ABSTRACT
Traditional disciplines have frequently dealt with complex phenomena from a given level of analysis, be that molecular, cellular, organ system, or organismic level. This can yield highly valuable information on biological and psychological processes. There is an explanatory value added, however, by an integrative multilevel approach, in which different levels of analysis and different levels of the neural organization are considered in the models and theories of psychological functions. This is the essence of the emerging discipline of social neuroscience, promoted by John Cacioppo and Gary Berntson, which seeks to inform the interactions between social psychological and biological processes.
Introduction & early history
Gary Berntson and John Cacioppo first met at Ohio State University in 1976, when Berntson was a relatively new assistant professor (from a postdoc at Rockefeller University) and Cacioppo was a senior graduate student in the Social Psychology program. We crossed paths in the laboratory of Curt Sandman, a clinical psychophysiologist, who both of us were working with at the time. At this encounter, Berntson was wearing a dress shirt and tie whereas Cacioppo was adorned in farmer’s denim bib overalls – no shirt or t-shirt, and no socks. But we hit it off, if only briefly, because he graduated the next year. The next time we crossed paths was in 1988, when OSU was soliciting his wife at the time, Barb Andersen, for our health psychology position, and in the course of that solicitation we were able to obtain funds to also extend an offer to John Cacioppo, as a spousal hire opportunity, for the social psychology program. Both came to Ohio State in 1989, in view of mutual interests we initiated a number of collaborative research efforts.
At that point, the Berntson lab had a long history of research on multiple levels of behavioral/affective regulation, in studies ranging from learning capacities in decerebrate infants and animals (Berntson et al., Citation1983; Ronca et al., Citation1985; Tuber et al., Citation1980) to stimulation and lesion studies of the hierarchical re-representation of function in neurobehavioral systems at multiple levels of the neuraxis (Berntson & Micco, Citation1976; Berntson & Torello, Citation1981). A central focus of these studies was how multiple levels of neurobehavioral systems synergistically interact to yield adaptive behavioral/affective outputs. An illustration of this is our work on the cerebellum, a structure that is comprised of a larger number of neurons than all other brain areas combined, and issues vast ascending and descending projections to widespread areas of the nervous system. The cerebellum has been traditionally implicated solely in motor coordination, although there early voices to the contrary. Flourens (Citation1824) for example asserted “the cerebral lobes do not act in the same way as the cerebellum, nor the cerebellum like the spinal cord, not the cord absolutely like the nerves. But it is a single system, all of its parts concur, consent and are in accord; what distinguishes them is the appropriate and determined manner of acting; what unites them is a reciprocal action through their common energy” (Flourens, Citation1824, p. 368).
Our studies and others revealed a far broader integrative role of the cerebellum, including the regulation of cognitive, emotional, and motivational processes (Berntson & Torello, Citation1981). More recent imaging studies, for example, have revealed that the cerebellum is activated by a range of social cognitive processes, displaying specific functional connectivity with cerebral structures implicated in social processes (Van Overwalle et al., Citation2015). The cerebellum has also been shown to project to the ventral tegmentum, triggering the release of dopamine and associated reward processes, which have been shown to be critical for aspects of social preference and sociability (Carta et al., Citation2019). In this regard, cerebellar dysfunctions have been implicated in a wide variety of social and psychiatric disturbances (for review, see Adamaszek et al., Citation2017).
This literature illustrates the multiple levels of neural functions underlying cognitive and behavioral processes. John Hughlings Jackson noted that with neural damage to higher structures, functions often do not disappear, but may revert to a more restricted, primitive form. In his “Evolution and Dissolution of the Nervous System” he argues that this reflects a progressive evolutionary re-representation of function at higher levels of the nervous system, and its dissolution with the injury of higher parts of the system (Jackson, Citation1884/1958).
The multi-level perspective in Berntson’s work resonated with Cacioppo when he returned to Ohio State as a professor in the late 1980s. Although more on a functional than a neurological level, Cacioppo and Rich Petty had developed the elaboration likelihood model of attitudes, which entails higher cognitive (central, more elaborated) and lower (peripheral, more automatic, reflexive) routes to persuasion (Petty & Cacioppo, Citation1986). Clearly, we reasoned that there must be a neural hierarchy that that supports these multi-level cognitive processes. This common perspective led to a joint writing effort on the Cardinal Principle of Evaluative Bivalence, which outlined the multiple levels of neural organization in evaluative systems, and the fundamental bivalence in positive and negative neural substrates (Berntson, Boysen & Cacioppo, Citation1993). In contrast to the circumplex model of affect, positive and negative disposition are not exclusively reciprocally regulated. Borrowing from our bivariate autonomic space model of ANS regulation (see below), we developed a model of bivariate evaluative space, in which positive and negative dispositions can vary reciprocally, independently, or co-actively (e.g., in ambivalence) (Cacioppo & Berntson, Citation1994; Norman, Norris et al., Citation2011).
Another point of convergence between Berntson and Cacioppo was our mutual interest in psychophysiology. Both of our labs had been employing physiological measures in studies of social psychological processes, including patients with “locked-in” syndrome, as well as investigations of social recognition in chimpanzees. In view of these mutual interests, Cacioppo & Berntson pursued a social psychophysiology training program (1992–1997), supported by an NIMH training grant, attended by researchers of many disciplines from across the country. This was an important effort that brought the field of social psychophysiology and multilevel analysis into prominence for many senior researchers in the field. These perspectives were featured in our 1992 American Psychologist piece “Social psychological contributions to the decade of the brain: Doctrine of multilevel analysis” (Cacioppo & Berntson, Citation1992). It was here that we formally introduced the term Social Neuroscience, which helped establish a disciplinary identity for those working at the intersection of social psychology and neuroscience. Our subsequent efforts, along with those of many others, continued to emphasize the importance of multi-level perspective and analysis in social neuroscience (Berntson & Cacioppo, Citation2000, Citation2004; Cacioppo, Berntson, & Decety, Citation2010; Cacioppo, Berntson et al., Citation2000), and this applies not only to human but also non-human animal research (Norman, Hawkley, Cole et al., Citation2012). We will return below to the value of integration of human and animal research. These efforts ultimately contributed to the establishment of the Society for Social Neuroscience in 2010, spearheaded by Cacioppo and Jean Decety.
An illustration of this collaborative effort in psychophysiology was the confirmation of multiple levels of organization in the autonomic nervous system (ANS). The historical perspectives on the ANS, from the Walter Cannon era, considered the ANS to be a) a predominantly homeostatic system, that was b) largely automatic and independent of the cerebral cortex, and c) whose sympathetic and parasympathetic divisions were subject to reciprocal central control. In a 1991 Psychological Review piece, and follow-up studies, we (along with Karen Quigley) demonstrated that each of these assumptions were incorrect or at least not comprehensive (Berntson et al., Citation1991). The baroreceptor-heart rate reflex, for example, has been considered an “automatic,” reciprocally regulated, homeostatic reflex organized at a brainstem level. But we now know that lower ANS substrates receive extensive connections from the cerebral cortex, which can hierarchically modulate the lower lever representations. Indeed, it was Cannon’s student, Philip Bard, who noted that the baroreflex could be inhibited by psychological stressors of higher-level origin. In contrast to the classical reciprocal model of ANS control, these considerations led to the development of the autonomic space model of bivariate autonomic control, and the recognition that there are multiple levels of representation (including cortical) in ANS regulatory substrates. It further clarified some of the principles of an organization in such multi-level systems that we will return to below. An important perspective in multilevel processes is that regulatory influences are not only top-down, but also bottom-up (see Berntson et al., Citation1998, Citation2003), as lower visceral activity and afference can powerfully modulate basic cognitive and affective states. This is in keeping with a now-growing recognition of the important role of interoception, and visceral afference more generally, in higher-level systems and processes (G. G. Berntson et al., Citation2018; Critchley & Harrison, Citation2013; Eisenberger et al., Citation2017; Norman et al., Citation2014; Tsakiris & De Preester, Citation2018).
The above discussion illustrates some of the origins of our work on the concept of multilevel analyses and its implications for psychophysiology and more broadly for research and theory in social and behavioral processes. Below are detailed some of the subsequent developments emerging from these early studies. Although Cacioppo left Ohio State for the University of Chicago in 1999, collaborations between the Berntson & Cacioppo labs continued for over 15 years, including multiple research efforts as well as joint editorships of a number of scholarly books, including the Handbook of Neuroscience for the Behavioral Sciences (Berntson & Cacioppo, Citation2009), Essays in Social Neuroscience (Cacioppo & Berntson, Citation2004), Social Neuroscience (Cacioppo & Berntson, Citation2005) and several issues of the Handbook of Psychophysiology (Cacioppo, Tassinary & Berntson, 2000, Berntson & Cacioppo, Citation2007; Cacioppo et al., Citation2016).
Continuing research efforts
Continuing collaborative, multilevel efforts are illustrated by related lines of development on loneliness and its health consequences. Loneliness is a growing problem with the current structure of society, together with an aging population. Our interest in loneliness arose out of the then-emerging literature on the effects of loneliness on morbidity and mortality (Holt-Lunstad et al., Citation2017; House et al., Citation1988), together with our investigations on the relations and mechanisms underlying the links between stress and health (Cacioppo et al., Citation1998). A focus on loneliness grew out of a MacArthur Foundation sponsored project at Ohio State (1995–2000; Cacioppo, PI) on social neuroscience (see Cacioppo, Ernst et al., Citation2000; Cacioppo, Hawkley, Berntson et al., Citation2002).
CAR vs. CAB and health
These studies continued after Cacioppo left for Chicago, highly facilitated by Louise Hawkley, who had worked with Cacioppo and Berntson at OSU, and ultimately received her Ph.D. with Berntson, before joining Cacioppo in Chicago, as a post-doc (e.g., Cacioppo et al., Citation2003; Cacioppo, Hawkley, Berntson et al., Citation2002; Cacioppo, Hawkley, Crawford et al., Citation2002; Cacioppo et al., Citation2011). Of relevance was the development of the Chicago Health, Aging, and Social Relations Study (CHASRS), funded by a Program Project Grant to Cacioppo, with Hawkley as a central figure in this effort (e.g., Hawkley et al., Citation2011). CHASRS was a population-based longitudinal study, entailing the mapping of multiple, behavioral, personality, physiological, endocrinological, and health status. In the context of that project, Cacioppo, Hawkley, Berntson, and a senior Ph.D. candidate at the time, Greg Norman (now a Professor at U Chicago), were examining the potential links between autonomic function and health, with a particular focus on the bivariate autonomic space model. As discussed above, we had observed notable individual differences in activities of the autonomic branches, likely arising from the operations of rostral neural systems in psychological contexts. At a group level, the heart rate responses of human subjects to orthostatic stress and to standard psychological stressors (mental arithmetic, speech stress, reaction time task) were similar (Berntson et al., Citation1994). Analysis of the separate contributions of the two autonomic branches by the use of single and dual pharmacological blockades revealed that the orthostatic stress (transition from sitting to standing) yielded a rather consistent response across subjects, characterized by a highly correlated sympathetic activation and parasympathetic withdrawal. In contrast, psychological stressors resulted in a more varied pattern of response across subjects, with no overall correlation between the responses of the autonomic branches. Some subjects showed a predominant sympathetic activation, others a predominant parasympathetic withdrawal, and others varying combinations of these responses (co-activation or co-inhibition). Although there were considerable individual differences in the responses, individual response patterns were stable across the three psychological stressors (Berntson et al., Citation1994). The question arose as to whether these distinct patterns may have different health implications. We addressed that question in the context of the CHASRS sample (Berntson, Norman, Hawkley & Cacioppo, Citation2008a).
In addition to separate measures of sympathetic and parasympathetic activities, we sought to capture the orthogonal dimensions of reciprocity and co-activity in ANS space . A measure of sympathetic activity was taken as the cardiac pre-ejection period (PEP, measured by impedance cardiography), which is the period between the electrical invasion of the ventricular myocardium and the development of sufficient ventricular pressure to yield ventricular ejection. This has been shown to be modulated by sympathetic control, which increases myocardial contractility (and hence reduces PEP), and is minimally sensitive to parasympathetic control. Respiratory sinus arrhythmia (RSA) was used as an index of parasympathetic control, as has been widely validated. RSA is a rhythmic (high, respiratory, frequency, HF) variation in heart rate coupled to respiratory variations in parasympathetic (vagal) control, and is largely insensitive to sympathetic control. These metrics provide estimates of independent activities of the ANS branches, representing the two axes in . But health relevancy may relate to the pattern of activity between the branches. Consequently, in addition to the independent measures, we sought to capture the relations between the ANS branches along the diagonals of . Based on a reciprocal, bipolar model of autonomic control, a cardiac autonomic balance (CAB) metric was derived as a scale extending from maximal sympathetic activation on one end of a continuum to maximal parasympathetic activation at the other extreme (i.e., along the reciprocity diagonal of ). Although this index was not correlated with many aspects of health and disease in the CHASRS sample, it was predictive of diabetes mellitus, independent of demographics and health behaviors.
Figure 1. CAB and CAR metrics in bivariate autonomic space. CAB captures the classical reciprocal model of autonomic control, extending from parasympathetic to sympathetic dominance (low levels of CAB are reflective of high sympathetic control). CAR captures the coactivity dimension of autonomic control (high levels of CAR are reflective of an overall higher level of autonomic control)
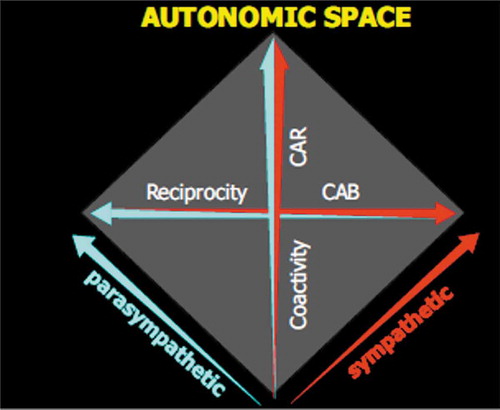
In contrast to bipolar models of autonomic control, as discussed above, recent conceptualizations of psychosomatic relations have emphasized the overall capacity for autonomic control as indexed by autonomic flexibility and variability (Friedman, Citation2007; Thayer & Sternberg, Citation2006). This concept, together with the demonstration of the basic bivariate structure of autonomic control, provided an alternative metric to CAB. An index of the aggregate Cardiac Autonomic Regulation (CAR) was derived as the sum of activities of the autonomic branches (normalized HF and PEP measures). In contrast to the reciprocal diagonal represented by CAB, CAR captures the co-activity diagonal of . Analysis of the CHASRS sample revealed that CAR was a better predictor of overall health status than was CAB, and was a significant (negative) predictor of the prior occurrence of a myocardial infarction (MI, heart attack), independent of demographics and health behaviors. Participants with lower CAR scores (lower overall ANS cardiac control) showed a progressive increase in the probability of an MI. As a retrospective marker, however, it is not clear if lower CAR was a cause or consequence of MI. The former, however, was suggested by the fact that several participants in the longitudinal study had ANS measures taken before the occurrence of an MI. As in the retrospective cases, these subjects had lower CAR scores prior to the MI, indicating that low CAR may be a prospective predictor as well. Subsequent work has supported the view that patterns of CAR and CAB, beyond simply the individual metrics of sympathetic and parasympathetic control, may be predicting of metabolic and cardiovascular disease (Licht et al., Citation2010, Citation2013; Mitchell et al., Citation2017). These results suggest that distinct patterns of autonomic control may be associated with distinct health dimensions. A bipolar conception of autonomic control, however, admits theory and measurement only of CAB and would occlude the relationships between CAR and health. In contrast, the broader and more comprehensive bivariate model of autonomic control subsumes CAB as one diagonal (reciprocal diagonal of ) and captures CAR as the co-activity diagonal. Such findings illustrate the fact that theories are constrained by the measurement models used to describe the phenomena they attempt to explain, and specious or oversimplified theories may obscure lawful relationships.
The richness of central neural control of the stress response coupled and the reciprocal interactions among the central and peripheral systems are only now being fully appreciated. As described below, the nervous system is a potent modulator of immune function through both the neuroendocrine and the autonomic nervous system (Pavlov & Tracey, Citation2017). Appreciation of the integrated nature of autonomic, neuroendocrine, and immune systems continues to be biased by reflexive depictions of the ANS and HPA axis function. The heterarchical organization of the CNS allows for more rostral neuraxial systems to increase the complexity of neuroendocrine and autonomic regulation of immune parameters. There is significant inter-individual variability in autonomic and neuroendocrine response which may have important implications for health that would not be readily apparent when the systems are studied in isolation. Despite powerful homeostatic controls over many immune parameters, even regulated dimensions of immunity are not characterized by fixed, invariant levels. Rather, it is alterations in these dimensions (e.g., pro-inflammatory or anti-inflammatory profile, glucocorticoid sensitivity) that permit an adaptive, immune response to environmental perturbations and immune challenges. Higher-level behavioral systems may, in fact, powerfully modulate immune function and health status. Results of the CHASRS study, for example, suggest that social connectedness may be an important modulator of patterns of ANS control and health. Levels of social support and connectedness (e.g., UCLA, ISEL) appeared to moderate the low parasympathetic control (indexed by RSA) and associated low CAR in MI patients. MI subjects with low levels of social support displayed exaggerated decreases in RSA (and CAR). This may be relevant to health. This possibility was followed up in an animal model of cardiac arrest.
Social support and health
To investigate potential pathways between autonomic modes of control and cardiovascular health, and the role of social factors, a mouse model of cardiac arrest was pursued in collaboration with Courtney DeVries and Greg Norman at OSU (Norman et al., Citation2010). Cardiac arrest (CA) was induced (by potassium infusion), followed by a cardiopulmonary resuscitation (CPR) regimen (epinephrine, 100% O2, and chest compressions). Sympathetic and parasympathetic measures of cardiac control were obtained before and after the procedure. Two groups of animals were tested: one group was pair‐housed, and the other was socially isolated for a 2‐week period prior to CA/CPR. Similar to results from the human CHASRS study, CA resulted in a significant and sizable decrease in parasympathetic control and CAR. Also, as in the CHASRS study, social support (single vs. pair housing) was a potent moderator of outcome after CA. Mice who were isolated prior to CA showed a much larger decrease in autonomic control than pair-housed animals. They also showed a two‐fold increase in mortality from the CA procedure (Norman et al., Citation2010). Moreover, those who survived had a significantly higher level of parasympathetic control 24 hours after the CA procedure than those who ultimately died. Consistent with human studies, this suggests that MI outcome is positively correlated with the level of vagal cardiac control.
A possible mediating pathway may be related to parasympathetic modulation of inflammatory processes, through parasympathetic (cholinergic α7‐nicotinic) signaling mechanisms (Tracey, Citation2009). Neural injury from inflammatory reactions, following CA/CPR, results from a reduction in cerebrovascular blood flow and subsequent reperfusion injury. Proinflammatory cytokines increase dramatically following cerebral ischemia, resulting in the activation of the resident immune cells of the brain (microglia), and ultimately cell death. Animals undergoing CA/CPR showed evidence of neuroinflammation as evidenced by microglial activation and by Fluoro‐Jade staining of dying neurons. These inflammatory reactions were substantially increased in socially isolated animals. This may have been related to the greater reduction in parasympathetic control after CA/CPR in socially isolated animals, as suggested by the significant negative correlation between parasympathetic control and proinflammatory cytokines. There was also a significant negative correlation between parasympathetic control and the inflammatory markers of microglial activation and cell death. The causal role of reduced parasympathetic control is suggested by the fact that the effects of social isolation on inflammatory markers are significantly reduced by GTS21 (a specific α7‐nicotinic parasympathetic cholinergic receptor agonist), which increased the survival rate of socially isolated mice after CA/CPR was increased comparable to that of pair‐housed animals (Karelina et al., Citation2011).
Oxytocin (OT) represents one potential mechanism through which social status may modulate autonomic control and thus health. OT has been shown to restore the decrease in RSA associated with social isolation in voles (Grippo et al., Citation2009). Pair‐housing in mice increases OT gene expression, and OT reduces neuroinflammation in socially isolated mice following cerebral ischemia (Karelina et al., Citation2011). In follow‐up studies in humans, intranasal administration of oxytocin resulted in a significant increase in parasympathetic and sympathetic cardiac control, and thus CAR, without an effect on CAB (Norman, Cacioppo et al., Citation2011). The effects of OT on autonomic control, however, were moderated by social connectedness (UCLA). Increases in RSA and CAR were only seen in participants who were in the top half of the UCLA scores. Those in the lower half failed to show an RSA increase to oxytocin, while still showing a sympathetic increase. Consequently, oxytocin may be a positive health modulator (increased parasympathetic control and CAR) in socially connected individuals, but less so in the lonely (increased sympathetic activation and CAB). This may be one mechanism by which social status can impact health. This is further suggested by our finding in the CHASRS sample that variation in the oxytocin receptor gene influences neurocardiac reactivity to social stress and HPA function (Norman, Hawkley, Luhmann et al., Citation2012).
Current status of multilevel analysis
As a result of the development of fields such as social neuroscience, psychoneuroimmunology, and psychophysiology, the explanatory power of integrative multilevel analysis is evident when attempting to model complex phenomena that develop over disparate levels of neural organization. Multilevel analyses represent a subset of interdisciplinary approaches where the measures, constructs, and theories extend across levels of the organization. Efforts to integrate information across levels of analyses are especially challenging given the inherent complexity of biological and social systems, but it is only through such research that a complete understanding of mind-body issues will be possible.
Given the recent unprecedented growth in scientific understanding of biological processes, the breadth of scope is fitting. Remarkably, only 50 years after the elucidation of the double helix structure of DNA, scientists succeeded in decoding the 3 billion base pairs constituting the human genome. We now have a basic understanding of the molecular basis of inheritance and how the symptoms of certain diseases are caused by changes in the action of one or more proteins caused by mutations in the genes encoded by DNA. Such developments represented a significant milestone in what was considered by many to be the ultimate goal of biological research, namely the ability to explain biological processes in terms of physics and chemistry (Crick, Citation1966).
Similarly, advancements in psychology and neuroscience have begun to unlock the mysteries of the human mind. Imaging technology has made it increasingly possible to investigate the differential activation of particular brain regions in normal and disordered thought, capturing the imagination of the public and the scientific community. Additionally, the brain-machine interface has advanced to the state where surgically implanted microchips are capable of translating thought-generated brain activation into movement in external devices (see Rajangam et al., Citation2016). These and other developments represent a new era in the understanding of both the biological and psychological underpinning of human existence. However, similar to how the observations of wave-particular duality and quantum entanglement demonstrated the inadequacy of classical physics and engendered the quantum revolution, it has become increasingly apparent that conventional models in both biology and psychology are inadequate, or at least not comprehensive, in explaining the complexity of human behavior.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adamaszek, M., D’Agata, F., Ferrucci, R., Habas, C., Keulen, S., Kirkby, K. C., Leggio, M., Mariën, P., Molinari, M., Moulton, E., Orsi, L., Van Overwalle, F., Papadelis, C., Priori, A., Sacchetti, B., Schutter, D. J., Styliadis, C., & Verhoeven, J. (2017). Consensus paper: Cerebellum and emotion. Cerebellum, 16(2), 552–576. https://doi.org/10.1007/s12311-016-0815-8
- Berntson, G. G., & Cacioppo, J. T. (2004). Multilevel analyses and reductionism: Why social psychologists should care about neuroscience and vice versa. In J. T. Cacioppo & G. G. Berntson (Eds.), Essays in social neuroscience (pp. 107–120). MIT Press.
- Berntson, G. G., Gianaros, P. J., & Tsakiris, M. (2018). Interoception and the autonomic nervous system: Bottom-up meets top-down. In M. Tsakiris & H. De Preester (Eds.), Mind and Interoception (pp. 3-26). Oxford University Press.
- Berntson, G. G., Boysen, S. T., & Cacioppo, J. T. (1993). Neurobehavioral organization and the cardinal principle of evaluative bivalence. Annals of the New York Academy of Sciences, 702(1 Brain Mechani), 75–102. https://doi.org/10.1111/j.1749-6632.1993.tb17243.x
- Berntson, G. G., & Cacioppo, J. T. (2000). Psychobiology and social psychology: Past, present, and future. Personality and Social Psychology Review, 4(1), 3–15. https://doi.org/10.1207/S15327957PSPR0401_2
- Berntson, G. G., & Cacioppo, J. T. (2007). Integrative physiology: Homeostasis, allostasis and the orchestration of systemic physiology. In Cacioppo, J. T., TassELinary, L. G., & Berntson, G. G. (eds.), Handbook of psychophysiology (Vol. 3, pp. 433–452).
- Berntson, G. G., & Cacioppo, J. T. (Eds.). (2009). Handbook of neuroscience for the behavioral sciences. John Wiley & Sons.
- Berntson, G. G., Cacioppo, J. T., Binkley, P. F., Uchino, B. N., Quigley, K. S., & Fieldstone, A. (1994). Autonomic cardiac control: III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology, 31(6), 599–608. https://doi.org/10.1111/j.1469-8986.1994.tb02352.x
- Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint.. Psychological Review, 98(4), 459–487. https://doi.org/10.1037/0033-295X.98.4.459
- Berntson, G. G., & Micco, D. J. (1976). Organization of brainstem behavioral systems. Brain Research Bulletin, 1(5), 471–483. https://doi.org/10.1016/0361-9230(76)90117-9
- Berntson, G. G., Norman, G. J., Hawkley, L. C., & Cacioppo, J. T. (2008a). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. https://doi.org/10.1111/j.1469-8986.2008.00652.x
- Berntson, G. G., Sarter, M., & Cacioppo, J. T. (1998). Anxiety and cardiovascular reactivity: The basal forebrain cholinergic link. Behavioural Brain Research, 94(2), 225–248. https://doi.org/10.1016/S0166-4328(98)00041-2
- Berntson, G. G., Sarter, M., & Cacioppo, J. T. (2003). Ascending visceral regulation of cortical affective information processing. European Journal of Neuroscience, 18(8), 2103–2109. https://doi.org/10.1046/j.1460-9568.2003.02967.x
- Berntson, G. G., & Torello, M. W. (1981). The paleocerebellum and the integration of behavioral function. Physiological Psychology, 10(1), 2–12. https://doi.org/10.3758/BF03327003
- Berntson, G. G., Tuber, D. S., Ronca, A. E., & Bachman, D. S. (1983). The decerebrate human: Associative learning. Experimental Neurology, 81(1), 77–88. https://doi.org/10.1016/0014-4886(83)90158-9
- Cacioppo, J. T., & Berntson, G. G. (1992). Social psychological contributions to the decade of the brain: Doctrine of multilevel analysis. American Psychologist, 47(8), 1019–1028. https://doi.org/10.1037/0003-066X.47.8.1019
- Cacioppo, J. T., & Berntson, G. G. (1994). Relationship between attitudes and evaluative space: A critical review with emphasis on the separability of positive and negative substrates. Psychological Bulletin, 115(3), 401–423. https://doi.org/10.1037/0033-2909.115.3.401
- Cacioppo, J. T., & Berntson, G. G. (Eds.). (2004). Essays in social neuroscience. MIT Press.
- Cacioppo, J. T., & Berntson, G. G. (Eds.). (2005). Social neuroscience: Key readings. Psychology Press.
- Cacioppo, J. T., Berntson, G. G., & J. Decety. (2010).Social neuroscience and its relationship to social psychology. Social Cognition,28, 675–684. doi: 10.1521/soco.2010.28.6.675. PMID: 24409007
- Cacioppo, J. T., Berntson, G. G., Sheridan, J. F., & McClintock, M. K. (2000). Multilevel integrative analyses of human behavior: Social neuroscience and the complementing nature of social and biological approaches. Psychological Bulletin, 126(6), 829. https://doi.org/10.1037/0033-2909.126.6.829
- Cacioppo, J. T., Ernst, J. M., Burleson, M. H., McClintock, M. K., Malarkey, W. B., Hawkley, L. C., Kowalewski, R. B., Paulsen, A., Hobson, J. A., Hugdahl, K., Spiegel, D., & Berntson, G. G. (2000). Lonely traits and concomitant physiological processes: The MacArthur social neuroscience studies. International Journal of Psychophysiology, 35(2–3), 143–154. https://doi.org/10.1016/S0167-8760(99)00049-5
- Cacioppo, J. T., Hawkley, L. C., & Berntson, G. G. (2003). The anatomy of loneliness. Current Directions in Psychological Science, 12(3), 71–74. https://doi.org/10.1111/1467-8721.01232
- Cacioppo, J. T., Hawkley, L. C., Berntson, G. G., Ernst, J. M., Gibbs, A. C., Stickgold, R., & Hobson, J. A. (2002). Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychological Science, 13(4), 384–387. https://doi.org/10.1111/j.0956-7976.2002.00469.x
- Cacioppo, J. T., Hawkley, L. C., Crawford, L. E., Ernst, J. M., Burleson, M. H., Kowalewski, R. B., Malarkey, W. B., VanCauter, E., & Berntson, G. G. (2002). Loneliness and health: Potential mechanisms. Psychosomatic Medicine, 64(3), 407–417. https://doi.org/10.1097/00006842-200205000-00005
- Cacioppo, J. T., Hawkley, L. C., Norman, G. J., & Berntson, G. G. (2011). Social isolation. Annals of the New York Academy of Sciences, 1231(1), 17–22. https://doi.org/10.1111/j.1749-6632.2011.06028.x
- Cacioppo, J. T., Poehlmann, K. M., Kiecolt-Glaser, J. K., Malarkey, W. B., Burleson, M. H., Berntson, G. G., & Glaser, R. (1998). Cellular immune responses to acute stress in female caregivers of dementia patients and matched controls. Health Psychology, 17(2), 182–189. https://doi.org/10.1037/0278-6133.17.2.182
- Cacioppo, J. T., Tassinary, L. G., & Berntson, G. G. ( 2000, 2007, 2016). Handbook of Psychophysiology. Cambridge University Press.
- Carta, I., Chen, C. H., Schott, A. L., Dorizan, S., & Khodakhah, K. (2019). Cerebellar modulation of the reward circuitry and social behavior. Science, 363(6424), 248. https://doi.org/10.1126/science.aav0581
- Crick, F. H. (1966). Of molecules and man. University of Washington Press.
- Critchley, H. D., & Harrison, N. A. (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. https://doi.org/10.1016/j.neuron.2013.02.008
- Eisenberger, N. I., Moieni, M., Inagaki, T. K., Muscatell, K. A., & Irwin, M. R. (2017). In sickness and in health: The co-regulation of inflammation and social behavior. Neuropsychopharmacology, 42(1), 242. https://doi.org/10.1038/npp.2016.141
- Flourens, P. (1824). Recherches expérimentales sur les propriétés et les fonctions du système nerveux dans les animaux vertébrés. Crevot.
- Friedman, B. H. (2007) An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74, 185–99. doi:10.1016/j.biopsycho.2005.08.009. PMID: 17069959
- Grippo, A. J., Trahanas, D. M., Zimmerman, R. R. I. I., Porges, S. W., & Carter, C. S. (2009). Oxytocin protects against negative behavioral and autonomic consequences of long‐term social isolation. Psychoneuroendocrinology, 34(10), 1542–1553. https://doi.org/10.1016/j.psyneuen.2009.05.017
- Hawkley, L. C., Lavelle, L. A., Berntson, G. G., & Cacioppo, J. T. (2011). Mediators of the relationship between socioeconomic status and allostatic load in the Chicago Health, Aging, and Social Relations Study (CHASRS). Psychophysiology, 48(8), 1134–1145. https://doi.org/10.1111/j.1469-8986.2011.01185.x
- Holt-Lunstad, J., Robles, T. F., & Sbarra, D. A. (2017). Advancing social connection as a public health priority in the United States. American Psychologist, 72(6), 517–530. https://doi.org/10.1037/amp0000103
- House, J. S., Landis, K. R., & Umberson, D. (1988). Social relationships and health. Science, 241(4865), 540–545. https://doi.org/10.1126/science.3399889
- Jackson, J. H. (1884/1958). Evolution and dissolution of the nervous system. In J. Taylor (Ed.), Selected writings of John Hughlings Jackson (pp. 3–92). Basic Books.
- Karelina, K., Stuller, K. A., Jarrett, B., Zhang, N., Wells, J., Norman, G. J., & DeVries, A. C. (2011). Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke, 42(12), 3606–3611. https://doi.org/10.1161/STROKEAHA.111.628008
- Licht, C. M. M., de Geus, E. J. C., & Penninx, B. W. J. H. (2013). Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. Journal of Clinical Endocrinology & Metabolism, 98(6), 2484–2493. https://doi.org/10.1210/jc.2012–3104
- Licht, C. M. M., Vreeburg, S. A., Dortland, A. K. B. V., Giltay, E. J., & Penninx, B. W. J. H. (2010). Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. Journal of Clinical Endocrinology & Metabolism, 95(5), 2458–2466. https://doi.org/10.1210/jc.2009-2801
- Mitchell, J. C., Paulson, J., Cannarozzi, M., Neer, S. M., & Cassisi, J. E. (2017). Maladaptive cardiac autonomic control during a stress reactivity assessment among primary care patients with metabolic syndrome. Applied Psychophysiology and Biofeedback, 42(2), 97–105. https://doi.org/10.1007/s10484-017-9355-3
- Norman, G. J., Berntson, G. G., & Cacioppo, J. T. (2014). Emotion, somatovisceral afference, and autonomic regulation. Emotion Review, 6(2), 113–123. https://doi.org/10.1177/1754073913512006
- Norman, G. J., Cacioppo, J. T., Morris, J. S., Malarkey, W. B., Berntson, G. G., & DeVries, A. C. (2011). Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biological Psychology, 86(3), 174–180. https://doi.org/10.1016/j.biopsycho.2010.11.006
- Norman, G. J., Hawkley, L., Luhmann, M., Ball, A. B., Cole, S. W., Berntson, G. G., & Cacioppo, J. T. (2012). Variation in the oxytocin receptor gene influences neurocardiac reactivity to social stress and HPA function: A population based study. Hormones and Behavior, 61(1), 134–139. https://doi.org/10.1016/j.yhbeh.2011.11.006
- Norman, G. J., Hawkley, L. C., Cole, S. W., Berntson, G. G., & Cacioppo, J. T. (2012). Social neuroscience: The social brain, oxytocin, and health. Social Neuroscience, 7(1), 18–29. https://doi.org/10.1080/17470919.2011.568702
- Norman, G. J., Norris, C. J., Gollan, J., Ito, T. A., Hawkley, L. C., Larsen, J. T., & Berntson, G. G. (2011). Current emotion research in psychophysiology: The neurobiology of evaluative bivalence. Emotion Review, 3(3), 349–359. https://doi.org/10.1177/1754073911402403
- Norman, G. J., Zhang, N., Karelina, K., Morris, J. S., Berntson, G. G., & DeVries, A. C.(2010). Social interaction modulates autonomic, inflammatory and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proceedings of the National Academy of Sciences, 107, 16342–16347. doi:10.1073/pnas.1007583107. PMID: 20805484
- Pavlov, V. A., & Tracey, K. J. (2017). Neural regulation of immunity: Molecular mechanisms and clinical translation. Nature Neuroscience, 20(2), 156. https://doi.org/10.1038/nn.4477
- Petty, R. E., & Cacioppo, J. T. (1986). The elaboration likelihood model of persuasion. Advances in Experimental Social Psychology, 19, 123–205. doi:10.1016/S0065-2601(08)60214-2
- Rajangam, S., Tseng, P. H., Yin, A., Lehew, G., Schwarz, D., Lebedev, M. A., & Nicolelis, M. A. (2016). Wireless cortical brain-machine interface for whole-body navigation in primates. Scientific Reports, 6(1), 22170. https://doi.org/10.1038/srep22170
- Ronca, A. E., Berntson, G. G., & Tuber, D. A. (1985). Cardiac orienting and habituation to auditory and vibrotactile stimuli in the infant decerebrate rat. Developmental Psychobiology, 18(6), 79–83. doi:10.1002/dev.420180610.PMID: 4092841
- Thayer, J. F., & Sternberg, E. (2006). Beyond heart rate variability: Vagal regulation of allostatic systems. Annals of the New York Academy of Sciences, 1088(1), 361–372. https://doi.org/10.1196/annals.1366.014
- Tracey, K. J. (2009). Reflex control of immunity. Nature Reviews Immunology, 9(6), 418–428. https://doi.org/10.1038/nri2566
- Tsakiris, M., & De Preester, H. (Eds.). (2018). Mind and interoception. Oxford University Press.
- Tuber, D. S., Berntson, G. G., Bachman, D. S., & Allen, J. N. (1980). Associative learning in premature hydranencephalic and normal twins. Science, 210(4473), 1035–1037. https://doi.org/10.1126/science.7192015
- Van Overwalle, F., D’aes, T., & Mariën, P. (2015). Social cognition and the cerebellum: A meta-analytic connectivity analysis. Human Brain Mapping, 36(12), 5137–5154. https://doi.org/10.1002/hbm.23002
