ABSTRACT
Introduction
Argon laser peripheral iridoplasty (ALPI) is an effective and safe procedure for relieving appositional angle closure. While commonly performed, accurate diagnosis for the indications, and attention to procedural details, would be essential for successful outcome.
Areas covered
The article reviewed the indications and contraindications to ALPI and discussed the details and common pitfalls regarding the procedure.
Expert opinion
Argon laser peripheral iridoplasty is efficacious in acute primary appositional angle closure, and secondary causes of appositional angle closure such as plateau iris syndrome (PIS), and phacomorphic glaucoma. Its efficacy in chronic angle closure is variable. Contraindications, e.g. flat anterior chamber and synechial angle closure, should be identified. The proper technique, including longer duration, lower laser power settings, larger spot size, and placement of burns over the extreme periphery of the iris, are crucial to the success of the procedure.
1. Introduction
Argon laser peripheral iridoplasty (ALPI) is a simple and effective means of opening an appositionally closed angle by placing contraction burns of longer duration, lower power, and larger spot size in the iris periphery to contract the stroma at the site of the burn, compacting the iris stroma and physically pulling open the angle () [Citation1–5]. While ALPI was initially described as a treatment to abort medically unresponsive attacks of angle closure glaucoma [Citation6], it is now a commonly performed procedure in situations where laser iridotomy was not feasible, or could not eliminate appositional closure due to mechanisms other than pupillary block. Accurate diagnosis for the indications for ALPI and attention to procedural details are essential for successful outcome, and require the clinician’s ability to differentiate subtle gonioscopic findings associated with the underlying anatomic mechanism of the angle closure. The examiner must be facile with darkroom indentation gonioscopy and with the anatomic causes of angle closure and the means of diagnosing these clinically [Citation7].
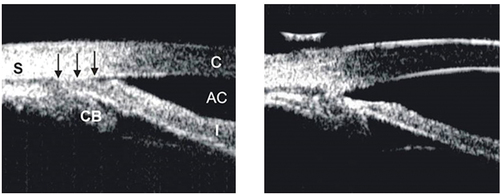
Figure 1. Ultrasound biomicroscopic (UBM) images illustrating how ALPI compacts the peripheral iris stroma, creating a space between the anterior iris surface and the trabecular meshwork, thus opening the angle. Left: appositionally closed angle in an eye with plateau iris syndrome (S: sclera, CB: ciliary body, AC: anterior chamber, C: cornea). Right: open angle after ALPI.
2. Indications
2.1. Acute primary angle closure (APAC)
Definitive treatment for APAC is to eliminate any pupillary block with laser peripheral iridotomy (LPI), even if other mechanisms are present. Argon laser peripheral iridoplasty may be helpful in aborting an APAC attack when it is unresponsive to medical therapy, when immediate iridotomy is precluded by corneal edema, shallow anterior chamber, or marked inflammation, or when an attack is unresponsive despite patent iridotomy [Citation1,Citation8–11], by treating the iris circumferentially and opening up the angle in areas without peripheral anterior synechiae (PAS). Since ALPI does not eliminate pupillary block, LPI is still required once IOP is controlled with sufficient corneal clarity to prevent the angle from re-closing due to continued posterior pressure against the iris.
The reported success rate of ALPI in APAC was reported to be as high as 100% [Citation12]. In a prospective study of 20 eyes with pre-laser IOP of 24 to 72 mmHg after several hours of medical therapy, the IOP was lowered to 16.7 mmHg (range of 5 to 35 mmHg) two hours after iridoplasty, allowing sufficient corneal clarity for subsequent laser iridotomy to be performed [Citation12].
ALPI may also be used as initial therapy for APAC, with or without any preliminary medical treatment [Citation1,Citation11,Citation13–18]. In the randomized controlled studies by Lam et al. [Citation19] and Lai et al. [Citation20], immediate ALPI for acute attacks after initial treatment with 4% pilocarpine and 0.5% timolol was compared to conventional medications of intravenous then oral acetazolamide, until IOP normalized, with the use of intravenous mannitol if IOP was greater than 60 mmHg at presentation. In the study by Lam et al., the mean IOP in the ALPI-treated group was reduced from 60.8 ± 11.6 mmHg at presentation to 20.6 ± 10.1 mmHg one hour after ALPI. The ALPI-treated group had significantly lower IOP than the medically treated group at 15 minutes, 30 minutes, and 1 hour after initiation of treatment, but the difference in IOP levels became statistically insignificant from 2 hours onward. The duration of the attack did not affect the efficacy of ALPI in reducing IOP [Citation19]. In the study by Lai et al., longer follow-up (mean 15.7 months) of patients randomized to ALPI or medical therapy revealed no significant differences between the two groups in mean IOP or requirement for glaucoma medications [Citation20].
A recent meta-analysis of four RCTs that assessed IOP reduction following ALPI treatment versus medical therapy further demonstrated the efficacy of ALPI in APAC. In the total 183 eyes (92 randomized to ALPI, 91 to medical therapy), the weighted mean differences of the percent reduction in IOP following ALPI versus medical therapy were 30.0 at 15 minutes, 27.4 at 30 minutes, 18.2 at 1 hour, and 12.9 at 2 hours. There was no statistically significant difference at 24 hours or more than six months. Hence, ALPI is a more effective treatment option for immediately bringing down the IOP in an APAC attack [Citation21]. Out of the four RCTs [Citation19,Citation20,Citation22,Citation23], only one could demonstrate change in anterior segment optical coherence tomography parameters. Compared to medical therapy, APAC eyes receiving ALPI had larger increase in anterior chamber volume, anterior chamber area, angle opening distance and trabecular iris space area at 750 um from scleral spur, smaller increase in iris area and decreased iris curve in ALPI group at one hour post treatment compared to pre-treatment [Citation23]. It should be stressed that since ALPI does not eliminate pupillary block, LPI is still required in APAC eyes once IOP is controlled and the cornea has cleared sufficiently. As shown in a recent 10-year report on post-APAC eyes receiving either phacoemulsification versus LPI, early phacoemulsification, whether as a primary procedure or after LPI, would also be beneficial for opening up the angle, thereby preventing irreversible damage to the trabecular meshwork and IOP elevation [Citation24].
2.2. Primary angle closure disease (PACD)
Argon laser peripheral iridoplasty has been studied in eyes with primary angle closure disease and a combination of PAS and appositional closure, as it may theoretically open up the appositionally closed portions of the angle.
Effect of ALPI on lowering the IOP in primary angle closure glaucoma (PACG) patients has been widely studied but with variable results. In the study by Rosman et al., the long-term clinical course in eyes with optic nerve head and visual field damage in the presence of an angle at least partially closed due to PAS were compared between patients in New York and Singapore. While all eyes underwent laser iridotomy and the vast majority in both groups required further treatment for IOP control, 31.3% of the eyes from New York patients went on to filtering surgery, compared to 53.0% of the Singapore eyes. Seven eyes in the New York group underwent ALPI, after which IOPs were controlled and surgery was not required, while no similar subgroup was encountered in the Singapore group as patient went directly onto filtering surgery [Citation25]. In a prospective interventional study of 24 eyes in 24 German patients with non-acute primary angle closure (PAC) and PACG without peripheral iridotomy, ALPI showed a significant reduction of IOP and appositional angle closure [Citation26]. The IOP reduced from 18.8 ± 3.6 to 14.7 ± 3.1 mmHg (p < 0.001), with 87.5% of patients showing IOP reduction. There was also significant angle widening on gonioscopy in all quadrants, with 74% of the angles showing increase in Shaffer grading, which was found in each single eye. However, Pentacam did not show any significant increase in anterior chamber depth, volume, or angle at three months after ALPI [Citation26]. Few RCTs were conducted to study the efficacy of ALPI in PACD patients. In IMPACT study, 22 patients with PAC or primary angle closure suspect (PACS) having occludable angles on gonioscopy post-LPI were randomized to ALPI treatment (n = 11) or no treatment (n = 11). Diurnal IOP at 3 months following ALPI, 5.0 mmHg ±1.6 mmHg, was significantly lower compared to 6.6 mmHg ±1.6 mmHg in the control group, explained by the significantly higher maximum IOP in the non-ALPI group [Citation27]. In another RCT comparing iridotomy versus iridotomy plus iridoplasty for PACG patients, both groups showed significant drop in IOP at one year [Citation28]. A similar RCT was performed in PACS patients, with study period of 3 months [Citation29]. In both studies, there were no significant differences in IOP between the treatment and control groups at the end of study period. In the pooled analysis of these two studies, there was no evidence of effect by ALPI on the final IOP [Citation30]. The efficacy of ALPI in PAC and PACG with persistent appositional closure and elevated IOP after iridotomy was studied in another RCT, with control being travoprost 0.004% and a topical prostaglandin analogue (PGA). In the 51 and 55 eyes randomized to ALPI and PGA, respectively, overall success rate (IOP ≤21 mmHg with or without medication) was seen in 70.0% of eyes treated with ALPI compared to 92.5% of eyes treated with PGA (p = 0.01). The IOP reduction was also greater in the PGA group, 25.5% or 6.1 mmHg (95% C.I., 5.1–7.1 mmHg, p < 0.001) versus 19.3% or 4.9 mmHg (95% C.I. 3.5–6.3 mmHg, p < 0.001) in the ALPI group (p = 0.04) [Citation31].
In addition to its IOP-lowering effect, individual studies have also shown the reduction in PAS and change in angle parameters in PAC eyes receiving ALPI. A prospective, observational case control study showed that ALPI reduced the extent of PAS in eyes with PAC that were not responsive to laser peripheral iridotomy (LPI) treatment [Citation32]. Of the 16 eyes with PAC that underwent ALPI, the median PAS extent decreased from 3.5 (quartile range, 1.5 to 6.0) to 2.0 (0.5 to 4.0) clock hours (p < 0.001). Also, anterior segment optical coherence tomography (ASOCT) angle parameters increased after ALPI treatment: angle opening distance at 500um (AOD500) increased from 0.132 ± 0.016 to 0.179 ± 0.062 mm (p < 0.001), trabecular iris space area at 500um (TISA500) from 0.085 ± 0.012 to 0.104 ± 0.051 mm2 (p < 0.001), and scleral spur angle from 19.5 ± 2.4 to 26.8 ± 4.5 degrees (p < 0.001) [Citation32]. In the IMPACT study, ALPI increased angle parameters on ASOCT in eyes with occludable angles post-LPI [Citation27]. Angle opening distance, trabecular-iris angle, angle recess area, and TISA at 500um over seven out of eight sections on ASOCT were all shown to be significantly increased three months after ALPI [Citation27]. Among PACS patients, Lee et al. was able to demonstrate more significant increase in mid-peripheral anterior chamber depth in iridotomy plus iridoplasty group, compared to iridotomy group [Citation29]. On the contrary, in the RCT comparing ALPI and PGA among PACG patients, despite the significant increase in mean angle width from baseline to 1 year, eyes treated with ALPI had significant progression of PAS from 1.7 to 2.6 clock hours, compared to no significant change in the PGA arm. The cause of this unexpected result was postulated to be the combination of the inadequate angle widening effect of ALPI in eyes with persistent iridotrabecular contact, as well as low-grade inflammation induced by laser treatment [Citation31].
With the significant 5-year incidence rate of PAC at 22%, and of PACG at 28.5% among PACS and PAC eyes respectively [Citation33,Citation34], further studies of longer duration would be needed to assess the long term IOP control after ALPI in patients with persistent appositional angle closure despite LPI in PACG [Citation35]. Indeed, multiple studies have demonstrated the effect of phacoemulsification in significantly lowering IOP in PACG eyes in mid to long terms, postulated to be due to the angle-opening effect of lens extraction [Citation36–38]. The benefit of mechanical opening of the angle by ALPI in PACG would be better gauged by taking gonioscopic changes into account in assessing treatment success [Citation35].
2.3. Plateau iris syndrome
Plateau iris is a configuration where a large or anteriorly positioned ciliary body keeps the iris root in proximity to the trabecular meshwork [Citation39,Citation40]. On gonioscopy, the iris root angulates forward and then centrally. Pupillary block and plateau iris configuration often coexist, and persistent angle closure or narrowing following a patent iridotomy is often the reason plateau iris is suspected clinically [Citation41]. Before iridotomy, the anterior chamber is usually of medium depth and the iris surface mildly convex. If pupillary block is either not a component mechanism of the angle closure, or has been eliminated by iridotomy, ALPI may help eliminate the physical blockade of the angle by compressing the iris root and therefore widening up the angle.
Compared to APAC, ALPI in PIS was less supported by high level evidence e.g. RCTs, and most studies were limited by small sample size and retrospective design [Citation42]. In a series of 23 eyes with a mean follow-up of 79 months, Ritch et al. reported long term success of ALPI among eyes with PIS for a period of ≥6 years. The angle in 20 eyes (87.0%) remained open throughout follow-up after only one treatment [Citation43]. There was gradual re-closure of the angles in 3 eyes years later, but a single repeat treatment was sufficient to re-open the angle and maintain its status. No patient required filtration surgery during follow-up in this series.
One should be reminded that patients with plateau iris should be followed closely for recurrence of appositional closure with regular gonioscopic examination despite high success rate of ALPI. Any component of lens-related mechanisms of glaucoma should be observed. While retreatment is occasionally needed in patients with plateau iris, those with intumescent lenses usually benefit from cataract extraction. In some cases, drainage angles remain narrow despite cataract extraction, leading to IOP elevation. Repeat ALPI could be considered in this situation, although the long-term efficacy is unknown [Citation44].
2.4. Phacomorphic angle closure
ALPI is also effective as initial treatment to break attacks of acute phacomorphic angle closure [Citation6,Citation22,Citation45,Citation46]. In a small prospective case series of 10 consecutive patients with acute phacomorphic glaucoma [Citation45], topical atropine (1%), timolol (0.5%), and immediate ALPI were given as initial treatment. At 15 minutes after ALPI, the mean IOP dropped 19.3% from 56.1 ± 12.5 mmHg to 45.3 ± 14.5 mmHg, and a further 17.0% reduction at 30 minutes post ALPI to 37.6 ± 7.5 mmHg. By 4 hours after ALPI, all eyes returned to IOP of less than 25 mmHg without need for intravenous mannitol for IOP control. All 10 patients had uncomplicated cataract extraction soon after ALPI. None had irreversible peripheral corneal edema.
Argon laser peripheral iridoplasty (ALPI) would be helpful in breaking the attack in acute phacomorphic angle closure, where the eye is often severely inflamed, to allow time for the inflammation and corneal edema to clear up, providing more optimal conditions for the definitive treatment of cataract extraction. Any element of pupillary block is treated with LPI as soon as possible (usually within two to three days) after breaking the attack.
3. Contraindications
3.1. Severe and extensive corneal edema or opacification
In APAC, moderate degrees of corneal edema are not a contraindication to ALPI. Since 180 degrees of treatment may be sufficient to abort an attack, partial obscuration of view of the iris is not a contraindication to use of ALPI as treatment of APAC. The presence of an arcus senilis should be ignored.
3.2. Flat anterior chamber
The laser burns from ALPI may heat up the aqueous nearby, and the resultant current may cause corneal endothelial burn. Damage to the corneal endothelium may also occur with attempt of performing the laser while the iris was apposed to the cornea. Laser applications should be timed apart enough for the heat to dissipate if the anterior chamber is very shallow.
3.3. Synechial angle closure
Although ALPI was reported to be successful in breaking peripheral anterior synechiae in a small series of six eyes [Citation47], no further evidence was available to support its use in reopening PAS due to uveitis, neovascular glaucoma, or iridocorneal-endothelial syndrome, and appositional angle closure remain the major indication for ALPI.
4. Complications
The incidence of severe complications from ALPI is rare [Citation30]. While some studies reported no complications from ALPI [Citation48], transient iritis and potential risk of transient IOP spike are to be expected with the laser [Citation32,Citation49]. There was one reported case of persistent uveitis beyond two weeks post-procedure and one case of iris hemorrhage, both of which recovered conservatively [Citation32]. Another case of malignant glaucoma was reported after iridotomy and iridoplasty [Citation28].
As ALPI is often performed in a shallow anterior chamber, peripheral corneal burns may occur, though these burns would usually resolve within days. One case of corneal decompensation following ALPI had been reported in a patient with preexisting Fuchs dystrophy [Citation49]. A meta-analysis of ALPI versus systemic medical therapy among Asian patients with APAC showed no difference in the endothelial cell count between two groups postoperatively [Citation21].
Espano et al. reported a case series of eight patients who developed symptomatic mydriatic and fixed pupil post uneventful ALPI not responding to pilocarpine, which gradually resolved spontaneously over 3–12 months. This complication was reported to occur in < 1% of the patients treated [Citation50].
5. Laser procedure
5.1. Pre-laser preparations
Before ALPI, 1 to 4% pilocarpine is given to constrict the pupil and to stretch the iris [Citation49,Citation51]. Perioperative brimonidine or timolol maleate 0.5% may be given to blunt any post-laser IOP spikes. In cases of corneal edema, topical glycerin may be given to clear the cornea temporarily to facilitate the procedure [Citation49].
5.2. Laser parameters
The argon laser is set to produce contraction burns (500 µm spot size, 0.5 to 0.7 second duration, and initially, 200 mW power). Using Abraham iridotomy contact lens, the beam is aimed at the portion of iris as peripheral as possible () [Citation49,Citation51]. Treatment consists of placing approximately 20 to 24 spots over 360°, leaving approximately 2 spot-diameters between each spot and avoiding large visible radial vessels if possible. Contraction of the iris should be seen during the laser procedure, which is often accompanied by noticeable deepening of peripheral anterior chamber at the site of the burn. Power should be titrated up if no contraction is noted, especially when the patient does not experience discomfort from the laser. It is necessary to use enough power for the patient to feel the burns in order to achieve successful contraction. The power should be reduced if there is bubble formation or pigment release into anterior chamber. As the procedure is repeated, one may notice laser burns may be placed more peripheral to where it was initially possible. This is due to the exposure of iris stroma further peripherally by previous contraction burns that pulled open the angle [Citation49].
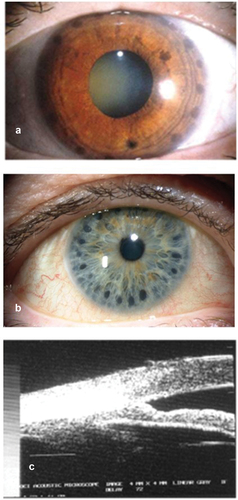
Figure 2. (a) Slit lamp photograph of an eye with plateau iris syndrome after ALPI. The dark, round laser marks can be clearly seen on the peripheral iris. (b) ALPI burns being placed too centrally, and thus ineffective. (c) UBM showing ALPI being placed too centrally, and the angle remains closed.
5.3. Post-laser treatment
Topical brimonidine may be given immediately after the laser, and IOP is monitored post-laser as in other anterior segment laser procedures, with treatment given for IOP spike if necessary. Topical steroid is used four to six times daily for 3 to 5 days [Citation49]. Espana et al. reported a small series of 12 eyes of 8 patients who experienced persistent mydriasis after the procedure, which gradually resolved spontaneously over a period of 3 to 12 months. While pilocarpine was initially prescribed but discontinued in most patients for lack of response, the authors suggested pilocarpine should be given at least as a brief trial [Citation50].
5.4. Pitfalls
One of the most common errors resulting in failure of the procedure is the application of laser spots not peripherally enough on the iris. One may consider allowing a thin crescent of aiming beam to overlap onto the sclera at the limbus, with the patient looking into the direction of the beam to achieve more peripheral laser spot placement. The aiming of the beam peripherally may be further facilitated in the lower 240 degrees of angle, where one can often see the peripheral iris insertion into the angle wall directly through the button on the lens [Citation49].
To achieve adequate iris contraction, the foot pedal should be pressed for the entire duration of the burn, i.e. 0.5 to 0.7 seconds. Light blue and dark brown irides often require higher energy than medium brown irides for contraction to occur. While a 200 um spot size may be more effective in light irides, that may mean a larger number of burns needed, which may lead to stromal destruction and pigment release. Confluent spots should be avoided to prevent iris necrosis. More spots may be given at a later sitting if the initial treatment is insufficient [Citation49].
6. Conclusion
In conclusion, ALPI is a safe, effective, and simple out-patient laser procedure that effectively opens appositionally closed portions of the drainage angle. Evidence has shown its efficacy as an alternative first-line treatment for APAC, giving a more rapid IOP reduction compared to systemic IOP-lowering medications. Since it does not eliminate pupillary block, laser peripheral iridotomy is still indicated if pupillary block is present. Further studies will be needed to ascertain whether ALPI can reduce the conversion rate of APAC to PACG and its efficacy in plateau iris syndrome and phacomorphic angle closure. It is essential to keep in mind that peripheral placement of laser spots on the iris, lower power, longer duration, and larger spot sizes (contraction burns) are essential for success in this procedure.
7. Expert opinion
Despite the increased availability of phacoemulsification and IOP-lowering medications, argon laser peripheral iridoplasty (ALPI) remains an indispensible option in managing conditions with appositional angle closure, which are often acute and preclude safe cataract extraction or laser iridotomy. In the past decade, treatment of PACD has gained much attention via high-level evidence on its treatment with early lens extraction, e.g. EAGLE study, that supported clear lens extraction for PACG over peripheral iridotomy [Citation52]. On the other hand, laser peripheral iridotomy was shown to have modest, albeit significant, prophylactic effect among primary angle closure suspect patients, although widespread prophylactic LPI for PACS was not recommended [Citation53]. However, management of PACD would not be complete without discussion on the treatment of APAC, which requires treatment that could lower the IOP in a timely fashion to prevent irreversible optic nerve damage. While large-scale study on the treatment of APAC is lacking, probably due to relative difficulty for recruitment of cases in an urgent condition, there is still high-level evidence that supports ALPI treatment over medical therapy in APAC.
It is still controversial whether the success of ALPI in APAC can be replicated in chronic angle closure disease. Despite strong evidence from the EAGLE study and other studies like-wise that demonstrated the cost-effectiveness of clear lens extraction to PACG patients, this treatment also brings concern to surgeons as well as patients, especially in the younger age group, due to its invasive nature and accompanied loss of accommodation. With small studies of relatively short-term follow-up, ALPI was shown to be a noninvasive means to lower the IOP and limit the extent of peripheral anterior synechiae for eyes with PACD. While phacoemulsification has increasingly become the mainstream treatment for PACD, it would be worthwhile to compare ALPI with phacoemulsification in this group of patients in studies with larger sample size and longer follow up.
Treatment goal of PACD would be to reduce the IOP, as well as to prevent further progression of angle closure, as intermittent angle closure may lead to undetected IOP spikes and hence disease progression. While medical therapy with prostaglandin analogue showed lower failure rate and higher IOP reduction compared with ALPI in eyes with persistent appositional angle closure and raised IOP post-iridotomy in an RCT, a more detailed examination of the structural changes of the drainage angle and visual field changes would be beneficial in understanding the effect of each treatment modality on eyes with PACD.
It is important to know the principles, settings, and techniques of ALPI in achieving the intended therapeutic effect, as well as the few contraindications to performing this procedure. While argon laser machine is commonly available in ophthalmology departments, the correct laser lens should also be made readily available in acute settings. Education effort should be directed not only to glaucoma specialists, but more importantly to general ophthalmologists and ophthalmology trainees, as they are often the first to encounter patients with acute glaucoma who would benefit most from a timely ALPI.
Article highlights
This article highlights the indications and contraindications of argon laser peripheral iridoplasty (ALPI), as well as proper techniques for performing it.
ALPI is effective and safe for relieving appositional angle closure. Indications include acute primary angle closure (APAC), primary angle closure disease, plateau iris syndrome, and phacomorphic angle closure. In particular, ALPI is a viable alternative first-line treatment for APAC in place of systemic IOP-lowering medications.
Proper technique (longer duration, lower laser power, larger burn size, placement of burns over extreme periphery of the iris) is crucial to the success of ALPI.
Declaration of interest
C.C.Y. Tham has served as a consultant or advisor for Alcon Laboratories, Inc., Allergan, Inc., Bausch+Lomb, C-MER Eye Care Holdings Ltd., IOPtima Ltd., Merck & Co., Inc., Pfizer, Inc., Santen Pharmaceutical Co., Ltd; Grant support: Aeon Astron Corporation, Alcon Laboratories, Inc., AMO Asia Ltd, ICare Finland, Novartis, Pfizer, Inc., Santen Pharmaceutical Co. Ltd., Sensimed; Lecture fees: Alcon Laboratories, Inc., Allergan, Inc., Merck & Co., Inc., Novartis, Pfizer, Inc., Santen Pharmaceutical Co., Ltd; Travel support: Alcon Laboratories, Inc., Allergan, Inc., Merck & Co., Inc., Pfizer, Inc., Santen Pharmaceutical Co., Ltd.
R. Ritch has served as a consultant or advisor for: C-MER, Diopsys Inc, Emerald Bioscience Inc., Glauconix, Inc., Guardion Health Sciences, Intelon Optics, Sanoculis, Sensimed, Xoma; Patents/Royalty: Guardion Health Sciences, Ocular Instruments Inc.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Ritch R. Argon laser treatment for medically unresponsive attacks of angle-closure glaucoma. Am J Ophthalmol. 1982;94(2):197. doi: 10.1016/0002-9394(82)90075-7
- Ritch R. Techniques of argon laser iridectomy and iridoplasty. Palo Alto: Coherent Medical Press; 1983.
- York K, Ritch R, Szmyd LJ. Argon laser peripheral iridoplasty: Indications, techniques and results. Invest Ophthalmol Vis Sci. 1984;25(Suppl):94.
- Ritch R, Solomon IS. Laser treatment of glaucoma. In: L’Esperance FA Jr, editor. Ophthalmic lasers. 3rd ed. Vol. 2. St. Louis: CV Mosby Co; 1989. p. 650–748.
- Ritch R. Argon laser peripheral iridoplasty: an overview. J Glaucoma. 1992;1(3):206–213. doi: 10.1097/00061198-199201030-00012
- Ritch R. Argon laser treatment for medically unresponsive attacks of angle-closure glaucoma. Am J Ophthalmol. 1982 Aug;94(2):197–204. doi: 10.1016/0002-9394(82)90075-7
- Ritch R, Liebmann JM, Tello C. A construct for understanding angle-closure glaucoma: the role of ultrasound biomicroscopy. Ophthalmol Clin N Amer. 1995;8:281–293.
- Matai A, Consul S. Argon laser iridoplasty. Indian J Ophthalmol. 1987;35:290–292.
- Malis V. Iridoplasty and primary angle-closure glaucoma. Ceska a Slovenska Oftalmolgie. 2001;57:22–26.
- Lim AS, Tan A, Chew P, et al. Laser iridoplasty in the treatment of severe acute angle closure glaucoma. Int Ophthalmol. 1993;17(1):33–36. doi: 10.1007/BF00918865
- Chew P, Chee C, Lim A. Laser treatment of severe acute angle-closure glaucoma in dark Asian irides: the role of iridoplasty. Lasers And Light In Ophthalmol. 1991;4:41–42.
- Lim ASM, Tan A, Chew P, et al. Laser iridoplasty in the treatment of severe acute angle closure glaucoma. Int Ophthalmol. 1993 Feb 01;17(1):33–36. doi: 10.1007/BF00918865
- Agarwal HC, Kumar R, Kalra VK, et al. Argon laser iridoplasty: a primary mode of therapy in primary angle closure glaucoma. Indian J Ophthalmol. 1991;39(3):87–90.
- Lam DSC, Lai JSM, Tham CCY. Immediate argon laser peripheral iridoplasty as treatment for acute attack of primary angle-closure glaucoma. A preliminary study. Ophthalmol. 1998;105(12):2231–2236. doi: 10.1016/S0161-6420(98)92237-0
- Lam DSC, Lai JSM, Tham CCY, et al. Argon laser peripheral iridoplasty versus conventional systemic medical therapy as the first line treatment of acute angle closure: a prospective randomized controlled trial. Ophthalmol. 2002;109(9):1591–1596. doi: 10.1016/S0161-6420(02)01158-2
- Lai JSM, Tham CCY, Lam DSC. Limited argon laser peripheral iridoplasty as immediate treatment for an acute attack of angle-closure glaucoma: a preliminary study. Eye. 1999;13(1):26–30. doi: 10.1038/eye.1999.5
- Lai JSM, Tham CCY, Chua JKH, et al. Laser peripheral iridoplasty as initial treatment of acute attack of primary angle-closure: a long-term follow-up study. J Glaucoma. 2002;11(6):484–487. doi: 10.1097/00061198-200212000-00005
- Tham CCY, Lai JSM, Lam DSC. Immediate ALPI for acute attack of angle-closure glaucoma (addendum to previous report)(letter). Ophthalmol. 1999;106(6):1042–1043. doi: 10.1016/S0161-6420(99)90275-0
- Lam DSC, Lai JSM, Tham CCY, et al. Argon laser peripheral iridoplasty versus conventional systemic medical therapy in treatment of acute primary angle-closure glaucoma: a prospective, randomized, controlled trial. Ophthalmology. 2002 Nov 1;109(9):1591–1596. doi: 10.1016/s0161-6420(02)01158-2
- Lai JS, Tham CC, Chua JK, et al. To compare argon laser peripheral iridoplasty (ALPI) against systemic medications in treatment of acute primary angle-closure: mid-term results. Eye (Lond). 2006 Mar;20(3):309–314. doi: 10.1038/sj.eye.6701867
- Cai W, Lou Q, Fan J, et al. Efficacy and safety of argon laser peripheral iridoplasty and systemic medical therapy in asian patients with acute primary angle closure: a meta-analysis of randomized controlled trials. J Ophthalmol. 2019;2019:7697416. doi: 10.1155/2019/7697416
- Lee JW, Lai JS, Yick DW, et al. Argon laser peripheral iridoplasty versus systemic intraocular pressure-lowering medications as immediate management for acute phacomorphic angle closure. Clin Ophthalmol. 2013;7:63–69. doi: 10.2147/OPTH.S39503
- Sng CC, Aquino MC, Liao J, et al. Anterior segment morphology after acute primary angle closure treatment: a randomised study comparing iridoplasty and medical therapy. Br J Ophthalmol. 2016 Apr;100(4):542–548. doi: 10.1136/bjophthalmol-2015-307087
- Chan PP, Tang FY, Leung DY, et al. Ten-year clinical outcomes of acute primary angle closure randomized to receive early phacoemulsification versus laser peripheral iridotomy. J Glaucoma. 2021 Apr 1;30(4):332–339. doi: 10.1097/IJG.0000000000001799
- Rosman M, Aung T, Ang LP, et al. Chronic angle-closure with glaucomatous damage: long-term clinical course in a North American population and comparison with an Asian population. Ophthalmol. 2002 Dec;109(12):2227–2231. doi: 10.1016/S0161-6420(02)01275-7
- Pillunat KR, Spoerl E, Orphal J, et al. Argon laser peripheral iridoplasty for chronic primary angle-closure and angle-closure glaucoma in caucasians. Acta Ophthalmol. 2019 Mar;97(2):e225–e230. doi: 10.1111/aos.13878
- Bourne RRA, Zhekov I, Pardhan S. Temporal ocular coherence tomography-measured changes in anterior chamber angle and diurnal intraocular pressure after laser iridoplasty: IMPACT study. Br J Ophthalmol. 2017 Jul;101(7):886–891. doi: 10.1136/bjophthalmol-2016-308720
- Sun X, Liang YB, Wang NL, et al. Laser peripheral iridotomy with and without iridoplasty for primary angle-closure glaucoma: 1-year results of a randomized pilot study. Am J Ophthalmol. 2010 Jul;150(1):68–73. doi: 10.1016/j.ajo.2010.02.004
- Lee JR, Choi JY, Kim YD, et al. Laser peripheral iridotomy with iridoplasty in primary angle closure suspect: anterior chamber analysis by pentacam. Korean J Ophthalmol. 2011 Aug;25(4):252–256. doi: 10.3341/kjo.2011.25.4.252
- Bayliss JM, Ng WS, Waugh N, et al. Laser peripheral iridoplasty for chronic angle closure. Cochrane Database Syst Rev. 2021 Mar 23;3(3):CD006746. doi: 10.1002/14651858.CD006746.pub4
- Narayanaswamy A, Baskaran M, Perera SA, et al. Argon laser peripheral iridoplasty for primary angle-closure glaucoma: a randomized controlled trial. Ophthalmol. 2016 Mar;123(3):514–521. doi: 10.1016/j.ophtha.2015.11.002
- Ramakrishnan R, Mitra A, Abdul Kader M, et al. To study the efficacy of laser peripheral iridoplasty in the treatment of eyes with primary angle closure and plateau iris syndrome, unresponsive to laser peripheral iridotomy, using anterior-segment OCT as a Tool. J Glaucoma. 2016 May;25(5):440–446. doi: 10.1097/IJG.0000000000000307
- Thomas R, George R, Parikh R, et al. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. Br J Ophthalmol. 2003 Apr;87(4):450–454. doi: 10.1136/bjo.87.4.450
- Thomas R, Parikh R, Muliyil J, et al. Five-year risk of progression of primary angle closure to primary angle closure glaucoma: a population-based study. Acta Ophthalmol Scand. 2003 Oct;81(5):480–485. doi: 10.1034/j.1600-0420.2003.00135.x
- Almeida I, Ushida M, Dias DT, et al. Re: Narayanaswamy et al.: Argon laser peripheral iridoplasty for primary angle-closure glaucoma: a randomized controlled trial (Ophthalmology. 2016;123: 514-521). Ophthalmology. 2017 Apr;124(4):e34. doi: 10.1016/j.ophtha.2016.05.055
- Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmol. 2008 Dec;115(12):2167–2173.e2. doi: 10.1016/j.ophtha.2008.06.016
- Tham CC, Kwong YY, Baig N, et al. Phacoemulsification versus trabeculectomy in medically uncontrolled chronic angle-closure glaucoma without cataract. Ophthalmol. 2013 Jan;120(1):62–67. doi: 10.1016/j.ophtha.2012.07.021
- Hansapinyo L, Choy BNK, Lai JSM, et al. Phacoemulsification versus phacotrabeculectomy in primary angle-closure glaucoma with cataract: long-term clinical outcomes. J Glaucoma. 2020 Jan;29(1):15–23. doi: 10.1097/IJG.0000000000001397
- Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol. 1992 Apr 15;113(4):390–395. doi: 10.1016/S0002-9394(14)76160-4
- Ritch R. Plateau iris is caused by abnormally positioned ciliary processes. J Glaucoma. 1992;1(1):23–26. doi: 10.1097/00061198-199204000-00006
- Mandell MA, Pavlin CJ, Weisbrod DJ, et al. Anterior chamber depth in plateau iris syndrome and pupillary block as measured by ultrasound biomicroscopy. Am J Ophthalmol. 2003 Nov;136(5):900–903. doi: 10.1016/S0002-9394(03)00578-6
- Bourdon H, Aragno V, Baudouin C, et al. Iridoplasty for plateau iris syndrome: a systematic review. BMJ Open Ophthalmol. 2019;4(1):e000340. doi: 10.1136/bmjophth-2019-000340
- Ritch R, Tham CC, Lam DS. Long-term success of argon laser peripheral iridoplasty in the management of plateau iris syndrome. Ophthalmol. 2004 Jan;111(1):104–108. doi: 10.1016/j.ophtha.2003.05.001
- Choy BN, Chan JC, Chien CP, et al. Recurrent acute angle-closure attack due to plateau iris syndrome after cataract extraction with or without argon laser peripheral iridoplasty: a case report. BMC Ophthalmol. 2016 May 26;16(1):64. doi: 10.1186/s12886-016-0244-y
- Tham CC, Lai JS, Poon AS, et al. Immediate argon laser peripheral iridoplasty (ALPI) as initial treatment for acute phacomorphic angle-closure (phacomorphic glaucoma) before cataract extraction: a preliminary study. Eye (Lond). 2005 Jul;19(7):778–783. doi: 10.1038/sj.eye.6701651
- Yip PP, Leung WY, Hon CY, et al. Argon laser peripheral iridoplasty in the management of phacomorphic glaucoma. Ophthalmic Surg Lasers Imaging. 2005 Jul;36(4):286–291. doi: 10.3928/1542-8877-20050701-06
- Wand M. Argon laser gonioplasty for synechial angle closure. Arch Ophtalmol. 1992;110(3):363–367. doi: 10.1001/archopht.1992.01080150061029
- Ritch R, Tham CCY, Lam DSC. Long-term success of argon laser peripheral iridoplasty in the management of plateau iris syndrome. Ophthalmol (Rochester, Minn). 2004;111(1):104–108. doi: 10.1016/j.ophtha.2003.05.001
- Ritch R, Tham CC, Lam DS. Argon laser peripheral iridoplasty (ALPI): an update. Surv Ophthalmol. 2007 May;52(3):279–288. doi: 10.1016/j.survophthal.2007.02.006
- Espana EM, Ioannidis A, Tello C, et al. Urrets-Zavalia syndrome as a complication of argon laser peripheral iridoplasty. Br J Ophthalmol. 2007 Apr;91(4):427–429. doi: 10.1136/bjo.2006.105098
- Chan PP, Pang JC, Tham CC. Acute primary angle closure-treatment strategies, evidences and economical considerations. Eye (Lond). 2019 Jan;33(1):110–119. doi: 10.1038/s41433-018-0278-x
- Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016 Oct 1;388(10052):1389–1397. doi: 10.1016/S0140-6736(16)30956-4
- He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet. 2019 Apr 20;393(10181):1609–1618. doi: 10.1016/S0140-6736(18)32607-2
