1. Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in older patients. The non-neovascular form of AMD, accounting for approximately 90% of cases, is characterized by the accumulation of drusen in the macula; the advanced form is characterized by geographic atrophy, thus atrophy of the outer retina and the retinal pigment epithelium. While recently approved invasive treatments for non-neovascular AMD focus on slowing the progression of the disease in advanced patients, they do not address intermediate stages of the disease, nor do they restore lost vision. Photobiomodulation (PBM), a noninvasive therapy that uses low-level light to stimulate cellular processes and mitochondrial function, has emerged as a promising new approach for treating non-neovascular AMD. This editorial summarizes the growing body of evidence supporting the use of PBM in non-neovascular AMD, highlighting its potential to improve visual function and to reduce disease progression.
PBM is the use of light of particular wavelengths (i.e. yellow (590 nm), red (660 nm), and near-infrared (NIR; 850 nm) with the intention to induce a positive therapeutic effect. It was invented in the 1960’s when researchers discovered that the treatment with low-level light from a laser source improves wound healing and tissue regeneration. Since then, PBM has been shown to have a wide range of therapeutic applications. PBM has evolved into a versatile therapeutic modality with applications extending from pain management to neurological disorders, with multiple applications in retinal disease [Citation1,Citation2].
2. Mechanism of action
The exact mechanism by which PBM works in non-neovascular AMD is still being investigated, but it is known to involve several factors. Intensive research has been conducted using PBM in the past decades but mostly focused on non-ophthalmic indications like pain, dermatologic and orthopedic pathologies, but ophthalmology and macular degeneration have only recently emerged as an area of intense research. PubMed is listing 9,490 results (as of June 2024) for the search term photobiomodulation, but only 78 of them are related to macular degeneration [Citation3]. Application of light with wavelengths in the red and NIR spectrum can increase mitochondrial activity, which is essential for cellular energy production. Respective wavelengths activate the two copper sites of the cytochrome C oxidase, a mitochondrial protein which is the main photoacceptor in animals and humans (see ). The binding enhances ATP production by restoring and increasing the proton gradient. This can improve the overall health and function of retinal cells, particularly in disease. It also leads to a more optimized visual circle, leading to less developments of byproducts such as ROS and A2E. Second, PBM can reduce oxidative stress, which can damage cells and contribute to the progression of non-neovascular AMD. Third, PBM can modulate the release of cytokines and other soluble mediators, which may promote the repair, healing, and regeneration of damaged retinal cells. Last but not least, sustained cellular changes are seen through activation of transcription factors. PBM exhibits promising benefits for degenerative eye diseases, including the reduction of inflammation in the retina, improvement of blood flow for adequate oxygen and nutrient supply, and enhancement of mitochondrial function. Clinical evidence exists for various eye diseases, including non-neovascular AMD, diabetic retinopathy and macular edema, retinopathy of prematurity, retinitis pigmentosa, Stargardt’s Disease, myopia, and glaucoma [Citation4–10]. Notably, PBM has demonstrated the ability to improve vision, slow disease progression, enhance blood flow, and reduce the risk of vision loss.
Figure 1. Photons are absorbed by photoacceptors in the targeted tissue mitochondrial protein, cytochrome C oxidase to restore energy production.
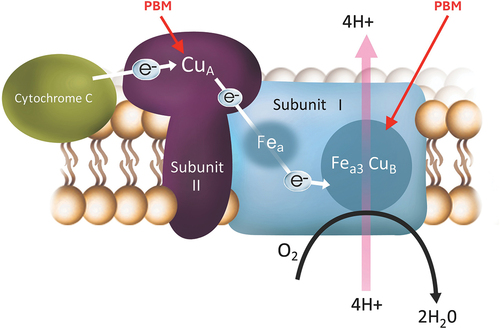
PBM is a therapeutic modality for treating non-neovascular AMD. Its mechanism is part of ongoing studies to better define its clinical use.
3. Clinical evidence in non-neovascular AMD
Clinical data support the use of PBM in non-neovascular AMD. Several studiesin particular, studies sponsored by LumiThera (Seattle, WA, U.S.A. [Citation11]) including the LIGHTSITE I to III studies [Citation5–7] have shown that PBM can improve visual function in patients with non-neovascular AMD. In addition, PBM can slow the accumulation of drusen, and the onset of advanced non-neovascular AMD.
What is noteworthy, is that these three trials included different severity of non-neovascular AMD. The majority of included patients in the LIGHTSITE I trial had advanced non-neovascular AMD AREDS category 4, thus, already center involving GA. Sub-analyses revealed that patients with earlier stages of AMD profit the most in terms of BCVA improvement. The LIGHTSITE III trial focused on early to intermediate dry AMD patients with BCVA ranging from 20/30 to 20/100 with 70% of the including patients having a BCVA of 20/40 or better and mainly drusen maculopathy with complete absence of Geographic Atrophy (GA). Many of these patients gained >5 letters in the LIGHTSITE trials, while patients with foveola involving GA only showed a mean increase of 2 letters at the end of the 24 M study. Also, the LIGHTSITE I trial had a treatment schedule of every 6 months. However, here a slight decrease in the gained BCVA was seen prior to the next treatment cycle in some patients. This led to an adapted trial design with treatment cycles every 4 months in the subsequent trials. In this setting, long-lasting and sustained BCVA benefit was seen over the course of 24 months and patients revealed sustained BCVA improvement.
The interpretation of studies like LIGHTSITE I and II requires caution. For instance, LIGHTSITE I was a pilot study and included 46 eyes of 30 patients, 1:1 randomized into either the active sham or the PBM treatment group. While there was a statistically significant improvement in BCVA in the PBM treated group and in contrast no significant change in the sham treated group, the study was the first to look at repeat PBM treatments and how long the benefits lasted versus comparisons between PBM and sham treatments. Similarly, LIGHTSITE II’s findings did not significantly distinguish between the efficacy of PBM and the active sham treatments in improving visual function, unless you focused on the patients that completed all rounds of treatment, underscoring the necessity for a nuanced understanding of these results. It has to be highlighted here that the LIGHTSITE II trial was designed to evaluate the potential difference between sham and PBM treatment, however due to COVID the study was truncated before the required sample size was achieved and the number of treatments and intervals were impacted. LIGHTSITE III’s improvement of 2.4 letters in vision between the PBM and sham treatment groups at 13 months, while statistically significant, prompts further discussion on clinical relevance and the comparative effectiveness of PBM. However, these results must be seen in the context of the natural progression of vision loss of intermediate dry AMD. Patients with none or few and large drusen at baseline with no progression to late stage AMD exhibit a mean letter loss of about 2 letters after 2 years, while the same population who develop late stage AMD after 2 years reveal a men letter loss of 30 letters [Citation12]. In this sense, stability of visual function in a patient with non-neovascular AMD should already be seen as a benefit, not to mention an improvement of visual function. An expanded separation of vision between treatment groups was further seen at the 24-month time point with sustained vision benefits and more vision losses in the sham group with disease progression.
The functional benefits taken together with the slowing of disease progression from intermediate AMD to GA of the LIGHTSITE III trial have to be seen as valuable offers to patients.
PBM as a routine clinical therapy option in non-neovascular AMD was introduced by LumiThera with the VALEDA Device in 2018. Since then, more than 20.000 patients with non-neovascular AMD/GA have been treated with VALEDA in Europe and South America. The data were recently submitted for approval to the FDA. The PBM therapy is applied in short settings (less than 5 min per eye) for 9 applications over a period of 3 weeks. For the best efficacy, these therapy settings should be repeated every 4 months.
While PBM holds promise, and patients are welcoming this new treatment opportunity for previously untreatable early and intermediate non-neovascular AMD, critics emphasize key considerations. Some retina specialists claim limited evidence strength and the lack of larger, long-term studies. Often the patient population of the LT trials are compared to the number of included patients in the recent pivotal GA trials assessing the inhibition of the complement pathway. It has to be emphasized here that the LIGHTSITE trials chose a different predefined endpoint, i.e. BCVA improvement and not GA growth rate. The size of the patient population was similar to that in any other randomized masked controlled trial chosen based on statistical power calculation. Thus, the sample size of the LIGHTSITE III trial was sufficient to meet the predefined primary end point parameter. It must be emphasized as well that this therapy is the first to offer patients with non-neovascular AMD a proven visual function improvement without any intravitreal injection associated risks.
The treatment with PBM is considered very safe, and PBM may have side effects which are usually mild and mostly limited in some patients to eye pain directly after treatment, redness, and irritation of the eye.
The future of PBM in eye care holds great promise, fostering advancements in understanding and treating various ocular conditions. While more research is ongoing, the growing body of evidence suggests that PBM may be a valuable addition to the treatment of non-neovascular AMD and other ocular pathologies. As research continues, we can expect to see even more exciting developments in the field of PBM in Ophthalmology in the years to come.
4. Expert opinion
In conclusion, while PBM presents a novel approach to managing non-neovascular AMD, it is imperative to approach its current evidence base with a balanced perspective, acknowledging both its potential and the limitations of existing studies. In conjunction with already established interventions like nutritional supplements such as antioxidants, lutein, and zeaxanthin, PBM may help to manage and slow the impairment of activities of daily life with AMD. Often patients report visual benefits that are hard to quantify via established functional outcome measures, such as the capacity of reading display boards on the train or being able to read with less light. From a physician’s perspective it is sometimes hard to trust and believe in the benefit of something new, especially when there is no strong correlation to quantifiable morphological changes. Hopefully, the implementation of high-resolution OCT and adaptive optics OCT will help in the future to measure and understand the functional benefits. This will help us to accept this potential new treatment modality. From a personal perspective, as someone who started to use PBM in patients with non-neovascular AMD, one has to say that although some patients experience no subjective or objective improvement after their first treatment cycle, the majority is very satisfied and reports visual function benefits in their everyday life. Most patients continue with the treatment because of the subjective improvements they have experienced in their daily life. But for sure, further clinical trials are essential to fully understand PBMs efficacy, mechanism of action, and potential side effects.
Declaration of interests
Both authors are consultants for Lumithera, a company that developed and markets the PBM device ‘Valeda’ in several countries in Europe, Asia, and South America. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Siqueira RC. Photobiomodulation using light-emitting diode (LED) foR treatment of retinal diseases. Clin Ophthalmol. 2024 Jan 22;18:215–225. doi: 10.2147/OPTH.S441962
- Zhang CX, Lou Y, Chi J, et al. Considerations for the use of photobiomodulation in the treatment of retinal diseases. Biomol. 2022 Dec 3;12(12):1811. doi: 10.3390/biom12121811
- [cited 2024 Jun 5]. Available from: https://pubmed.ncbi.nlm.nih.gov/?term=photobiomodulation;https://pubmed.ncbi.nlm.nih.gov/?term=%27photobiomodulation+AND+macular+degeneration
- Merry GF, Munk MR, Dotson RS, et al. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmologica. 2017;95(4):e270–e277. doi: 10.1111/aos.13354
- Markowitz SN, Devenyi RG, Munk MR, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina. 2020 Aug;40(8):1471–1482. doi: 10.1097/IAE.0000000000002632
- Burton B, Parodi MB, Jürgens I, et al. LIGHTSITE II randomized multicenter trial: evaluation of multiwavelength photobiomodulation in non-exudative age-related macular degeneration. Ophthalmol Ther. 2023 Apr;12(2):953–968. doi: 10.1007/s40123-022-00640-6
- Boyer D, Hu A, Warrow D, et al. LIGHTSITE III: 13-month efficacy and safety evaluation of multiwavelength photobiomodulation in nonexudative (dry) age-related macular degeneration using the lumithera valeda light delivery system. Retina. 2024 Mar 1;44(3):487–497. doi: 10.1097/IAE.0000000000003980
- Kaymak H, Munk MR, Tedford SE, et al. Non-invasive treatment of early diabetic macular edema by multiwavelength photobiomodulation with the valeda light delivery system. Clin Ophthalmol. 2023 Nov 22;17:3549–3559. doi: 10.2147/OPTH.S415883
- Ivandic BT, Ivandic T. Low-level laser therapy improves vision in a patient with retinitis pigmentosa. Photomed Laser Surg. 2014;32(3):181–184. doi: 10.1089/pho.2013.3535
- Scalinci S, Valsecchi N, Pacella E, et al. Effects of photo-biomodulation in stargardt disease. Clin Ophthalmol 2022 Jan 10;16:85–91. doi: 10.2147/OPTH.S344378
- [cited 2024 Mar 30]. Available from: https://www.lumithera.com/
- Chew E, Traci C, Elvira A, et al. Age-Related Eye Disease Study Research Group Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol. 2014 Mar;132(3):272–277. doi: 10.1001/jamaophthalmol.2013.6636
