Abstract
Regret-related brain activity is dependent on free choice, but it is unclear whether this activity is a function of more subtle differences in the degree of responsibility a decision-maker exerts over a regrettable outcome. In this experiment, we show that trial-by-trial subjective ratings of regret depend on a higher subjective sense of responsibility, as well as being dependent on objective responsibility. Using fMRI we show an enhanced amygdala response to regret-related outcomes when these outcomes are associated with high, as compared to low, responsibility. This enhanced response was maximal in participants who showed a greater level of enhancement in their subjective ratings of regret engendered by an objective increase in responsibility. Orbitofrontal and cingulate cortex showed opposite effects, with an enhanced response for regret-related outcomes when participants were not objectively responsible. The findings indicate that the way the brain processes regret-related outcomes depends on both objective and subjective aspects of responsibility, highlighting the critical importance of the amygdala.
INTRODUCTION
Liberty means responsibility. That is why most men dread it. (George Bernard Shaw, 1856–1950)
Good or bad events in our lives can invite a comparison to what might have been, a psychological process referred to as “counterfactual thinking.” Outcomes that could have been better from the same choice tend to induce feelings of disappointment, while outcomes that could have been better from a different choice induce feelings of regret (Roese & Olson, Citation1995). A focus on this different choice, or a between-option counterfactual comparison, invokes the idea that regret depends on reproach, self-blame, and a desire to have acted differently, whereas disappointment is associated more with a sense of bad luck and/or powerlessness (Zeelenberg et al., Citation1998b). While it has been suggested that outcome regret can be experienced without a feeling of self-blame (Connolly & Zeelenberg, Citation2002; Simonson, Citation1992), the dominant view is that self-blame or responsibility is a necessary prerequisite in the experience of regret (see Connolly, Ordónez, & Coughlan, Citation1997; Ordonez & Connolly, Citation2000; and Zeelenberg, van Dijk, & Manstead, Citation1998a, Citation2000 for a thorough review and discussion).
Neurobiological studies provide compelling evidence for a role of the orbitofrontal cortex (OFC) in regret. Patients with orbitofrontal cortex lesions do not experience regret but show no impairment in experiencing disappointment (Camille et al., Citation2004). Using functional magnetic resonance imaging (fMRI), Coricelli et al. (Citation2005) reported activity in medial OFC associated with the experience of regretful outcomes as well as amygdala activity associated with a tendency to avoid choices that might engender regret. Others report a greater role of the OFC in regret compared to disappointment (Chua, Gonzalez, Taylor, Welsh, & Liberzon, Citation2009), regret compared to relief (Chandrasekhar, Capra, Moore, Noussair, & Berns, Citation2008), relative compared to absolute loss (Fujiwara, Tobler, Taira, Iijima, & Tsutsui, Citation2008), and evaluating incorrect compared to correct choices (Lui et al., Citation2007).
The role of agency in modulating brain activity associated with choice outcomes has been highlighted by findings that ventral striatal responses to absolute losses and gains depend on agency over the causal choice (Coricelli et al., Citation2005). We extended this finding to show that ventral striatal responses to outcomes that are relatively better or worse than what might have been from commitment to a different choice also depend on agency (Nicolle, Bach, Driver, & Dolan, Citationin press). However, self-blame regret involves more than just agency over a choice, depending also on social norms and decision justifiability (Connolly & Zeelenberg, Citation2002). For example, it is likely to be the case that we can more easily justify a bad decision, thus reducing feelings of self-blame, if we know others would have made a similar choice. Moreover, if others actually played a part in the bad decision, self-blame regret can be reduced by transferring a component of responsibility onto them. The likely importance of self-blame in regret led us to predict that regret-related responses would be modulated by the degree to which a decision-maker feels responsible for an outcome. Thus, we designed an experiment where participants experienced outcomes of “played” and “unplayed” gambles under various levels of responsibility (a task modified from a design of Mellers, Schwartz, & Ritov, Citation1999). While the act of making a choice between two gambles was constant across all trials, our paradigm created situations where participants' actual experienced sense of responsibility for outcomes was systematically varied. The latter manipulation was realized by informing participants that each of their played gambles would depend on their own choice along with the votes of varying numbers of additional individuals. Here, we predicted that participants' subjective sense of responsibility for gamble outcomes would decrease as a function of greater numbers of these “other voters.”
There are good grounds to expect an effect of responsibility being expressed in brain regions implicated in agency and motor control, including the insula and angular gyrus (Farrer & Frith, Citation2002; Farrer et al., Citation2003), although such regions are implicated more in being responsible, as opposed to feeling responsible. In relation to our central question we predicted regret-related brain activity to be modulated by the degree to which the outcome of the played gamble is worse than that of the unplayed gamble in regions previously implicated in regret (including OFC, amygdala, hippocampus, ACC, insula, and striatum), but only with higher levels of responsibility.
METHODS
Participants
We recruited 18 participants (10 female) for the experiment. All were right-handed with normal or corrected vision, and no history of neurological or psychiatric disorder. Participants' ages ranged between 19 and 30 years (mean = 23.67 years) and each gave informed consent, according to procedures approved by the UCL Research Ethics Committee. Due to incomplete behavioral data collection, one participant was removed from the behavioral analysis, but included in the imaging analysis. A second participant was removed from the imaging analysis due to scanner malfunction, but was included in the behavioral analysis. A further participant was excluded as an outlier, after showing a relationship between regret-related outcomes and subjective ratings of regret that was 2.5 standard deviations from the mean and negative.
Experimental procedure
Our central aim was to explore how subjective and neuronal responses to regret-related outcomes are modulated by responsibility. We used a task based on that of Mellers et al. (Citation1999). In brief, participants were instructed to choose between two “wheel-of-fortune” gambles on each trial, each incorporating a win and loss outcome with differing probabilities (25%, 50%, or 75%). We used 24 different gamble pairs, allowing for four different pairs per probability combination (e.g., 25% win against 50% win). Points allocated to the possible winning and loosing outcomes were such that the two gambles in the pair were of equal expected value (EV) (i.e., probability of win × magnitude of possible win). In order to enhance feelings of skill in the game, two further pair types made up 7% of trials and included one gamble of a clearly higher EV than the other. Details of the gamble pairs used are given in .
TABLE 1 Details of the gamble pairs used in the task, including the probabilities of each of the winning and losing outcomes (in points) of each gamble option
An exemplar trial timeline is shown in . Participants were told that their choice would count as one vote towards the gamble to be played. In mini-blocks of five trials, participants were informed that they were playing in a group alongside 0, 2, 4, 6, or 8 other voters, whom they believed to have performed the task in advance, but who were not real. After they selected their preferred gamble, the gamble receiving the highest number of votes from the group (including their own vote) was played. With more than 0 other voters, there was a chance that the played gamble would not be congruent with the participant's own choice. The probability of participants' chosen gamble being played varied probabilistically as a function of the number of voters, such that a greater number of voters meant an increased chance that their gamble would not be selected, while in situations of 0 other voters their chosen gamble was always played. After a 2 s delay, participants were then shown the points outcome of both the played and the unplayed gamble. The outcome of played gamble, whether congruent or incongruent with their own gamble choice, determined payment for the experiment. If the played gamble was incongruent with the participant's own gamble choice the lowest level of responsibility was predicted, as participants were not agents of the choice. If the played gamble was congruent with the participant's own gamble choice, however, we predicted that participants' subjective sense of responsibility for gamble outcomes would decrease as a function of the increased numbers of “other voters,” even though participants were still the agents of the choice. Participants received 50p for each percentage they won of the maximum points they could have won in their game. This encouraged participants to compare the received outcome with what might have been under the unplayed gamble, on a trial-by-trial basis. Participants were assumed to treat all trials as having an equal impact on their financial gain.
Figure 1. An exemplar trial timeline. Trials included a choice phase in which participants select their choice of gamble, with choice indicated by a blue box. For each gamble, outcome probabilities were indicated by wedge size, with corresponding outcomes written above in number of points. Number of other voters was also shown, but was constant for mini-blocks of 5 trials. A gamble selection stage followed, in which the gamble receiving the highest number of votes was indicated with a yellow box. This gamble was then played, with its outcome affecting participants' winnings whether chosen by them or not. Next, the outcome of both the played and the unplayed gambles was revealed.
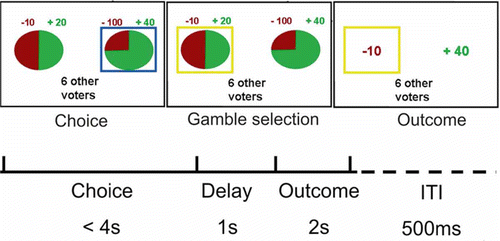
Participants performed both a behavioral session and a scanning session, occurring on separate days (order counterbalanced across participants). In the behavioral session, participants played 320 trials in four games and, after every trial, provided two subjective ratings on a 100-point horizontal visual analog scale (this was not practical in the scanning session). The starting position of the slider on each rating scale was random between the extremes of 1 and 100, in order to avoid anchoring effects. The rating questions comprised either a memory probe asking “How much bigger or smaller was the received gamble outcome than what might have been received from the unplayed gamble?” or a rating of subjective feeling comprising the following probes, “How positive or negative do you feel about the trial outcome?”, “How much regret do you feel for outcome?”, and “How responsible do you feel for the outcome?” It was randomly decided which two of these four ratings would be presented on each trial. We explained to participants that these ratings related to their response to the outcome of each trial. Only the memory test, and not the other ratings, was used during the scanning session (in 10% of trials) in order to enhance the tendency to think counterfactually. In the scanning session, participants also played 320 trials in four 8‐min games.
Imaging acquisition
We scanned participants in a 3 T Allegra scanner (Siemens, Erlangen, Germany) operated with its standard head transmit–receive coil. The manufacturer's standard automatic 3D-shim procedure was performed at the beginning of each experiment. Participants were scanned with a single-shot gradient-echo planar imaging (EPI) sequence optimized to reduce blood-oxygen-level-dependent (BOLD) sensitivity losses in the orbitofrontal cortex due to susceptibility artifacts, using a combination of increased spatial resolution in the readout direction and reduced echo time (Weiskopf et al., Citation2007). Imaging parameters were as follows: 48 oblique transverse slices tilted by 30°, resolution of 1.5 mm in the readout direction and 3 mm in the PE direction, slice thickness = 2 mm, gap between slices = 1 mm, repetition time TR = 3.12 s, α = 90°, echo time TE = 25 ms, bandwidth (BW) = 1953 Hz/pixel, phase-encoding (PE) direction anterior–posterior, field of view (FOV) = 192 × 192 mm2, matrix size 128 × 64, z-shim gradient pre-pulse moment = −1.4 mT/m × ms. EPI magnitude images were reconstructed from the complex k-space raw data using a generalized reconstruction method based on the measured EPI k-space trajectory to minimize ghosting (Josephs, Deichmann, & Turner, Citation2000). EPI data acquisition was monitored online using a real-time reconstruction and quality assurance system (Weiskopf et al., Citation2007). We acquired fieldmaps for each subject at the start of scanning (Siemens standard double echo gradient echo fieldmap sequence, echo time = 12.46 ms, TR = 10.2 ms, matrix size = 64 × 64, 64 slices covering the whole head, voxel size = 3 × 3 × 3 mm). These allowed for calculation of static geometric distortions caused by susceptibility-induced field inhomogeneities, which were used to correct EPI images for both these static distortions and any changes in these distortions due to head motion (Andersson, Hutton, Ashburner, Turner, & Friston, Citation2001; Hutton et al., Citation2002). We also recorded heart rate with a pulse oximeter, along with respiratory phase and volume using a breathing belt. At the end of the scanning session, we acquired a T1-weighted anatomical scan for each participant using a modified driven equilibrium Fourier transform (MDEFT) sequence (Uğurbil et al., Citation1993), with optimized parameters as described in the literature (Deichmann, Schwarzbauer, and Turner, Citation2004): for each volunteer, 176 sagittal partitions were acquired with an image matrix of 256 × 224 (Read × Phase).
Analysis of subjective ratings
We operationalized “regret-related outcomes” as those where the outcome of the played gamble showed a negative discrepancy with (i.e., was worse than) that of the unplayed gamble. Our design incorporated a continuous variable of this between-gamble negative outcome discrepancy (i.e., received outcome – foregone outcome). We predicted that subjective ratings of regret (as well as negative affect) would be enhanced with increasingly negative outcome discrepancy. We also predicted that subjective ratings of responsibility would decrease with increasing number of other voters. Finally, we predicted that the relationship between subjective regret and negative outcome discrepancy would depend on both subjective and objective measures of responsibility. We used linear regressions to test for continuous relationships between outcome discrepancy and subjective ratings of regret and negative affect, since the independent variables were on a continuous scale. To test the relationship between subjective responsibility, number of voters, and choice congruence we used a repeated-measures ANOVA. To assess our main hypothesis that regret depends on choice responsibility, and its degree, we tested a multiple regression model with subjective regret ratings as the dependent variable. The model entered as independent variables the outcome discrepancy (from extreme positive to extreme negative) along with the interaction of this discrepancy with a measure of responsibility. For the latter, we tested three distinct measures of responsibility comprising subjective ratings of responsibility, choice congruency, and the number of other voters. For descriptive purposes, we also performed an ANOVA, similar to that described above in the case of subjective responsibility, to test the relationship between subjective regret and number of voters and choice congruence for negatively discrepant outcomes only (i.e., only for those outcomes objectively thought to induce regret, rather than relief).
As we obtained multiple subjective ratings from each participant along parametric continua of regret, responsibility and negative feeling, these ratings were standardized for each participant, to avoid anchoring effects, and the standardized regression coefficients for all analyses were calculated on an individual subject basis. We report mean standardized regression coefficients from the between-subjects level of a hierachical linear model. Finally, with a one-sample t-test, we tested whether participants' answers on the post-trial memory tests showed performance significantly above chance. This enabled us to verify whether participants were taking into account both the outcomes received from the chosen and the alternative outcome of the unchosen gamble on each and every trial.
Imaging processing and analysis
For fMRI, we used a two-variable parametric design, with one factor for the number of other voters (0, 2, 4, 6, and 8 other voters), comprising our manipulation of responsibility, and one factor for level of outcome discrepancy, which ranged from −200 to +200 points. This factor was transformed such that positive numbers were regret-related (i.e., negative outcome discrepancy). An additional two-level factor was expected to influence sense of responsibility, and comprised whether a participant's choice of gamble was congruent or incongruent with the gamble selected by the majority vote.
Image preprocessing and data analysis were implemented using Statistical Parametric Mapping software in Matlab2009a (SPM8; Wellcome Trust Centre for Neuroimaging, Institute of Neurology, UCL). After discarding the first six volumes of each run, to allow for T1 equilibration, EPI images were corrected for geometric distortions caused by susceptibility-induced field inhomogeneities. Fieldmaps were processed for each participant using the FieldMap toolbox implemented in SPM8 (Hutton, Deichmann, Turner, & Andersson, Citation2004). The EPI images were then realigned and unwarped using SPM8 (Andersson et al., Citation2001). Each participant's structural image was coregistered to the mean of the motion-corrected functional images using a 12-parameter affine transformation, and was segmented according to the standard procedure in SPM8 (Ashburner & Friston, Citation2005). The spatial normalization parameters resulting from the previous step were then applied to the functional images to allow for intersubject analysis, and finally these images were smoothed using an 8 mm full width, half maximum (FWHM) Gaussian kernel.
For each participant, we constructed an event-related general linear model, including 9 regressors of interest. These included one regressor for the onsets of trial outcomes at each level of responsibility, separated out as full responsibility (i.e., 0 other voters), along with 2, 4, 6, and 8 other voters. Choice trials in which there were more than 0 other voters were further segregated as a function of whether participants' choices were congruent or incongruent, with the gamble selected by the majority vote. In trials with 0 other voters, participants' choices were always congruent with the gamble selected, as participants were the only voter. Each of these 9 regressors was parametrically modulated by a mean-corrected regressor of outcome discrepancy. Positive values of this parametric regressor were regret-related outcomes (i.e., negatively discrepant). Onsets were modeled with stick-functions at the time at which participants received the outcome feedback, convolved with a canonical hemodynamic response function and its temporal derivative. Motion parameters defined by the realignment procedure were entered as 6 regressors of no interest, along with 17 additional regressors of cardiac phase (10 regressors), respiratory phase (6 regressors) and respiratory volume (1 regressor).
We generated statistical parametric maps from contrasts of interest including the main effect of responsibility as measured by contrasts motivated by effects of responsibility seen in the subjective ratings results. Here, we were particularly interested in probing activity within the regions of interest (ROIs) reported by Farrer & Frith (Citation2002), as involved in decreased (the angular gyrus) and increased motor control (the insula). As mentioned above, while these regions are implicated in simple motor responsibility (or agency), they may also be involved in processing variations in blame based on how easily we can assign outcome responsibility externally (of key importance for decision justification models of regret; e.g., Connolly & Zeelenberg, Citation2002). Next we tested the main effect of negative outcome discrepancy (as a linear parametric effect) where we were interested in activity that increased with greater levels of negative outcome discrepancy independent of level of responsibility, as well as (using a conjunction analysis) brain activity that showed significantly increased response to negative outcome discrepancy across all levels of responsibility. We tested for these effects within anatomical ROIs in regions previously implicated in the experience of regret and regret-induced decision bias, including OFC, anterior cingulate cortex (ACC), amygdala, hippocampus, insula, and ventral and dorsal striatum (all defined anatomically and bilaterally through the WFU PickAtlas in SPM; Maldjian, Laurienti, Burdette, & Kraft, Citation2003; Tzourio-Mazoyer et al., Citation2002). To address our hypotheses about how responses to regret-related (i.e., negatively discrepant) outcomes are modulated by responsibility we compared the response to increasingly negative outcome discrepancy under full responsibility (i.e., 0 other voters) with that when other voters were present. Critically this contrast was performed only on trials where the participant's own chosen gamble was played (i.e., congruent choice). Additionally, we compared response to increasingly negative outcome discrepancy under congruent choice vs. incongruent choice. We implemented a group-level random-effects analysis using one-sample t-tests on the contrast images obtained from each contrast of interest for each participant. Within our regret-related ROIs, we report activity that survives family-wise error correction for multiple comparisons at a voxel-level significance of p < .05. For completeness, we also report any activity that survives whole-brain cluster-wise corrected significance of p < .05. All reported activity had a voxel-level uncorrected significance of at least p < .001.
Behavioral results
A one-sample t-test confirmed that participants had above-chance memory for whether the outcome received from a played gamble was better or worse than that which might have been received from the unplayed gamble, as determined by post-trial memory questions, t(15) = 15.09, p < .001, mean percent correct = 91.3%. This showed that participants were aware of both the received and the unplayed alternative outcomes on each trial. Mean earnings were £12.20 (SD £2.64) in the behavioral session, and £22.43 (SD £2.67) in the scanning session.
We found a linear effect of number of voters on subjective ratings of responsibility, F(1, 15) = 49.5, p < .001, reflected in an increased sense of responsibility with decreased number of other voters. The linear effect was also significant within congruent choice trials alone, F(1, 15) = 24.0, p < .001, i.e., when participants had chosen the played gamble, but not within incongruent choice trials, F(1, 15) = 0.6, ns. We also found significant quadratic, F(1, 15) = 9.1, p < .01, and cubic, F(1, 15) = 5.6, p < .05, effects of voters in the congruent choice condition. Participants showed significantly higher ratings of responsibility for congruent than incongruent choice trials, F(1, 15) = 46.9, p < .001 (, blue line).
Figure 2. (a) Gradually decreasing mean normalized ratings of subjective responsibility and of subjective regret under congruent and incongruent choices with 0, 2, 4, 6, and 8 other voters, otherwise considered objective responsibility. For clarity, subjective regret ratings here are shown only for negatively discrepant outcomes, i.e., where the outcome of the played gamble was worse than the outcome of the unplayed gamble. (b) The mean normalized subjective ratings of regret, displayed as a binary separation of low and high subjective responsibility, for negatively discrepant outcomes. (c) The mean regression coefficients for the three regression models used, each indicating that the predictive value of outcome discrepancy (Odiff) on subjective ratings of regret is greatly enhanced by multiplying this Odiff by one of the three measures of responsibility (number of voters, subjective rating of responsibility, or choice congruence). Error bars show the standard error of the mean across participants.
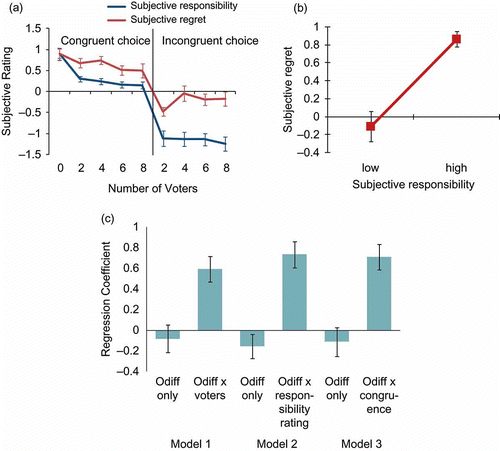
Single-subject standardized regression coefficients, taken forward to a between-subject one-sample t-test, showed that increasingly negative outcome discrepancy significantly predicted greater subjective ratings of regret, mean R = 0.52, t(15) = 8.04, p < .001. This showed a successful manipulation of subjective regret by outcome discrepancy. More negatively discrepant outcomes also significantly predicted increased negative affect, mean R = 0.71, t(15) = 14.19, p < .001.
A multiple regression analysis indicated that increased responsibility amplified the tendency for participants to report high subjective feelings of regret for outcomes with increasingly negative discrepancy. That is, outcomes were rated as more regretful when they were both more negative than what would have been from the alternative gamble and when participants felt more responsible. The three regression models used indicated that this effect was significant for all three measures of responsibility; namely, decreased number of voters, t(15) = 2.82, p < .02, increased subjective ratings of responsibility, t(15) = 3.83, p < .002, and choice congruency, t(15) = 3.29, p < .005. The regression coefficients for the three regression models are illustrated in . These regression models were not restricted to negatively discrepant outcomes (i.e., they include outcomes objectively likely to induce relief, as well as regret), and were performed using continuous functions of subjective responsibility and outcome discrepancy. To directly assess the direction of this effect on regret, we show subjective regret for negatively discrepant outcomes alone under different levels of objective responsibility, as seen in , and under high and low subjective responsibility in . We found a linear effect of number of voters on subjective ratings of regret for negatively discrepant outcomes, F(1, 15) = 18.7, p < .001, reflected in increased regret with decreased number of other voters. The linear effect was significant within congruent choice trials alone, F(1, 14) = 6.8, p < .05; i.e., when participants had chosen the played gamble, but not within incongruent choice trials, F(1, 14) = 0.3, ns. Participants also showed significantly higher ratings of regret for congruent than incongruent choice trials, F(1, 15) = 30.7, p < .001 (, red line), and for high compared to low subjective responsibility, t(13) = 4.5, p < .001 ().
FMRI results
Manipulation of responsibility
We examined the effect of linearly decreasing responsibility, following the pattern shown in . While no areas showed a significant effect of increasing responsibility, we found significant corrected level effects in superior frontal cortex (MNI 51, 26, 31), brainstem (MNI 0, −34, −26) and left insula (−45, 14, −8) for decreasing responsibility. In our a priori ROIs, right angular gyrus activity (within a 20 mm sphere radius of the coordinates reported by Farrer & Frith, Citation2002) significantly increased with decreasing responsibility (MNI 57, −43, 34), as did bilateral insula (MNI right 30, 20, −11 and left −45, 14, −8). No regions dissociated, at the time of outcome, between played gambles that were congruent or incongruent with the participant's own choice, at a whole-brain corrected level. However, right insula activity showed such an effect within our a priori ROI of bilateral insula (MNI 30, 20, −14).
Manipulation of regret
Averaged across all levels of responsibility we found significantly increased activity as outcomes became linearly more negatively discrepant in left angular gyrus and lateral OFC (). Activity in our other a priori ROIs, in the amygdala, hippocampus, ACC, and insula, did not show this effect. Crucially, no regions showed responsibility-invariant responses to negatively discrepant outcomes, as evident in a conjunction analysis, providing evidence against a neural representation of a purely outcome-based form of regret. Instead, regret-related responses in angular gyrus and lateral OFC appeared to be dependent on the level of responsibility. However, it is important to note that the reduced power inherent in such a conjunction analysis means that we cannot reject the null hypothesis that such responsibility-invariant responses do exist.
Figure 3. Group SPM data, thresholded at p < .005 for display purposes, shown on a normalized canonical template brain. (a) Activity in angular gyrus (peak at −42, −64, 25) and lateral OFC (peak at 51, 38, −11) associated with the average effect of linearly increasing negative outcome discrepancy. (b) Enhanced activity in amygdala (peak −18, −1, −20) associated with increasingly negatively outcome discrepancy during full responsibility (0 other voters) compared to other congruent choice trials in the presence of other voters. Plotted in (c) are the beta weights showing the same amygdala response to increasingly negative outcome discrepancy under the different numbers of other voters. (d) Right amygdala activity (peak 30, 5, −26) showing greater enhancement of its response (FWE corrected) to more negatively discrepant outcomes by increased responsibility in participants who showed a greater enhancement of subjective regret for increasingly negative outcome discrepancy by increased responsibility (decreased number of voters). Coordinates are in MNI space. Error bars show standard error of the mean.
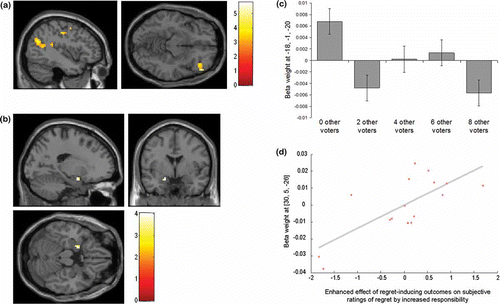
To address whether activity associated with negative outcome discrepancy is modulated by level of responsibility, we restricted the analysis to congruent choice trials, i.e., where the participant's chosen gamble was played. This was justified on the basis that negatively discrepant outcomes received in incongruent choice trials were associated with participants actually having been the agents of the post-hoc better choice. Within a priori anatomical ROIs implicated in regret, we found no regions showing an entirely linear enhanced response to more negatively discrepant outcomes by decreased number of other voters (i.e., increased responsibility). However, in keeping with the marked step-like decrease in rated responsibility from 0 to 2 or more other voters (shown in ), we found that left amygdala activity was enhanced for more negatively discrepant outcomes during full responsibility trials, and not when there were any number of other voters ( and ) (within anatomically specified bilateral ROI of the amygdala). A linear interaction of outcome discrepancy and responsibility (within congruent choice trials) was seen in right amygdala activity but only in participants who showed a greater enhancement of subjective regret by responsibility (in the form of decreased numbers of other voters) in the separate behavioral session (), an effect also seen in left amygdala at a trend level of significance.
By contrast, activity in middle cingulate cortex and angular gyrus showed an increased response to more negatively discrepant outcomes, when the played gamble was incongruent (whole-brain cluster corrected p < .05), compared to when it was congruent with the participant's own choice. This indicates a role of middle cingulate cortex and angular gyrus in the processing of regret-related outcomes that are externally enforced. Furthermore, activity in middle cingulate cortex, left angular gyrus, and lateral OFC responsive to average effect of regret-related outcomes () showed a greater response to more negatively discrepant outcomes on these incongruent trials compared to congruent trials (although this was only at p < .005 uncorrected level for the lateral OFC). This indicates that regret-related activity in these regions is not associated with self-blame.
DISCUSSION
In agreement with previous research (e.g., Zeelenberg et al., Citation1998b), we found that subjective ratings of regret depend both on the outcome being worse than what might have been and on having a sense of responsibility. We now provide new evidence that subjective regret depends on the level of subjective responsibility even though the individual's own choice or action directly contributed to the regret-related outcome (as in the case of congruent trials). These findings indicate that regret is not just a function of being an agent of a choice but also depends on subtle changes in sense of responsibility, or accountability, for the outcomes of our actions. In keeping with these behavioral effects we found that regret-related neuronal activity in the amygdala was enhanced by increased responsibility, suggesting a critical role in “self-blame regret.” This effect was magnified in participants who displayed a greater enhancement of their subjective ratings of regret by responsibility. Interestingly, we did not find any brain regions responding to what has been termed “outcome regret,” i.e., showing invariant responses to regret-related outcomes under all levels of responsibility. This suggests that, as for the psychological experience of regret, the way the brain processes regretful events may crucially depend on a sense of responsibility.
The human amygdala is known to be important in emotional memory (Cahill, Babinsky, Markowitsch, & McGaugh, Citation1995; Richardson, Strange & Dolan, Citation2004; Strange & Dolan, Citation2004) and in learning stimulus–reward associations (e.g., Buechel, Morris, Dolan, & Friston, 1997; Gottfried, O'Doherty, & Dolan, Citation2003; LaBar, Gatenby, Gore, LeDoux, & Phelps, Citation1998; Whalen et al., Citation2004). A model of amygdala function in our study relates to its putative role as a “relevance detector” (Sander, Grafman, & Zalla, Citation2003; Bach et al., Citation2008). This proposes that the amygdala is involved in focusing attentional and physiological resources towards cues that have special relevance for personal safety or success. Amygdala activation is found in socially relevant situations, such as viewing untrustworthy or novel faces (Winston, Strange, O'Doherty, & Dolan, Citation2002; Wright, Martis, Schwartz, Shin, Fischer, McMullin, et al., Citation2003) when following eye gaze (Kawashima et al., Citation1999), as well as for especially self-relevant situations, as when hearing one's own name being presented during sleep (Portas et al., Citation2000). Moreover, the amygdala is implicated in biasing future decisions based on previous regret (Coricelli et al., Citation2005), emphasizing its importance in goal-directed motivation. Regret associated with high responsibility is an experience with particular self-relevance, while also providing a strong motivation for future behavior, such as motivating active attempts to (or intentions to) undo unpleasant events (Zeelenberg et al., Citation1998b; Zeelenberg & Pieters, Citation1999). On the other hand, a failure to appropriately accept responsibility for our mistakes may interfere with learning accurate associations between our actions and their outcomes, which are vital in adapting future behavior. Specifically self-relevant information may be passed to the amygdala from other task-engaged cortical regions (for example, we have shown information about responsibility and between-option outcome comparisons to be associated with activity in the angular gyrus and prefrontal cortex), in order to allocate processing resources to control mechanisms driving adaptive behavior. In keeping with such a framework, there is good evidence that the amygdala imparts information necessary for acquiring stimulus–reward associations to the OFC, which uses this information to guide behavior (Pickens et al., Citation2003; Arana et al., Citation2003; Schoenbaum, Gottfried, Murray, & Ramus, Citation2007), allowing for behavioral flexibility in accordance with strategic goals (Morris & Dolan, Citation2004).
The possibility of medial–lateral differences of OFC involvement in regret is worthy of discussion. While the null effect in the medial OFC does not challenge the role of medial OFC involvement in the self-blame component of regret, it is surprising that we find lateral OFC involvement in negatively discrepant outcomes without responsibility. It is possible that this lateral OFC response reflects anger, frustration, or loss of control in the participant, on the realization that the other voters contributed to a bad outcome for the group. On the other hand, it may reflect the participant's rejoicing associated with the knowledge that they made the better choice on these incongruent trials, despite the received outcome being aversive. Our finding of strong negative affect associated with low responsibility in our task, along with reports that lateral OFC processes the unpleasantness of external stimuli (e.g., Anderson et al., Citation2003), support the former view. It is further possible that any medial OFC response associated with self-blame may have been overshadowed by more lateral OFC involvement in such externally attributed negative emotions.
Understanding the role of subjective responsibility and blame in regret may help in formulating therapies for painful and debilitating life regrets. An ability to externally shift responsibility for our bad choices can reduce feelings of regret (Zeelenberg & Pieters, Citation2007), while understanding that others have made similarly bad choices can provide justification for our actions by placing us within the social norm (Connolly & Zeelenberg, Citation2002). On the other hand, ability to accept responsibility for our actions can motivate adaptive future behaviors, leading to long-term improvements in quality of life. In real-life decision-making, anticipating future regret has positive influences on decisions in sexual behavior (e.g., Richard, Van der Pligt, & de Vries, Citation1996), consumer choices (e.g., Inman & Zeelenberg, Citation2002; Tsiros & Mittal, Citation2000), and health-related choices (e.g., Lechner, de Vries, & Offermans, Citation1997). Reb (Citation2008) also found that anticipating regret can improve the quality of the decision process by increasing vigilance and carefulness. Furthermore, decision-making in areas of business, politics, the legal world, health care, and personal relationships may also be improved by the ability to effectively anticipate levels of self-blame.
In summary, we show that in the amygdala—a region important in gathering personally relevant information, in forming stimulus–reward associations, and in guiding future behavior—responses to outcomes that could have been better from a different choice are enhanced by responsibility, even when a free choice has been made, highlighting a particular role of the amygdala in the self-blame component of our experiences of regret.
Acknowledgments
This work was supported by a Wellcome Trust Programme Grant to RJD and a Brain Research Trust Prize Studentship to AN. We wish to acknowledge the constructive comments of Jean Decety and two anonymous reviewers on an earlier version of this manuscript.
REFERENCES
- Anderson , A. K. , Christoff , K. , Stappen , I. , Panitz , D. , Ghahremani , D. G. Glover , G. 2003 . Dissociated neural representations of intensity and valence in human olfaction . Nature Neuroscience , 6 : 196 – 202 .
- Andersson , J. L. , Hutton , C. , Ashburner , J. , Turner , R. and Friston , K. 2001 . Modeling geometric deformations in EPI time series . NeuroImage , 13 ( 5 ) : 903 – 919 .
- Arana , F. S. , Parkinson , J. A. , Hinton , E. , Holland , A. J. , Owen , A. M. and Roberts , A. C. 2003 . Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection . Journal of Neuroscience , 23 ( 29 ) : 9632 – 9638 .
- Ashburner , J. and Friston , K. J. 2005 . Unified segmentation . NeuroImage , 26 ( 3 ) : 839 – 851 .
- Bach , D. R. , Schächinger , H. , Neuhoff , J. G. , Esposito , F. , Di Salle , F. Lehmann . 2008 . Rising sound intensity: An intrinsic warning cue activating the amygdala . Cerebral Cortex , 18 : 145 – 150 .
- Buechel , C. , Morris , J. , Dolan , R. J. and Friston , K. L. 1998 . Brain systems mediating aversive conditioning: An event-related fMRI study . Neuron , 20 : 947 – 957 .
- Cahill , L. , Babinsky , R. , Markowitsch , H. J. and McGaugh , J. L. 1995 . The amygdala and emotional memory . Nature , 377 : 295 – 296 .
- Camille , N. , Coricelli , G. , Sallet , J. , Pradat-Diehl , P. , Duhamel , J. and Sirigu , A. 2004 . The involvement of the orbitofrontal cortex in the experience of regret . Science , 304 ( 5674 ) : 1167 – 1170 .
- Chandrasekhar , P. V. , Capra , C. M. , Moore , S. , Noussair , C. and Berns , G. S. 2008 . Neurobiological regret and rejoice functions for aversive outcomes . NeuroImage , 39 ( 3 ) : 1472 – 1484 .
- Chua , H. F. , Gonzalez , R. , Taylor , S. F. , Welsh , R. C. and Liberzon , I. 2009 . Decision-related loss: Regret and disappointment . NeuroImage , 47 ( 4 ) : 2031 – 2040 .
- Connolly , T. , Ordónez , L. D. and Coughlan , R. 1997 . Regret and responsibility in the evaluation of decision outcomes . Organizational Behavior and Human Decision Processes , 70 ( 1 ) : 73 – 85 .
- Connolly , T. and Zeelenberg , M. 2002 . Regret in decision making . Current directions in psychological science , 11 ( 6 ) : 212 – 216 .
- Coricelli , G. , Critchley , H. D. , Joffily , M. , O'Doherty , J. P. , Sirigu , A. and Dolan , R. J. 2005 . Regret and its avoidance: A neuroimaging study of choice behavior . Nature Neuroscience , 8 ( 9 ) : 1255 – 1262 .
- Deichmann , R. , Schwarzbauer , C. and Turner , R. 2004 . Optimisation of the 3D MDEFT sequence for anatomical brain imaging: Technical implications at 1.5 and 3 T . NeuroImage , 21 : 757 – 767 .
- Farrer , C. , Franck , N. , Georgieff , N. , Frith , C. D. , Decety , J. and Jeannerod , M. 2003 . Modulating the experience of agency: A positron emission tomography study . NeuroImage , 18 ( 2 ) : 324 – 333 .
- Farrer , C. and Frith , C. D. 2002 . Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency . NeuroImage , 15 ( 3 ) : 596
- Fujiwara , J. , Tobler , P. N. , Taira , M. , Iijima , T. and Tsutsui , K. I. 2008 . Personality-dependent dissociation of absolute and relative loss processing in orbitofrontal cortex . European Journal of Neuroscience , 27 ( 6 ) : 1547
- Gottfried , J. A. , O'Doherty , J. and Dolan , R. J. 2003 . Encoding predictive reward value in human amygdala and orbitofrontal cortex . Science , 301 ( 5636 ) : 1104 – 1107 .
- Hutton , C. , Bork , A. , Josephs , O. , Deichmann , R. , Ashburner , J. and Turner , R. 2002 . Image distortion correction in fMRI: A quantitative evaluation . NeuroImage , 16 ( 1 ) : 217 – 240 .
- Hutton , C. , Deichmann , R. , Turner , R. and Andersson , J. L. R. 2004 . “ Combined correction for geometric distortion and its interaction with head motion in fMRI ” . In Proceedings of ISMRM 12 Kyoto, , Japan
- Inman , J. J. and Zeelenberg , M. 2002 . Regret repeat versus switch decisions: The attenuation role of decision justifiability . Journal of Consumer Research , 29 ( 1 ) : 116 – 128 .
- Janis , I. L. and Mann , L. 1977 . “ Anticipatory regret ” . In Decision making: A psychological analysis of conflict, choice, and commitment , 219 – 242 . New York : Free Press .
- Josephs , O. , Deichmann , R. and Turner , R. 2000 . Trajectory measurement and generalised reconstruction in rectilinear EPI . NeuroImage , 11 ( 5 ) : 543 – 543 .
- Kawashima , R. , Sugiura , M. , Kato , T. , Nakamura , A. , Hatano , K. Ito , K. 1999 . The human amygdala plays an important role in gaze monitoring: A PET study . Brain , 122 : 779 – 783 .
- LaBar , K. S. , Gatenby , J. C. , Gore , J. C. , LeDoux , J. E. and Phelps , E. A. 1998 . Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study . Neuron , 20 : 937 – 945 .
- Lechner , L. , de Vries , H. and Offermans , N. 1997 . Participation in a breast cancer screening program: Influence of past behavior and determinants on future screening participation . Preventive Medicine , 26 ( 4 ) : 473 – 482 .
- Liu , X. , Powell , D. K. , Wang , H. , Gold , B. T. , Corbly , C. R. and Joseph , J. E. 2007 . Functional dissociation in frontal and striatal areas for processing of positive and negative reward information . Journal of Neuroscience , 27 ( 17 ) : 4587 – 4597 .
- Maldjian , J. A. , Laurienti , P. J. , Burdette , J. B. and Kraft , R. A. 2003 . An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets . NeuroImage , 19 : 1233 – 1239 .
- Mellers , B. , Schwartz , A. and Ritov , I. 1999 . Emotion-based choice . Journal of Experimental Psychology: General , 128 : 332 – 345 .
- Morris , J. S. and Dolan , R. J. 2004 . Dissociable amygdala and orbitofrontal responses during reversal fear conditioning . NeuroImage , 22 ( 1 ) : 372 – 380 .
- Nicolle , A. , Bach , D. R. , Driver , J. and Dolan , R. J. in press . A role for the striatum in regret-related choice repetition . Journal of Cognitive Neuroscience. , Advance online publication. doi:10.1162/jocn.2010.21510
- Ordónez , L. D. and Connolly , T. 2000 . Regret and responsibility: A reply to Zeelenberg et al. (1998) . Organizational Behavior and Human Decision Processes , 81 ( 1 ) : 132 – 142 .
- Pickens , C. L. , Saddoris , M. P. , Setlow , B. , Gallagher , M. , Holland , P. C. and Schoenbaum , G. 2003 . Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task . Journal of Neuroscience , 23 ( 35 ) : 11078 – 11084 .
- Portas , C. M. , Krakow , K. , Allen , P. , Josephs , O. , Armony , J. L. and Frith , C. D. 2000 . Auditory processing across the sleep–wake cycle: Simultaneous EEG and fMRI monitoring in humans . Neuron , 28 : 991 – 999 .
- Reb , J. 2008 . Regret aversion and decision process quality: Effects of regret salience on decision process carefulness . Organizational Behavior and Human Decision Processes , 105 ( 2 ) : 169 – 182 .
- Richard , R. , Van der Pligt , J. and de Vries , N. 1996 . Anticipated regret and time perspective: Changing sexual risk-taking behavior . Journal of Behavioral Decision Making , 9 ( 3 ) : 185 – 199 .
- Richardson , M. P. , Strange , B. A. and Dolan , R. J. 2004 . Encoding of emotional memories depends on amygdala and hippocampus and their interactions . Nature Neuroscience , 7 ( 3 ) : 278 – 285 .
- Roese , N. J. and Olson , J. M. 1995 . “ Counterfactual thinking: A critical overview ” . In What might have been: The social psychology of counterfactual thinking , Edited by: Roese , N. J. and Olson , J. M. 1 – 55 . Mahwah, NJ : Lawrence Erlbaum Associates .
- Sander , D. , Grafman , J. and Zalla , T. 2003 . The human amygdala: An evolved system for relevance detection . Reviews in the Neurosciences , 14 ( 4 ) : 303 – 316 .
- Schoenbaum , G. , Gottfried , J. A. , Murray , E. A. and Ramus , S. J. 2007 . Preface . Annals of the New York Academy of Sciences , 1121 : xi – xiii .
- Simonson , I. 1992 . The influence of anticipating regret and responsibility on purchase decisions . Journal of Consumer Research , 19 ( 1 ) : 105 – 118 .
- Strange , B. A. and Dolan , R. J. 2004 . β-Adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses . Proceedings of the National Academy of Sciences of the United States of America , 101 ( 31 ) : 11454 – 11458 .
- Tsiros , M. and Mittal , V. 2000 . Regret: A model of its antecedents and consequences in consumer decision making . Journal of Consumer Research , 26 ( 4 ) : 401 – 417 .
- Tzourio-Mazoyer , N. , Landeau , B. , Papathanassiou , D. , Crivello , F. , Etard , O. Delcroix , N. 2002 . Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain . NeuroImage , 15 : 273 – 289 .
- Uğurbil , K. , Garwood , M. , Ellermann , J. , Hendrich , K. , Hinke , R. Hu , X. 1993 . Imaging at high magnetic fields: Initial experiences at 4 T . Magnetic Resonance Quarterly , 9 ( 4 ) : 259
- Weiskopf , N. , Sitaram , R. , Josephs , O. , Veit , R. , Scharnowski , F. Goebel , R. 2007 . Real-time functional magnetic resonance imaging: Methods and applications . Magnetic Resonance Imaging , 25 ( 6 ) : 989 – 1003 .
- Whalen , P. J. , Kagan , J. , Cook , R. G. , Davis , F. C. , Kim , H. Polis , S. 2004 . Human amygdala responsivity to masked fearful eye whites . Science , 306 ( 5704 ) : 2061
- Winston , J. S. , Strange , B. A. , O'Doherty , J. and Dolan , R. J. 2002 . Automatic and intentional brain responses during evaluation of trustworthiness of faces . Nature Neuroscience , 5 : 277 – 283 .
- Wrosch , C. and Heckhausen , J. 2002 . Perceived control of life regrets: Good for young and bad for old adults . Psychology and Aging , 17 ( 2 ) : 340 – 350 .
- Wright , C. I. , Martis , B. , Schwartz , C. E. , Shin , L. M. , Fischer , H. H. McMullin , K. 2003 . Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex . NeuroImage , 18 : 660 – 669 .
- Zalla , T. , Koechlin , E. , Pietrini , P. , Basso , G. , Aquino , P. Sirigu , A. 2000 . Differential amygdala responses to winning and losing: A functional magnetic resonance imaging study in humans . European Journal of Neuroscience , 12 ( 5 ) : 1764 – 1770 .
- Zeelenberg , M. and Pieters , R. 1999 . On serviced delivery that might have been: Behavioral responses to disappointment and regret . Journal of Service Research , 2 : 86 – 97 .
- Zeelenberg , M. and Pieters , R. 2007 . A theory of regret regulation 1.0 . Journal of Consumer Psychology , 17 : 3 – 18 .
- Zeelenberg , M. , van Dijk , W. W. and Manstead , A. S. 1998a . Reconsidering the relation between regret and responsibility . Organizational Behavior and Human Decision Processes , 74 : 254 – 272 .
- Zeelenberg , M. , van Dijk , W. W. and Manstead , A. S. 2000 . Regret and responsibility resolved? Evaluating Ordonez and Connolly's (2000) conclusions . Organizational Behavior and Human Decision Processes , 81 : 143 – 154 .
- Zeelenberg , M. , van Dijk , W. W. , van der Pligt , J. , Manstead , A. S. , van Empelen , P. and Reinderman , D. 1998b . Emotional reactions to the outcomes of decisions: The role of counterfactual thought in the experience of regret and disappointment . Organizational Behavior and Human Decision Processes , 75 : 117 – 141 .