmus has been extensively studied and is well documented in the literature. Extraocular muscle surgery to shift an eccentric null zone into primary position is one of the most effective treatments of nystagmus and can be performed in children and adults as well. The Kestenbaum–Anderson procedure and its modifications are the most popular surgical approach for nystagmus today. Nystagmus surgery can be performed to correct horizontal head turns, vertical head turns (chin-up or chin-down head postures), head tilts, or combined head postures. Apart from the alleviation of AHPs, nystagmus surgery has been shown to ameliorate VA, reduce nystagmus intensity, and improve cosmesis. In addition, nystagmus surgery should be performed in combination with strabismus surgery. For example, patients with MLN and strabismus may substantially benefit from strabismus correction (surgical or optical) because their nystagmus can decrease, and their vision can significantly improve [Citation56].
Recent approaches, such as biofeedback, acupuncture and gene replacement therapy, are still at experimental stage and have a limited role in the clinical setting. In conclusion, there is a need for larger randomized controlled clinical trials for nystagmus interventions, which will help in the development of therapeutic algorithms for INS. Future studies will have to address potential difficulties, such as inclusion of pediatric patients, lack of homogenous patient groups and small study samples.
Article highlights
Treatment options for INS are designed to optimize vision by increasing foveation duration, to reduce the intensity of nystagmus and correct AHP and associated strabismus.
Current interventions include optical, pharmacological, and surgical options, as well as novel approaches, such as acupuncture, biofeedback, and gene therapies.
Optical treatment includes correction of refractive errors with spectacles or contact lenses, and the use of prisms to induce convergence or correct small AHP.
Contact lenses may be helpful in INS patients with high refractive errors, because they do not have the problems of decentration and obstruction of the visual axis by spectacle frames.
Both rigid gas-permeable and soft CLs have been used in patients with INS; however, the relative benefits of CLs in comparison to spectacles still remain debatable.
Gabapentin and memantine are antiglutaminergic drugs with clinically proven efficacy in vision and eye movement recordings of INS patients.
The most common surgeries for nystagmus involve muscle recession, resection, tenotomy, or a combination of these, and their aim is to correct AHP or improve visual acuity by dampening nystagmus.
The use of acupuncture and biofeedback in INS is still limited due to lack of large randomized controlled trials, small study samples, and inconsistent standards of practice.
Gene interventions in albino animal models have already shown promising results and larger clinical trials are required in order to establish the safety and efficacy of these treatments in humans with INS.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Proudlock FA, Gottlob I. Nystagmus. In: editors, Taylor D, Hoyt CS. Paediatric ophthalmology and strabismus. 4th ed. Kidlington, UK: Elsevier B.V.; 2011. p. 909-923.
- Sarvananthan N, Surendran M, Roberts EO, et al. The prevalence of nystagmus: the Leicestershire nystagmus survey. Invest Ophthalmol Vis Sci. 2009;50(11):5201–5206.
- Chung SUSANATL, Bedell HE. Velocity criteria for ”Foveation periods” Determined from image motions simulating congenital Nystagmus. Optom Vis Sci. 1996;73(2):92–103.
- Hertle RW. Examination and refractive management of patients with nystagmus. Surv Ophthalmol. 2000;45(3):215–222.
- Ehrt O. Infantile and acquired nystagmus in childhood. Eur J Paediatr Neurol. 2012;16(6):567–572.
- Papageorgiou E, Gottlob I. The challenges faced by clinicians diagnosing and treating infantile nystagmus Part I: diagnosis. Expert Rev Ophthalmol. 2021;16(2):97–112.
- Papageorgiou E, Kapsalaki E, Tsironi EE. Disconjugate nystagmus in a child with chiasmal glioma. Pediatr Neurol. 2020;104:70–71.
- Lavery MA, O’Neill JF, Chu FC, et al. Acquired nystagmus in early childhood: a presenting sign of intracranial tumor. Ophthalmology. 1984;91(5):425–453.
- Estrada M, Kelly JP, Wright J, et al. Visual function, brain imaging, and physiological factors in children with asymmetric nystagmus due to chiasmal gliomas. Pediatr Neurol. 2019;97:30–37.
- Daroff RB. See-saw nystagmus. Neurology. 1965;15(9):874–877.
- Dell’Osso LF, Daroff RB. Two additional scenarios for see-saw nystagmus: achiasma and hemichiasma. J Neuroophthalmol. 1998;18:112–113.
- Sharpe JA, Fletcher WA. Saccadic intrusions and oscillations. Can J Neurol Sci. 1984;11(4):426–433.
- Abadi RV, Whittle J. The nature of head postures in congenital nystagmus. Arch Ophthalmol. 1991;109(2):216–220.
- Hertle RW, Dell’Osso LF. Clinical and ocular motor analysis of congenital nystagmus in infancy. J AAPOS. 1999;3(2):70–79.
- Abadi RV, Bjerre A. Motor and sensory characteristics of infantile nystagmus. Br J Ophthalmol. 2002;86(10):1152–1160.
- Lee H, Sheth V, Bibi M, et al., Potential of handheld optical coherence tomography to determine cause of infantile nystagmus in children by using foveal morphology. Ophthalmology. 2013;120(12):2714–2724.
- Lee H, Proudlock F, Gottlob I. Is handheld optical coherence tomography reliable in infants and young children with and without nystagmus? Invest Ophthalmol Vis Sci. 2013;54(13):8152–8159.
- McLean RJ, Windridge KC, Gottlob I. Living with nystagmus: a qualitative study. Br J Ophthalmol. 2012;96(7):981–986.
- Pilling RF, Thompson JR, Gottlob I. Social and visual function in nystagmus. Br J Ophthalmol. 2005;89(10):1278–1281.
- Decarlo DK, McGwin G Jr, Bixler ML, et al. Impact of pediatric vision impairment on daily life: results of focus groups. Optom Vis Sci. 2012;89(9):1409–1416.
- Surace EM, Domenici L, Cortese K, et al., Amelioration of both functional and morphological abnormalities in the retina of a mouse model of ocular albinism following AAV-mediated gene transfer. Mol Ther. 2005;12(4): 652–628. DOI:https://doi.org/10.1016/j.ymthe.2005.06.001. •• The authors found that adeno-associated viral vector-mediated OA1 gene transfer to the retina of an ocular albinism OA1 knockout mouse model rescues both functional and morphological abnormalities.
- Gottlob I. Is impaired emmetropization related to foveal hypoplasia or is it specific to albinism? Invest Ophthalmol Vis Sci. 2013;54(4):2940.
- Hertle RW, Zhu X. Oculographic and clinical characterization of thirty-seven children with anomalous head postures, nystagmus, and strabismus: the basis of a clinical algorithm. J AAPOS. 2000;4(1):25–32.
- Brodsky MC. Congenital nystagmus and its congeners. J Binocul Vis Ocul Motil. 2020;70(2):63–70.
- Hertle RW, Dell’Osso LF. Nystagmus in infancy and childhood: current concepts in mechanisms, diagnoses, and management. New York: Oxford University Press; 2013. p. 21–26.
- Fresina M, Benedetti C, Marinelli F, et al. Astigmatism in patients with idiopathic congenital nystagmus. Graefes Arch Clin Exp Ophthalmol. 2013;251(6):1635–1639.
- Yahalom C, Tzur V, Blumenfeld A, et al. Refractive profile in oculocutaneous albinism and its correlation with final visual outcome. Br J Ophthalmol. 2012;96(4):537–539.
- Healey N, McClelland JF, Saunders KJ, et al. Longitudinal study of spherical refractive error in infantile nystagmus syndrome. Ophthalmic Physiol Opt. 2014;34(3):369–375.
- Sampath V, Bedell HE. Distribution of refractive errors in albinos and persons with idiopathic congenital nystagmus. Optom Vis Sci. 2002;79(5):292–299.
- Reinecke RD. Costenbader Lecture. Idiopathic infantile nystagmus: diagnosis and treatment. J AAPOS. 1997;1(2):67–82.
- Wang J, Wyatt LM, Felius J, et al. Onset and progression of with-the-rule astigmatism in children with infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2010;51(1):594–601.
- Schweigert A, Lunos S, Connett J, et al. Changes in refractive errors in albinism: a longitudinal study over the first decade of life. J AAPOS. 2018;22:462–466.
- Brodsky MC, Fray KJ. The prevalence of strabismus in congenital nystagmus: the influence of anterior visual pathway disease. J AAPOS. 1997;1(1):16–19.
- Kumar A, Gottlob I, McLean RJ, et al., Clinical and oculomotor characteristics of albinism compared to FRMD7 associated infantile Nystagmus. Invest Ophthalmol Vis Sci. 2011;52(5):2306–2313.
- Hertle RW. Albinism: particular attention to the ocular motor system. Middle East Afr J Ophthalmol. 2013;20(3):248–255.
- Bagheri A, Abbasi H, Tavakoli M, et al. Effect of rigid gas permeable contact lenses on nystagmus and visual function in hyperopic patients with infantile nystagmus syndrome. Strabismus. 2017;25(1):17–22.
- Dell’Osso LF, Traccis S, Abel LA, et al. Contact lenses and congenital nystagmus. Clin Vision Sci. 1988;3:229–232.
- Abadi RV. Visual performance with contact lenses and congenital idiopathic nystagmus. Br J Physiol Opt. 1979;33:32–37.
- Rutner D, Ciuffreda K. Soft contact lenses to improve motor and sensory function in congenital nystagmus. J Behav Optom. 2005;16:17–20.
- Allen ED, Davies PD. Role of contact lenses in the management of congenital nystagmus. Br J Ophthalmol. 1983;67(12):834–836.
- Taibbi G, Wang ZI, Dell’Osso LF. Infantile nystagmus syndrome: broadening the high-foveation-quality field with contact lenses. Clin Ophthalmol. 2008;2:585–589.
- Hale JR. Contact-lens application in four cases of congenital nystagmus. Optom Wkly. 1962;53:1865–1868.
- Jayaramachandran P, Proudlock FA, Odedra N, et al., A randomized controlled trial comparing soft contact lens and rigid gas-permeable lens wearing in infantile nystagmus. Ophthalmology. 2014;121(9):1827–1836.
- Theodorou M, Quartilho A, Xing W, et al., Soft contact lenses to optimize vision in adults with idiopathic infantile nystagmus: a pilot parallel randomized controlled trial. Strabismus. 2018;26(1):11–21.
- Golubović S, Marjanović S, Cvetković D, et al. The application of hard contact lenses in patients with congenital nystagmus. Fortschr Ophthalmol. 1989;86:535–539.
- Matsubayashi K, Fukushima M, Tabuchi A. Application of soft contact lenses for children with congenital nystagmus. Neuro-Ophthalmology. 1992;12(1):47–52.
- Abel LA, Williams IM, Levi L. Intermittent oscillopsia in a case of congenital nystagmus. Dependence upon waveform. Invest Ophthalmol Vis Sci. 1991;32:3104–3108.
- Safran AB, Gambazzi Y. Congenital nystagmus: rebound phenomenon following removal of contact lenses. Br J Ophthalmol. 1992;76(8):497–498.
- Enoch JM, Windsor CE. Remission of nystagmus following fitting contact lenses to an infant with aniridia. Am J Ophthalmol. 1968;66(2):333–335.
- Dell’Osso LF, Jacobs JB. An expanded nystagmus acuity function: intra- and intersubject prediction of best-corrected visual acuity. Doc Ophthalmol. 2002;104(3):249–276.
- Self JE, Dunn MJ, Erichsen JT, et al., Nystagmus UK Eye research group (NUKE). Management of nystagmus in children: a review of the literature and current practice in UK specialist services. Eye (Lond). 2020;34(9):1515–1534.
- Serra A, Dell’Osso LF, Jacobs JB, et al. Combined gaze-angle and vergence variation in infantile nystagmus: two therapies that improve the high-visual-acuity field and methods to measure it. Invest Ophthalmol Vis Sci. 2006;47(6):2451–2460.
- Dell’Osso L, Gauthier G, Liberman G, et al. Eye movement recordings as a diagnostic tool in a case of congenital nystagmus. Am J Optom Arch Am Acad Optom. 1972;49(1):3–13.
- Dickinson CM. The elucidation and use of the effect of near fixation in congenital nystagmus. Ophthalmic Physiol Opt. 1986;6(3):303–311.
- Spielmann A. Nystagmus. Curr Opin Ophthalmol. 1994;5(5):20–24.
- Ospina LH. Dealing with nystagmus. J Binocul Vis Ocul Motil. 2018;68(4):99–109.
- Gauthier GM, Dell’Osso L, Liberman G, et al. Etude et traitement d’un cas de nystagmus congénital [Study and treatment of a case of congenital nystagmus]. Rev Electroencephalogr Neurophysiol Clin. 1972;2(3):263–266.
- Anderson JR. Causes and treatment of congenital eccentric nystagmus. Br J Ophthalmol. 1953;37(5):267–281.
- Dell’Osso LF. Development of new treatments for congenital nystagmus. Ann N Y Acad Sci. 2002;956(1):361–379.
- Stegall FW. Symposium: nystagmus. Orthoptic aspects of nystagmus. Am Orthopt J. 1973;23(1):30–34.
- DellOsso LF. Prism exploitation of gaze and fusional null angles in congenital nystagmus. In: Moore S, Mein J, Stockbridge L, editors. Orthoptics: past, present, future. Miami: Symposia Specialists; 1976. p. 135–142.
- Tang X, Wang X, Zeng T, et al. Preliminary observation on the effect of pressing triple prism in correcting residual compensatory head posture after congenital nystagmus surgery. Int J Ophthalmol. 2013;13:2012–2013.
- Dell’Osso LF, Hertle RW, Leigh RJ, et al. Effects of topical brinzolamide on infantile nystagmus syndrome waveforms: eyedrops for nystagmus. J Neuroophthalmol. 2011;31(3):228–233.
- Hertle RW, Yang D, Adkinson T, et al., Topical brinzolamide (Azopt) versus placebo in the treatment of infantile nystagmus syndrome (INS). Br J Ophthalmol. 2015;99(4):471–476.
- Aldskogius H, Arvidsson J, Hansson P. Carbonic anhydrase enzyme histochemistry of cranial nerve primary sensory afferent neurons in the rat. Histochemistry. 1988;88(2):151–154.
- Aygit ED, Ocak OB, İnal A, et al. The effects of topical carbonic anhydrase inhibitor in treatment of nystagmus. Int Ophthalmol. 2018;38:265–269.
- Mehta AR, Kennard C. The pharmacological treatment of acquired nystagmus. Pr Neurol. 2012;12(3):147–153.
- Kalla R, Strupp M. Aminopyridines and acetyl-DL-leucine: new therapies in cerebellar disorders. Curr Neuropharmacol. 2019;17(1):7–13.
- McLean RJ, Gottlob I. The pharmacological treatment of nystagmus: a review. Expert Opin Pharmacother. 2009;10(11):1805–1816.
- Solomon D, Shepard N, Mishra A. Congenital periodic alternating nystagmus: response to baclofen. Ann N Y Acad Sci. 2002;956(1):611–615.
- Comer RM, Dawson EL, Lee JP. Baclofen for patients with congenital periodic alternating nystagmus. Strabismus. 2006;14(4):205–209.
- Shery T, Proudlock FA, Sarvananthan N, et al. The effects of gabapentin and memantine in acquired and congenital nystagmus: a retrospective study. Br J Ophthalmol. 2006;90(7):839–843.
- McLean R, Proudlock F, Thomas S, et al., Congenital nystagmus: randomized, controlled, double-masked trial of memantine/gabapentin. Ann Neurol. 2007;61(2):130–138.
- Thurtell MJ, Dell’osso LF, Leigh RJ, et al. Effects of acetazolamide on infantile nystagmus syndrome waveforms: comparisons to contact lenses and convergence in a well-studied subject. Open Ophthalmol J. 2010;4(1):42–51.
- Kestenbaum A. [New operation for nystagmus]. Bull Soc Ophtalmol Fr. 1953;6:599–602.
- Hertle RW, Anninger W, Yang D, et al. Effects of extraocular muscle surgery on 15 patients with oculo-cutaneous albinism (OCA) and infantile nystagmus syndrome (INS). Am J Ophthalmol. 2004;138(6):978–987.
- Papageorgiou E, McLean RJ, Gottlob I. Nystagmus in childhood. Pediatr Neonatol. 2014;55(5):341–351.
- Liu S, Kuht HJ, Moon EH, et al. Current and emerging treatments for albinism. Surv Ophthalmol. 2021;66(2):362–377.
- Dell’Osso LF, Flynn JT. Congenital nystagmus surgery. A quantitative evaluation of the effects. Arch Ophthalmol. 1979;97(3):462–469.
- Flynn JT, Dell’Osso LF. The effects of congenital nystagmus surgery. Ophthalmology. 1979;86(8):1414–1427.
- Parks MM. Symposium: nystagmus. Congenital nystagmus surgery. Am Orthopt J. 1973;23(1):35–39.
- Pratt-Johnson JA. The surgery of congenital nystagmus. Can J Ophthalmol. 1971;6:268–272.
- De Decker W. Kestenbaum transposition in nystagmus therapy. Transposition in horizontal and torsional plane. Bull Soc Belge Ophtalmol. 1987;221–222:107–120.
- Nelson LB, Ervin-Mulvey LD, Calhoun JH, et al. Surgical management for abnormal head position in nystagmus: the augmented modified Kestenbaum procedure. Br J Ophthalmol. 1984;68(11):796–800.
- Calhoun JH, Harley RD. Surgery for abnormal head position in congenital nystagmus. Trans Am Ophthalmol Soc. 1973;71:70–83.
- Kang NY, Isenberg SJ. Kestenbaum procedure with posterior fixation suture for anomalous head posture in infantile nystagmus. Graefes Arch Clin Exp Ophthalmol. 2009;247(7):981–987.
- Lee IS, Lee JB, Kim HS, et al. Modified Kestenbaum surgery for correction of abnormal head posture in infantile nystagmus: outcome in 63 patients with graded augmentaton. Binocul Vis Strabismus Q. 2000;15:53–58.
- Taylor JN, Jesse K. Surgical management of congenital nystagmus. Aust N Z J Ophthalmol. 1987;15(1):25–34.
- Scott WE, Kraft SP. Surgical treatment of compensatory head position in congenital nystagmus. J Pediatr Ophthalmol Strabismus. 1984;21(3):85–95.
- Sandall GS. Surgical treatment of congenital nystagmus in patients with singular binocular vision. Ann Ophthalmol. 1976;8:227–238.
- Kraft SP, O’Donoghue EP, Roarty JD. Improvement of compensatory head postures after strabismus surgery. Ophthalmology. 1992;99(8): 1301–138.
- Chang YH, Chang JH, Han SH, et al. Outcome study of two standard and graduated augmented modified Kestenbaum surgery protocols for abnormal head postures in infantile nystagmus. Binocul Vis Strabismus Q. 2007;22:235–241.
- Spielmann A. Clinical rationale for manifest congenital nystagmus surgery. J AAPOS. 2000;4(2):67–74.
- Biglan AW, Hiles DA, Ying-Fen Z, et al. Results after surgery for null point nystagmus with abnormal head position. Am Orthop J. 1989;39(1):134–142.
- Arroyo-Yllanes ME, Fonte-Vázquez A, Pérez-Pérez JF. Modified Anderson procedure for correcting abnormal mixed head position in nystagmus. Br J Ophthalmol. 2002;86(3):267–269.
- Bishop JE. A novel new [yet again] procedure for correction of compensatory head posture in infantile nystagmus; augmented Anderson plus dell’osso-hertle. Binocul Vis Strabolog Q Simms Romano. 2011;26:37–42.
- Gupta R, Sharma P, Menon V. A prospective clinical evaluation of augmented Anderson procedure for idiopathic infantile nystagmus. J AAPOS. 2006;10(4):312–317.
- Pierse D. Operation on the vertical muscles in cases of nystagmus. Br J Ophthalmol. 1959;43(4):230–233.
- Hertle RW, Yang D, Adams K, et al. Surgery for the treatment of vertical head posturing associated with infantile nystagmus syndrome: results in 24 patients. Clin Exp Ophthalmol. 2011;39:37–46.
- Schild AM, Fricke J, Rüssmann W, et al. Kestenbaum procedure on the vertical rectus muscles with simultaneous compensation of the induced cyclodeviation for nystagmus patients with chin-up or chin-down head posture. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1395–1400.
- Escuder AG, Ranka MP, Lee K, et al. The role of superior oblique posterior tenectomy along with inferior rectus recessions for the treatment of chin-up head positioning in patients with nystagmus. J Pediatr Ophthalmol Strabismus. 2018;55(4):234–239.
- Lee J. Surgical management of nystagmus. J R Soc Med. 2002;95(5):238–241.
- Conrad HG, De Decker W. “Rotatorischer Kestenbaum” – umlagerungschirurgie bei Kopfzwangshaltung zur Schulter [“Kestenbaum’s surgical rotation of the eyes” in patients with head tipped to the shoulder (author’s transl)]. Klin Monbl Augenheilkd. 1978;173:681–690.
- Lueder GT, Galli M. Oblique muscle surgery for treatment of nystagmus with head tilt. J AAPOS. 2012;16(4):322–326.
- von Noorden GK, Jenkins RH, Rosenbaum AL. Horizontal transposition of the vertical rectus muscles for treatment of ocular torticollis. J Pediatr Ophthalmol Strabismus. 1993;30(1):8–14.
- De Castro-abeger A, Benegas NM, Kushner B, et al. Horizontal transposition of the vertical rectus muscles to correct a head tilt in 5 patients with idiopathic nystagmus syndrome. Am J Ophthalmol. 2020;217:68–73.
- Spielmann A. Surgical treatment of nystagmus. J Metabol Ophthalmol. 1978;2:157–163.
- von Noorden GK, Sprunger DT. Large rectus muscle recessions for the treatment of congenital nystagmus. Arch Ophthalmol. 1991;109(2):221–224.
- Helveston EM, Ellis FD, Plager DA. Large recession of the horizontal recti for treatment of nystagmus. Ophthalmology. 1991;98(8):1302–1305.
- Atilla H, Erkam N, Işikçelik Y. Surgical treatment in nystagmus. Eye (Lond). 1999;13(1):11–15.
- Dell’Osso LF, Hertle RW, Williams RW, et al. A new surgery for congenital nystagmus: effects of tenotomy on an achiasmatic canine and the role of extraocular proprioception. J AAPOS. 1999;3(3):166–182.
- Khanna S, Dell’Osso LF. The diagnosis and treatment of infantile nystagmus syndrome (INS). ScientificWorldJournal. 2006;6:1385–1397.
- Hertle RW, Dell’Osso LF, FitzGibbon EJ, et al., Horizontal rectus tenotomy in patients with congenital nystagmus: results in 10 adults. Ophthalmology. 2003;110(11):2097–2105.
- Hertle RW, Yang D. Clinical and electrophysiological effects of extraocular muscle surgery on patients with infantile nystagmus syndrome (INS). Semin Ophthalmol. 2006;21(2):103–110.
- Hertle RW, Dell’Osso LF, FitzGibbon EJ, et al. Horizontal rectus muscle tenotomy in children with infantile nystagmus syndrome: a pilot study. J AAPOS. 2004;8(6):539–548.
- Wang Z, Dell’Osso LF, Jacobs JB, et al. Effects of tenotomy on patients with infantile nystagmus syndrome: foveation improvement over a broadened visual field. J AAPOS. 2006;10(6):552–560.
- Richards MD, Wong A. Infantile nystagmus syndrome: clinical characteristics, current theories of pathogenesis, diagnosis, and management. Can J Ophthalmol. 2015;50(6):400–408.
- Sinskey RM, Eshete A. Maximal subtotal extirpation of the horizontal rectus extraocular muscles for the treatment of nystagmus with no null point. A report of four successful human cases. Binocul Vis Strabismus Q. 2002;17:297–302.
- Lingua RW, Liu CY, Gerling A, et al. Myectomy of the extraocular muscles without reattachment as a surgical treatment for horizontal nystagmus. J Pediatr Ophthalmol Strabismus. 2016;53(3):156–166.
- Dell’Osso LF, Hertle RW, Jacobs JB. Clinical and ocular motor complications of extraocular muscle extirpation for infantile nystagmus syndrome. J AAPOS. 2018;22(2):110–114.
- Cüppers C. Probleme der operativen Therapie des okulären Nystagmus [Problems in the surgery for ocular nystagmus]. Klin Monbl Augenheilkd. 1971;159:145–157.
- Zubcov AA, Stärk N, Weber A, et al. Improvement of visual acuity after surgery for nystagmus. Ophthalmology. 1993;100(10):1488–1497.
- Felius J, Muhanna ZA. Visual deprivation and foveation characteristics both underlie visual acuity deficits in idiopathic infantile nystagmus. Invest Ophthalmol Vis Sci. 2013;54(5):3520–3525.
- Dell’Osso LF, Traccis S, Abel LA. Strabismus-a necessary condition for latent and manifest latent nystagmus. Neuro-ophthalmol. 1983;3(4):247–257.
- Von Noorden GK, Avilla C, Sidikaro Y, et al. Latent Nystagmus and Strabismic Amblyopia. Am J Ophthalmol. 1987;103(1):87–89.
- Simonsz HJ. The effect of prolonged monocular occlusion on latent nystagmus in the treatment of amblyopia. Doc Ophthalmol. 1989;72(3–4):375–384.
- Siganos DS, Evangelatou KA, Papadaki TG, et al. Photorefractive keratectomy in eyes with congenital nystagmus. J Refract Surg. 1998;14(6):649–652.
- Konuk O, Bilgihan K, Hasanreisoğlu B. Laser in situ keratomileusis in an eye with congenital nystagmus. J Cataract Refract Surg. 2001;27(4):636–638.
- Soloway BD, Roth RE. Laser in situ keratomileusis in a patient with congenital nystagmus. J Cataract Refract Surg. 2002;28(3):544–546.
- Mahler O, Hirsh A, Kremer I, et al. Laser in situ keratomileusis in myopic patients with congenital nystagmus. J Cataract Refract Surg. 2006;32(3):464–467.
- Barbara A, Shehadeh-Masha’our R, Garzozi HJ. Laser ablation in eyes with congenital nystagmus. J Refract Surg. 2007;23(6):623–625.
- Bagheri A, Abbasi H, Tavakoli M, et al. Effect of photorefractive keratectomy on nystagmus and visual functions in myopic patients with infantile nystagmus syndrome. Am J Ophthalmol. 2016;162:167–172.
- Fresina M, Giannaccare G, Gizzi C, et al. Photorefractive keratectomy in 22 adult eyes with infantile nystagmus syndrome. J Cataract Refract Surg. 2015;41(7):1448–1453.
- Montecucco C, Molgó J. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol. 2005;5(3):274–279.
- Wan MJ, AlShaker S, Hunter DG. Use of botulinum toxin in ophthalmology. Handb Exp Pharmacol. 2021;263: 147–160. Erratum in: Handb Exp Pharmacol. 2021;263:283.
- Carruthers J. The treatment of congenital nystagmus with Botox. J Pediatr Ophthalmol Strabismus. 1995;32(5):306–308.
- Oleszczyńska-Prost E. Toksyna botulinowa A w leczeniu oczoplasu wrodzonego u dzieci [Botulinum toxin A in the treatment of congenital nystagmus in children]. Klin Oczna. 2004;106:625–628.
- Hernández-García E, Gómez-De-Liaño-Sánchez R. Empleo de toxina botulínica en paciente con nistagmus congénito de tipo pendular [Use of botulinum toxin in a patient with pendular congenital nystagmus]. Arch Soc Esp Oftalmol. 2012;87(10):330–332.
- Thurtell MJ, Leigh RJ. Treatment of Nystagmus. Curr Treat Options Neurol. 2012;14(1):60–72.
- Helveston EM, Pogrebniak AE. Treatment of acquired nystagmus with botulinum A toxin. Am J Ophthalmol. 1988;106(5):584–586.
- Thomas R, Mathai A, Braganza A, et al. Periodic alternating nystagmus treated with retrobulbar botulinum toxin and large horizontal muscle recession. Indian J Ophthalmol. 1996;44:170–172.
- Ruben S, Dunlop IS, Elston J. Retrobulbar botulinum toxin for treatment of oscillopsia. Aust N Z J Ophthalmol. 1994;22(1):65–67.
- Repka MX, Savino PJ, Reinecke RD. Treatment of acquired nystagmus with botulinum Neurotoxin A. Arch Ophthalmol. 1994;112(10):1320–1324.
- Tomsak RL, Remler BF, Averbuch-Heller L, et al. Unsatisfactory treatment of acquired nystagmus with retrobulbar injection of botulinum toxin. Am J Ophthalmol. 1995;119(4):489–496.
- Leigh RJ, Tomsak RL, Grant MP, et al. Effectiveness of botulinum toxin administered to abolish acquired nystagmus. Ann Neurol. 1992;32(5):633–642.
- Blekher T, Yamada T, Yee RD, et al. Effects of acupuncture on foveation characteristics in congenital nystagmus. Br J Ophthalmol. 1998;82(2):115–120.
- Blechschmidt T, Krumsiek M, Todorova MG. The effect of acupuncture on visual function in patients with congenital and acquired nystagmus. Medicines (Basel). 2017;4(2):33.
- Vingolo EM, Napolitano G, Fragiotta S. Microperimetric biofeedback training: fundamentals, strategies and perspectives. Front Biosci (Schol Ed). 2018;10(1):48–64.
- Ratra D, Gopalakrishnan S, Dalan D, et al. Visual rehabilitation using microperimetric acoustic biofeedback training in individuals with central scotoma. Clin Exp Optom. 2019;102(2):172–179.
- Ciuffreda KJ, Goldrich SG, Neary C. Use of eye movement auditory biofeedback in the control of nystagmus. Am J Optom Physiol Opt. 1982;59(5):396–409.
- Grenga PL, Trabucco P, Meduri A, et al. Microperimetric biofeedback in a patient with oculocutaneous albinism. Can J Ophthalmol. 2013;48(5):105–107.
- Caputo R, Febbrini Del Magro E, Amoaku WM, et al. The efficacy of biofeedback visual rehabilitation therapy in patients with infantile nystagmus syndrome: a retrospective study. Eur J Ophthalmol. 2020 Jul 6:1120672120940981. Epub ahead of print. DOI:https://doi.org/10.1177/1120672120940981.
- Daibert-Nido M, Pyatova Y, Markowitz M, et al. Post audio-visual biofeedback training visual functions and quality of life in paediatric idiopathic infantile nystagmus: a pilot study. Eur J Ophthalmol. 2021 Jan 26:1120672121991048. Epub ahead of print. DOI:https://doi.org/10.1177/1120672121991048.
- Lee JH, Wang JH, Chen J, et al. Gene therapy for visual loss: opportunities and concerns. Prog Retin Eye Res. 2019;68:31–53.
- Thomas MG, Maconachie G, Sheth V, et al., Development and clinical utility of a novel diagnostic nystagmus gene panel using targeted next-generation sequencing. Eur J Hum Genet. 2017;25(6):725–734.
- Gargiulo A, Bonetti C, Montefusco S, et al. AAV-mediated tyrosinase gene transfer restores melanogenesis and retinal function in a model of oculo-cutaneous albinism type I (OCA1). Mol Ther. 2009;17(8):1347–1354.
- Tomita Y, Takeda A, Okinaga S, et al. Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem Biophys Res Commun. 1989;164(3):990–996.
- Hori T, Fukutome M, Koike C. Adeno Associated Virus (AAV) as a tool for clinical and experimental delivery of target genes into the mammalian retina. Biol Pharm Bull. 2019;42(3):343–347.
- Song Y, Zhang Y, Chen M, et al. Functional validation of the albinism-associated tyrosinase T373K SNP by CRISPR/Cas9-mediated homology-directed repair (HDR) in rabbits. EBioMedicine. 2018;36:517–525.
- Russell-Eggitt I, Kriss A, Taylor DS. Albinism in childhood: a flash VEP and ERG study. Br J Ophthalmol. 1990;74(3):136–140.
- Wack MA, Peachey NS, Fishman GA. Electroretinographic findings in human oculocutaneous albinism. Ophthalmology. 1989;96(12):1778–1785.
- Self JE, Lee H. Novel therapeutics in nystagmus: what has the genetics taught us so far? Therapeutic Adv Rare Disease. 2021 Jan;2:263300402199871.