Abstract
Covert exchange of autonomic responses may shape social affective behavior, as observed in mirroring of pupillary responses during sadness processing. We examined how, independent of facial emotional expression, dynamic coherence between one's own and another's pupil size modulates regional brain activity. Fourteen subjects viewed pairs of eye stimuli while undergoing fMRI. Using continuous pupillometry biofeedback, the size of the observed pupils was varied, correlating positively or negatively with changes in participants’ own pupils. Viewing both static and dynamic stimuli activated right fusiform gyrus. Observing dynamically changing pupils activated STS and amygdala, regions engaged by non-static and salient facial features. Discordance between observed and observer's pupillary changes enhanced activity within bilateral anterior insula, left amygdala and anterior cingulate. In contrast, processing positively correlated pupils enhanced activity within left frontal operculum. Our findings suggest pupillary signals are monitored continuously during social interactions and that incongruent changes activate brain regions involved in tracking motivational salience and attentionally meaningful information. Naturalistically, dynamic coherence in pupillary change follows fluctuations in ambient light. Correspondingly, in social contexts discordant pupil response is likely to reflect divergence of dispositional state. Our data provide empirical evidence for an autonomically mediated extension of forward models of motor control into social interaction.
INTRODUCTION
Dynamic changes in facial features are powerful signals for the communication of emotion across individuals. Foremost among these are expressions determined by the facial musculature (Adolphs, Citation2002; Ekman & Friesen, Citation1971). Beyond the face, emotional state may also be inferred from body posture, gait, vocal expressions and prosodic elements of speech (de Gelder, Citation2006). Autonomic changes in both bodily and visceral arousal complement and contribute to these facial and non-facial emotional signals, consistent with the evolutionary grounding of emotion in homoeostatic processes (Darwin, Citation1872). In the face, changes such as blushing, pallor, sweating, salivation and pupillary constriction may complement and reinforce emotion-specific signals of the branchiomeric facial muscles (Darwin, Citation1872).
In two recent studies using static stimuli, we demonstrated that observed pupil size modulates the processing of expressions of sadness. Small pupils make sad faces appear more intense and more negative, yet pupil size has no effect on neutral, happy or angry expressions (Harrison, Singer, Rotshtein, Dolan, & Critchley, Citation2006; Harrison, Wilson, & Critchley, Citation2007). Importantly, we also observed a “contagion” of pupil change: When subjects viewed sad faces, their own pupils automatically mirrored the pupil size of the static face stimulus observed (Harrison et al., Citation2006). Again this effect was not associated with other primary emotions and reflected a functional interaction between the amygdala and brainstem autonomic nuclei controlling meiosis. We confirmed and extended these observations, using an independent set of stimuli in a separate population, showing that pupil size did not directly influence ratings of other primary emotions, including fear, disgust and surprise. Furthermore individual sensitivity to another's pupillary signals predicted scores of emotional empathy (Harrison et al., Citation2007). Together these observations highlight an implicit autonomic effect influencing the communication of distress, mediated through changes in pupil size.
Pupil size may influence perceived attractiveness, particularly in males viewing female faces (Tombs & Silverman, Citation2004). A recent study investigating this effect showed that amygdala activity in men viewing female faces was significantly greater if they were shown with large pupils, even though they were not explicitly rated as appearing more attractive (Demos, Kelley, Ryan, Davis, & Whalen, Citation2008). This was interpreted as indicating that large pupils in observed female faces (with neutral or happy expressions) induce an amygdala-based arousal or alerting response in the male observer even when they are apparently unaware of pupil size change. Together these studies illustrate a growing awareness of the importance of pupillary signals in social exchange and suggest a dynamic influence of pupillary signals in shaping social behavior.
In parallel with these findings a number of recent empirical studies have implicated the somatic motor system in action observation, imitation and social interaction, leading to the suggestion that others’ actions are decoded by activating our own somatic motor systems (Liberman & Whalen, Citation2000). It has been proposed that forward models of motor control, such as MOSAIC, that employ efference copies of motor commands to predict sensory consequences of our own motor commands may be extended to the social domain (Wolpert, Doya, & Kawato, 2003). These models propose that, in social contexts, people generate internal forward motor models to compare their own motor behavior with the observed motor behavior of others. Discrepancies in observed and predicted motor responses may then be used to predict others’ hidden (intentional) states. In the current study, we extended these models to the autonomic nervous system with the following predictions: first, we predicted that if pupillary signals provide salient information in social interactions independent of emotional facial expression, changes in observed pupil size will activate regions previously implicated in social and emotional processing; second, if other's pupillary responses are continuously monitored, discrepant (unpredicted) pupillary responses that signal a potential change in another's dispositional state will be detected and further modulate activity in these regions.
We therefore conducted a neuroimaging experiment to explore the neural substrates supporting the processing of interactions between changes in observer's and observed pupils. We used biofeedback techniques to provide a continuous real-time dynamic context where the participant/observer saw a pair of eyes with pupils that either accurately mirrored changes in their own pupils, or did the opposite. We were thus able to examine brain activity supporting the processing of naturalistic dynamic changes in pupil size and explore the neural substrates engaged when pupil size changed continuously in a coherent or incoherent manner with respect to changes in the viewer's own pupils. We hypothesized that the main effect of observed change in pupil size would engage regions previously shown to process other dynamic changes in facial features, including the cortex of the superior temporal sulcus (Puce, Allison, Bentin, Gore, & McCarthy, Citation1998; Perrett & Emery, Citation1994), and that activity within amygdala, anterior cingulate and anterior insula cortices, regions implicated in integrating emotional processing, subjective emotional states and empathetic understanding with autonomic control would differentially reflect the coherence between the participant's pupils and those observed.
METHODS
Subjects
Fourteen right-handed healthy volunteers (7 females, mean age [± SD] 22.0 [± 3.5] years) were recruited for the study. Each participant was screened to exclude those with a history of neurological or psychiatric illness or taking prescribed medication (other than oral contraceptive). No one reported recreational or other drug use within two weeks of scanning. Informed consent was obtained in accordance with the Declaration of Helsinki (1991) and the procedures were approved by the joint Ethics Committee of the National Hospital for Neurology & Neurosurgery and Institute of Neurology, London. Participants were instructed to look at the eyes of the individuals shown as stimuli.
Stimuli
The stimuli were high-definition digital photographs taken of neutral facial expressions of one man and one woman, who both had blue irises (accentuating pupil contrast). The images were cropped to reveal only the eye region (a). Iris size was digitally manipulated using Adobe Photoshop® (Adobe Systems) to produce two pairs of images with maximally dilated (8.2 mm male, 8.0 mm female) and maximally constricted (pupil diameter = 2 mm) pupils. These values were selected to ensure pupil size remained within the physiological range. Morpher® (M.Fujimiya) was used to produce a total of 24 images with pupil sizes between these extremes.
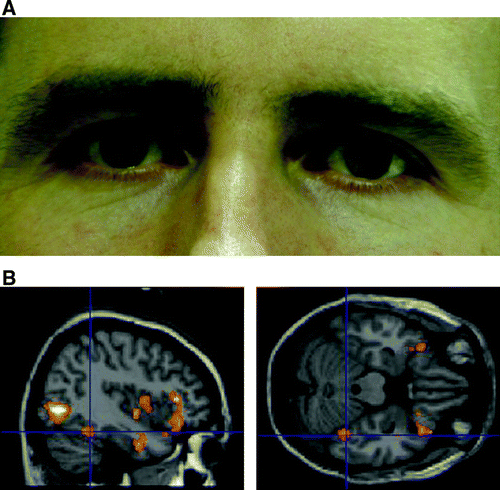
Figure 1. (A) Example of male face stimulus illustrating mild pupillary dilatation (image 13 of 24). (B) Right fusiform face area activation in response to the main effect of viewing faces. Plotted at p=.005 for illustration, only clusters of 50 or more voxels shown.
All images were displayed in a 400×200 pixel array, back-projected onto a mirror mounted on the magnetic resonance imaging (MRI) head coil. Each set of eyes was shown centrally on the screen for epochs of mean duration 22 s. The pupil size of the stimuli was updated at a frequency of 60 Hz, driven directly by changes in the subject's own pupil size. Each set of eye stimuli was shown four times, twice each in the positive and negative feedback conditions, resulting in a total of four positive and negative feedback epochs per subject. Tasks were written and presented using Cogent 2000 (VisLab, FIL, UCL London) software on a Matlab platform (Mathwork, Nantick MA).
Physiological data recording and analysis
Pupil diameter was monitored online throughout the functional magnetic resonance imaging (fMRI) scanning sessions using an infra-red eye tracker (Applied Sciences Laboratories, Waltham MA, Model 504) recording at 60 Hz. Each participant's pupil sizes were measured in response to dark (black) and bright (white) screens displayed for 5 s prior to alternate feedback epochs and used to calculate subject- and epoch-specific pupillary dynamic ranges. Maximal pupil size was defined as the maximum pupil size recorded to the black screen and minimal pupil size as the mean of all non-zero recordings to the gray screen. This method accurately recorded maximal pupil size, though it is likely to have produced a slight underestimate of minimal pupil size (estimated to be less than 0.2 mm by analyzing raw and blink removed data in the bright screen condition). Maximal and minimal pupil sizes were re-measured on alternate presentations to accommodate for drift in pupil size. Positive and negative conditions were presented in random order. Prior to each trial, the participant's initial pupil size was measured in response to a gray screen of luminance equal to the mean pupil image. Initial pupil size was defined as the mean of all non-zero recordings. Initial pupil size of the dynamic stimulus was selected to produce a discrepancy between observed and observer's pupil size determined by the formula:
In the positive feedback condition, changes in the participant's pupil size were directly mirrored by an equal fractional change in the pupil size of the eyes viewed. In the negative feedback condition, changes in the participant's pupil size were associated with an equal and opposite fractional change in the size of the observed pupil. During analysis, blinks were identified as pupillometry recordings below the minimum observed pupil size; these were removed and replaced by interpolation of neighboring values. These blink-free pupillometry recordings were then used to reconstruct the images observed and the variance in these images in the positive and negative feedback epochs used to inform the subsequent SPM analysis.
Scanning and imaging data analysis
Whole-brain fMRI data were acquired on a 1.5 T Siemens Sonata magnetic resonance scanner equipped with a standard head coil. Functional images were obtained with a gradient echo-planar T2* sequence using blood-oxygenation level-dependent (BOLD) contrast, each comprising a full brain volume of 44 contiguous slices (2 mm slice thickness, 1 mm interslice gap) in a −30° tilted plane acquisition sequence to minimize signal dropout in the orbitofrontal, medial temporal and brainstem regions (Deichmann, Gottfried, Hutton, & Turner, Citation2003). Volumes were acquired continuously with a repetition time (TR) of 3.96 s. A total of 79 volumes were acquired for each participant in a single session, with the first five volumes subsequently discarded to allow for T1 equilibration effects.
Functional MRI data were analyzed using the general linear model for event-related designs in SPM2 (Wellcome Trust Centre for Neuroimaging; www.fil.ion.ucl.ac.uk/spm). Individual scans were realigned and unwarped, normalized and spatially smoothed with an 8-mm FWHM Gaussian kernel using standard SPM methods. A high-pass frequency filter (cutoff 120 s) and corrections for autocorrelation between scans (AR1) were applied to the time series. Each event was modeled by a standard synthetic hemodynamic response function at each voxel across the whole brain. The gray, white, and black images together with the positive and negative feedback blocks were modeled as separate regressors, with variance in observed pupil size modeled as a parametric regressor for the positive and negative feedback conditions. Parameter estimates using a block design were obtained at each voxel, for each condition and subject. Statistical parametric maps of the t-statistic (SPM {t}) were generated for each condition and transformed to a normal distribution (SPM {Z}) for each individual participant.
Second-level random effects analysis of the main effects of (i) observing face images and (ii) change in observed pupil size were then performed using single-sample t-tests on subject-specific contrast images. The interaction between feedback condition (positive and negative) and variance in observed pupil size was performed by means of a one-way repeated measures ANOVA using the positive and negative feedback contrast images for each subject. Participants were treated as the random variable. Results for the group analysis for the main effect of viewing eye stimuli is reported at a whole brain corrected cluster threshold of p<.05 and for effect of change in pupil size for clusters of 10 or more contiguous voxels surviving an uncorrected threshold of p<.001. Results for the predicted regions of interest (ROI): fusiform face area (FFA) (coordinates taken from Grill-Spector, Knouf, & Kanwisher, 2004), bilateral superior temporal sulci, amygdala, cingulate, anterior insula and frontal operculum (coordinates taken from Tomaiuolo et al., Citation1999) are reported for clusters of six or more contiguous voxels surviving an uncorrected threshold of p<.005. FFA and frontal operculum ROI were defined as 10-mm diameter spheres centered on the peak voxels from the above references. All other regions of interest were defined using masks from the image analysis package MarsBaR (Brett, Anton, Valabregue, & Poline, Citation2002), with anterior insula defined as insula mask voxels anterior to y>0.
RESULTS
Pupillometry data
Mean pupil size, change in pupil size across blocks and variance in subjects’ pupil size during blocks did not differ between the two feedback conditions (mean pupil size (±SD) positive feedback 3.91 (± 0.44) mm, negative feedback 3.92 (±0.44 mm). A illustrates the change in a single subject's pupil size (and corresponding image observed for coherent condition) for both coherent and incoherent feedback.
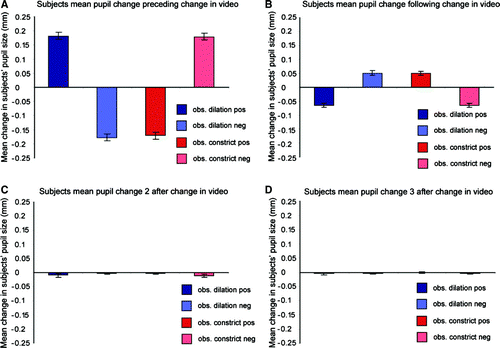
Analysis of subjects’ pupil data immediately preceding a change in observed pupil size confirmed that observed pupillary constrictions and dilations were preceded by congruent (positive feedback) and incongruent (negative feedback) pupillary changes in the observer, repeated measures ANOVA, interaction between factors feedback type (positive or negative) and pupillary change (constriction or dilation), F(1, 13) = 190.2, p<.001 (a). The mean change in subjects’ pupil size driving a change in the observed stimulus was 0.18 mm and did not differ between feedback conditions, paired t-test t(13) = 1.05, p=.31, or between constrictions or dilations, t(13) = − 0.43, p=.67. There was also a significant interaction between feedback type and subjects’ pupillary response (dilation or constriction) immediately following an observed pupil size change, F(1, 13) = 66.6, p<.001 (b). Interestingly, this showed the opposite interaction, i.e., rather than mimicking the observed pupil size change subjects’ pupils showed a small (mean 0.06 mm) constriction if the previous change was a dilation and vice versa. The direction of this effect argues against a direct mimetic response to the observed stimulus and, further, the timescale (<17 ms) is too rapid for this or an effect driven by the pupillary light reflex (latency ∼180 ms). Again there was no significant difference in the size of this pupillary response between feedback types (p=.88) or between constrictions or dilations (p=.72). Change in observed stimuli did not have a significant effect on subjects’ subsequent pupillary responses (c and 2d). Together this suggests a compensatory constriction following a previous dilation and vice versa that is not influenced by feedback condition.
Figure 2. (A) Mean change in observer's pupil size preceding an observed pupil size change. (B) Mean change in observer's pupil size following an observed pupil size change. (C) Change in observer's pupils 2 frames (33 ms) after an observed change in pupil size. (D) Change in observer's pupils 3 frames (50 ms) after an observed change in pupil size.
Functional imaging data
Viewing the eye stimuli was associated, as a main effect, with enhanced activation within the right fusiform cortex, [(42, −46, −22) t=3.07, Z=3.15, p<.001] (b, ). This region is associated with processing of invariant facial features including internal face parts and includes a “fusiform face area” (FFA). Additionally, other regions of extrastriate visual cortex were also activated as a main effect of the eye stimuli.
Table 1. Main effect of viewing eyes
Activity within a discrete set of brain regions also responded to the magnitude of dynamic changes in participant's own pupil size and the corresponding changes observed in the pupil stimuli across positive and negative feedback conditions (see ). These regions included dorsolateral pons bilaterally, left amygdala [(−12, −2, −16) t=6.24, Z=4.17, p<.001] and left superior temporal sulcus (see b, c), a region implicated in processing changeable facial features such as eye gaze, expression and lip movements [(−60, −42, 4) t=4.09, Z=3.22, p<.001]. Right STS activity was also observed [(64 −30 −10) t=3.71, Z=3.01, p<.001, k=5], though this did not survive our contiguity threshold.
Figure 3. (A) Mean change in pupil size in observed image across time for coherent and incoherent feedback. (B) Main effect of increasing variance in observed pupil size in the left amygdala. (C) Right superior temporal sulcus. Activations plotted at p=.005 for illustration, only clusters of 50 or more voxels shown.
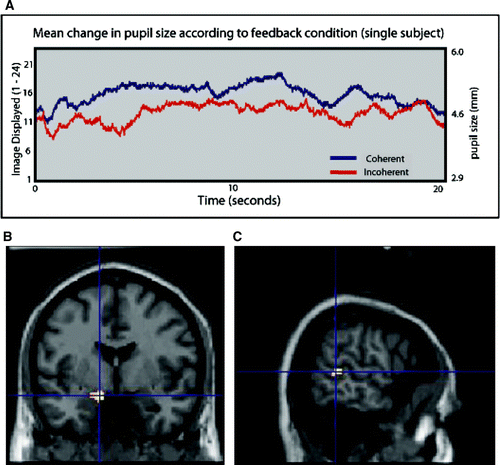
Table 2. Main effect of change in observed and observer's pupil size
We also examined the context-specific engagement of different brain systems by positive and negative feedback by testing for an interaction between feedback condition (coherent or incoherent) and the magnitude of change in observed pupil size. Pupil change in the positive feedback condition was associated with increasing activity within right precentral gyrus, right frontal operculum and mid cingulate cortex (see ). Region-of-interest analysis revealed no significant activation within superior temporal sulcus (STS), insula or amygdala. Pupil change during the negative (incoherent) feedback conditions, where the observed pupils reacted in a directly opposite manner to the participant's own pupils, was also associated with mid cingulate activation. However, in contrast to the coherent feedback condition, there was additional activation in the left amygdala and bilateral anterior insula, regions implicated in social and emotional processing (see ).
Figure 4. Interaction between feedback condition and variance in pupil size showing significant activation in: (A) anterior cingulated; (B) left amygdala; (C) bilateral anterior insula for negative > positive feedback. Activations plotted at p=.05 for illustration, only clusters of 50 or more voxels shown.
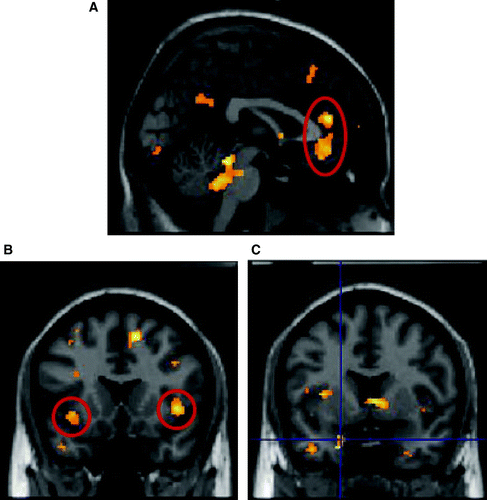
Table 3. Interaction between feedback condition and variance in pupil size
DISCUSSION
Eyes are highly salient facial features with particular importance in social perception, verbal and non-verbal communication. Eye signals serve as potent cues to the direction of another's attention (Perrett & Emery, Citation1994), convey intimacy (Kleinke, Citation1986), regulate turn-taking in conversation and are central to displays of fear and sadness (Smith, Cottrell, Gosselin, & Schyns, Citation2005). The ability to perceive eyes and eye-like stimuli develops very early in humans. By two months of age, infants show a preference for looking at eyes over other regions of the face (Maurer, Citation1985) and by four months of age they are able to differentiate direct from averted gaze (Vecera SP, Citation2006).
Sympathetic arousal resulting in lid retraction (and pupillary dilatation) is characteristic of the expression of fear (Adolphs et al., Citation2005). Small pupils enhance the negative intensity of the expression of sadness (Harrison et al., Citation2006, Citation2007), while large pupils in women result in men rating them as more attractive (Tombs & Silverman, Citation2004). Unlike other primate species which typically have dark-colored irises and sclera, humans have evolved eyes with bright white sclera and variable iris pigmentation that facilitate perception of both of these components. Speculatively, this uniquely enhanced sclera–iris–pupil contrast may have evolved to augment our ability to detect the direction of other people's eye gaze (Kobayashi & Kohshima, Citation1997; Adolphs, Citation2006). The sharing of focused attention at close range and recognition of subtle emotional signals (e.g., distress or sexual attractiveness) may enhance affiliative behavior and social bonding between conspecifics.
Our current study shows that perception of changes in another's pupils while performing an implicit eye observation task engages cortical and subcortical regions involved in face processing and emotion recognition (fusiform cortex, superior temporal sulcus, amygdala). This is particularly striking as our stimuli were carefully cropped to reveal only the eye region, removing all other facial and emotional contextual information. Further subjects were not requested to explicitly evaluate the eye stimuli, suggesting that regions implicated in the implicit evaluation of emotional facial features governed by the facial musculature (Winston, O'Doherty, & Dolan, Citation2003; Critchley et al., Citation2000) also play a role in the implicit evaluation of salient autonomically governed facial features such as pupil size. Previous studies using intracranial recordings in humans (McCarthy, Puce, Belger, & Allison, Citation1999) have also shown that internal face parts such as isolated eyes, mouth or nose in addition to activating the fusiform face area (FFA) activate a more lateral fusiform region. Interestingly our activations to isolated eye stimuli also lie slightly lateral to the FFA reported by Grill-Spector et al. (Citation2004), which is in keeping with activity within a more lateral internal face part sensitive cortical region. Note, however, that we did not employ a whole face localizer in the current study so are unable to conclusively distinguish activity in FFA and more lateral face part sensitive fusiform cortex.
Also noteworthy is the laterality of our activations. Activations to the eye stimuli within fusiform cortex showed a right lateralization (though left-sided activations were observed when the activation threshold was dropped). These findings of greater right- than left-sided FFA activity are in keeping with the literature on prosopagnosia, in which right lateralized lesions are both necessary and sufficient to cause deficits in face recognition (Barton, Press, Keenan, & O'Connor, Citation2002). In contrast, our STS and amygdala activations to changes in observed pupil size were associated with a preferential left lateralized response (though again right-sided activations were observed at a lower threshold). Accumulating evidence suggests a differential role for right and left amygdala in processing emotional and arousing stimuli, with right amygdala sensitive to diverse course arousing stimuli, regardless of their emotional and behavioral significance, which correlates with the overall level of physiological arousal (Glascher & Adolphs, Citation2003). In contrast, the left amygdala may better discriminate between emotional and arousing stimuli (Hardee, Thompson, & Puce, Citation2008) and better differentiate the magnitude of associated arousal (Davidson, Fedio, Smith, Aureille, & Martin, Citation1992). Greater left amygdala (and STS) activity in our current study to observation of varying pupil size may therefore represent a greater sensitivity to a dynamically varying arousal. Previous suggestions of faster right than left amygdala habituation (Wright et al., Citation2001) may also underlie the (relative) lack of right amygdala activity observed in our study, which was modeled as a block design of 22 s epochs.
The stimuli in our study were cropped to reveal the eye region, removing all other contextual emotional information.
Subjective context also affected processing of pupillary signals. Negative versus positive coherence between observers’ and observed pupils evoked different activity patterns. While the magnitude of change in observers’ and observed pupil size correlated with activity within mid cingulate across conditions, negative feedback further engaged affective and social processing centers within amygdala and insula. We have previously shown using static stimuli a coherent mirroring of pupillary signals during implicit processing of sad (but not other) facial expressions (Harrison et al., Citation2006). However, in the present study, to exclude contamination from other emotional cues, the face images were posed with neutral expressions and cropped to exclude much of the face. We therefore did not predict the presence of an automatic tendency to mirror pupil responses within the present study, and correspondingly our data showed no significant difference between feedback conditions in mean pupil size, change in pupil size or variance of pupil size across conditions. Analysis of our pupillometry data in a frame-by-frame manner also failed to show an entrainment effect of observed pupillary changes on subjects’ own subsequent constricting or dilatory pupillary responses. This observation also means that an absolute change in participants’ pupil size did not directly account for the activity differences observed between positive and negative feedback conditions.
Nevertheless, positive coherence in dynamic pupillary signals enhanced activity within pre-motor and parietal regions implicated in imitative and “mirroring” behavioral responses. In contrast, in the incoherent (negative feedback) condition changes in pupil size activated the amygdala and insula cortices, regions typically associated with processing emotional salience and social information encoded in facial expressions. These regions represent core areas for the evaluation of emotional expressions and behaviors of others and experience of emotion in self, including the specialized visual representation of emotional valence and threat (within amygdala), feeling states and affective reactions (insula) (Wicker et al., Citation2003; Winston, Strange, O'Doherty, & Dolan, Citation2002; Critchley et al., Citation2005).
This discrepancy in functional neuroanatomy for processing positive and negative pupil coherence suggests different meanings within motivational behavior. For example, in naturalistic settings, the overriding influence on pupil size is ambient light. Thus in social exchanges (sadness excluded) coherent pupillary change occurs predominantly as a consequence of changes in environmental luminance that influence participant members of the social exchange to a similar degree. Coherence in pupil size could therefore be viewed as the default condition in social behavior that therefore need not, in general, convey strong emotional or motivational information. The relative absence of activity across the distributed neural system for face and emotional processing may reflect the relative insignificance of coherent pupillary responses in general social behavior. Nevertheless, it is clear that this information does not go unnoticed, evoking activity within the frontal operculum, a region associated with both performance and observation of actions (Grezes & Decety, Citation2001), including mimicry or passive viewing of emotional facial expressions.
Similar to ongoing motor control, social exchanges involve a series of sensorimotor interactions. However, when interacting with another person, sensory feedback comes not from our own body but from observation of the other's own motor responses. Forward motor control models suggest that discrepancies between expected and observed responses to our own motor behavior may be used to predict others’ hidden (intentional) states (Wolpert et al., Citation2003). In the current experiment, differential responses to incongruent (unpredicted) and congruent (predicted) feedback provide empirical evidence for an autonomically mediated extension of forward models of motor control into social interaction. Interestingly, this occurred outside of conscious awareness: while participants were aware of the changes in pupil size in the observed stimuli, none reported that the observed pupillary changes were driven by their own pupillary responses or recognized a relationship with their own arousal/bodily state). Incoherent changes in pupil size across individuals may therefore represent a salient social signal, revealing a change in the internal dispositional and cognitive state unique to the person observed. Non-conforming and oddball pupil responses may betray covert intentions, mental effort, recognition and emotional likes and dislikes ( Hess & Polt, Citation1960; Kahneman & Beatty, Citation1966; Steinhauer & Hakerem, Citation1992). There is adaptive value to being attuned to such subtle psychophysiological information: an effect recognized by poker players, who may wear dark glasses to mask a pupillary flare signaling a winning hand to other players. Nevertheless, this interpretation needs to be viewed with some caution. While eye stimuli and changes in pupil size produced robust activations in regions shown to respond to both static and dynamic facial features (e.g. fusiform gyrus and STS), differential activation in these regions was not seen in the two feedback conditions.
In conclusion, by using biofeedback of pupil size in the context of a functional brain imaging experiment we have shown that observed pupil size is continuously monitored during a social exchange. The study also suggests that only antagonistic changes in observed and observer's pupil size result in activity change in brain regions involved in the encoding and analysis of another's mental state. This is consistent with our contention that incongruent pupillary changes primarily signal a change in another's dispositional state and highlights brain regions implicated in processing this emotionally, motivationally and attentionally meaningful information.
Acknowledgements
This work was supported by a programme grant from the Wellcome Trust to HDC. The authors declare that they have no competing financial interests.
References
- Adolphs , R. 2002 . Neural systems for recognizing emotion . Current Opinion in Neurobiology , 12 : 169 – 177 .
- Adolphs , R. 2006 . A landmark study finds that when we look at sad faces, the size of the pupil we look at influences the size of our own pupil . Social Cognitive and Affective Neuroscience , 1 : 3 – 4 .
- Adolphs , R. , Gosselin , F. , Buchanan , T. W. , Tranel , D. , Schyns , P. G. and Damasio , A. R. 2005 . A mechanism for impaired fear recognition after amygdala damage . Nature , 433 : 68 – 72 .
- Barton , J. J. S. , Press , D. Z. , Keenan , J. P. and O'Connor , M. 2002 . Lesions of the fusiform, face area impair perception of facial configuration in prosopagnosia . Neurology , 58 : 71 – 78 .
- Brett , M. Anton , J.-C. Valabregue , R. & Poline , J.-B. 2002 . Region of interest analysis using an SPM toolbox . NeuroImage , 16 .
- Critchley , H. D. , Daly , E. M. , Bullmore , E. T. , Williams , S. C. R. , van Amelsvoort , T. Robertson , D. M. 2000 . The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions . Brain , 123 : 2203 – 2212 .
- Critchley , H. D. , Rotshtein , P. , Nagai , Y. , O'Doherty , J. , Mathias , C. J. and Dolan , R. J. 2005 . Activity in the human brain predicting differential heart rate responses to emotional facial expressions . NeuroImage , 24 : 751 – 762 .
- Darwin , C. 1872 . The expression of the emotions in man and animals , London : John Murray .
- Davidson , R. A. , Fedio , P. , Smith , B. D. , Aureille , E. and Martin , A. 1992 . Lateralized mediation of arousal and habituation: Differential bilateral electrodermal activity in unilateral temporal lobectomy patients . Neuropsychologia , 30 : 1053 – 1063 .
- de Gelder , B. 2006 . Towards the neurobiology of emotional body language . Nature Reviews Neuroscience , 7 : 242 – 249 .
- Deichmann , R. , Gottfried , J. A. , Hutton , C. and Turner , R. 2003 . Optimized EPI for fMRI studies of the orbitofrontal cortex . NeuroImage , 19 : 430 – 441 .
- Demos , K. E. , Kelley , W. M. , Ryan , S. L. , Davis , F. C. , & Whalen , P. J. 2008 . Human amygdala sensitivity to the pupil size of others . Cerebral Cortex Epub, doi: 10.1093/cercor/bhn034.
- Ekman , P. and Friesen , W. V. 1971 . Constants across cultures in face and emotion . Journal of Personality and Social Psychology , 17 : 124 – 129 .
- Glascher , J. and Adolphs , R. 2003 . Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala . Journal of Neuroscience , 23 : 10274 – 10282 .
- Grezes , J. and Decety , J. 2001 . Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis . Human Brain Mapping , 12 : 1 – 19 .
- Grill-Spector , K. , Knouf , N. and Kanwisher , N. 2004 . The fusiform face area subserves face perception, not generic within-category identification . Nature Neuroscience , 7 : 555 – 562 .
- Hardee , J. E. , Thompson , J. C. and Puce , A. 2008 . The left amygdala knows fear: laterality in the amygdala response to fearful eyes . Social Cognitive and Affective Neuroscience , 3 : 47 – 54 .
- Harrison , N. A. , Singer , T. , Rotshtein , P. , Dolan , R. J. and Critchley , H. D. 2006 . Pupillary contagion: Central mechanisms engaged in sadness processing . Social Cognitive and Affective Neuroscience , 1 : 5 – 17 .
- Harrison , N. A. , Wilson , C. E. and Critchley , H. D. 2007 . Processing of pupil size modulates perception of sadness and predicts empathy . Emotion , 7 : 724 – 729 .
- Hess , E. H. and Polt , J. M. 1960 . Pupil size as related to interest value of visual stimuli . Science , 132 : 349 – 350 .
- Kahneman , D. and Beatty , J. 1966 . Pupil diameter and load on memory . Science , 154 : 1583 – 1585 .
- Kleinke , C. L. 1986 . Gaze and eye contact: A research review . Psychological Bulletin , 100 : 78 – 100 .
- Kobayashi , H. and Kohshima , S. 1997 . Unique morphology of the human eye . Nature , 387 : 767 – 768 .
- Liberman , A. M. and Whalen , D. H. 2000 . On the relation of speech to language . Trends in Cognitive Sciences , 4 : 187 – 196 .
- Maurer , D. 1985 . “ Infants’ perception of facedness ” . In Social perception in infants , Edited by: Field , T. and Fox , N. Norwood, NJ : Ablex .
- McCarthy , G. , Puce , A. , Belger , A. and Allison , T. 1999 . Electrophysiological studies of human face perception. II: Response properties of face-specific potentials generated in occipitotemporal cortex . Cerebral Cortex , 9 : 431 – 444 .
- Perrett , D. I. and Emery , N. J. 1994 . Understanding the intentions of others from visual signals: Neurophysiological evidence . Cahiers de Psychologie Cognitive–Current Psychology of Cognition , 13 : 683 – 694 .
- Puce , A. , Allison , T. , Bentin , S. , Gore , J. C. and McCarthy , G. 1998 . Temporal cortex activation in humans viewing eye and mouth movements . Journal of Neuroscience , 18 : 2188 – 2199 .
- Smith , M. L. , Cottrell , G. W. , Gosselin , F. and Schyns , P. G. 2005 . Transmitting and decoding facial expressions . Psychological Science , 16 : 184 – 189 .
- Steinhauer , S. R. and Hakerem , G. 1992 . The pupillary response in cognitive psychophysiology and schizophrenia . Annals of the New York Academy of Sciences , 658 : 182 – 204 .
- Tomaiuolo , F. , MacDonald , J. D. , Caramanos , Z. , Posner , G. , Chiavaras , M. Evans , A. C. 1999 . Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: An in vivo MRI analysis . European Journal of Neuroscience , 11 : 3033 – 3046 .
- Tombs , S. and Silverman , I. 2004 . Pupillometry: A sexual selection approach . Evolution and Human Behavior , 25 : 221 – 228 .
- Vecera , S. P. 2006 . Gaze detection and the cortical processing of faces: Evidence from infants and adults . Visual Cognition , 2 : 59 – 87 .
- Vogt , B. A. 2005 . Pain and emotion interactions in subregions of the cingulate gyrus . Nature Reviews Neuroscience , 6 : 533 – 544 .
- Wicker , B. , Keysers , C. , Plailly , J. , Royet , J. P. , Gallese , V. and Rizzolatti , G. 2003 . Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust . Neuron , 40 : 655 – 664 .
- Winston , J. S. , O'Doherty , J. and Dolan , R. J. 2003 . Common and distinct neural responses during direct and incidental processing of multiple facial emotions . NeuroImage , 20 : 84 – 97 .
- Winston , J. S. , Strange , B. A. , O'Doherty , J. and Dolan , R. J. 2002 . Automatic and intentional brain responses during evaluation of trustworthiness of faces . Nature Neuroscience , 5 : 277 – 283 .
- Wolpert , D. M. , Doya , K. and Kawato , M. 2003 . A unifying computational framework for motor control and social interaction . Philosophical Transactions of the Royal Society of London Series B: Biological Sciences , 358 : 593 – 602 .
- Wright , C. I. , Fischer , H. , Whalen , P. J. , McInerney , S. , Shin , L. M. and Rauch , S. L. 2001 . Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli . NeuroReport , 12 : 379 – 383 .