ABSTRACT
Introduction
Perfluorohexyloctane (PFHO) ophthalmic solution (brand name, MIEBO) was recently approved by the United States Food and Drug Administration to treat the signs and symptoms of dry eye disease (DED). Unlike most DED treatments, PFHO addresses evaporative DED, which represents the vast majority of DED cases. PFHO may function as a surrogate for the tear film’s lipid layer, inhibiting evaporation.
Areas covered
This article summarizes data – found via PubMed and clinicaltrials.gov (May 30-1 September 2023) – surrounding PHFO and provides some considerations for its use. Preclinically, PFHO’s unique molecular properties facilitate formation of a layer at the tear film’s air-liquid interface and inhibit saline evaporation by 81%. These properties translate to improved clinical outcomes in DED: in phase 3 studies, 40% to 50% of PFHO-group patients had a ≥ 3-step total corneal fluorescein staining improvement at day 57, and approximately 60% of patients had ≥ 30% reduction in visual analog scale dryness scores, improvements that persisted to 52 weeks. Additionally, PFHO demonstrated a robust safety profile.
Expert opinion
PFHO is the first prescription eye drop that directly addresses hyperevaporation in DED. PFHO is poised to help fill the large unmet need of patients with evaporative DED, potentially improving patient outcomes and quality of life.
1. Introduction
Dry eye disease (DED) is a common ocular surface disorder that affects approximately 344 million people worldwide and can lead to reduced vision [Citation1,Citation2]. DED typically affects adults, with increasing incidence with age; DED is particularly prevalent among older women [Citation1,Citation3]. If left untreated, DED can progress in severity. Recently reviewed by Tsubota and colleagues (2020), DED symptoms – most commonly ocular surface discomfort – often worsen as the day goes on or with prolonged reading or digital device use [Citation4,Citation5].
DED is characterized by loss of tear film homeostasis, which can be caused by either inadequate aqueous tear production or hyper-evaporation of the tear film. Often, DED involves both aqueous-deficient and evaporative components [Citation6]. In both cases, loss of tear film homeostasis leads to inflammation and ocular tissue damage, further exacerbating tear film dysfunction [Citation5]. In evaporative dry eye, the primary cause is meibomian gland dysfunction [Citation7]. Meibomian glands produce lipids that protect the aqueous portion of the tear film from evaporation, but in meibomian gland dysfunction, reduced or altered tear film lipids result in a changed tear film lipid layer, leading to tear film instability and, thus, more rapid evaporation [Citation8–11].
Prescription treatment for DED is aimed at restoring the tear film lipid layer and addressing inflammation, thereby reducing ocular signs and symptoms of the disease. Management of DED often follows a stepwise approach that starts with eyelid hygiene, warm compresses, and ocular lubricants. When the initial steps fail to provide adequate improvement, management progresses to the use of office-based therapies and prescription medications. For a detailed look at current management strategies please see the recent cited articles [Citation12,Citation13].
It is now known that meibomian gland dysfunction may contribute to as much as 86% of DED cases [Citation7]. Though meibum inhibits evaporation by only 8% [Citation14], it may still provide significant contributions to tear film stability, which is characteristically disrupted in DED. Holding on to the aqueous component of tears minimizes desiccant stress and inflammation, allowing the ocular surface time for repair. Despite the known role of meibum in DED, many current dry eye treatments primarily focus on tear replacement, which only addresses aqueous deficiency or underlining inflammation; tear replacement therapies do not address excess evaporation, a component of the vast majority of dry eye cases [Citation2]. Additionally, tear film replacers have several limitations, including short ocular surface residence times, visual disturbances such as blurring, and preservative-related toxicity [Citation15,Citation16]. Treatments that attempt to reduce the inflammation cycle, such as cyclosporine drops, have recently been shown to be less effective than initially observed [Citation12,Citation13]. Additionally, other anti-inflammatory treatments such as corticosteroids are problematic, with long-term use associated with increased risk of intraocular pressure (IOP) and cataract formation.
Until recently, there were no US Food & Drug Administration (FDA)-approved prescription pharmacotherapies for DED that directly target tear film evaporation [Citation7,Citation17,Citation18]. This review provides an overview of perfluorohexyloctane (PFHO) ophthalmic solution (previously known as the investigational agent NOV03, which will be marketed under the brand name MIEBO, Bausch + Lomb, Bridgewater, NJ, U.S.A.), the recently approved first-in-class, water-free, non-steroidal, single-component, preservative-free topical drop indicated for treatment of the signs and symptoms of DED. The data discussed were found via PubMed searches for ‘perfluorohexyloctane,’ ‘dry eye disease,’ and related terms (May 30 through 1 September 2023). PubMed was also searched for the names of leaders in ophthalmology known to participate in research on DED and/or PFHO. Additionally, clinical trial information was, where needed, found using searches of clinicaltrials.gov.
2. The unique properties of perfluorohexyloctane
PFHO has undergone a thorough study and comprehensive characterization within the Novaliq (Heidelberg, Germany) non-clinical and pre-clinical testing program, then progressing to an extensive clinical study effort in collaboration with Bausch + Lomb (Bridgewater, NJ, U.S.A.). This partnership has brought to light the unique properties and attributes of PFHO. PFHO (C14H17F13) is a semifluorinated alkane characterized by its molecular structure, which imparts a distinct set of properties [Citation12,Citation19,Citation20]. It comprises a lipophobic/aerophilic fluorinated segment and a lipophilic hydrocarbon segment [Citation12,Citation20]. This structure potentially enables PFHO to emulate crucial functions of meibum by forming a liquid layer at the air-liquid interface of the tear film, effectively inhibiting evaporation [Citation19]. Furthermore, its bipartite molecular structure grants PFHO a reduced surface tension, facilitating swift spreading across the ocular surface [Citation12,Citation21]. This spreading property also aids in minimizing friction, ultimately contributing to reduced discomfort during blinking. The lower surface tension enables PFHO to yield smaller drop sizes compared to aqueous drops, potentially mitigating the issue of drop overflow after instillation.
Like other semifluorinated alkanes, PFHO is clear [Citation12,Citation20], with a refractive index similar to that of water, which helps to minimize vision blurring after instillation [Citation16]. In a clinical study of 57 patients with low lipid layer thickness (≤75 interferometric color units), the use of PFHO was not associated with higher-order visual aberrations but was linked to improved noninvasive-tear-breakup-time, indicating that PFHO may help stabilize the tear film in patients with thinner lipid layer thicknesses [Citation22]. As PFHO contains no water, it cannot support the microbial growth that can lead to ocular infections, allowing it to be preservative-free and thereby avoid concerns about the preservative-related ocular toxicity seen with aqueous drops [Citation12,Citation20]. Additionally, because PFHO is non-aqueous, it has no pH, a common source of ocular irritation in other topical drops in the physiological range. As with other semifluorinated alkanes, PFHO is chemically and physiologically inert, which can further reduce the risk for ocular irritation.
The extent of PFHO’s systemic availability following its application to the eye’s surface has been meticulously examined in a single pharmacokinetic investigation, revealing significantly limited absorption. In experimental animal models, it was observed that the majority of PFHO, when administered to the eye, undergoes evaporation from the ocular area into the atmosphere. Any minute quantity finding its way into the digestive tract through tear duct drainage is excreted within a span of 24 hours via fecal matter [Citation23]. The fractional PFHO that undergoes systemic absorption through topical application is subsequently expelled through exhalation. In an environmental analysis conducted for the US FDA approval of PFHO ophthalmic solution, it was estimated that the concentration of PFHO at the point of entry into the aquatic environment would be < 1 part per billion [Citation23]. Thus, while minimal PFHO quantities may enter the environment, those levels are expected to be extremely low, and the non-hazardous nature and low bioavailability of PFHO indicate that its effects in the environment would likely be negligible.
Another potential benefit of PFHO is that, like other semifluorinated alkanes, it can act as an O2 carrier, as semifluorinated alkanes are known to transport O2 [Citation24]. Using 19F-nuclear magnetic resonance spectroscopy, PFHO was found to have an O2-carrying capacity of about 260 mmHg (O2 partial pressure), 62% higher than that of air. Therefore, PFHO layered on the surface of the eye is not a barrier to O2 but facilitates O2 transport to the corneal surface, which is of vital importance for maintaining the health of the avascular corneal epithelium, promoting corneal wound healing, and preventing corneal edema [Citation25,Citation26].
3. Preclinical data on perfluorohexyloctane
Regarding the PFHO mechanism of action, it is thought that PFHO helps improve tear film stability by inhibiting evaporation: in vitro data show that PFHO decreases the evaporation rate of saline [Citation14]. In this study, evaporation rates were measured gravimetrically at 25°C for saline alone and with various volumes of PFHO layered on top. PFHO in volumes ≥50 μL inhibited saline evaporation by approximately 80% (). In an experiment more closely resembling its clinical use, a single, 11-μL drop of PFHO inhibited saline evaporation by 28% [Citation14]. When comparing evaporation rates of saline alone or saline with PFHO, meibum, or both at 37°C, 100 μL PFHO alone inhibited the rate of saline evaporation by 88% (P < 0.0001); adding a 125-nm-thick layer of meibum alone inhibited the rate of saline evaporation by only 8%, while adding meibum along with 100 μL PFHO resulted in an 83% inhibition of the saline evaporation rate (P < 0.0001) (). Thus, in vitro, PFHO is significantly more effective than meibum at inhibiting evaporation.
Figure 1. Mean evaporation rates of saline alone or overlaid with PFHO, meibum, or commercially available artificial tears. (a) saline alone and with increasing amounts of PFHO at 25°C. (b) saline alone, saline + meibum, and saline overlaid with human meibum with and without the addition of either 100 μL PFHO layered on top at 35°C. (c) saline alone and with 100 μL of various over-the-counter eye drops and PFHO at 25°C. *P<.0001. Error bars represent the standard error of the mean. PFHO, perfluorohexyloctane.
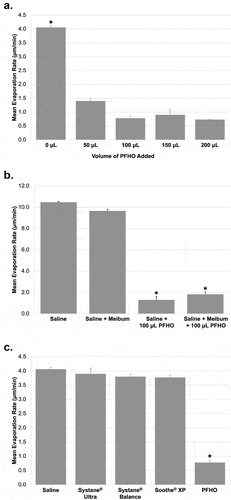
The study also examined how adding commerciallyavailable over-the-counter tear replacers affects saline evaporation rates () [Citation14]. While PFHO reduced the rate of saline evaporation by 81% (P < 0.0001) at 25°C, none of the over-the-counter drops significantly inhibited saline evaporation (P ≥0.13 versus saline alone).
PFHO can also be distinguished from aqueous-based drops by its longer residence time. The pharmacokinetic properties of PFHO were assessed in rabbit eyes after bilateral topical 14Cradiolabeled PFHO (14C-PFHO) administration followed by liquid scintillation counting to measure radioactivity levels [Citation25]. Eyes had high 14C-PFHO levels in tears for at least six hours. 14C-PFHO also had extended residence in the meibomian glands, suggesting that meibomian glands may act as depot sites. Negligible amounts of 14C-PFHO were observed in the posterior segment tissues or absorbed systemically. In line with preclinical data, in vivo pharmacokinetics data and clinical pharmacokinetics data indicate that there is very little systemic PFHO following ocular instillation. In the phase 2 SEECASE study (N = 336), which is discussed below in more detail, PFHO was not detectable in > 70% of blood samples [Citation25].
4. Clinical research
The clinical development program for PFHO consisted of a comprehensive set of studies conducted to characterize the tolerability, efficacy, and safety of PFHO (). The FDA approval of PFHO ophthalmic solution was supported by a randomized, controlled, phase 2 study (SEECASE), 2 randomized, controlled phase 3 clinical trials (GOBI and MOJAVE), and an open-label safety extension study (KALAHARI) of a subset of patient from the GOBI study. Each of these studies demonstrated that PFHO is well tolerated and associated with reduced dry eye signs and symptoms [Citation12,Citation21,Citation27]. Additionally, because PFHO ophthalmic solution is 100% active drug molecule – there are no solvents or excipients [Citation19]—the clinical program could not have assessments by dose level and, thus, no safety or efficacy concerns related to changes in drug concentration.
Table 1. Clinical program studies featuring PFHO [Citation12,Citation21,Citation27,Citation28].
The SEECASE (phase 2) and GOBI and MOJAVE (phase 3) studies were each multicenter, randomized, double-masked, saline-controlled studies designed to evaluate the effect of PFHO on the signs and symptoms of DED. Included patients (SEECASE, N = 336; GOBI, N = 597; MOJAVE, N = 620) had a self-reported history of DED in both eyes for ≥6 months with a tear film breakup time (TFBUT) ≤5 seconds, Ocular Surface Disease Index (OSDI) score ≥ 25, unanesthetized Schirmer I test results of ≥5 mm, total meibomian gland dysfunction score of ≥ 3, and total corneal fluorescein staining (tCFS) score ≥ 4 and ≤ 11 [Citation12,Citation21]. For the GOBI and MOJAVE (phase 3) studies, patients were randomized to receive either PFHO or hypotonic saline (0.6%). In SEECASE, patients were dosed four times daily (QID) or twice daily (BID), and in GOBI and MOJAVE, patients were dosed QID. Demographics and baseline clinical characteristics were similar between treatment groups.
4.1. Efficacy of perfluorohexyloctane in clinical trials
In SEECASE, the primary endpoint was the change from baseline (CFB) in investigator-rated tCFS, while patient-reported visual analog scale (VAS) dryness score and OSDI score were secondary endpoints, all at 8 weeks [Citation28]. SEECASE met its primary and secondary endpoints, with PFHO instilled QID demonstrating significant decreases at 8 weeks in tCFS (P < 0.001) and VAS dryness score (P < 0.001) versus saline; CFB in OSDI score was numerically decreased (P = 0.087). Additionally, significantly greater reductions for all three assessments were observed at week 2 compared to the saline control.
For GOBI and MOJAVE, the primary efficacy sign and symptom endpoints were investigator-rated CFB in tCFS and patient-reported VAS dryness score at day 57 [Citation12,Citation21]. Secondary efficacy endpoints included CFB in VAS dryness score and tCFS (National Eye Institute scale) at day 15, and VAS burning/stinging score and central corneal fluorescein staining (cCFS) at day 57.
GOBI and MOJAVE met both their signs and symptoms endpoints, with significantly greater CFBs in the PFHO group versus the saline group in tCFS and VAS dryness score at day 57 (P < 0.001) () [Citation12,Citation21]. Statistically significant between-group differences in tCFS and VAS dryness score were observed as early as week 2. In GOBI, more than 40% of patients receiving PFHO had a ≥ 3-step improvement in tCFS score, and 57% patients receiving PFHO had ≥ 30% reduction in VAS dryness scores [Citation21]. These rates were similar in MOJAVE, with 50% of patients in the PFHO group showing a ≥ 3-step improvement in tCFS score, and more than 60% of PFHO-group patients showing ≥ 30% reduction in VAS dryness scores [Citation12]. Additionally, all key secondary endpoints were met in GOBI and MOJAVE. At day 15, tCFS was significantly improved (mean treatment differences CFB [95% confidence interval (CI)] in GOBI: −0.6 [−0.9, −0.2], P < 0.01; in MOJAVE: −0.6 [−1.0, −0.2]; P < 0.001) as was VAS dryness score (mean treatment differences CFB [95% CI] in GOBI: −4.7 [−8.2, −1.2]; in MOJAVE: −7.8 [−11.3, −4.3]; P < 0.001 for both) [Citation12,Citation21]. At day 57, VAS burning/stinging scores improved by −5.5 (95% CI: −9.5, −1.6; P < 0.01) in GOBI and −7.3 (95% CI: −11.3, −3.4; P < 0.001) in MOJAVE. cCFS at day 57 improved significantly by −0.2 (95% CI: −0.4, −0.1; P < 0.01) in GOBI and −0.3 (95% CI: −0.5, −0.2;P < 0.001) in MOJAVE.
Figure 2. CFB at day 57 in tCFS (a) and VAS dryness scores (b) in the PFHO and saline control groups from the GOBI and MOJAVE studies [Citation12,Citation20]. *P<.001. Error bars represent the SEM. CFB, change from baseline; PFHO, perfluorohexyloctane; SEM, standard error of the mean; tCFS, total corneal fluorescein staining; VAS, visual analog scale.
![Figure 2. CFB at day 57 in tCFS (a) and VAS dryness scores (b) in the PFHO and saline control groups from the GOBI and MOJAVE studies [Citation12,Citation20]. *P<.001. Error bars represent the SEM. CFB, change from baseline; PFHO, perfluorohexyloctane; SEM, standard error of the mean; tCFS, total corneal fluorescein staining; VAS, visual analog scale.](/cms/asset/2d411e56-efeb-42cd-b865-de32eff4eab8/ierl_a_2275586_f0002_b.gif)
The third phase 3 study, KALAHARI, was a multicenter, open-label, single-arm extension safety study of a subset of patients who completed the GOBI study, with data collected for up to 52 weeks [Citation27]. Data from KALAHARI, recently presented at the annual meetings of the Association for Research in Vision and Ophthalmology and the American Optometric Association, indicate that PFHO remained effective in reducing the signs and symptoms of DED with long-term use.
In addition to clinical studies conducted in the US, a study similar to GOBI and MOJAVE was conducted in China: patients with DED (N = 312) received PFHO QID for 57 days and assessed primarily for tCFS and VAS dryness score [Citation29]. Consistent with GOBI and MOJAVE, patients in this study had significant reductions from baseline compared to saline in both mean (standard deviation [SD]) tCFS (−3.8 [2.7] versus −2.7 [2.8]; P < 0.001) and VAS dryness score (−38.6 [21.9] versus −28.3 [20.8]; P < 0.001) at day 57, with significant effects seen as early as day 15. In addition to the primary assessments, the study also examined a number of factors that were not included in the primary GOBI and MOJAVE analyses. At day 57, patients receiving PFHO had significantly reduced scores for pain, DED symptom awareness, and frequency of ocular dryness.
In addition to these larger studies, a small (N = 72) 6- to 8-week, observational, prospective, multicenter study conducted in Germany examined the ability of PFHO QID to improve tear film stability by recording the mean TFBUT in each eye [Citation30]. At 6 to 8 weeks, patients receiving PFHO had significantly improved mean TFBUT versus baseline in both eyes by approximately 3 seconds (P < 0.0001). In addition, OSDI values significantly decreased from a mean (SD) of 37 (13) to 26 (16). The four adverse events (AEs) related to study drug were considered mild to moderate. Best-corrected visual acuity (BCVA) and IOP did not change over the study period.
4.2. Safety of perfluorohexyloctane in phase 3 trials
Throughout clinical testing, PFHO demonstrated a low incidence of AEs – most of which were considered mild or moderate in severity – out to 52 weeks. This was true for patients who received PFHO in SEECASE, who had a low incidence of AEs and similar AE rates across all treatment groups [Citation28]. Treatment-emergent ocular AEs were reported by 4.5% in the PFHO BID group, 11.4% in the PFHO QID group, and 11.7% in the saline group (BID and QID), and most events were mild or moderate in severity. Ocular AEs that occurred in > 2% of PFHO-treated eyes were blurred vision and eye irritation. Blurred vision occurred in 2.6% of PFHO QID patients, compared to 0% of PFHO BID patients, while 0.9% experienced blurred vision in the saline group. Eye irritation occurred in 0.9% of patients in the QID group, 2.7% of patients in the BID group, and 0% of patients in the saline group. There were no meaningful changes in slit-lamp biomicroscopy, dilated fundoscopy, mean visual acuity, or mean IOP.
In GOBI and MOJAVE, PFHO instilled QID had a favorable safety profile [Citation12,Citation21]. In the PFHO and saline control groups, AEs occurred at similar frequencies. In GOBI, ocular AEs occurred in 9.6% of patients in the PFHO group and 7.5% of patients in the saline control group. In MOJAVE, these rates were 12.9% in the PFHO group and 12.3% in the saline control group.
In both GOBI and MOJAVE, most ocular AEs were considered mild in severity [Citation12,Citation21]. In GOBI, the ocular AEs occurring in ≥ 1% of patients in the PFHO group were blurred vision, instillation site pain, and eye discharge [Citation21]; in MOJAVE, these were blepharitis, conjunctival hyperemia, conjunctival papillae, ocular hyperemia, blurred vision, hordeolum (stye), and visual acuity reduction () [Citation12]. There were no serious ocular AEs reported in either study.
Table 2. Most common ocular AEs reported in GOBI, MOJAVE [Citation12,Citation21].
Across both GOBI and MOJAVE, only one patient experienced an AE leading to discontinuation [Citation12,Citation21]. There was one severe AE of bilateral eye irritation in GOBI, which was considered suspected related or related to treatment and led to treatment discontinuation. Beyond AEs, there were no clinically significant changes in other safety measures – BCVA, slit-lamp examination, intraocular pressure, and dilated fundoscopy – in either GOBI or MOJAVE.
Similar to GOBI and MOJAVE, AE rates were low in KALAHARI, and most ocular AEs reported were considered mild in severity [Citation27]. There were no new safety signals in the year-long KALAHARI safety extension study.
The PFHO safety profile also extended to the studies conducted in China and Germany [Citation29,Citation30]. The study conducted in China had similar rates of ocular AEs compared to GOBI and MOJAVE, and the US, Chinese, and German studies all maintained favorable safety profiles [Citation12,Citation21,Citation29,Citation30].
5. Conclusion
Current therapies for DED may target aqueous-deficient dry eye or inflammation, yet the vast majority of DED cases stem from hyper-evaporation, such as in meibomian gland dysfunction [Citation6]. With its recent FDA approval, however, PFHO ophthalmic solution can potentially address a previously unmet need for pharmacotherapeutics specifically indicated to treat the signs and symptoms of DED, including cases stemming from hyperevaporation. Because PFHO likely acts as a surrogate for the lipid layer of the tear film, it significantly inhibits evaporation of saline in vitro and may provide even better inhibition of evaporation than that provided by meibum lipids, positioning it as a potential option for the millions of patients with evaporative dry eye [Citation24]. PFHO will likely help patients with aqueous-deficient dry eye as well, as it will help them hold on to the aqueous component of the tear film and, indirectly, address ocular inflammation.
Across the two pivotal safety and efficacy trials, PFHO consistently provided significant improvements in the signs and symptoms of DED compared to hypotonic saline control [Citation12,Citation21]. tCFS and VAS dryness scores improved significantly compared to saline over these 8-week studies, with significant differences detected as early as day 15 [Citation12,Citation21]. PFHO has a favorable safety profile, with low rates of ocular AEs—none serious—and only one ocular AE leading to study discontinuation; the KALAHARI long-term safety extension study further demonstrated that PFHO is effective and maintains a low rate of AEs over the long term [Citation12,Citation21,Citation27].
With its unique physical and chemical properties, PFHO is the first prescription eye drop that directly addresses the evaporative component of DED, representing a new type of eye drop altogether. As a first-in-class prescription therapy indicated to treat the signs and symptoms of DED by inhibition of tear film hyper-evaporation, PFHO is poised to serve the large unmet need of patients with evaporative DED. We hope that highlighting some of the practical considerations of PFHO use () will help clinicians think preemptively about incorporating PFHO into their armamentariums and help lessen any barriers to its adoption for the treatment of DED in clinical practice.
Table 3. Practical questions and responses for eye care professionals when using PFHO (investigational name NOV03; brand name MIEBO).
6. Expert opinion
The dry eye space has long focused on aqueous deficiency despite the fact that 86% of dry eye cases stem, at least in part, from excess evaporation [Citation7].
It is well known that most current prescription DED treatments primarily focus on addressing the tear film lipid layer or underlying inflammation associated with DED. This is compounded by over-the-counter tear replacers, which are used ubiquitously but are limited by their short residence times and ineffectiveness in addressing tear film evaporation [Citation15], resulting in either continual re-administration or the return of ocular discomfort. PFHO is the first prescription eye drop that directly addresses the evaporative component of DED. PFHO is thought to act as a surrogate for the tear film lipid layer [Citation33]. In doing so, PFHO can help inhibit tear film evaporation [Citation14] and potentially help increase tear film stability. Additionally, because tear film instability is inherent to both evaporative and aqueous-deficient DED, PFHO’s ability to support the function of the lipid layer of the tear film has the potential to address signs and symptoms of all forms of DED.
PFHO demonstrated significant improvements in the signs and symptoms of DED compared to hypotonic saline control across two pivotal safety and efficacy trials (GOBI and MOJAVE) [Citation12,Citation21]. tCFS and VAS dryness scores improved significantly compared with saline over the 8-week studies, with significant differences detected as early as day 15. Low rates of ocular AEs were also seen in the large pivotal trials, indicating a favorable safety profile for PFHO. In the open-label safety extension study (KALAHARI), PFHO was observed to be effective while maintaining a low rate of AEs [Citation27].
Just as DED is a multifactorial disease, the possibilities for future experiments on PFHO – both at the bench and in the clinic – are myriad. While PFHO is known to develop a liquid layer atop the tear film, many details of its interaction with meibum lipids and the mucoaqueous tear film layer are yet to be determined. Additionally, it will be interesting to explore how PFHO behaves and redistributes in the tear film during and after blinking. Clinically, it would be very valuable to understand where PFHO sits in pre- and post-operative treatment for patients who will undergo ocular surgery. It will also be useful to determine the effectiveness of PFHO when used in combination with instrumental therapies, such as intense pulsed light. There are many questions to be answered regarding PFHO; for this reason, dry eye research has ample opportunity to explore the chemical and clinical effects of PFHO over the next several years.
Adoption of PFHO into clinical practice will likely have minimal hurdles. PFHO is a water-free, preservative-free, steroid-free, single-ingredient formulation. As PFHO is among the first treatments to address excess evaporation in DED, it could become clinicians’ first choice for DED therapy. Self-administration by patients will be similar to how many other eye drop treatments that have been widely adopted, and its QID dosing will not be exceedingly burdensome. This has been reinforced by the comfort and satisfaction data collected during the PFHO clinical study program. Overall, PFHO is well-positioned to meet the significant, unmet needs of patients suffering from evaporative DED, offering the promise of enhancing both patient outcomes and their overall quality of life.
Article highlights
While prescription DED treatments often aim at restoring the tear film lipid layer and addressing inflammation, many patients first treat their dry eye using aqueous tear replacement, which is typically ineffective for the majority of patients with DED, who have evaporative dry eye; the recently US Food & Drug Administration (FDA)-approved perfluorohexyloctane (PFHO) ophthalmic solution, however, is a water- and preservative-free topical drop specifically indicated to treat both signs and symptoms of DED by inhibiting tear film hyper-evaporation
PFHO ophthalmic solution is thought to act as a meibum surrogate, addressing the major root cause of dry eye: evaporation.
Preclinical data have demonstrated that following PFHO administration, PFHO is retained in tears for at least 6 hours and in meibomian glands for up to 24 hours, while it inhibits the evaporation of saline by approximately 80% in vitro.
Clinically, PFHO has consistently been shown across two pivotal safety and efficacy trials to significantly reduce signs, such as total corneal fluorescein staining, and symptoms, as indicated by the visual analog scale (VAS) dryness score, of DED. Moreover, PFHO’s robust and well-characterized tolerability profile further enhance its promise as an option for patients with DED.
As a new type of DED therapy, there are ample opportunities to explore its chemical properties, interactions with the tear film, and clinical effects. Additional areas of interest include effects of PFHO on tear film stability, ocular surface healing, and quality of life.
Declaration of interests
Consulting Fees— AdOM, Alcon, Aldeyra, Allergan/Abbvie, Apellis, Atlas, Aurion, Avellino, Azura, Bausch + Lomb, BioTissue, Bruder, Bruno, Dompe, Eyedetec, Healthe, Horizon, Imprimis, Iveric, Konan, Neurolens, Oasis Medical, Ocuphire, Oculus, OcuMedic, OcuSoft, Olympic Ophthalmics, Orasis, RegenerEyes, Rendia, Reichert, RVL Pharmaceuticals, RxSight, Santen, Science Based Health, Scope, Sentiss, Sight Sciences, Silk Technologies, Sun Pharmaceuticals, Surface, Sydnexis, Tarsus, TearClear, Thea, Vial, Viatris, Visant Medical, Vital Tears, WebMD; Payment or Honoraria—Bausch + Lomb, Dompe, Iveric, Mallinckrodt, Sun Pharmaceuticals, Viatris; Stock or stock options—AdOM, Aviana, AI Optics, Azura, Barti, Danelli Ocular Creations, Enchroma, IKKDA, Eyedaptec, Eyedetec, Eyesafe, Hui.AI, iOR Holdings, Iveena, LacriSciences, LeGrande, LenTechs, Lubris, Mati Therapeutics, New Sight Reality, Ocular Science, OcuMedic, OcuPhire, OM Solutions, Omega Ophthalmics, Omera Medical, Ophthalmic Resources, Orasis, Percept, Tarsus, Healthe, Cambium, Olympic Ophthalmics, RegenerEyes, Silk Technologies, Stuart Therapeutics, TearClear, TearSolutions, TecLens, Visant Medical, Vision Path.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Each author made substantial contributions to the conception and design of the article. Each author reviewed the literature, provided substantive input and edits to this article, and gave final approval of the article.
Acknowledgments
Editorial and medical writing assistance was provided under the direction of the authors by Hayley Clay, PhD (Ethis, Inc) and funded by Bausch + Lomb, Inc.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Morthen MK, Magno MS, Utheim TP, et al. The vision-related burden of dry eye. Ocul Surf. 2022;23:207–215. doi: 10.1016/j.jtos.2021.10.007
- Sun M, Moreno IY, Dang M, et al. Meibomian gland dysfunction: what have animal models taught us? Int J Mol Sci. 2020;21(22):8822. doi: 10.3390/ijms21228822
- Dana R, Bradley JL, Guerin A, et al. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age United States health care system. Am J Ophthalmol. 2019;202:47–54. doi: 10.1016/j.ajo.2019.01.026
- Tsubota K, Pflugfelder SC, Liu Z, et al. Defining dry eye from a clinical perspective. Int J Mol Sci. 2020;21(23):9271. doi: 10.3390/ijms21239271
- Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017 Oct;15(4):802–812. doi: 10.1016/j.jtos.2017.08.003
- McMonnies CW. Aqueous deficiency is a contributor to evaporation-related dry eye disease. Eye Vis (Lond). 2020;7(1):6. doi: 10.1186/s40662-019-0172-z
- Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi: 10.1097/ICO.0b013e318225415a
- Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmol. 2017;124(11S):S20–6. doi: 10.1016/j.ophtha.2017.05.031
- Covita A, Chen MH, Leahy C. Correlation between meibomian gland appearance and tear breakup time using a slit scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 2019;60(9):6793.
- Kim WJ, Ahn YJ, Kim MH, et al. Lipid layer thickness decrease due to meibomian gland dysfunction leads to tear film instability and reflex tear secretion. Ann Med. 2022;54(1):893–899. doi: 10.1080/07853890.2022.2056238
- Li J, Ma J, Hu M, et al. Assessment of tear film lipid layer thickness in patients with Meibomian gland dysfunction at different ages. BMC Ophthalmol. 2020;20(1):394. doi: 10.1186/s12886-020-01667-8
- Sheppard JD, Kurata F, Epitropoulos AT, et al. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 MOJAVE study. Am J Ophthalmol. 2023;252:265–274. doi: 10.1016/j.ajo.2023.03.008
- Mason L, Jafri S, Dortonne I, et al. Emerging therapies for dry eye disease. Expert Opin Emerg Drugs. 2021 Dec;26(4):401–413. doi: 10.1080/14728214.2021.2011858
- Vittitow J, Kissling R, DeCory H, et al. In vitro inhibition of evaporation with perfluorohexyloctane, an eye drop for dry eye disease. Curr Ther Res. 2023;98:100704. doi: 10.1016/j.curtheres.2023.100704
- Hall JQ Jr., Ridder WH 3rd, Nguyen AL, et al. Visual effect and residence time of artificial tears in dry eye subjects. Optom Vis Sci. 2011;88(7):872–880. doi: 10.1097/OPX.0b013e31821b0b2c
- Agarwal P, Craig JP, Rupenthal ID. Formulation considerations for the management of dry eye disease. Pharmaceutics. 2021;13(2):207. doi: 10.3390/pharmaceutics13020207
- White DE, Zhao Y, Ogundele A, et al. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin Ophthalmol. 2019;13:2285–2292. doi: 10.2147/OPTH.S226168
- Bausch + Lomb. Bausch + Lomb and Novaliq announce FDA approval of MIEBO™ (perfluorohexyloctane ophthalmic solution) for the treatment of the signs and symptoms of dry eye disease. Bausch + Lomb News Releases. 2023 [cited 2023 May 28]. Available from: https://www.bausch.com/news/releases/?id=156
- MIEBO [package insert]. Bridgewater NJ: Bausch & Lomb Incorporated. 2023. https://www.bausch.com/globalassets/pdf/packageinserts/pharma/miebo-package-insert.pdf
- Steven P, Scherer D, Krosser S, et al. Semifluorinated alkane eye drops for treatment of dry eye disease—a prospective, multicenter noninterventional study. J Ocul Pharmacol Ther. 2015;31(8):498–503. doi: 10.1089/jop.2015.0048
- Tauber J, Berdy GJ, Wirta DL, et al. NOV03 for dry eye disease associated with meibomian gland dysfunction: results of the randomized phase 3 GOBI study. Ophthalmol. 2023;130(5):516–524. doi: 10.1016/j.ophtha.2022.12.021
- Habbe KJ, Frings A, Saad A, et al. The influence of a mineral oil cationic nanoemulsion or perfluorohexyloctane on the tear film lipid layer and higher order aberrations. PLoS One. 2023;18(1):e0279977. doi: 10.1371/journal.pone.0279977
- Drug approval package: MIEBO: United States Food and drug administration. 2023 [updated 2023 Jun 20; cited 2023 Sep 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/216675Orig1s000TOC.cfm
- Stolowich N, Vittitow J, Kissling R, et al. Oxygen-carrying capacity of perfluorohexyloctane, a novel eye drop for dry eye disease. Curr Ther Res Clin Exp. 2023;98:100705. doi: 10.1016/j.curtheres.2023.100705
- Krösser S, Spencer E, Grillenberger R, et al. Ocular and systemic distribution of 14C-perfluorohexyloctane following topical ocular administration to rabbits. Invest Ophthalmol Vis Sci. 2018;59:2656.
- Li S, Tian Q, Ding G, et al. The impact of different oxygen delivery methods on corneal epithelial repair after injury. J Ophthalmol. 2022;2022:3260087. doi: 10.1155/2022/3260087
- Vittitow J, Protzko E, Segal BA, et al. Long-term safety and efficacy of NOV03 (perfluorohexyloctane) for the treatment of patients with dry eye disease associated with meibomian gland dysfunction: the KALAHARI study. Invest Ophthalmol Vis Sci. 2023;64:3995.
- Tauber J, Wirta DL, Sall K, et al. A randomized clinical study (SEECASE) to assess efficacy, safety, and tolerability of NOV03 for treatment of dry eye disease. Cornea. 2021;40(9):1132–1140. doi: 10.1097/ICO.0000000000002622
- Tian L, Gao Z, Zhu L, et al. Perfluorohexyloctane eye drops for dry eye disease associated with meibomian gland dysfunction in Chinese patients: a randomized clinical trial. JAMA Ophthalmol. 2023;141(4):385–392. doi: 10.1001/jamaophthalmol.2023.0270
- Schmidl D, Bata AM, Szegedi S, et al. Influence of perfluorohexyloctane eye drops on tear film thickness in patients with mild to moderate dry eye disease: a randomized controlled clinical trial. J Ocul Pharmacol Ther. 2020;36(3):154–161. doi: 10.1089/jop.2019.0092
- Agarwal P, Khun D, Krosser S, et al. Evaluating the lubricating effect of semifluorinated alkanes on the ocular surface. Invest Ophthalmol Vis Sci. 2018;59:3282.
- Novel drug approvals for 2023: the United States Food & drug administration. 2023 [updated 2023 Jul 25; cited 2023 Jul 27]. Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-431therapeutic-biological-products/novel-drug-approvals-2023
- Borchman D, Vittitow J, Ewurum A, et al. Spectroscopic study of perfluorohexyloctane—human meibum interactions. Ophthalmol Vis Sci. 2022;63(7):1525–A0250.
