ABSTRACT
The mirroring of actions is performed by a specialized system of neurons found in the sensorimotor cortex, termed the mirror neuron system. This system is considered an important mechanism that facilitates social understanding. We present a pre-registered experiment that used EEG to investigate whether short-term training via physical rehearsal or observational learning elicit distinct changes in mirror neuron activity for unfamiliar hand actions, and whether these training effects are influenced by the degree of familiarity (i.e., the frequency of action repetitions during training). Sixty adults completed a pre- and post-training EEG action observation task. Half of the participants completed 30 min of execution training (i.e., observing and performing unfamiliar hand actions), and half completed observation-only training (i.e., observing unfamiliar hand actions being performed). Post-training familiarity was manipulated by varying the number of training repetitions for each hand action (from 0 to 50 repetitions). Results revealed that sensorimotor cortex activity to the observation of hand actions increased following execution training, but did not change when training was simply observational. Moreover, the frequency of training repetitions did not modulate sensorimotor cortex activation after training, suggesting that short-term physical rehearsal enhances general processes involved in action understanding, rather than specific motor representations.
When a person executes or observes another person performing a motor action, a specialized system of neurons in the sensorimotor cortex fires to “mirror” this action and form a motor representation of the action. This system is considered an important mechanism for understanding others’ actions and intentions (Gallese, Keysers, & Rizzolatti, Citation2004), and is therefore crucial to understanding how humans succeed in the social world. For example, the mirror system has been implicated as a key mechanism in social cognitive processes, such as imitation (Catmur, Walsh, & Heyes, Citation2009), theory of mind (Gallese & Goldman, Citation1998), self-awareness (Oberman & Ramachandran, Citation2008) and empathy (Gallese, Citation2001). There is now abundant evidence for a mirror neuron system in humans (Fox et al., Citation2016), wherein only motor actions that are part of the person’s motor repertoire activate the mirror system (Calvo-Merino, Glaser, Grezes, Passingham, & Haggard, Citation2005; Calvo-Merino, Grezes, Glaser, Passingham, & Haggard, Citation2006). Experience with motor actions also has a crucial role in modulating the function of the mirror system (e.g., Cannon et al., Citation2014). The current study therefore investigated whether short-term training of unfamiliar hand actions via physical rehearsal or observational learning modulate distinct changes in the activity of the mirror system, thus reflecting social learning. In addition, we directly manipulated the degree of post-training familiarity with hand actions by varying the number of training repetitions for each hand action. This allowed us to test whether short-term training effects on the mirror system are operationalized by enhancements in general sensorimotor experience or specific motor representations.
The current study uses electroencephalography (EEG) methods to investigate the mirror system (see Fox et al., Citation2016). At rest, the sensorimotor cortex fires in synchrony, but during both action execution and observation, the firing of neurons in the sensorimotor cortex becomes desynchronized, reflecting cortical activity (Fox et al., Citation2016). This leads to the desynchronization of the mu rhythm (an EEG oscillation between 8 and 13 Hz; Hari, Salmelin, Makela, Salenius, & Helle, Citation1997) over sensorimotor areas, which is thought to reflect the activation of the mirror system (Pineda, Citation2005). Mirror neuron studies have also considered beta oscillations from 13 to 35 Hz (e.g., Hobson & Bishop, Citation2017; Puzzo, Cooper, Cantarella, & Russo, Citation2011), as the mu rhythm appears to consist of two spectral peaks at ~10 Hz and ~20 Hz (Hari, Citation2006). There has been a recent debate in the mirror neuron literature regarding the distinction between mu rhythm and alpha activity, since both are composed of the same frequency bands (Bowman et al., Citation2017; Fox et al., Citation2016; Hobson & Bishop, Citation2017). Therefore, this study considered both alpha and beta desynchronization as a proxy of mirror system activation and distinguished mu and alpha based on their topography, with mu originating from central areas overlying the sensorimotor cortex, and alpha originating from occipital areas.
The mirroring of others’ actions involves activating the corresponding motor representation in the sensorimotor cortex when observing another person’s action. Heyes and colleagues have suggested that the mirror system is configured through sensorimotor learning, i.e., through the repeated co-occurrence between a sensory input and motor output (Catmur et al., Citation2008; Catmur & Heyes, Citation2013; Catmur, Walsh, & Heyes, Citation2007; Catmur et al., Citation2009; Heyes, Citation2001). Infants as young as 8 months old have been found to be sensitive to contingencies between actions and effects (e.g., Paulus, Hunnius, van Elk, & Bekkering, Citation2012). Paulus et al. (Citation2012) trained 8-month-olds to use a novel rattle that produced a specific sound when rattled. After 1 week’s training, the action-related sound of the trained rattle and two sounds of untrained rattles were played. The infants displayed increased mu desynchronization when listening to the action-related sound as compared to the other two sounds. This finding suggests that through active experience with contingencies between actions and effects, infants map others’ actions onto their own motor representations in the sensorimotor cortex.
The strong top-down effect of experience with motor actions has also been shown among adults, with fMRI studies revealing that experts show greater activation in the mirror system to the actions that they are an expert in performing, such as in dance (Calvo-Merino et al., Citation2005, Citation2006; Cross, Hamilton, & Grafton, Citation2006; Cross, Hamilton, Kraemer, Kelley, & Grafton, Citation2009a; Cross, Kraemer, Hamilton, Kelley, & Grafton, Citation2009b) and sports (Abreu et al., Citation2012; Kim et al., Citation2011; Wright, Bishop, Jackson, & Abernethy, Citation2010). The majority of this research has focused on individuals with established levels of expertise for specific motor actions, with this expertise typically acquired over prolonged periods of training and experience. For example, in Calvo-Merino et al. (Citation2006)’s study, ballet dancers showed greater activation in the mirror neuron system compared to novices while watching videos of ballet moves. Moreover, this activation was greater when participants were viewing dance moves from their own motor repertoire, i.e., female dancers showed greater mirror system activation to female-specific dance moves compared to male-specific dance moves. In another study, Liew, Han, and Aziz-Zadeh (Citation2010) used fMRI to show that distinct brain responses are activated when viewing actors perform symbolic hand gestures, depending on participants’ familiarity with that motor action. Specifically, although both familiar and unfamiliar gestures activated the mirror system relative to still images, the mentalizing system (particularly the posterior cingulate cortex, involved in reasoning of others’ perspectives) was preferentially activated during the observation of familiar gestures (likely reflecting the process of inferring intentions), and the mirror system (involved in automatic motor simulations of observed actions) was preferentially activated during observation of unfamiliar gestures.
Much less research has explored the impact of shorter-term training interventions among non-expert participants. In one study, Cross et al. (Citation2009b) employed a 5-day dance training procedure, and found that highly familiar stimuli that was either executed or observed during training elicited greater activation in a subset of the mirror system compared to unfamiliar, untrained stimuli, suggesting that both active and passive experience with actions modulates activity over a common network in the mirror system. Similarly, Cannon et al. (Citation2014) tested the impact of action training over a 9-month period, comparing a group of expert performers who were trained to use and perform an action using a tool with a group of expert observers who simply watched and coded videos of actions performed with the tool, and a group of untrained novices. Though all groups showed mu desynchronization over the sensorimotor cortex when viewing the tool-use action, this effect was significantly larger in the group who physically performed this action during training, suggesting that mirror system activity is enhanced by active experience performing an action. However, this study suffered from numerous limitations. It was largely underpowered (N = 33 across three between-groups training conditions), and the robustness of the design can be disputed since no pre-training baseline was measured, experience with the action was not directly manipulated, and only one action was used. Therefore, the current study makes an important contribution to our understanding of how active versus passive experience with actions influences sensorimotor cortex activation by employing a tightly controlled experimental design, and a much shorter and more intensive training period than previously used (30 min vs. 9 months in Cannon et al., Citation2014, or 5 days in Cross et al., Citation2009b). A related, and to date overlooked, question is whether activity in the mirror neuron system is sensitive to the degree of familiarity/expertise that an individual has for a specific motor action, rather than a binary contrast in familiarity/expertise. The current study addressed this question directly by manipulating the frequency with which participants were trained on a specific action (between 0 and 50 repetitions).
In sum, the current study explored whether and how sensorimotor cortex activity to action observation is modulated by experience. We used EEG to compare activity over the sensorimotor cortex before and after a 30-min training task, where individuals either executed unfamiliar hand actions (execution training) or observed unfamiliar hand actions being performed (observation-only training). The number of training repetitions was manipulated to test whether action familiarity has a graded effect on the mirror system. First, we predicted that there would be greater sensorimotor alpha and beta desynchronization during hand action observation compared to static hand observation for both training conditions, consistent with the mirror neuron hypothesis. Second, in line with research that has shown increased mirror system activity for familiar/trained actions (e.g., Calvo-Merino et al., Citation2005, Citation2006; Cross et al., Citation2006, Citation2009a, Citation2009b), we predicted that alpha and beta desynchronization to hand action observation would increase from pre- to post-training. Since these previous studies revealed contrasting influences of active and passive training, we tested whether short-term training effects are evident following both physical execution and observational learning (as in Cross et al., Citation2009b), or whether training only emerges following execution training (as in Cannon et al., Citation2014). Finally, based on expertise studies that have demonstrated increased mirror system activation to the observation of familiar actions, we predicted that there would be an interaction between the number of training repetitions and training group for the action-static difference in alpha and beta power in the post-training task to the observation of these actions. Specifically, we predicted a greater action-static difference in the post-training task for actions that were repeated at a higher frequency only in the execution training group.
Method
The Methods and Analyzes for this study were pre-registered at https://osf.io/2upbj.
Participants
In total, 67 participants completed this study. Participants were recruited from the university’s research participation scheme and through email advertisements. All participants were fluent English-speakers, had a normal or corrected-to-normal vision, had no known neurological disorders, and had no mental health or autism spectrum disorder diagnoses. The participants’ consent was obtained according to the Declaration of Helsinki, and the study was approved by the Ethical Committee of the School of Psychology, University of Kent.
From the original sample, three participants were excluded due to excessive noise on the EEG recordings and four participants were excluded due to too few segments (less than two-thirds of segments remaining). The final sample consisted of 60 participants in total, with 30 participants in the execution training group [age range 18–30 years, mean age 20.70 years; 22 females] and 30 participants in the observation training group [age range 18–39 years, mean age 22.13 years; 20 females].
Stimuli
For the training tasks, stimuli consisted of six 10-s video clips depicting a novel hand action (see for descriptions) and an image of a static hand. These video clips were reduced to 3 s in length for the pre- and post-training tasks. All videos were presented in color with a resolution of 320 × 240 pixels and a frame rate of 25 frames per second.
Table 1. A description of the unfamiliar hand actions performed in the videos and performed by the participants in the execution group.
During the training-execution task, the objects used in the videos were available to the participant. These objects were a yellow spongy ball, a desk bell, a small replica rugby ball, a pair of scissors and a long, caterpillar toy. These objects were placed on an A4 image of the object stimuli so that participants could locate and put back the object for each training repetition.
Procedure
After reading the information sheet and signing the consent form, the Acticap was applied and set up for recording. Participants completed three tasks: pre-training, training and post-training, with EEG recorded during the pre- and post-training tasks only.
Pre-training task
Initially, participants performed a resting EEG as a baseline for 2 min which involved fixating a central cross on a gray screen. After a self-directed break, participants watched video clips, with a total of 90 experimental trials. Trials consisted of a 1000 ms fixation cross; then, a 3000 ms video clip, ending with a 1000–3000 ms blank screen (the inter-trial interval was variable to prevent expectancy effects on mu rhythm). Each hand action video clip was shown 10 times with a total of 60 hand action trials. The static hand video clip was shown 30 times with a total of 30 static hand trials. Trials were presented in a randomized order over three blocks of 30 trials, each with a self-directed break in-between blocks.
Training task
Half of the participants completed a training-execution task and the other half completed a training-observation task.
For the training-execution task, trials consisted of a 1000 ms fixation cross, then a 10,000 ms video clip, ending with a 2000 ms blank screen. The objects were placed in front of the participant for this task only. Participants were instructed to physically perform the hand action that was executed in the video for the duration of the trial.
For the training-observation task, trials consisted of a 1000 ms fixation cross, then a 10,000 ms video clip, ending with a 2000 ms blank screen. On 10% of trials, the trial ended with a 500 ms blank screen, followed by a 1000 ms screen showing three stars (***), and a 500 ms blank screen. On another 10% of trials, the trial ended with a 500 ms blank screen, followed by a 1000 ms screen showing two arrows (< >), and a 500 ms blank screen. Participants were told that they would see something after some videos and would be asked a question about this following the task. This “light” cognitive task was utilized to keep participants engaged with the videos in the observation task (see Hobson & Bishop, Citation2017).
For both training tasks, the repetition frequency for each hand action video was varied (either 50 trials, 40 trials, 30 trials, 20 trials, 10 trials, or not at all). Participants were assigned to one of six versions of the experiment, which ensured that the combination of hand action and training frequency was counterbalanced. Trials were presented in a randomized order, across five blocks of 30 trials, with a self-directed break in-between blocks. In total, there were 150 training trials, and both training tasks lasted approximately 30 min.
Post-training task
The post-training task was identical to the pre-training task.
EEG recording and analysis
Electroencephalographic (EEG) activity was recorded from 30 active electrodes using a Brain Vision Quickamp amplifier system with an ActiCap cap referenced to FCz, and Ground placed at AFz. Vertical electrooculogram (VEOG) was recorded from an electrode below the right eye, and horizontal electrooculogram (HEOG) was recorded from an electrode to the left of the left eye. EEG and EOG recordings were sampled at 1000 Hz, and electrode impedance was kept below 10kΩ.
First, a vertical ocular calculation was applied (1*Fp2+(−1*VEOG)), then all data were re-referenced to a common average reference. EEG and EOG activity were band-pass filtered (0.1–70 Hz, notch filter at 50 Hz). Data were visually inspected for noisy sections or channels, and for other general artifacts. EEG activity containing blinks was corrected using a semi-automatic ocular ICA correction approach (Brain Vision Analyzer 2.1). An average of three ICA components were removed in both the pre- and post-test per individual dataset.
The 2 min resting EEG data pre- and post-training was then cut into 2 s epochs (starting 0–2000 ms). Semi-automatic artifact detection software (Brain Vision Analyzer 2.1) was run, to identify and discard segments with non-ocular artifacts (drifts, channel blockings, EEG activity exceeding ± 50µV). A fast-Fourier transformation was then applied to the segments, with a 10% Hanning window. The average alpha (8–13 Hz) and beta power (13–35 Hz) at rest pre- and post-training was then calculated for each electrode of interest. Overall, there was an average trial loss of 3.4% for the pre-test resting EEG and 6.5% for the post-test resting EEG, with an average of 58 (out of 60) pre-test baseline segments and 56 (out of 60) post-test baseline segments retained per participant.
The pre- and post-training task trial data segments (all hand actions and static hand trials) were cut into 2 s epochs (500–2500 ms from stimulus onset), and further divided by the number of repetitions that hand action received in the training task (0, 10, 20, 30, 40, 50 repetitions). Semi-automatic artifact detection software was run to identify and discard segments with non-ocular artifacts (drifts, channel blockings, EEG activity exceeding ± 50µV). A fast-fourier transformation was then applied to the segments, with a 10% Hanning window. There was an overall data loss of 6.1% for the pre-test hand action trials and 5.8% for the post-test hand action trials, with an average of 56 (out of 60) pre-test hand action trial segments and 57 (out of 60) post-test hand action trial segments retained per participant. There was an overall data loss of 4.7% for the pre-test static hand trials and 6.2% for the post-test static hand trials, with an average of 29 (out of 30) pre-test hand action trial segments and 28 (out of 30) post-test hand action trial segments retained per participant.
For the power analyzes, the average alpha (8–13 Hz) and beta (13–35 Hz) power for each condition was calculated for the electrodes of interest over the sensorimotor (C3, Cz, C4) and occipital electrodes (O1, Oz, O2). A measure of the percentage change in power for each condition (test: hand action or static hand) relative to the pre/post resting EEG (reference) was calculated for each electrode of interest for both alpha and beta bands. The following formula was used (reference-test/reference) x 100. Positive values indicate alpha and beta desynchronization and negative values indicate alpha and beta synchronization.
Results
All data and code used for these analyzes are available at https://osf.io/2upbj.
First, to investigate whether training elicited distinct brain responses to action observation, we ran a pre-registered 2 × 2 × 2 × 2 repeated-measures ANOVA (https://osf.io/6rbu8/), with time (pre, post), condition (hand action, static hand), and electrode site (central, occipital) as within-subjects variables, and training group (observation, execution) as a between-subjects variable. The dependent variables were alpha and beta desynchronization values (as described in the Methods). The resulting mean percentage change in power from pre- to post-test for hand action observation and static hand observation over the central and occipital electrode sites for both training groups can be seen in .
Figure 1. Percentage change from pre- to post-test for hand action observation and static hand observation in a) central alpha, b) occipital alpha, c) central beta, and d) occipital beta power for both training groups.
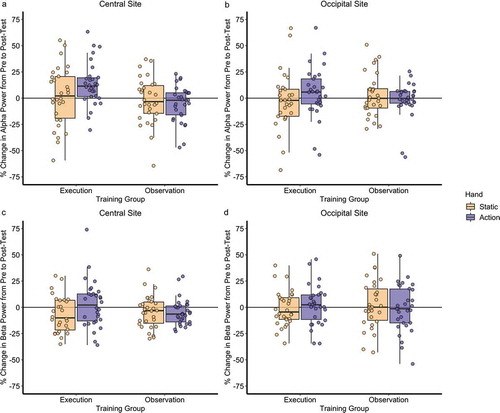
Results revealed a significant main effect of condition in both the alpha (F (1, 58) = 202.57, p < .001, ηp2 = .777) and beta bands (F (1, 58) = 225.21, p < .001, ηp2 = .795), showing significantly greater alpha and beta desynchronization during hand action observation (alpha M = 24.7%; beta M = 15.5%) compared to static hand observation (alpha M = 5.8%; beta M = 2.0%). A significant main effect of electrode site was found for both the alpha (F (1, 58) = 19.78, p < .001, ηp2 = .254) and beta band (F (1, 58) = 8.15, p = .006, ηp2 = .123), indicating greater power over the occipital electrodes (alpha M = 21.5%; beta M = 11.5%) compared to the central electrodes (alpha M = 9.0%; beta M = 6.0%). There was also a significant main effect of group for the alpha band only (F (1, 58) = 4.40, p = .040, ηp2 = .070), with the execution training group exhibiting significantly greater alpha power overall (alpha M = 21.1%) than the observation training group (alpha M = 9.4%). All other main effects were non-significant (all ps > .113).
There was a significant two-way interaction between condition and electrode in the beta band only (F (1, 58) = 10.78, p = .002, ηp2 = .157). The action-static difference was significantly greater over the central site (beta M = 15.5%) than over the occipital site (beta M = 11.5%; t (59) = 3.30, p < .001). There was also a significant two-way interaction between time and condition in the alpha band only (F (1, 58) = 4.96, p = .030, ηp2 = .079). This interaction was subsumed under a significant three-way interaction between time, condition and group in the alpha band (F (1, 58) = 12.25, p = .001, ηp2 = .174), which was also significant in the beta band (F (1, 58) = 4.13, p = .047, ηp2 = .066). All other interactions were not significant (all ps > .104).
To further examine the source of the three-way interaction between time, condition and group, two follow-up exploratory one-way ANCOVA analyses were performed for each condition with training group as a between-subjects variable, and pre-training task alpha or beta power as a covariate. The dependent variable was a difference score, calculated for each participant by subtracting the percentage change in power during the pre-training period from the percentage change in power during the post-training period, separately for alpha and beta power bands, averaged across the central and occipital electrodes.
Results for alpha power in the hand action condition revealed a significant difference in pre- to post-training between the training groups (F (1, 58) = 6.81, p = .012, ηp2 = .105), when adjusting for pre-training task alpha power. One-sample t-tests confirmed that alpha power activity to the hand action significantly increased from pre- to post-training in the execution training group (M = 7.8%; t (29) = 2.33, p = .027), but did not change in the observation training group (M = −3.4%; t (29) = −1.27 p = .251). For the static hand condition, pre- to post-training alpha power did not differ between the training groups (F (1, 58) = 0.08, p = .782, ηp2 = .001), and neither showed a significant change from pre- to post-training (execution M = −2.7%; observation M = −1.1%; all ps > .564).
Results for beta power revealed no significant difference in pre- to post-training task between the training groups for either condition (hand action: F (1, 58) = 1.97, p = .166, ηp2 = .033; static hand: F (1, 58) = 0.01, p = .919, ηp2 < .001), when adjusting for pre-training task beta power. Thus, to further examine the source of the three-way interaction for beta power, two 2 (condition: hand action, static hand) × 2 (group: execution training, observation training) ANOVAs were performed separately for the pre- and post-training task. Results revealed that the condition by group interaction was significant only at post-training (pre: F (1, 58) = 0.62, p = .805, ηp2 = .001; post: F (1, 58) = 4.55, p = .037, ηp2 = .073), indicating that the post-training action-static difference was significantly greater in the execution training group (beta M = 15.8%) than the observation training group (beta M = 11.5%; t (58) = 2.13, p = .037).
Number of action repetitions during training
To examine whether and how the number of action repetitions during training influences sensorimotor activity, a difference score was calculated for each participant by subtracting the percentage change in power for the static hand condition from the percentage change in power for each hand action repetition frequency (0, 10, 20, 30, 40, 50) during the post-training task, separately for alpha and beta power bands and across the central electrodes, as per the pre-registered analyses (https://osf.io/6rbu8/). Mean action-static difference scores per participant in each training group are displayed in for each of the six repetition frequencies.
Figure 2. Mean action-static difference across the central electrodes in the post-training task for each training repetition frequency for both training groups in a) alpha and b) beta power. The square point represents the mean data for each training repetition frequency by training group and the circle point represents individual data.
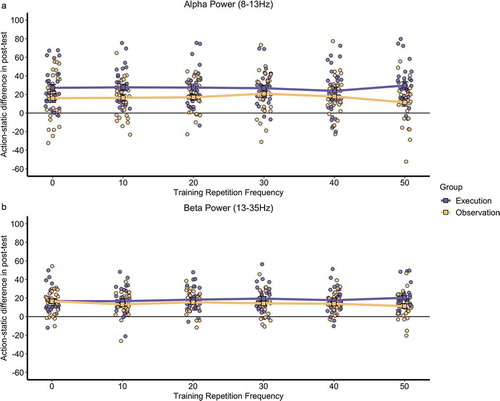
Two repeated-measures ANOVAs were conducted separately for each power band (alpha, beta), with the mean action-static difference score as the dependent variable, action repetition frequency as a within-subjects factor (6 levels; 0, 10, 20, 30, 40, 50 repetitions during training), and training group as a between-subjects factor (execution, observation).Footnote1 The main effect of repetition frequency was not significant for either power band (alpha: F (5, 346) = 0.19, p = .966, ηp2 = .002; beta: F (5, 347) = 0.22, p = .954, ηp2 = .003). The main effect of training group was significant (alpha: F (1, 346) = 26.77, p< .001, ηp2 = .069; beta: F (1, 347) = 9.62, p = .002, ηp2 = .027) indicating that the execution group had significantly greater action-static difference in power during the post-training task (alpha M = 27.1%, beta M = 18.2%) as compared to the observation group (alpha M = 16.5%, beta M = 14.2%). Importantly, the interaction between repetition frequency and training group was not significant for either power band (alpha = F (5, 346) = 0.71, p = .616, ηp2 = .009; beta = F (5, 347) = 0.83, p = .532, ηp2 = .011), indicating no modulation of repetition frequency during training on mirror neuron activation to action observation.
Discussion
The current pre-registered study sought to examine whether short-term training via physical rehearsal or observational learning elicit distinct changes in mirror neuron activity for unfamiliar hand actions, and whether these training effects are influenced by degree of familiarity (i.e., the frequency of action repetitions during training). Given that the mirror neuron system has been proposed as an important mechanism to facilitate social cognition (Gallese, Keysers & Rizzolatti, Citation2004), learning effects would provide new insights into the neural basis of understanding others’ actions and intentions. Thirty individuals completed execution training and 30 individuals completed observation-only training. We measured pre- and post-training alpha (8–13 Hz) and beta (13–35 Hz) desynchronization to the observation of short video clips depicting hand actions or a static hand as an EEG marker of mirror neuron activity across the sensorimotor cortex. Results revealed greater alpha and beta desynchronization across the sensorimotor cortex during hand action observation compared to static hand observation, in support of our predictions and the mirror neuron hypothesis. More importantly, this activation was modulated by training. Specifically, alpha desynchronization to hand action observation increased from pre- to post-training in the execution training group only; the observation group showed no change from pre- to post-training. In addition, the action-static difference in beta power was greater after training in the execution training group compared to the observation training group. Finally, post-training alpha and beta desynchronization were not influenced by the number of training repetitions, suggesting that the training effects described above reflect an enhancement in general action processing, rather than specific motor representations.
Previous studies that have examined the effect of expertise on mirror neuron activity have reported greater activation during action observation when the observer is an expert in performing that action as compared to a novice, such as for dancers (Calvo-Merino et al., Citation2005, Citation2006). In addition, two studies have examined the effects of training interventions among non-expert participants, and have revealed contrasting findings following physical rehearsal and observational learning. Using fMRI, Cross et al. (Citation2009b) showed that active and passive training over a five-day period elicited overlapping increases in activation over the mirror system compared to unfamiliar, untrained stimuli. In contrast, Cannon et al. (Citation2014) found that active experience using a tool modulates the mirror neuron system (alpha activity) more than passive observation experience. The current study adopted a more strictly controlled design, and a shorter and more intensive training period, to distinguish effects from active versus passive motor action training on sensorimotor cortex activity. Overall, our findings complement these previous findings by showing that activity in the mirror neuron system can be “trained” through experience. Moreover, we have provided the first evidence that these training enhancement effects can be elicited following just 30 min of training with unfamiliar actions. Our results also make an important contribution to debates about whether active and passive experience with actions modulates activity over a common network in the mirror system. Here we showed that short-term training effects on the mirror neuron system emerge only following physical experience with goal-directed actions, but not when participants merely observed the action being performed. This finding is consistent with the effects found in Cannon et al. (Citation2014), despite the very different designs and controls. It provides support for a mirror system expertise account in which action experience increases mu desynchronization to actions signifying greater expertise in action understanding even after a single training session. Moreover, when considered alongside Cross et al. (Citation2009b) who showed comparable effects following 5 days of active and passive training, our specific training effects suggest that observation training effects take longer to emerge than physical training effects. Thus, a testable prediction for future work is that specialization of the mirror system to new actions can be sped up through physical experience with motor actions, though the same level of expertise can be reached through sustained observational learning.
Another important finding from the current study is that the number of repetitions during the training period did not modulate the activation of the mirror system after training for either execution or observation-only training. In fact, even an action that was never presented during training elicited comparable sensorimotor activity at post-test to an action that was repeated 50 times. This finding of an overall increase in activation in the mirror neuron system after executing the unfamiliar actions therefore suggests that physical rehearsal of actions during training elicits an enhancement of general processes involved in action understanding, rather than encoding specific motor representations. Thus, activity in the mirror neuron system (at least, recorded via EEG) does not appear to be sensitive to the degree of familiarity/expertise that an individual has for a specific motor action. In addition, the fact that activation across the sensorimotor cortex increased after execution training for untrained actions suggests that physical rehearsal initiated a transfer of training effect. An outstanding question is whether this transfer of training effect also generalizes to completely novel actions (i.e., unfamiliar actions not shown in the pre-training task).
Effects of execution training can be more closely compared with those reported by Cannon et al. (Citation2014). Specifically, we showed that active training modulated sensorimotor activity in both the alpha and beta bands, while Cannon et al. found that effects of active training were specific to the alpha rhythm, and not the beta rhythm. They suggested that this was due to the short-term exposure to training that the beta rhythm is sensitive to more complex actions, or that the beta rhythm is too localized and motor learning depends on a more global network. Our results contradict this proposal by showing effects in the beta band with an even shorter exposure duration (i.e., both studies included ~150 action repetitions, but either spread over 9 months or 30 min). We relate our results in the beta band to motor preparation and selection processes (Doyle, Yarrow, & Brown, Citation2005). Consequently, the more rigorous execution training in this study provided specific motor experience about when and what to move to perform the action, and this intensive motor training caused motor selection processes to be more activated when observing these actions after training.
The current study has clear implications for the sensorimotor learning hypothesis. The sensorimotor learning hypothesis suggests that the development of the human mirror system is experience-dependent; we learn to understand the actions of others through experiencing sensory and motor representations of those actions in an associative manner (Heyes, Citation2001). This study provides support for the sensorimotor learning hypothesis as experience with both sensory input and motor output in the execution training, as compared to sensory-only in the observation training, led to an increase in activity in the sensorimotor cortex. Therefore, experiencing these sensory inputs and motor outputs in an associative way led to increased activity in the mirror system, suggesting that sensorimotor learning took place over the training period. This finding is also consistent with a study by Catmur et al. (Citation2007) in which they measured the functioning of the mirror system before and after compatible (i.e., observe index-finger movement, perform index-finger movement) and incompatible sensorimotor training (observe index-finger movement, perform little-finger movement). In support of the sensorimotor learning hypothesis, this incompatible training altered the brain’s response to action observation after training, yet the compatible training produced an unaltered response. Taken together, these two studies suggest that sensorimotor learning depends on the contingency between action observation and execution. Nevertheless, we did not find that the number of training repetitions impacted on sensorimotor cortex activation, somewhat against the predictions of the sensorimotor learning hypothesis. Conceivably, the general experience of a large number of contingencies between action observation and execution during training may have led to an overall increase in sensorimotor cortex activation. We propose that the development of the mirror system depends on experiencing a vast number of sensory-motor associations and that these learned associations can then be applied to understanding numerous actions. However, a limitation of the current study is that the desynchronization to hand actions was measured immediately after training, and as such, we cannot infer the longevity of the training effect.
In conclusion, we have provided evidence in support of the mirror neuron hypothesis, as sensorimotor activation in the alpha and beta bands was greater when observing hand actions compared to static hands. More importantly, we have shown that short-term active experience with hand actions enhances activity over the sensorimotor cortex, whereas this activity is not altered by passively observing actions. In addition, degree of familiarity did not modulate sensorimotor cortex activation after training, suggesting that training enhances general processes involved in action understanding, rather than specific motor representations. These findings show that sensorimotor experiences are crucial for the development of the mirror system, and even a mature mirror system is experience-dependent. Thus, the mechanisms that underlie social understanding in the real world can be enhanced through active learning.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes
1 The pre-registered analyses specified bivariate Spearman’s correlations to examine the relationship between repetition frequency and the action-static difference in power during the post-training task for each training group. However, an ANOVA approach was deemed more appropriate for the types of variable (i.e., categorical and continuous). To note, the same data analyzed with Spearman’s correlations indicated no relationship between repetition frequency and the action-static difference in the post-training task for either training group in both power bands. These analyses can be found at https://osf.io/6rbu8/.
References
- Abreu, A. M., Macaluso, E., Azevedo, R. T., Cesar, P., Urgesi, C., & Aglioti, S. M. (2012). Action anticipation beyond the action observation network: A functional magnetic resonance imaging study in expert basketball players. European Journal of Neuroscience, 35, 1646–1654.
- Bowman, L. C., Bakermans-Kranenburg, M. J., Yoo, K. H., Cannon, E. N., Vanderwert, R. E., Ferrari, P. F., … Fox, N. A. (2017). The mu-rhythm can mirror: Insights from experimental design, and looking past the controversy. Cortex, 96, 121–125.
- Calvo-Merino, B., Glaser, D. E., Grezes, J., Passingham, R. E., & Haggard, P. (2005). Action observation and acquired motor skills: An fMRI study with expert dancers. Cerebral Cortex, 15(8), 1243–1249.
- Calvo-Merino, B., Grezes, J., Glaser, D. E., Passingham, R. E., & Haggard, P. (2006). Seeing or doing? Influence of visual and motor familiarity in action observation. Current Biology, 16(19), 1905–1910.
- Cannon, E., Yoo, K. H., Vanderwert, R. E., Ferrari, P. F., Woodward, A. L., & Fox, N. A. (2014). Action experience, more than observation, influences mu rhythm desynchronization. PloS One, 9(3), e92002.
- Catmur, C., Gillmeister, H., Bird, G., Liepelt, R., Brass, M., & Heyes, C. (2008). Through the looking glass: counter-mirror activation following incompatible sensorimotor learning. European Journal of Neuroscience, 28(6), 1208–1215.
- Catmur, C., & Heyes, C. (2013). Is it what you do, or when you do it? The roles of contingency and similarity in pro‐social effects of imitation. Cognitive Science, 37, 1541–1552.
- Catmur, C., Walsh, V., & Heyes, C. (2007). Sensorimotor learning configures the human mirror system. Current Biology, 17(17), 1527–1531.
- Catmur, C., Walsh, V., & Heyes, C. (2009). Associative sequence learning: the role of experience in the development of imitation and the mirror system. Philosophical Transactions of the Royal Society B – Biological Sciences, 364(1528), 2369–2380.
- Cross, E. S., Hamilton, A. F. D. C., & Grafton, S. T. (2006). Building a motor simulation de novo: Observation of dance by dancers. Neuroimage, 31, 1257–1267.
- Cross, E. S., Hamilton, A. F. D. C., Kraemer, D. J. M., Kelley, W. M., & Grafton, S. T. (2009a). Dissociable substrates for body motion and physical experience in the human action observation network. European Journal of Neuroscience, 30, 1383–1392.
- Cross, E. S., Kraemer, D. J. M., Hamilton, A. F. D. C., Kelley, W. M., & Grafton, S. T. (2009b). Sensitivity of the action observation network to physical and observational learning. Cerebral Cortex, 19, 315–326.
- Doyle, L. M. F., Yarrow, K., & Brown, P. (2005). Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clinical Neurophysiology, 116(8), 1879-1888.
- Fox, N. A., Bakermans-Kranenburg, M. J., Yoo, K. H., Bowman, L. C., Cannon, E. N., Vanderwert, R. E., … van IJzendoorn, M. H. (2016). Assessing human mirror activity with EEG mu rhythm: A meta-analysis. Psychological Bulletin, 142(3), 291–313.
- Gallese, V. (2001). The ‘shared manifold’ hypothesis - From mirror neurons to empathy. Journal of Consciousness Studies, 8, 33–50.
- Gallese, V., & Goldman, A. (1998). Mirror neurons and the simulation theory of mind reading. Trends in Cognitive Science, 2, 493–501.
- Gallese, V., Keysers, C., & Rizzolatti, G. (2004). A unifying view of the basis of social cognition. Trends in Cognitive Science, 8, 396–403.
- Hari, R. (2006). Action-perception connection and the cortical mu rhythm. Event-Related Dynamics of Brain Oscillations, 159, 253–260.
- Hari, R., Salmelin, R., Makela, J. P., Salenius, S., & Helle, M. (1997). Magnetoencephalographic cortical rhythms. International Journal of Psychophysiology, 26(1–3), 51–62.
- Heyes, C. (2001). Causes and consequences of imitation. Trends in Cognitive Science, 5(6), 253–261.
- Hobson, H. M., & Bishop, D. V. M. (2017). The interpretation of mu suppression as an index of mirror neuron activity: Past, present and future. Royal Society Open Science, 4(3), 160662.
- Kim, Y.-T., Seo, J.-H., Song, H.-J., Yoo, D.-S., Lee, H. J., Lee, J., … Chang, Y. (2011). Neural correlates related to action observation in expert archers. Behavioural Brain Research, 223(2), 342–347.
- Liew, S.-L., Han, S., & Aziz-Zadeh, L. (2010). Familiarity modulates mirror neuron and mentalizing regions during intention understanding. Human Brain Mapping, 32(11), 1986–1997.
- Oberman, L. M., & Ramachandran, V. S. (2008). Reflections on the mirror neuron system: Their evolutionary functions beyond motor representation. In J. A. Pineda (Ed.), Mirror neuron systems (pp. 39-59). Contemporary Neuroscience. New Jersey, NJ: Humana Press.
- Paulus, M., Hunnius, S., van Elk, M., & Bekkering, H. (2012). How learning to shake a rattle affects 8-month-old infants’ perception of the rattle’s sound: Electrophysiological evidence for action-effect binding in infancy. Developmental Cognitive Neuroscience, 2(1), 90–96.
- Pineda, J. A. (2005). The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Research Reviews, 50(1), 57–68.
- Puzzo, I., Cooper, N. R., Cantarella, S., & Russo, R. (2011). Measuring the effects of manipulating stimulus presentation time on sensorimotor alpha and low beta reactivity during hand movement observation. Neuroimage, 57(4), 1358–1363.
- Wright, M. J., Bishop, D. T., Jackson, R. C., & Abernethy, B. (2010). Functional MRI reveals expert-novice differences during sport-related anticipation. Neuroreport, 21, 94–98.
