Abstract
Repetition priming can be driven by the encoding and retrieval of stimulus–response (S–R) bindings. When a previously encoded S–R binding is retrieved, and is congruent with the response currently required, it can bias response-selection processes towards selecting the retrieved response, resulting in facilitation. Previous studies have used classification tasks at retrieval. Here, two (or more) response options are competing, and it is likely that any evidence (e.g., an S–R binding) in favour of one option will be utilized to effect a decision. Thus, S–R effects are likely to be seen when using such a task. It is unclear whether such effects can be seen under conditions of higher response certainty, when participants are explicitly cued to make a response. Across two experiments, evidence for a modulating influence of S–R bindings is seen despite using a response cueing method at retrieval to minimize response uncertainty and despite stimuli being task irrelevant. Finally, the results suggest that responses within these S–R bindings are coded at the level of left versus right hand, and not a more fine-grained within-hand thumb versus index finger. The results underline the resilience of S–R effects, suggesting that they are present even under conditions where no explicit object-oriented decision is required.
Our interactions with everyday objects are strongly modulated by experience. We are often faster and more accurate at interacting with familiar than with novel objects. In an experimental setting, this experience-dependent change in reaction time (RT) or accuracy is referred to as repetition priming (Richardson-Klavehn & Bjork, Citation1988). Multiple learning mechanisms are thought to contribute to repetition priming (Henson, Citation2003); however, it is clear that the encoding and retrieval of stimulus–response (S–R) bindings play a significant role in object-related repetition priming (see Henson, Eckstein, Waszak, Frings, & Horner, Citation2014, for a review).
S–R theories of repetition priming propose that when a stimulus is first encountered, a direct association or binding forms between the stimulus presented (e.g., a common object such as a mug) and the response made to the object (e.g., a reaching action with the right hand; Hommel, Citation2004; Logan, Citation1990). When the stimulus is encountered for a second time, the previously encoded S–R binding is rapidly retrieved. This retrieval is thought to either bypass the processes required on first presentation (speeding RTs relative to first presentation; Logan, Citation1988, Citation1990) or bias response-selection processes towards performing the action previously made (Horner & Henson, Citation2009; Race, Badre, & Wagner, Citation2010; Race, Shanker, & Wagner, Citation2009). This bias to perform a specific action can lead to facilitation when the same action is required (e.g., reaching with the right hand) but not when a different action is required (e.g., reaching with the left hand). Indeed, when a different response is required, interference effects can be seen where RTs are slower than if the object was experimentally novel (e.g., Horner & Henson, Citation2011, Citation2012; Waszak & Hommel, Citation2007). Thus, in situations where responses are consistent across repetitions, S–R learning allows for rapid and efficient interactions with common objects. Though S–R theories typically relate to associations between task-relevant stimuli and the response made in relation to those stimuli, here I use the term “S–R binding” more broadly to refer to any association between a stimulus (regardless of task relevance) and a response executed in the presence of that stimulus.
S–R contributions to repetition priming have been shown across multiple experimental methodologies. Much of the literature has focused on S–R effects for attended task-relevant stimuli. At one end of the spectrum, they have been shown to last across many minutes and intervening stimuli in the case of perceptual or semantic classification of objects (e.g., Denkinger & Koutstaal, Citation2009; Dobbins, Schnyer, Verfaellie, & Schacter, Citation2004; Horner & Henson, Citation2008; Soldan, Clarke, Colleran, & Kuras, Citation2012; Waszak & Hommel, Citation2007), words (e.g., Dennis & Schmidt, Citation2003; Race et al., Citation2009), and faces (Valt, Klein, & Boehm, Citation2014). At the other end, S–R effects have been shown under immediate repetition conditions (i.e., no intervening stimuli) for simplistic stimuli (e.g., single X or O letters) under simple classification tasks (e.g., is the stimulus an X or O; Hommel, Citation1998; Hommel & Colzato, Citation2004). Importantly, despite differences in stimuli, lag, and task, both approaches have typically utilized a (binary) object-oriented classification task at retrieval.
More recently, attention has turned to whether bindings can be formed between responses and task-irrelevant or unattended stimuli (e.g., Hommel, Citation2005; Hommel & Colzato, Citation2004; Rothermund, Wentura, & De Houwer, Citation2005). Here, multiple stimuli are simultaneously presented at encoding (and retrieval), and the participant is required to pay attention and respond to the task-relevant stimulus whilst ignoring the task-irrelevant stimulus (the “distractor”). Interestingly, S–R effects can be seen when stimuli presented as distractors at encoding are attended and task relevant at retrieval (Rothermund et al., Citation2005). In other words, on first presentation, an S–R binding is formed between the distractor stimulus and the response made to the simultaneously presented attended stimulus. Attention and task relevance have also been manipulated at retrieval. Following the findings of Rothermund et al., it has been shown that distractor stimuli at encoding can modulate repetition priming at retrieval even when the stimulus remains a distractor (Frings, Moeller, & Rothermund, Citation2013; Frings, Rothermund, & Wentura, Citation2007). Such distractor-to-distractor effects suggest that, under certain experimental conditions, attention and task relevance are not necessary for both the encoding and the retrieval of S–R bindings (though see Moeller & Frings, Citation2014). As in the literature for attended stimuli, these studies typically utilize a classification task at retrieval (e.g., the colour of a word), typically binary (e.g., Rothermund et al., Citation2005), though also four distinct response options have been used (e.g., Frings et al., Citation2007).
Here I asked whether S–R effects can be seen when not using an object-oriented classification task at encoding or retrieval. Classification of a stimulus at retrieval (e.g., is the object man-made or natural?) may increase the probability of seeing S–R effects due to increased response uncertainty. If a decision between two (or more) competing response options is required, the retrieval of a previous S–R binding may have a larger effect on RTs than in situations where there is little response competition/uncertainty (e.g., if the response is given to the participant). Hommel (Citation1998) introduced a manipulation in which, prior to stimulus onset, a response cue is presented (e.g., an arrow pointing to the right). The participant is required to make this cued response when the main stimulus is presented (the response trigger). This manipulation allows for the encoding of arbitrary S–R bindings where there is no obvious relationship between the stimulus presented and response made. Importantly, it means no classification task is made to the stimulus itself. Although this manipulation has been previously used at encoding, it has not been used at retrieval. Here, I used a response cueing method at both encoding and retrieval to avoid the presence of a classification task. The presence of S–R effects in a situation of high response certainty (using response cues) would provide stronger evidence that the automatic retrieval of S–R bindings can modulate behaviour even in situations where there would appear to be little behavioural benefit in retrieving a prior response.
Further to testing for S–R effects without the use of a classification task, the present studies focused on whether S–R effects can be seen across longer lags (e.g., Horner & Henson, Citation2009), despite objects being task-irrelevant at both encoding and retrieval (e.g., Frings et al., Citation2007). Here, task relevance was defined as objects not being informative with regard to what response to make and when to make it. Objects were simply on the screen at the same time as responses were made at encoding and retrieval. The majority of studies manipulating task relevance have used an immediate repetition design (i.e., there was a short lag between repetitions with no intervening stimuli). Here, experiments used a study–test design more similar to long-lag repetition priming experiments (e.g., Horner & Henson, Citation2009) where objects are first presented in a single “study” block and then repeated in a “test” block with other novel stimuli. Repetition priming is defined as the difference between the repeated and novel stimuli in the test phase. Note that although the current experiments used a study–test design, relatively few stimuli were presented during each study–test block. Therefore the lag between repetitions of stimuli was relatively short compared to long-lag paradigms (though longer than immediate repetition paradigms).
In Experiment 1, a novel repetition priming paradigm is presented where visual objects are task irrelevant at both study and test (see ). At study, participants are cued to prepare a response by the location of a red square. An object is then centrally presented; however, participants are only required to respond when a red square is presented around the object. Thus, the object neither cues nor triggers the response but is simply concomitant with the response. At test, a single object is shown, and following a brief period of time a red arrow both cues and triggers a response. Again, this means the object does not tell the participant either how or when to respond. For the repeated stimuli, responses either were the same (congruent) or were switched (incongruent) between study and test. RTs for these conditions were compared to RTs for novel objects at test. Faster RTs were seen for congruent than for incongruent and novel objects, consistent with the encoding and retrieval of S–R bindings. In Experiment 2, I manipulated the incongruent condition such that participants either switched hand (e.g., left to right hand index finger press) or switched digit (e.g., index to thumb right hand press). S–R effects were only seen when switching hand (not digit within hand) suggesting that S–R bindings in the present paradigm are coded at the level of hand but perhaps not digit within hand. Thus, across two experiments I present novel evidence for (a) the encoding of S–R bindings between task-irrelevant objects and arbitrary responses, (b) the retrieval of such S–R bindings when objects at test are task irrelevant, (c) the presence of S–R effects without using an object-oriented classification task at encoding or retrieval, and (d) the representational level of responses within such arbitrary S–R bindings.
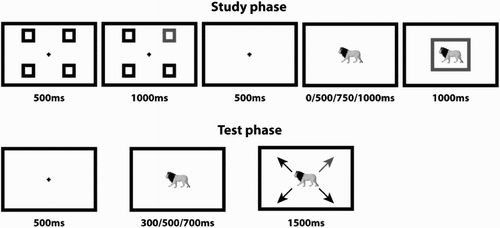
Figure 1 Trial structure for study and test phases of Experiments 1–2. During the study phase, participants prepared a response according to the location of the red square (one of four response options). They were then presented with a single object and, following a certain interval, were required to respond when the object was surrounded by a red square. During the test phase, participants were shown an object for a variable period of time and were required to respond when cued by a red arrow. Each study and test trial ended with a 1000-ms blank screen. The test phase trial is an example of a congruent item, as the participant responded “top right” at both study and test. Red squares and arrows are shown here in light grey.
EXPERIMENT 1
Method
Participants
A total of 33 participants (22 female) were recruited through the online UCL Psychology Subject Pool. All participants gave informed consent and were reimbursed for their time (£7.50). They had a mean age of 23.5 years (SD = 3.5). By self-report, one participant was left-handed and the remainder right-handed. The experiment was approved by the UCL Institute of Cognitive Neuroscience Departmental Research Ethics Committee (ICH-AH-PWB-2-10-13a).
Materials
A total of 240 colour images of common objects were used, as in Horner and Henson (Citation2008). The images subtended approximately 3° of visual angle when presented on the computer screen. They were divided into three sets of 80 images. Each set was assigned to one of the three conditions (congruent, incongruent, and novel), with the assignment of sets to conditions counterbalanced across participants in a Latin square design. Stimulus presentation was controlled by the Cogent toolbox within MATLAB. Responses were made on a QWERTY keyboard.
Procedure
The experiment consisted of 40 study–test blocks. Stimuli in each study–test block were unique (i.e., stimuli were not repeated across blocks). At study, four object images were paired with one of the four response options: top left, top right, bottom left, bottom right. Thus, for each study–test block there was a one-to-one stimulus-to-response mapping. The study phase was split into three mini-blocks, with each object shown once in each mini-block (presentation order of objects was random within each mini-block). The stimulus-to-response relationship was constant across study. As such, each stimulus–response pairing was repeated three times at study. There were a total of 12 trials in each study phase.
Study trials began with a centrally placed fixation cross for 500 ms (see ). Surrounding the fixation cross were four black squares, shown to the upper left, upper right, lower left, and lower right of fixation. One of the squares then turned red for 1000 ms, whilst the others remained black. The location of the red square told the participant which of the four possible response options they should prepare to make (i.e., they didn't respond at this point). The top left response (Q) was mapped to the left index finger, top right (]) to the right index finger, bottom left (Z) to the left thumb, and bottom right (/) to the right thumb.
The squares were removed from the screen, and a fixation cross was shown for a further 500 ms. A single object was then presented in the middle of the screen (participants were required to fixate centrally throughout). Participants were told to “pay attention to the object”; however, it was made clear to them that the objects were not task relevant (i.e., the objects had no bearing on when or how the participants should respond). For the first two presentations of the object, after 500 ms, 750 ms, or 1000 ms (randomly selected on each trial), a red square surrounding the object appeared. Participants were instructed to make the prepared response as soon as the red square appeared “as quickly and as accurately as possible”. On the third object presentation, the surrounding red square appeared at the same time as object presentation. Participants were given up to 1000 ms to respond following the presentation of the surrounding red square. On button press, the object and surrounding red square were replaced by a blank screen lasting 1000 ms.
This red square response trigger was used for three reasons: (a) to ensure that participants were not responding to the presentation of the object (i.e., to make objects task irrelevant), (b) to delay response on the first two trials to ensure that participants had adequate time to recognize each object, and (c) to ensure that the final response was triggered at the same time as object presentation. The idea was to increase the probability that an arbitrary S–R binding would form between the object presented and response made despite the object being task irrelevant.
At test, each of the four objects were presented once, as well as two further novel objects. Two of the “old” objects were assigned to the congruent condition and two to the incongruent condition. For congruent objects, participants were required to make the same response at test as that made at study. For incongruent objects, participants were required to make the opposite response at test to that made at study. For example, if the object was associated with the “top left” response at study, participants would be required to make a “bottom right” response at test (left index finger to right thumb). The two novel objects were randomly assigned to one of the four possible response options.
Test trials began with a 500-ms fixation cross followed by the presentation of one of the six objects at fixation (see ). After 300 ms, 500 ms, or 700 ms (randomly selected on each trial), four arrows appeared pointing to the top left, top right, bottom left, and bottom right of the screen. One of the arrows was in red, the others in black. The red arrow told the participant which of the four responses to make. Arrows were used at test to minimize the perceptual overlap between response cues at study and test. The jitter between object presentation and response cue (i.e., the arrows) was to ensure that participants were responding to the response cue onset (i.e., they could not predict on a trial-to-trial basis when they would be required to respond). Participants were required to respond as soon as the response cue appeared “as quickly and as accurately as possible”, up to a maximum of 1500 ms. On button press, the object and arrows were replaced by a blank screen lasting 1000 ms. Prior to the experiment, participants performed a single study–test block as practice.
Statistical analyses
Prior to calculating RTs (ms), for each individual participant, I excluded trials that were incorrect, or were faster or slower than 2 standard deviations from the condition-specific mean RT. For study and test phases, I report within-subject analyses of variance (ANOVAs) for both RT and accuracy. For the main effects and interactions within each ANOVA, I report partial eta squared effects sizes . Significant main effects with more than two conditions, and interactions, from ANOVAs were further interrogated with paired t tests. For these t tests, I report Cohen's d as the mean difference between conditions divided by the mean standard deviation across conditions (dav; Cumming, Citation2012; Lakens, Citation2013).
Results
Study phase
Study phase accuracy and RT data are shown in . At study I analysed accuracy and RTs for objects that were assigned to the congruent and incongruent conditions (i.e., that subsequently differ at test). I performed 2 × 3 within-subject ANOVAs with factors congruency (congruent vs. incongruent) and repetition (first–third object presentation). A 2 × 3 ANOVA on accuracy failed to reveal any significant effects or an interaction, Fs < 0.40, ps > .61, . Following exclusion of incorrect trials, a further 5% of trials were excluded as RT outliers (see Method for exclusion criteria) prior to analysis of the RT data. A similar ANOVA on RTs revealed a main effect of repetition, F(1.1, 36.1) = 97.23, p < .001,
. This effect was characterized by slower RTs for Presentation 3 than for Presentation 1, t(32) = 10.09, p < .001, d = 1.30, and Presentation 2, t(32) = 10.05, p < .001, d = 1.42, with no difference between Presentations 1 and 2, t(32) = 1.44, p = .16, d = 0.08. This is probably a function of the decreased time between the initial response cue and the appearance of the response trigger (i.e., the red square surrounding the object; see Method). Importantly, no main effect of congruency nor an interaction between congruency and repetition were seen, Fs < 1.4, ps > .27,
. As such, no accuracy or RT difference was seen between congruent and incongruent objects at study.
Table 1. Mean for accuracy and RTs during the study phase of Experiments 1 and 2 across the congruent and incongruent conditions and Presentations 1–3
Test phase
Test phase accuracy and RT data are shown in and . Accuracy and RT data were analysed with a one-way within-subjects ANOVA with the conditions congruent, incongruent, and novel. No significant effect was seen in the accuracy data, F(2.0, 62.4) = 1.62, p = .21, . Following exclusion of incorrect trials, a further 5% of trials were excluded as RT outliers prior to analysis of the RT data. A significant effect was seen in the RT data, F(1.9, 59.3) = 4.59, p < .05,
. Whereas RTs in the congruent condition were significantly faster than those in the incongruent, t(32) = 2.67, p < .05, d = 0.09, and novel, t(32) = 2.31, p < .05 , d = 0.08, conditions, no difference was seen between the incongruent and novel conditions, t(32) = 0.10, p = .92, d < 0.01. Thus, participants were faster when making the same response to a specific object at study and test than when they were either required to switch response to a specific object or required to respond to a novel object.
Figure 2 Repetition priming (repeated–novel; ms) for congruent and incongruent conditions across Experiments 1–2. *p < .05. **p < .01. ns = not significant.
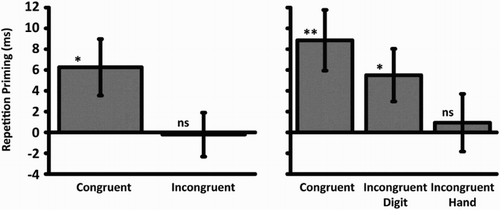
Table 2. Mean for accuracy and RTs during the test phase of Experiments 1 and 2 across the congruent and incongruent and novel conditions and response cue to object presentation intervals
To ensure that participants were not predicting when to respond at test, I varied the time between object presentation and response cue (red arrow). The interval was 300 ms, 500 ms, or 700 ms, randomly selected on each trial regardless of condition. To ensure that the differences seen between the congruent condition and both the incongruent and the novel condition were consistent across the three object presentation to response cue intervals, I conducted a 3 (congruency: congruent, incongruent, and novel) × 3 (interval: 300 ms, 500 ms, and 700 ms) ANOVA . For accuracy, no main effects or interactions were seen, Fs < 2.0, ps > .15, . For RTs, although a significant effect of interval was seen, F(1.8, 58.4) = 57.40, p < .001,
, revealing slower RTs for shorter intervals, no interaction was seen, F(3.0, 96.3) = 1.82, p = .15,
; the main effect of congruency was again significant, F(1.8, 58.8) = 4.09, p < .05,
. Thus, the difference between the three main conditions did not significantly differ as a function of the object presentation to response cue interval. However, caution is warranted given that the interval time was selected randomly on each trial, regardless of condition. As such, trials for each condition may not have been evenly allocated to the three interval times within each participant.
Discussion
Experiment 1 introduced a novel experimental paradigm to test for S–R contributions to repetition priming. The objects at study and test were entirely task irrelevant in that they did not inform the participants as to how or when to respond (though participants were told to “attend to the objects”). Further, the paradigm avoids the use of an object-oriented classification task at both encoding and retrieval. Despite this, participants still showed an S–R congruency effect. Participants reacted more quickly to objects seen at study if the same response was required at test as that at study than if a different response was required. Further, no difference was seen between previously seen objects that required a response switch between study and test relative to novel objects at test.
EXPERIMENT 2
Experiment 2 sought to replicate and extend the findings of Experiment 1. Specifically, I was interested in the level of response representation encoded within S–R bindings. Given the task has no semantic or classification component, responses being cued simply by squares or arrows, the “response” that becomes associated with each object at study is likely to be relative low level—that is, closely associated with a specific motoric action. In Experiment 1, incongruent responses at test switched both the hand (e.g., left to right) and the digit (e.g., finger to thumb). In Experiment 2, I introduced two incongruent conditions: (a) incongruent hand and (b) incongruent digit. In the incongruent hand condition, responses between study and test switched hand but not digit, whereas for the incongruent digit condition, responses switched digit but not hand.
Method
Experiment 2 was identical to Experiment 1 with the following exceptions.
Participants
A total of 32 participants (22 female) participated. They had a mean age of 22.2 years (SD = 2.9). By self-report, three participants were left-handed and the remainder right-handed.
Materials
The 240 object images were split into four sets of 60. Each set was assigned to one of the four conditions—congruent, incongruent hand, incongruent digit, and novel—with the assignment counterbalanced across participants in a Latin square design.
Procedure
The experiment consisted of 60 study–test blocks. For each study phase, three objects were presented three times each. Each object was assigned to one of the four possible response options (ensuring each stimulus–response pairing was unique). At test, each object from study was presented once, as well as a single novel trial. One of the objects from study required the same response at test (congruent), one required a switch in the hand, but not digit (e.g., from left to right hand, using the index finger; incongruent hand), and one required a switch in digit, but not hand (e.g., from index finger to thumb, using the left hand; incongruent digit). The novel object was randomly assigned one of the four response options.
Results
Study phase
Study phase accuracy and RT data are shown in . Accuracy and RT data were analysed using 3 × 3 within-subject ANOVAs with factors congruency (congruent, incongruent hand, incongruent digit) and repetition (first, second, third). The accuracy data revealed a main effect of repetition, F(1.9, 59.8) = 3.30, p < .05, , with decreasing accuracy across presentations. No main effect of congruency nor an interaction was present, Fs < 1.2, ps > .33,
. Following exclusion of incorrect trials, a further 5% of trials were excluded as RT outliers prior to analysis of the RT data. The RT data also revealed a main effect of repetition, F(1.1, 34.9) = 96.78, p < .001,
. Similar to Experiment 1, slower RTs were seen for the third presentation than for the first, t(31) = 9.18, p < .001, d = 1.64, and second, t(31) = 10.89, p < .001, d = 1.89, presentation. However, unlike Experiment 1, we also saw faster RTs for the second than for the first presentation, t(31) = 3.70, p < .01, d = 0.27. Despite a trend, the main effect of congruency was not significant, F(2.0, 61.8) = 3.08, p = .053,
, and no interaction was present, F(3.5, 108.4) = 1.11, p = .35,
. Therefore, replicating Experiment 1, we saw no differences between the main conditions of interest at study—as shown by a lack of a main effect of congruency in both the accuracy and the RT analyses.
Test phase
Test phase accuracy and RT data are shown in and . A one-way ANOVA across the four main conditions—congruent, incongruent hand, incongruent digit, and novel—for the accuracy data failed to reveal a main effect, F(2.6, 80.0) = 0.66, p = .56, . Following exclusion of incorrect trials, a further 5% of trials were excluded as RT outliers prior to analysis of the RT data. A main effect was seen when analysing the RT data, F(2.8, 86.7) = 4.26, p < .01,
. Faster RTs were seen in the congruent condition than in the incongruent hand, t(31) = 2.57, p < .05, d = 0.17, and novel, t(31) = 3.04, p < .01, d = 0.20, conditions. However, no difference was seen between the congruent and incongruent digit conditions, t(31) = 1.32, p = .20, d = 0.08. Replicating Experiment 1, the congruent condition differed from the incongruent and novel conditions. However, this only occurred in the incongruent condition that required a switch in hand (but not digit) and was not seen when switching digits (but not hands). Finally, faster RTs were seen in the incongruent digit than in the novel condition, t(31) = 2.17, p < .05, d = 0.12, whereas no difference was seen between the incongruent hand and novel conditions, t(31) = 0.34, p = .74, d = 0.02. However, the final comparison between incongruent hand and incongruent digit did not reach significance, t(31) = 1.48, p = .15, d = 0.09.
As in Experiment 1, I split the accuracy and RT data according to the object presentation to response cue interval (300 ms, 500 ms, and 700 ms). A 4 × 3 (Congruency × Interval) within-subjects ANOVA on the accuracy data failed to reveal any main effects or interactions, Fs < 1.7, ps > .19, . A similar ANOVA on the RT data revealed a main effect of interval, F(1.8, 56.7) = 28.86, p < .001,
, revealing slower RTs for shorter intervals, and a main effect of congruency, F(2.3, 72.7) = 2.97, p < .05,
. Unexpectedly, I also saw a Congruency × Interval interaction, F(4.8, 150.3) = 2.37, p < .05,
. This effect appears to be driven by a greater main effect of congruency for the 300-ms than for the 700-ms interval (see interexperiment analyses for further discussion of this issue).
Discussion
Experiment 2 replicated Experiment 1: I saw significantly faster RTs for objects that required the same response at study and test than for objects that required a switch in response (and relative to novel objects). Extending these results, I show that this slowing of RT (relative to the congruent condition) when participants are required to switch response to a specific object is only seen when switching hands (e.g., from left to right hand) but not when switching digits (e.g., from index finger to thumb).
Interexperimental analyses
I performed one final analysis to address two issues: (a) whether the congruency effect was consistent across experiments, and (b) whether the Congruency × Interval interaction seen in Experiment 2 (but not in Experiment 1) was consistent across experiments. To address this, I performed a 2 × 3 × 2 (Congruency × Interval × Experiment) mixed ANOVA using the congruent and incongruent conditions from Experiment 1 and the congruent and incongruent hand conditions from Experiment 2 (given that the results from Experiment 2 suggest that responses are coded at the level of hand, but not digit). A main effect of congruency, with no interaction with experiment or interval, would suggest that the congruency effect was consistent across experiments. Further, if no Congruency × Interval or Congruency × Interval × Experiment interaction is seen, this would suggest that the Congruency × Interval interaction seen in Experiment 2 was perhaps a Type I error (particularly given the low p-value and effect size associated with this interaction; see Experiment 2, Results section).
This ANOVA produced a main effect of congruency, F(1, 63) = 6.02, p < .05, , as well as a main effect of interval, F(1.9, 120.7) = 30.76, p < .001,
. Importantly, no further main effects or interactions were present, Fs < 1.14, ps > .32,
. To achieve a power of .8, with the sample size for this analysis, the effect size would need to be f = 0.22 (equivalent to
; a small-to-medium effect size). Thus, the interexperimental analysis has the required power to reveal relatively small effect sizes. The congruency effect therefore did not significantly differ across experiments, and no evidence could be found that this congruency effect differed as a function of the object presentation to response cue interval (at least not consistently across experiments). Therefore, across experiments there was a consistent, though relatively small (∼10 ms;
), RT difference between the congruent and incongruent conditions.
GENERAL DISCUSSION
Across two experiments, I have provided evidence for the encoding and retrieval of stimulus–response (S–R) bindings between a response and a task-irrelevant stimulus. In Experiment 1, I presented a novel paradigm that ensured a one-to-one mapping between stimuli (common objects) and responses. At encoding and retrieval, objects were task irrelevant as they were not informative with regard to what response the participant should make or when they should make it. Despite this, RTs were faster for repeated objects when the same response was made at encoding and retrieval. When participants were required to switch responses between study and test, RTs did not differ relative to when novel objects were presented. In Experiment 2, this response congruency effect was replicated but only when participants were required to switch hands (e.g., from left to right hand). When switching digit within hand (e.g., from index finger to thumb) RTs did not differ from when exactly the same response was made between encoding and retrieval. Thus, I provide novel evidence for the encoding and retrieval of stimulus to hand-response mappings during task-irrelevant conditions.
Whereas the majority of studies investigating S–R learning have used an object-oriented (binary) classification task at retrieval (and encoding), here using a response cueing method similar to Hommel (Citation1998), participants were never required to actively decide how to respond to a stimulus. Under such response cue conditions, uncertainty between possible response options is presumably low. Despite this, the retrieval of S–R bindings still facilitated behaviour when the response was repeated between encoding and retrieval. A similar effect has also been shown when responses for both the prime and probe stimulus in an immediate repetition paradigm are cued at the beginning of the prime–probe trial, presumably also resulting in conditions of high response certainty (Giesen & Rothermund, Citation2014, Experiment 3). These results suggest that S–R effects are a ubiquitous phenomenon that can manifest even under conditions where the retrieval of a previous response would appear to offer little behavioural benefit. It should be noted that the difference in RTs between congruent and incongruent stimuli in the present experiments is small (∼10 ms) relative to paradigms that use semantic classification of objects (∼60 ms in the case of Horner & Henson, Citation2009). This decreased congruency effect may be a result of the faster RTs in the present studies (<500 ms) relative to semantic classification studies (∼600 to 1000 ms), with less time for retrieved S–R bindings to modulate response-selection processes. Importantly, S–R effects were still seen despite such fast RTs, suggesting that S–R bindings can be retrieved rapidly. It is also possible that (separate to RT differences) increasing response certainty at retrieval decreases (though does not eliminate) S–R effects. Further work directly manipulating response certainty/competition at retrieval (e.g., by varying the coherence in moving random dot patterns used as response cues) under otherwise identical encoding and retrieval conditions is needed to address this possibility.
Another critical manipulation in the present studies was to ensure that objects were task irrelevant at both encoding and retrieval. Showing S–R effects under such conditions further demonstrates the ubiquity of this form of object-oriented learning. Previous research has provided evidence for mappings between responses and unattended or task-irrelevant stimuli present during response onset (e.g., Frings et al., Citation2007; Rothermund et al., Citation2005). However, these experiments only assessed S–R effects across immediate or short lags between encoding and retrieval. The exception to this is Experiment 2b of Frings and Rothermund (Citation2011), which presented a yellow circle between presentations of letters (the letters being the stimuli of experimental interest). Thus, despite the presence of an intervening stimulus, it was consistent across trials and had little perceptual similarity to the main experimental stimuli. Here, the number of intervening trials between the last study presentation and test presentation ranged from 0 to 8 in Experiment 1 and 0 to 5 in Experiment 2, with the time between presentation ranging from 1500 ms to ∼15 s in Experiment 1 and 1500 ms to ∼10 s in Experiment 2. This suggests that the S–R bindings formed in the present experiments can, at least, last for several seconds and are not completely disrupted by several intervening stimuli.
One key difference between the present studies and those of Rothermund, Frings and colleagues is that although the objects in the present studies were task irrelevant, they were not unattended. Manipulating attention was not a key aim in the present studies, and it is probable that attending to task-irrelevant stimuli increases the likelihood of seeing S–R effects relative to a strong attentional manipulation. Although S–R effects can be seen for unattended stimuli, these effects appear more short-lived. For example, distractor-to-distractor effects in an immediate repetition paradigm were seen with a 500-ms lag between encoding and retrieval, but not when the lag was increased to 2000 ms (Frings, Citation2011). Thus, under strong attentional conditions, S–R bindings might be relatively short-lived relative to those formed in the present attended but task-irrelevant conditions.
One might reasonably ask: “What were participants doing with the objects, given they were task irrelevant?” One possibility is that they were completely ignoring the objects and solely focusing on the response-cueing task. If so, this would provide even stronger evidence that S–R effects can manifest when stimuli are unattended at both encoding and retrieval. However, it is equally possible that participants were attending to the objects and explicitly trying to learn the S–R relationships. Although a possibility, it is perhaps unlikely given that participants were told to respond as fast as possible when the response trigger was presented. Thus, although the task was relatively easy, participants will have been highly focused on preparing to respond on trigger onset (as evidenced by the relatively fast RTs across conditions—i.e., <500 ms). Further, this possibility would not undermine the main argument relating to response uncertainty—response congruency effects were seen despite the use of highly salient response cues at retrieval.
S–R effects were seen only when participants were required to switch between hands (whilst keeping the digit constant) but not when required to switch between digits (whilst keeping the hand constant). These results are consistent with previous research suggesting that responses within S–R bindings can be coded at the level of a motoric action (e.g., Dennis & Perfect, Citation2013; Horner & Henson, Citation2009). They also relate to findings that show that retrieval of S–R bindings can modulate the onset of lateralized readiness potentials, thought to reflect preparation of a left- or right-hand movement (Frings, Bermeitinger, & Gibbons, Citation2011; Gibbons & Stahl, Citation2008). However, previous research did not investigate the specificity of these action representations. The results here suggest that responses within S–R bindings can be represented at the level of right/left hand, but perhaps not a more fine-grained index finger/thumb within hand, at least in the context of task-irrelevant conditions. Though the pairwise comparisons between each incongruent condition and the congruent and novel conditions were consistent with facilitation only in the incongruent digit condition, caution is warranted as the comparison between incongruent hand and incongruent digit did not reach significance.
It is possible that different digit arrangements could produce S–R effects (e.g., from thumb to ring finger); however, it is noteworthy that a recent study found thumb representations in primary motor cortex to be most distinct from any of the individual finger representations (Diedrichsen, Wiestler, & Ejaz, Citation2013). Thus, the thumb to index finger manipulation is likely to be one of the least fine-grained within-hand manipulations. Note that it is not that responses in S–R bindings are solely coded at the level of a motoric hand action. Many studies have provided evidence for multiple, more abstract, levels of response representation (Denkinger & Koutstaal, Citation2009; Dennis & Perfect, Citation2013; Horner & Henson, Citation2009; Race et al., Citation2009; Schnyer et al., Citation2007). Further, there is ample evidence for bindings between the stimulus presented and task performed during stimulus presentation (e.g., Moutsopoulou & Waszak, Citation2012; Waszak, Hommel, & Allport, Citation2003). However, the current results suggest that the hand might be the lower representational limit for responses within S–R bindings.
Finally, the response congruency effects in the present studies were primarily driven by a speeding of RTs in the congruent condition, with no clear differences present between the incongruent and novel conditions. Both facilitation (i.e., faster RTs for congruent than for novel trials) and interference (i.e., slower RTs for incongruent than for novel trials) effects have been seen in the literature (e.g., Hommel, Citation1998; Horner & Henson, Citation2009, Citation2011; Rothermund et al., Citation2005), and the circumstances under which these opposing effects emerge appears complex (Waszak & Hommel, Citation2007). It is worth noting that other sources of positive priming, for example perceptual/conceptual priming, may also modulate RTs, diminishing the likelihood of seeing interference effects. This unresolved issue would benefit from computational modelling approaches (e.g., Saggar, Miikkulainen, & Schnyer, Citation2010), whereby specific predictions can be made about the conditions under which facilitation and/or interference effects emerge.
In sum, I have presented novel evidence for S–R effects despite using a response cue manipulation that minimizes response uncertainty/competition at retrieval. Also, objects were task irrelevant at both encoding and retrieval, further minimizing the likelihood of seeing such effects. Thus, S–R effects can manifest even under conditions where the encoding and retrieval of S–R bindings would not appear to be behaviourally beneficial. The results also provide the first evidence that responses within S–R bindings can be coded at the level of left/right hand but perhaps not at more fine-grained motoric representations. The results therefore underline the automaticity, ubiquity, and resilience of S–R contributions to repetition priming.
REFERENCES
- Cumming, G. (2012). Understanding the new statistics: Effect sizes, confidence intervals and meta-analysis. New York, NY: Routledge.
- Denkinger, B., & Koutstaal, W. (2009). Perceive-decide-act, perceive-decide-act: How abstract is repetition-related decision learning? Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(3), 742–756.
- Dennis, I., & Perfect, T. J. (2013). Do stimulus-action associations contribute to repetition priming? Journal of Experimental Psychology: Learning, Memory, and Cognition, 39(1), 85–95.
- Dennis, I., & Schmidt, K. (2003). Associative processes in repetition priming. Journal of Experimental Psychology: Learning, Memory, and Cognition, 29(4), 532–538.
- Diedrichsen, J., Wiestler, T., & Ejaz, N. (2013). A multivariate method to determine the dimensionality of neural representation from population activity. NeuroImage, 76, 225–235. doi: 10.1016/j.neuroimage.2013.02.062
- Dobbins, I. G., Schnyer, D. M., Verfaellie, M., & Schacter, D. L. (2004). Cortical activity reductions during repetition priming can result from rapid response learning. Nature, 428(6980), 316–319. doi: 10.1038/nature02400
- Frings, C. (2011). On the decay of distractor-response episodes. Experimental Psychology, 58(2), 125–131. doi: 10.1027/1618-3169/a000077
- Frings, C., Bermeitinger, C., & Gibbons, H. (2011). Prime retrieval of motor responses in negative priming: Evidence from lateralized readiness potentials. Brain Research, 1407, 69–78. doi: 10.1016/j.brainres.2011.06.037
- Frings, C., Moeller, B., & Rothermund, K. (2013). Retrieval of event files can be conceptually mediated. Attention, Perception & Psychophysics, 75(4), 700–709. doi: 10.3758/s13414-013-0431-3
- Frings, C., & Rothermund, K. (2011). To be or not to be … included in an event file: Integration and retrieval of distractors in stimulus-response episodes is influenced by perceptual grouping. Journal of Experimental Psychology: Learning, Memory, and Cognition, 37(5), 1209–1227.
- Frings, C., Rothermund, K., & Wentura, D. (2007). Distractor repetitions retrieve previous responses to targets. The Quarterly Journal of Experimental Psychology, 60(10), 1367–1377. doi: 10.1080/17470210600955645
- Gibbons, H., & Stahl, J. (2008). Early activity in the lateralized readiness potential suggests prime-response retrieval as a source of negative priming. Experimental Psychology, 55(3), 164–172. doi: 10.1027/1618-3169.55.3.164
- Giesen, C., & Rothermund, K. (2014). Distractor repetitions retrieve previous responses and previous targets: Experimental dissociations of distractor-response and distractor-target bindings. Journal of Experimental Psychology: Learning, Memory, and Cognition, 40(3), 645–659.
- Henson, R. N. (2003). Neuroimaging studies of priming. Progress in Neurobiology, 70(1), 53–81. doi: 10.1016/S0301-0082(03)00086-8
- Henson, R. N., Eckstein, D., Waszak, F., Frings, C., & Horner, A. J. (2014). Stimulus-response bindings in priming. Trends in Cognitive Sciences, 18(7), 376–384. doi: 10.1016/j.tics.2014.03.004
- Hommel, B. (1998). Event files: Evidence for automatic integration of stimulus-response episodes. Visual Cognition, 5, 183–216. doi: 10.1080/713756773
- Hommel, B. (2004). Event files: Feature binding in and across perception and action. Trends in Cognitive Sciences, 8(11), 494–500. doi: 10.1016/j.tics.2004.08.007
- Hommel, B. (2005). How much attention does an event file need? Journal of Experimental Psychology: Human Perception and Performance, 31(5), 1067–1082.
- Hommel, B., & Colzato, L. (2004). Visual attention and the temporal dynamics of feature integration. Visual Cognition, 11(4), 483–521. doi: 10.1080/13506280344000400
- Horner, A. J., & Henson, R. N. (2008). Priming, response learning and repetition suppression. Neuropsychologia, 46(7), 1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018
- Horner, A. J., & Henson, R. N. (2009). Bindings between stimuli and multiple response codes dominate long-lag repetition priming in speeded classification tasks. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(3), 757–779.
- Horner, A. J., & Henson, R. N. (2011). Stimulus-response bindings code both abstract and specific representations of stimuli: Evidence from a classification priming design that reverses multiple levels of response representations. Memory & Cognition, 39(8), 1457–1471. doi: 10.3758/s13421-011-0118-8
- Horner, A. J., & Henson, R. N. (2012). Incongruent abstract stimulus-response bindings result in response interference: fMRI and EEG evidence from visual object classification priming. Journal of Cognitive Neuroscience, 24(3), 760–773. doi: 10.1162/jocn_a_00163
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. doi:10.3389/fpsyg.2013.00863
- Logan, G. D. (1988). Toward an instance theory of automatization. Psychological Review, 95(4), 492–527. doi: 10.1037/0033-295X.95.4.492
- Logan, G. D. (1990). Repetition priming and automaticity: Common underlying mechanisms? Cognitive Psychology, 22, 1–35. doi: 10.1016/0010-0285(90)90002-L
- Moeller, B., & Frings, C. (2014). Attention meets binding: Only attended distractors are used for the retrieval of event files. Attention, Perception & Psychophysics, 76, 959–978. doi: 10.3758/s13414-014-0648-9
- Moutsopoulou, K., & Waszak, F. (2012). Across-task priming revisited: Response and task conflicts disentangled using ex-Gaussian distribution analysis. Journal of Experimental Psychology. Human Perception and Performance, 38(2), 367–74. doi: 10.1037/a0025858
- Race, E. A., Badre, D., & Wagner, A. D. (2010). Multiple forms of learning yield temporally distinct electrophysiological repetition effects. Cerebral Cortex, 20(7), 1726–1738. doi: 10.1093/cercor/bhp233
- Race, E. A., Shanker, S., & Wagner, A. D. (2009). Neural priming in human frontal cortex: Multiple forms of learning reduce demands on the prefrontal executive system. Journal of Cognitive Neuroscience, 21(9), 1766–1781. doi: 10.1162/jocn.2009.21132
- Richardson-Klavehn, A., & Bjork, R. A. (1988). Measures of memory. Annual Review of Psychology, 39(1), 475–543. doi: 10.1146/annurev.ps.39.020188.002355
- Rothermund, K., Wentura, D., & De Houwer, J. (2005). Retrieval of incidental stimulus-response associations as a source of negative priming. Journal of Experimental Psychology: Learning, Memory, and Cognition, 31(3), 482–495.
- Saggar, M., Miikkulainen, R., & Schnyer, D. M. (2010). Behavioral, neuroimaging, and computational evidence for perceptual caching in repetition priming. Brain Research, 1315, 75–91. doi: 10.1016/j.brainres.2009.11.074
- Schnyer, D. M., Dobbins, I. G., Nicholls, L., Davis, S., Verfaellie, M., & Schacter, D. L. (2007). Item to decision mapping in rapid response learning. Memory & Cognition, 35(6), 1472–1482. doi: 10.3758/BF03193617
- Soldan, A., Clarke, B., Colleran, C., & Kuras, Y. (2012). Priming and stimulus–response learning in perceptual classification tasks. Memory, 20(4), 400–413. doi: 10.1080/09658211.2012.669482
- Valt, C., Klein, C., & Boehm, S. G. (2014). Dissociation of rapid response learning and facilitation in perceptual and conceptual networks of person recognition. British Journal of Psychology. Advance online publication. doi:10.1111/bjop.12095
- Waszak, F., & Hommel, B. (2007). The costs and benefits of cross-task priming. Memory & Cognition, 35(5), 1175–1186. doi: 10.3758/BF03193487
- Waszak, F., Hommel, B., & Allport, A. (2003). Task-switching and long-term priming: Role of episodic stimulus-task bindings in task-shift costs. Cognitive Psychology, 46(4), 361–413. doi: 10.1016/S0010-0285(02)00520-0
