 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Parent and child have been shown to synchronize their behaviors and physiology during social interactions. This synchrony is an important marker of their relationship quality and subsequently the child’s social and emotional development. Therefore, understanding the factors that influence parent–child synchrony is an important undertaking. Using EEG hyperscanning, this study investigated brain-to-brain synchrony in mother-child dyads when they took turns performing a visual search task and received positive or negative feedback. In addition to the effect of feedback valence, we studied how their assigned role, i.e., observing or performing the task, influenced synchrony. Results revealed that mother-child synchrony was higher during positive feedback relative to negative feedback in delta and gamma frequency bands. Furthermore, a main effect was found for role in the alpha band with higher synchrony when a child observed their mother performing the task compared to when the mother observed their child. These findings reveal that a positive social context could lead a mother and child to synchronize more on a neural level, which could subsequently improve the quality of their relationship. This study provides insight into mechanisms that underlie mother-child brain-to-brain synchrony, and establishes a framework by which the impact of emotion and task demand on a dyad’s synchrony can be investigated
Introduction
Extensive developmental research points toward the vital role of parents in the development of their child’s social, cognitive, and affective skills (Davis et al., Citation2018; Feldman, Citation2012). From early years on, children observe their parents to learn how to behave through imitation (Jones, Citation2009). This can result in interpersonal coordination where each individual’s behavior synchronizes unconsciously and spontaneously (Louwerse et al., Citation2012). Synchrony, the extent to which parent and child coordinate their behaviors and physiology during social interactions (Davis et al., Citation2018; Leclère et al., Citation2014), could be a reliable indicator of the quality of a parent–child relationship (Czeszumski et al., Citation2020).
Advances in neuroimaging techniques such as electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS) have enabled hyperscanning; a technique that allows simultaneous recording of brain activity in dyads, which has increased our understanding of the neural mechanism underlying parent–child synchrony (Nguyen et al., Citation2020; Turk et al., Citation2022). Using fNIRS hyperscanning for instance, parent–child inter-brain synchrony has been studied in a variety of tasks such as engaging in conversations (Nguyen et al., Citation2021), solving puzzles (Nguyen et al., Citation2020), and cooperative or competitive games (Reindl et al., Citation2018). Through these different tasks, multiple factors modulating parent–child synchrony have been recognized. For instance, higher brain-to-brain synchrony has been found to relate to more turn-taking during conversations (Nguyen et al., Citation2021), less maternal stress (Azhari et al., Citation2019; Nguyen et al., Citation2020), mutual gaze (Leong et al., Citation2017), and higher social connectedness (Czeszumski et al., Citation2020; Kinreich et al., Citation2017).
Another important factor that could play a role in parent–child synchrony but has received less attention is emotional processing (Czeszumski et al., Citation2020). An fNIRS hyperscanning study found that parent–child synchrony in the frontal brain regions mediates the relationship between a parent’s and child’s emotion regulation (Reindl et al., Citation2018). Such parent–child biobehavioral synchrony could be the mechanism by which children learn to regulate their emotions during social interactions (Feldman, Citation2015), which is an important skill for their social growth (Feldman, Citation2003). While other physiological measures (e.g., heart rate, skin temperature) have shown parent–child synchrony as an indicator of shared emotional experiences (Birk et al., Citation2022; Davis et al., Citation2018), hyperscanning studies have scarcely focused on the role of emotions on neural synchrony during parent–child tasks (Czeszumski et al., Citation2020). In a review by Czeszumski et al. (Citation2020), it was suggested that the level of a dyad’s synchrony is modulated by the closeness of their relationship (e.g., romantic couples) and the emotional intensity of their interaction (e.g., scenarios involving active emotional components), however, they also emphasized that more research is required to elucidate the effect of emotional valence (negative or positive) on neural synchrony.
When investigating parent–child brain synchrony, past studies have mostly employed a task that involves some form of social interaction or behavioral coordination; for instance the parent and child talking to each other, gazing at one another, or acting together (Leong et al., Citation2017; Nguyen et al., Citation2020). These actions are all known to increase mother-child synchrony (Burgess, Citation2013; Y. Hu et al., Citation2018), making it difficult to isolate the role of affect in the study. Other studies used a paradigm that elicited directional inter-brain networking. For instance, Santamaria et al. (Citation2020) measured EEG synchrony when mothers acted out positive and negative emotions while their infant observed them. They found higher mother-child synchrony in the alpha band when mothers expressed positive emotions compared to negative emotions. However, since the emotions were only posed by the mothers in this study, the results cannot be interpreted in the context of mutual emotional processing.
To determine whether and how emotional valence plays a role in mother-child brain synchrony, it is crucial to select a task that eliminates the impact of bio-behavioral synchrony while activating emotional processes in both mother and child. A possible solution is to employ a joint attention task with positive or negative feedback in which mother and child direct their gaze toward the same stimuli but interact as little and natural as possible (Azhari et al., Citation2019; Szymanski et al., Citation2017). Sharing attention toward a common stimulus can result in a shared awareness of events (Czeszumski et al., Citation2020), which in turn informs mother and child of each other’s responses (Nguyen et al., Citation2020). Previous fNIRS studies show that compared to no-feedback conditions, providing positive or negative feedback on tasks induces higher synchrony in stranger dyads (Balconi & Vanutelli, Citation2017; Lu et al., Citation2019). In parent–child dyads, the role of external feedback is not considered enough, while it is important to a child’s early development and can be influenced by parental presence (Kawamoto & Hiraki, Citation2019).
Using a joint attention task furthermore offers the unique opportunity to study synchrony when dyads engage in different behaviors (Santamaria et al., Citation2020). Indeed, studies in which one participant acts and the other observes, have found an influence of the individual’s role on the dyad’s EEG synchrony (Dumas et al., Citation2010; Ménoret et al., Citation2014). Such asymmetric patterns of synchrony could be explained by the different expectations that emerge with the role each individual takes on during the interaction (Liu et al., Citation2018). To date, the effect of role-taking and task demands on inter-brain synchrony has not been explored in mother–child interactions. This is particularly important to address as children learn to regulate their emotions through observation of their parents, and parents in turn guide their children through specific behaviors and responses (Morris et al., Citation2007). Therefore, just as it has been examined in stranger dyads (Dumas et al., Citation2010; Ménoret et al., Citation2014), differential role-taking in mother-child synchrony presents an important avenue for further exploration.
Considering the above-mentioned gaps in the literature, this study aimed to explore the effect of emotional valence and role-taking on parent–child synchrony in a hyperscanning framework when the dyad engaged in a visual search task. We employed an action-observation paradigm with minimal social interaction and without any explicit instructions to the subjects to engage in similar behaviors. That is, in one session the mother conducted the task (i.e., locate a target animal on a computer screen) while the child observed, and in the following session the roles were reversed. To introduce an emotional context, positive or negative feedback on their performance was provided. Since presenting feedback on a joint task alone (regardless of its valence) could impact a dyad’s synchrony, we also considered the task timeline (before vs. after feedback presentation) as a factor in our research. Taken together, this study examines whether brain-to-brain synchrony in mother-child dyads (1) changes in response to feedback presentation, (2) is modulated by the emotional valence of feedback, and (3) depends on the role a mother and child take during a joint task. Based on our literature review, we hypothesized that mothers and children would show higher neural synchrony after feedback presentation (Hypothesis 1), their synchrony would be higher after positive feedback relative to negative feedback (Hypothesis 2), and that the mother-child synchrony would be higher when a mother observes their child versus when the child observes their mother, regardless of the emotional context (Hypothesis 3).
Methods
Participants
A total of 38 participants, 19 children (Mage = 5.3, SD = 0.6) with their mothers (Mage = 40.2, SD = 1.1), took part in this study. After the experiment, one mother-child dyad was excluded due to missing sessions. Two more dyads were excluded due to excessive artifacts and bad channels. Thus, a total of 16 mothers and their children remained in the analysis. Mothers confirmed that their child could understand the experiment and instructions. Consent was given after informing mother and child about the study and the dyads received a monetary reward for their participation. The experiment was approved by the Ethics Committee of the University of Tokyo.
Experimental procedure
The procedure was adapted from Kawamoto and Hiraki (Citation2019). The mother and child were seated next to each other in front of a display screen () to perform a child-friendly search task while their EEG brain activity was recorded from 65 electrode sites (). There were two role-taking conditions: either the child performed the task, while the mother observed (Mother Observes); or the child observed their mother performing the task (Child Observes). The timeline of the task in one trial is depicted in . First, participants saw a picture of an animal at the top of the screen with the text “Where is it?” in Japanese (1500 ms). Then, five different animals appeared below the text on the right and another five on the left. Participants had to press a left or right button on the response pad before them (Cedrus, RB-844) to indicate on which side of the screen the target animal was located. They were verbally instructed that the goal of the game was to find as many animals as possible within the given time limit and, because animals are quick at running away, they must press the button as soon as they located the target animal. After their response, a blank screen was shown (1000–1500 ms), followed by positive (O) or negative (X) feedback (1500 ms). The time limit to respond was automatically adjusted depending on the performance in previous trials so that participants could only answer (approximately) half of the trials correctly. This was done so that participants received an equal amount of positive and negative feedback, balancing the dataset for further analysis. After an inter-trial interval of 2000 ms, the next trial started. These trials were referred to as GO trials.
Figure 1. Experimental setting. A) the mother and child sat next to each other in front of a screen and took turns to conduct the visual search task while their brain activity was recorded in a hyperscanning framework. B) EEG signals were collected from 65 electrodes according to the EGI sensor net layout. C) the visual search task started with a question “[animal image] where is it” on top of the screen followed by two groups of options displayed below it on the right or left side of the screen. Participants had to press a left or right button to indicate their response. This was followed by positive (O) or negative (X) feedback.
![Figure 1. Experimental setting. A) the mother and child sat next to each other in front of a screen and took turns to conduct the visual search task while their brain activity was recorded in a hyperscanning framework. B) EEG signals were collected from 65 electrodes according to the EGI sensor net layout. C) the visual search task started with a question “[animal image] where is it” on top of the screen followed by two groups of options displayed below it on the right or left side of the screen. Participants had to press a left or right button to indicate their response. This was followed by positive (O) or negative (X) feedback.](/cms/asset/9b44cd3e-e83c-4e8e-bfb6-0e23766234e0/psns_a_2228545_f0001_c.jpg)
In addition to the GO trials, there were also NO-GO trials where the target animal was not presented on either side and therefore participants should not have pressed any key. NO-GO trials were included to enhance attentional requirements of the task and prevent premature responses, and thus were not included in the analysis. In each condition, there were a total of 80 GO trials (around 40 with positive feedback and 40 with negative feedback) and 20 NO-GO trials. To reduce fatigue effects, especially for the young children, each condition was split into two separate sessions. Each session lasted six to eight minutes and contained 40 GO trials and 10 NO-GO trials. Thus, there were a total of four sessions, alternating between the child performing the task while the mother observed and vice versa. For instance, the child performed the task in session one and three, while the mother performed the task in session two and four. This order was counterbalanced for the dyads to avoid order effects.
EEG recording and processing
EEG was recorded simultaneously from the scalp of mother and child using two HydroCel Geodesic Sensor Nets and Net Amps amplifiers (Electrical Geodesics Inc, USA). Signals were recorded for each session at a sampling rate of 1000 Hz over 65 channels referenced to Cz (). The data was preprocessed in MATLAB (The MathWorks Inc, US) using the EEGLAB toolbox (Delorme & Makeig, Citation2004).
In this study, we examined synchrony in five frequency bands; delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and low gamma (30–45 Hz). First, a band-pass filter of 1–45 Hz was applied to EEG signals to remove baseline drifts and improve the signal-to-noise ratio. Bad channels were removed automatically in EEGLAB using the Clean Raw Data plugin. Channels were removed when they were poorly correlated with nearby channels (less than 0.7) or when they had a large amount of noise (more than four standard deviations relative to the signal). No automatic or manual rejection of data was performed. Instead, bad bursts of data were corrected using Artifact Subspace Reconstruction (Kothe & Makeig, Citation2013) when the segment exceeded 20 times the standard deviation of cleaner data segments. The value of 20 has been shown to be optimal to balance the amount of removed noise and retained brain signals (Chang et al., Citation2019). Sessions in which either the mother or child had more than 16 channels (25%) removed, were excluded from further analysis, leading to the exclusion of two mother-child dyads from the study as indicated in section 2.1. On average 4 to 5 channels were removed. Removed channels were then spherically interpolated (Perrin et al., Citation1989). This step ensures there is no bias of certain brain areas during re-referencing, e.g., when there are more left hemisphere channels.
Next, the data was re-referenced to the average of all channels for two reasons: first, this reconstructed the signal in channel Cz, which was a channel of interest, and second, to avoid the effect of a common reference like Cz which can lead to spurious synchrony values, known as the common source problem (Bastos & Schoffelen, Citation2016; S. Hu et al., Citation2010; Zhang et al., Citation2020). Finally, Independent Component Analysis (ICA) was performed to identify noisy components. Components were automatically rejected using ICLabel (Pion-Tonachini et al., Citation2019) if they were classified as being eye or muscle artifacts with more than 0.9 probability. On average, 1.8 components were rejected for the child and 3.3 for the mother.
Synchrony measurement
To measure brain-to-brain synchrony, metrics from intra-brain connectivity have been adjusted to inter-brain connectivity. Commonly used in EEG analysis are functional connectivity measures such as the phase-locking value (PLV; Dumas et al., Citation2010) and the phase-lag index (PLI; Czeszumski et al., Citation2020). While these phase synchrony measures are each calculated differently, their results have been shown to be similar in hyperscanning studies (Aydore et al., Citation2013). In this study, the PLV metric was used, which measures how phase-locked two EEG signals are across specific frequencies in a specific time window (Lachaux et al., Citation1999). In this case, the two EEG signals came from the same electrode location on mother and child. Intuitively, the PLV measures how much the timing of the dyad’s brain activity varies across each trial, being calculated as follows:
where is the number of time-frequency points,
is Euler’s number,
is the complex operator,
is the trial, and
is the phase angle of channel
(from mother’s brain) and channel
(from child’s brain) at time point
. The PLV value obtained from EquationEquation 1
(1)
(1) ranges from 0 to 1, with 0 indicating the two channels are unsynchronized, while 1 indicates complete synchrony.
As the PLV measure only uses a signal’s phase and ignores amplitude, it is much clearer to understand the contribution of the signal’s timing to any coupling of brain activity. The PLV is robust to time lags and non-stationarities, and can detect even small effects in comparison to other measures such as the PLI (Cohen, Citation2015). While the PLV is sensitive to volume conduction artifacts, this is mainly an issue in connectivity analysis between channels within one person’s brain. In hyperscanning, this is no issue as the two different brains each have their separate source (Czeszumski et al., Citation2020).
As we were interested in synchrony across the whole brain, we calculated the average PLV across all 65 channels. This resulted in a “global” synchrony value (global PLV), which was the dependent variable in this study. PLV values were calculated per frequency band. To obtain phase angles in each frequency band, the signal was first band-pass filtered in the frequency range of interest, e.g., 1 to 4 Hz for delta band. As the extracted phase angle at each time point belongs to the frequency with the highest amplitude, phases are best interpretable for narrowband signals. Also, it is recommended that each epoch contains at least three cycles of the lowest frequency examined (Sun et al., Citation2012). Thus, with 1 Hz as the lowest frequency in this study, the suitable epoch duration for phase synchrony analysis was decided to be 3 s long.
While inter-brain PLV is interpretable on its own and can be directly compared across conditions without a baseline (Vijver & Cohen, Citation2019), it was important to consider the potential synchrony between mother and child that could have emerged throughout the session due to joint task-solving. Therefore, global synchrony before feedback onset, that is during the search task, was compared to global synchrony after feedback onset to ensure that the measured effect was truly feedback related and not a byproduct of the naturally arising synchrony in the dyad. Thus, each trial was split into two epochs: i) the pre-feedback search task which ranged from 3000 ms to 200 ms before feedback onset and ii) post-feedback epochs starting from feedback onset to 3000 ms afterward. While the search task segment varied due to the reaction times (), the pre-feedback epochs always contained the search task itself. Furthermore, the cutoff was set to 200 ms before feedback onset to avoid temporal smearing of feedback onset.
Although epoching is often done during the pre-processing stage, we opted to do it after band-pass filtering, as filtering epochs introduces edge effects. Phase angles were extracted by applying a Hilbert transform to the bandpass-filtered epochs. These phase angles were then used to calculate a PLV value for each epoch between the same EEG channel of mother and child. To statistically validate each epoch’s synchrony against synchrony caused by background fluctuations or random noise, it was compared to a series of 200 surrogate PLVs as described by Lachaux et al. (Citation1999). This procedure identified on average 4.42% of PLV values as non-significant, subsequently excluding them in the calculation of a global synchrony value. The excluded amounts were similar across levels of each factor and thus no cause for concern.
In sum, for each dyad, we obtained eight sets of global PLV values based on (1) the role they played during the task (MO: Mother Observes vs. CO: Child Observes), (2) whether they received the feedback or not during the trial (pre- vs. post-feedback) and (3) the valence of the feedback they received (positive vs. negative). The PLV values in each set were then averaged to obtain one global PLV value per participant for each of the eight conditions (i.e., MO-pre-positive, MO-post-positive, MO-pre-negative, MO-post-negative, CO-pre-positive, CO-post-positive, CO-pre-negative, and CO-post-negative). This process was performed separately for each of the five frequency bands.
Statistical analysis
All statistical tests were performed in SPSS 27 (IBM, United States). To answer our research questions, we had to test for significant differences in global synchrony for three factors (1) Feedback presentation: how global synchrony differed in pre- versus post-feedback epochs, (2) Feedback valence: how global synchrony differed between trials with positive and negative feedback, and (3) Role during the task: how global synchrony differed when the mother was observing the child versus when the child was observing their mother. Furthermore, how these factors interacted was also of interest. As we had three within-subjects factors, with two levels each, a repeated measures ANOVA was chosen for statistical comparison of global synchrony per frequency band. However, phase-related information such as the PLV is circular and uniformly distributed instead of normally distributed, which is an assumption required to run the parametric ANOVA. Therefore, the global PLV values were first Fisher-Z transformed (the inverse hyperbolic tangent) to approximate a normal distribution (Zhang et al., Citation2020).
In all bands except gamma, the Fisher-Z transform succeeded in approximating a normal distribution, as indicated by Shapiro – Wilk tests. Therefore, in all bands except gamma, the Fisher-Z transformed global synchrony was statistically tested for differences using separate three-way repeated measures ANOVAs with Feedback presentation (Pre- versus Post-feedback), Feedback valence (Positive versus Negative), and Role (Mother Observes versus Child Observes) as within-subjects factors. In the gamma band, however, non-parametric Wilcoxon signed-rank tests were run on the non-transformed global PLV to test for significant main effects of Feedback presentation, Feedback valence, and Role.
Significant interactions were further examined for simple effects using two- or one-way repeated measures ANOVA for each level of the interacting factors. There was one exception where a two-way repeated measures ANOVA was directly applied to examine the impact of Role and Feedback valence on global synchrony: since logically we can only compare positive to negative feedback after the feedback was presented and received by the dyad, only post-feedback epochs were considered to examine the effects of Feedback valence. Consequently, to reduce the risk of false positives as much as possible, the conservative Bonferroni correction was applied to adjust the p-values for multiple comparisons of simple effects. As each factor had two levels, we always performed two follow-up simple contrasts. Thus, simple effects were significant with p-values less than 0.025. Finally, as none of the factors had more than two levels, the assumption of sphericity was always met. Henceforth, the results from these ANOVA tests are presented by factor, i.e., Feedback presentation, Feedback valence and Role.
Results
Effects of feedback presentation on mother-child synchrony
illustrates the global synchrony in pre- and post-feedback epochs for each frequency band. Three-way repeated measures ANOVAs revealed a significant main effect of Feedback presentation on global synchrony in all bands, that is, delta (F(1, 15) = 116.48, p < .001, ηp2 = .89), theta (F(1, 15) = 25.49, p < .001, ηp2 = .63), alpha (F(1, 15) = 100.62, p < .001, ηp2 = .87), and beta (F(1, 15) = 47.55, p < .001, ηp2 = .76) bands. The Wilcoxon signed-rank test also showed a significant main effect of Feedback presentation on global synchrony in the gamma band (Z = −3.36, p < .001). Global synchrony was on average higher in pre-feedback than post-feedback epochs in all bands (). In the delta band, the main effect of Feedback presentation was qualified by a significant interaction between Feedback presentation and Feedback valence (F(1, 15) = 5.61, p = .032, ηp2 = .27). This interaction was not significant in the theta (F(1, 15) = 1.47, p = .244, ηp2 = .09), alpha (F(1, 15) = .06, p = .818, ηp2 < .01), and beta (F(1, 15) = .49, p = .495, ηp2 = .03) bands.
Figure 2. Global synchrony in mother-child dyads obtained in pre- and post-feedback epochs per frequency band. Means are indicated by white circles and * denotes significant differences (p < .05).
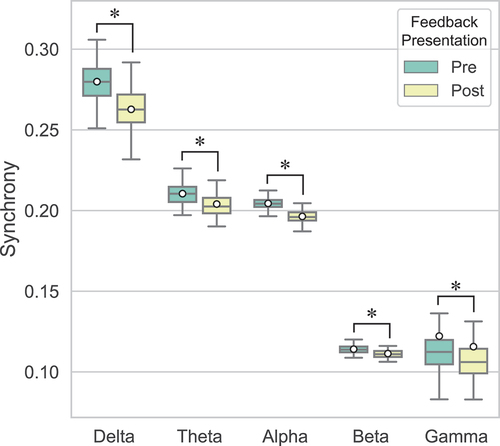
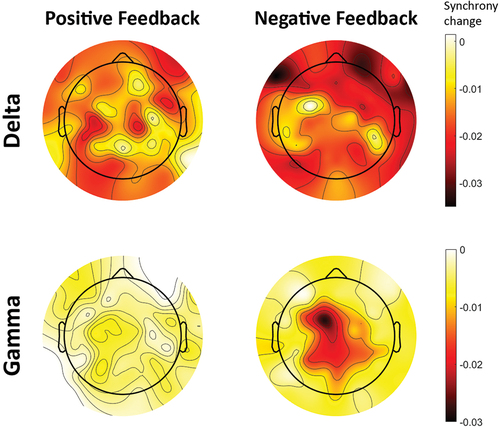
To further investigate this interaction in the delta band, a one-way ANOVA with Feedback presentation as within-subjects factor was performed on the global synchrony in Positive and Negative feedback trials separately. In the delta band, the simple main effect of Feedback presentation was significant for both Positive (F(1, 15) = 73.51, p < .001, ηp2 = .83) and Negative feedback (F(1, 15) = 84.30, p < .001, ηp2 = .85). There was higher synchrony pre-feedback than post-feedback for both Positive (M = .280, SD = .011 vs M = .266, SD = .010) and Negative feedback (M = .279, SD = .011 vs M = .260, SD = .013). illustrates the change in synchrony from pre- to post-feedback (ΔPLV = PLVpost − PLVpre) for both types of feedback in the delta and gamma bands. Across the brain, synchrony decreased for both Positive and Negative feedback after feedback presentation.
Figure 3. Change in dyads’ inter-brain synchrony across the brain from pre- to post-feedback epochs (ΔPLV = PLVpost − PLVpre). Each topographic map illustrates the region-wise change in mother-child synchrony after presentation of feedback. The plots are obtained separately for Positive and Negative feedback trials, and in the delta and gamma frequency bands. Lower values (darker color) indicate a larger reduction of synchrony between mother and child after presentation of feedback.
Finally, the three-way ANOVA indicated that Feedback presentation did not significantly interact with Role in any of the frequency bands, including delta (F(1, 15) = .71, p = .412, ηp2 = .05), theta (F(1, 15) = .80, p = .385, ηp2 = .05), alpha (F(1, 15) = 3.23, p = .093, ηp2 = .18), and beta (F(1, 15) = .09, p = .771, ηp2 = .01) bands. This suggests that the synchrony difference of pre- and post-feedback was similar for the two conditions regardless of the mother and child’s role during the task.
Effects of feedback valence on mother-child synchrony
To investigate the impact of feedback valence on mother-child synchrony, a two-way ANOVA with Role and Feedback valence was conducted on global synchrony of post-feedback epochs. Results showed a significant main effect of Feedback valence on global synchrony in only the delta band (F(1, 15) = 19.42, p < .001, ηp2 = .56). In this band, the dyad’s global synchrony was significantly higher for Positive feedback (M = .266, SD = .010) than Negative feedback (M = .260, SD = .013) (). In contrast, Feedback valence had no significant main effect on global synchrony in the theta (F(1, 15) = 2.24, p = .155, ηp2 = .13), alpha (F(1, 15) < .01, p = .970, ηp2 < .01), and beta (F(1, 15) = .24, p = .630, ηp2 = .02) bands. In these bands, global synchrony was not significantly different between Positive and Negative feedback when averaged across Role (). However, in the gamma band, Wilcoxon signed-rank tests revealed a significant effect of Feedback valence on global synchrony (Z = −2.59, p = .010). In this band, the dyad’s global synchrony was higher for Positive feedback (M = .118, SD = .047) than Negative feedback (M = .113, SD = .035) (). Furthermore, as illustrated in , synchrony across the brain decreased more after presentation of Negative feedback than Positive feedback, in both the delta and gamma bands.
Figure 4. Global synchrony in mother-child dyads after presentation of Positive and Negative feedback in each frequency band. Means are indicated by white circles and * denotes significant differences (p < .05).
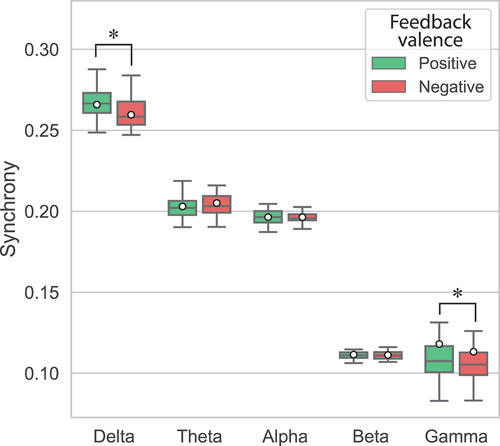
Finally, Feedback valence did not interact significantly with Role in any of the frequency bands during post-feedback epochs [delta: F(1, 15) = .57, p = .461, ηp2 = .04; theta: F(1, 15) = 3.53, p = .080, ηp2 = .19; alpha: F(1, 15) = 1.15, p = .301, ηp2 = .07; beta: F(1, 15) = 1.16, p = .299, ηp2 = .07]. Therefore, after feedback was presented, the effect of Feedback valence did not differ significantly whether a mother observed their child or vice versa.
Effects of role-taking on mother-child synchrony
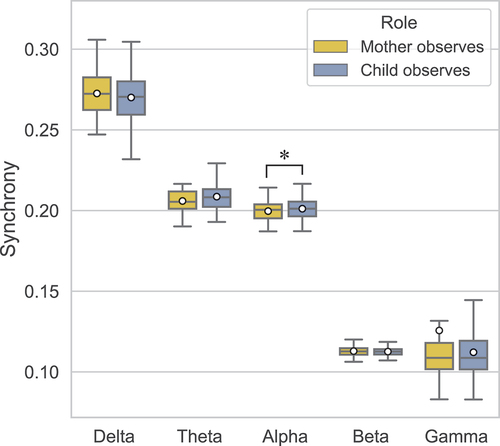
A three-way ANOVA showed that the main effect of Role on global synchrony was only significant in the alpha band (F(1, 15) = 4.62, p = .048, ηp2 = .24). The global synchrony in this band was significantly higher in the Child Observes condition (M = .201, SD = .003) than Mother Observes condition (M = .200, SD = .004) (). There was no significant effect of Role in the delta (F(1, 15) = 1.37, p = .259, ηp2 = .08), theta (F(1, 15) = 3.71, p = .073, ηp2 = .20), beta (F(1, 15) = .51, p = .488, ηp2 = .03), and gamma (Z = −.36, p = .717) bands. Therefore, the global synchrony in these bands was not significantly different whether a mother observed their child or vice versa ().
Figure 5. Global synchrony in mother-child dyads obtained during the Mother Observes and Child Observes conditions in each frequency band. Means are indicated by white circles and * denotes significant differences (p < .05).
None of the two-way interactions of Role with Feedback presentation or Feedback valence were significant in any of the bands, as mentioned in their respective sections above. This suggests that the global synchrony difference between the Role conditions was similar pre- and post-feedback. Similarly, the simple effect of Role did not differ significantly for Positive versus Negative feedback after presenting the feedback. Thus, global synchrony during the two conditions did not depend on Feedback presentation nor Feedback valence.
Lastly, the three-way interaction between Feedback presentation, Feedback valence, and Role on global synchrony was not significant in any bands (delta: F(1, 15) = .06, p = .806, ηp2 < .01; theta: F(1, 15) = 4.52, p = .050, ηp2 = .52; alpha: F(1, 15) = .81, p = .383, ηp2 = .05; beta: F(1, 15) = .42, p = .528, ηp2 = .03).
Discussion
The aim of this study was to investigate the factors that play a role in the brain-to-brain synchronization of mother-child dyads. Using the hyperscanning method, we studied EEG inter-brain synchronization between a mother and her child while they performed a visual search task and received feedback. In addition to feedback presentation (pre vs. post-feedback), we studied the impact of feedback valence (positive vs. negative) and each person’s role during the task (mother observed their child performing the task vs. child observed their mother performing the task). The results showed that in both role-taking conditions, brain-to-brain synchrony between the mother and child decreased in all frequency bands after receiving feedback regardless of its valence. However, this reduction was lower for the trials with positive feedback in the delta and gamma bands, revealing significantly higher mother-child synchrony in these bands following positive feedback as compared to negative feedback. Additionally, we found significantly higher mother-child synchrony in the alpha band when the child observed their mother conducting the task compared to when the role was reversed.
Against our expectations, mother-child synchrony was lower after presenting feedback than for the task-solving interval (pre-feedback). This was observed in all frequency bands, and also for both types of feedback (negative and positive) separately in the delta and gamma bands (). This observation lends support to the notion that feedback itself, regardless of its valence, has an effect on mother-child synchrony, albeit it is a diminishing effect relative to task-solving. This is inconsistent with previous work that found providing feedback leads to higher synchrony than no feedback intervals (Balconi & Vanutelli, Citation2017; Lu et al., Citation2019).
Multiple explanations can be provided for this contrasting result; first, these previous studies employed fNIRS (Balconi & Vanutelli, Citation2017; Lu et al., Citation2019), which measures brain activity indirectly through hemodynamic activity, while EEG measures brain activity directly through electrophysiological activity. The coupling of these two types of activity might differ during feedback processing. Second, in our study, the pre-feedback epochs contained a joint attention task where both mother and child attended the visual search but only one provided responses (the performer). As dyads synchronize more when cooperating on tasks (Liu et al., Citation2018; Sinha et al., Citation2016), the reduced synchrony after feedback presentation suggests that feedback processing may not require the same collaborative attitude that task solving does. Since the feedback reflected the performance of only one person, i.e., the performer, the observer might have disengaged from the task, either due to actual disinterest or to avoid a potentially negative outcome outside of their control (Andreatta et al., Citation2017; Eppinger & Kray, Citation2011). In this study, negative feedback avoidance is a more likely explanation than temporary disengagement when we consider the results on feedback valence.
Namely, avoidance of negative feedback might be evident from the finding that mother-child synchrony was higher during positive feedback compared to negative feedback trials. This effect of feedback valence is in line with previous hyperscanning studies that found mother-child inter-brain synchrony during a positive social context (Kinreich et al., Citation2017), and when the dyad attended positive stimuli in the form of audio (Azhari et al., Citation2020) or video (Levy et al., Citation2017). In our study, this effect was confirmed for the slowest and fastest brain oscillations, namely delta and gamma band. Gamma band activity has been previously associated with emotional processing in both individual (Headley & Paré, Citation2013; Luo et al., Citation2009; Yang et al., Citation2020) and hyperscanning studies (Deng et al., Citation2022; Kinreich et al., Citation2017; Levy et al., Citation2017; Mu et al., Citation2017; Zhu et al., Citation2018). For instance, Deng et al. (Citation2022) reported higher gamma band inter-brain synchrony in adolescent-parent dyads when they viewed positive pictures together relative to negative pictures. Mu et al. (Citation2017) also associated gamma-band synchrony with higher social coordination during threatening situations and interpreted this synchrony in terms of shared emotions between individuals under threat. The larger gamma synchrony after positive feedback in our study is consistent with these reports and could indicate that mother and child jointly process emotions on a neural level even in the absence of social interaction.
In addition to the gamma band, this study found an effect of feedback valence on delta oscillations, which are involved in motivational processes and increase in response to rewards (Knyazev, Citation2007, Citation2012). Synchrony in lower frequency bands has also been reported in tasks with a cooperative nature. Szymanski et al. (Citation2017) compared inter-brain synchronization in a joint-attention task (cooperative condition) to an individual attention task and observed increased inter-brain synchrony in delta and alpha bands. They argued that this increase in neural phase synchrony was due to general heightening of attention in social settings and thus reflected cognitive processes underlying social facilitation. Taken together, these observations support the notion that the emotional valence of feedback modulates inter-brain synchrony and in turn a dyad’s shared attention and social facilitation.
In the alpha band, there was an influence of role on mother-child synchrony, suggesting that mother and child synchronize to a different extent depending on whether the mother or the child is the task performer. This influence of role is consistent with previous studies that employed an actor-observer or leader-follower paradigm (Dumas et al., Citation2010; Ménoret et al., Citation2014). However, contrary to our hypothesis, there was higher synchrony when the child observed their mother performing the task, instead of when the mother observed. These results can be explained by the choice of methods in this study. First, this study did not employ a directional measure of synchrony and therefore it remains unknown whether the higher synchrony when the child observed was due to the information transfer initiated by the child or their mother. Directional measures such as the partial directed coherence (Leong et al., Citation2017; Li et al., Citation2021) can be employed in future research to elucidate the causal links between the pair particularly when they take up different roles in the task. Indeed, a recent study investigating adult-child cooperative puzzle-solving where the child and adult each took turns to move the pieces of the puzzle (Li et al., Citation2021) found elevated inter-brain connectivity in the alpha and theta bands that displayed directed flow patterns; when the child was watching the adult playing, the inter-brain connections emerged in Adult→Child directionality and vice versa when the adult was watching.
Second, the effect of role in our study was only found in the alpha band, which is known to facilitate selective attention to environmental stimuli by inhibiting distracting stimuli (Foxe & Snyder, Citation2011; Knyazev, Citation2007). In hyperscanning research, alpha-band synchrony is believed to reflect a dyad’s social coordination across different paradigms (Ahn et al., Citation2018; Astolfi et al., Citation2011; Dumas et al., Citation2010; Mu et al., Citation2018), including joint attention tasks (Liu et al., Citation2018; Szymanski et al., Citation2017). This suggests that perhaps mother and child attended the task more similarly when children observed their mother than when mothers observed. It is difficult to determine why the child might have paid as much attention as their mother when observing the task but a possible reason could be that the child tried to learn from their mother’s responses (Czeszumski et al., Citation2020; Morris et al., Citation2007; Nguyen et al., Citation2020). Future studies can incorporate behavioral measures of visual attention such as eye-tracking to provide answers to these questions.
The current study is limited in several ways. First, we did not control for the child’s gender and only examined mothers. Both the gender of the parent and the child have been shown to influence their synchrony in fNIRS hyperscanning (Nguyen et al., Citation2020; Reindl et al., Citation2018), which might also apply to EEG hyperscanning. Furthermore, same-gender dyads synchronize differently compared to when the gender of parent and child differs (Feldman, Citation2003; Miller et al., Citation2019). Second, the sample only consisted of Japanese mothers and children, thus the findings are difficult to generalize to other cultures. Extensive research validates cultural differences in the perception and expression of emotions (Matsumoto, Citation1992). Most relevant to this study, Japanese tend to display positive emotions less than North Americans (Safdar et al., Citation2009). Therefore, we recommend replications of this study in other countries to compare the findings across cultures. To our knowledge, there has been no study yet on cross-cultural differences in brain synchrony of dyads, let alone parent–child dyads. Finally, brain coupling alone may not be sufficient to determine mechanisms behind mother-child synchrony. It is difficult to ascertain how the participants responded to the feedback visibly e.g., smiling when receiving positive feedback. Keeping track of such behaviors through video recordings and coding scales could improve future studies by relating synchronous behaviors to synchronous brain activity (Endevelt-Shapira et al., Citation2021; Kinreich et al., Citation2017; Norton et al., Citation2022). Further recommendations for future research to determine mechanisms behind mother-child synchrony are to examine specific brain regions and use directional synchrony measures such as partial directed coherence.
To conclude, this study used mutual performance of a visual search task to demonstrate the influence of role and feedback on mother-child synchrony. By measuring the phase synchrony between mother and child’s EEG activity across the brain, we have shown that the emotional valence of feedback modulated mother-child synchrony. Corroborating previous research, mother-child dyads synchronized more in response to positive feedback, confirming that a positive social context enhances inter-brain synchrony. In addition, the influence of role suggests that dyads synchronize differently depending on their assigned role and task demands during activities. These observations are the first step toward enhancing our understanding of feedback and role in parent–child relationships. Furthermore, inter-brain synchrony can prove to be a useful measure of a mother and child’s relationship quality, reflecting mechanisms by which they learn to co-regulate their emotions. In practice, promoting positive social environments wherever caregiver and child interact may be beneficial to a child’s development. Caution must be taken though, as synchronous emotional regulatory mechanisms are not always beneficial to a child’s development, for instance when they observe their mother respond to feedback maladaptively (Azhari et al., Citation2020). These observations might extend to other environments where dyads display a similar relation to caregiver and child, such as in education.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahn, S., Cho, H., Kwon, M., Kim, K., Kwon, H., Kim, B. S., & Jun, S. C. (2018). Interbrain phase synchronization during turn‐taking verbal interaction—A hyperscanning study using simultaneous EEG/MEG. Human Brain Mapping, 39(1), 171–188. https://doi.org/10.1002/hbm.23834
- Andreatta, M., Michelmann, S., Pauli, P., & Hewig, J. (2017). Learning processes underlying avoidance of negative outcomes. Psychophysiology, 54(4), 578–590. https://doi.org/10.1111/psyp.12822
- Astolfi, L., Toppi, J., Borghini, G., Vecchiato, G., Isabella, R., Fallani, F. D. V., & Babiloni, F. (2011). Study of the functional hyperconnectivity between couples of pilots during flight simulation: An EEG hyperscanning study. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 2338–2341.
- Aydore, S., Pantazis, D., & Leahy, R. M. (2013). A note on the phase locking value and its properties. Neuroimage, 74, 231–244. https://doi.org/10.1016/j.neuroimage.2013.02.008
- Azhari, A., Leck, W. Q., Gabrieli, G., Bizzego, A., Rigo, P., Setoh, P., Bornstein, M. H., & Esposito, G. (2019). Parenting stress undermines mother-child brain-to-brain synchrony: A hyperscanning study. Scientific Reports, 9(1), 1–9. https://doi.org/10.1038/s41598-019-47810-4
- Azhari, A., Lim, M., Bizzego, A., Gabrieli, G., Bornstein, M. H., & Esposito, G. (2020). Physical presence of spouse enhances brain-to-brain synchrony in co-parenting couples. Scientific Reports, 10(1), 1–11. https://doi.org/10.1038/s41598-020-63596-2
- Balconi, M., & Vanutelli, M. E. (2017). Interbrains cooperation: Hyperscanning and self-perception in joint actions. Journal of Clinical and Experimental Neuropsychology, 39(6), 607–620. https://doi.org/10.1080/13803395.2016.1253666
- Bastos, A. M., & Schoffelen, J. M. (2016). A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Frontiers in Systems Neuroscience, 9, 175. https://doi.org/10.3389/fnsys.2015.00175
- Birk, S. L., Stewart, L., & Olino, T. M. (2022). Parent–child synchrony after early childhood: A systematic review. Clinical Child and Family Psychology Review, 25(3), 529–551. https://doi.org/10.1007/s10567-022-00383-7
- Burgess, A. P. (2013). On the interpretation of synchronization in EEG hyperscanning studies: A cautionary note. Frontiers in Human Neuroscience, 7, 881. https://doi.org/10.3389/fnhum.2013.00881
- Chang, C. Y., Hsu, S. H., Pion-Tonachini, L., & Jung, T. P. (2019). Evaluation of artifact subspace reconstruction for automatic artifact components removal in multi-channel EEG recordings. IEEE Transactions on Biomedical Engineering, 67(4), 1114–1121. https://doi.org/10.1109/TBME.2019.2930186
- Cohen, M. X. (2015). Effects of time lag and frequency matching on phase-based connectivity. Journal of Neuroscience Methods, 250, 137–146. https://doi.org/10.1016/j.jneumeth.2014.09.005
- Czeszumski, A., Eustergerling, S., Lang, A., Menrath, D., Gerstenberger, M., Schuberth, S., Schreiber, F., Rendon, Z. Z., & König, P. (2020). Hyperscanning: A valid method to study neural inter-brain underpinnings of social interaction. Frontiers in Human Neuroscience, 14, 39. https://doi.org/10.3389/fnhum.2020.00039
- Davis, M., West, K., Bilms, J., Morelen, D., & Suveg, C. (2018). A systematic review of parent–child synchrony: It is more than skin deep. Developmental Psychobiology, 60(6), 674–691. https://doi.org/10.1002/dev.21743
- Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
- Deng, X., Chen, X., Zhang, L., Gao, Q., Li, X., & An, S. (2022). Adolescent social anxiety undermines adolescent-parent interbrain synchrony during emotional processing: A hyperscanning study. International Journal of Clinical and Health Psychology, 22(3), 100329. https://doi.org/10.1016/j.ijchp.2022.100329
- Dumas, G., Nadel, J., Soussignan, R., Martinerie, J., Garnero, L., & Lauwereyns, J. (2010). Inter-brain synchronization during social interaction. PloS One, 5(8), 12166. https://doi.org/10.1371/journal.pone.0012166
- Endevelt-Shapira, Y., Djalovski, A., Dumas, G., & Feldman, R. (2021). Maternal chemosignals enhance infant-adult brain-to-brain synchrony. Science Advances, 7(50), 6867. https://doi.org/10.1126/sciadv.abg6867
- Eppinger, B., & Kray, J. (2011). To choose or to avoid: Age differences in learning from positive and negative feedback. Journal of Cognitive Neuroscience, 23(1), 41–52. https://doi.org/10.1162/jocn.2009.21364
- Feldman, R. (2003). Infant–mother and infant–father synchrony: The coregulation of positive arousal. Infant Mental Health Journal: Official Publication of the World Association for Infant Mental Health, 24(1), 1–23. https://doi.org/10.1002/imhj.10041
- Feldman, R. (2012). Parent–infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development, 77(2), 42–51. https://doi.org/10.1111/j.1540-5834.2011.00660.x
- Feldman, R. (2015). The adaptive human parental brain: Implications for children’s social development. Trends in Neurosciences, 38(6), 387–399. https://doi.org/10.1016/j.tins.2015.04.004
- Foxe, J. J., & Snyder, A. C. (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Frontiers in Psychology, 2, 2. https://doi.org/10.3389/fpsyg.2011.00154
- Headley, D. B., & Paré, D. (2013). In sync: Gamma oscillations and emotional memory. Frontiers in Behavioral Neuroscience, 7, 170. https://doi.org/10.3389/fnbeh.2013.00170
- Hu, Y., Pan, Y., Shi, X., Cai, Q., Li, X., & Cheng, X. (2018). Inter-brain synchrony and cooperation context in interactive decision making. Biological Psychology, 133, 54–62. https://doi.org/10.1016/j.biopsycho.2017.12.005
- Hu, S., Stead, M., Dai, Q., & Worrell, G. A. (2010). On the recording reference contribution to EEG correlation, phase synchrony, and coherence. IEEE Transactions on Systems, Man, and Cybernetics, 40(5), 1294–1304. https://doi.org/10.1109/TSMCB.2009.2037237
- Jones, S. S. (2009). The development of imitation in infancy. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1528), 2325–2335. https://doi.org/10.1098/rstb.2009.0045
- Kawamoto, T., & Hiraki, K. (2019). Parental presence with encouragement alters feedback processing in preschoolers: An ERP study. Social Neuroscience, 14(4), 499–504. https://doi.org/10.1080/17470919.2018.1527250
- Kinreich, S., Djalovski, A., Kraus, L., Louzoun, Y., & Feldman, R. (2017). Brain-to-brain synchrony during naturalistic social interactions. Scientific Reports, 7(1), 1–12. https://doi.org/10.1038/s41598-017-17339-5
- Knyazev, G. G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience & Biobehavioral Reviews, 31(3), 377–395. https://doi.org/10.1016/j.neubiorev.2006.10.004
- Knyazev, G. G. (2012). EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neuroscience & Biobehavioral Reviews, 36(1), 677–695. https://doi.org/10.1016/j.neubiorev.2011.10.002
- Kothe, C. A., & Makeig, S. (2013). BCILAB: A platform for brain–computer interface development. Journal of Neural Engineering, 10(5), 056014. https://doi.org/10.1088/1741-2560/10/5/056014
- Lachaux, J. P., Rodriguez, E., Martinerie, J., & Varela, F. J. (1999). Measuring phase synchrony in brain signals. Human Brain Mapping, 8(4), 194–208. https://doi.org/10.1002/SICI1097-019319998:4<194:AID-HBM4>3.0.CO;2-C
- Leclère, C., Viaux, S., Avril, M., Achard, C., Chetouani, M., Missonnier, S., Cohen, D., & Dekel, S. (2014). Why synchrony matters during mother-child interactions: A systematic review. PloS One, 9(12), 113571. https://doi.org/10.1371/journal.pone.0113571
- Leong, V., Byrne, E., Clackson, K., Georgieva, S., Lam, S., & Wass, S. (2017). Speaker gaze increases information coupling between infant and adult brains. Proceedings of the National Academy of Sciences, 114(50), 13290–13295. https://doi.org/10.1073/pnas.1702493114
- Levy, J., Goldstein, A., & Feldman, R. (2017). Perception of social synchrony induces mother–child gamma coupling in the social brain. Social Cognitive and Affective Neuroscience, 12(7), 1036–1046. https://doi.org/10.1093/scan/nsx032
- Liu, D., Liu, S., Liu, X., Zhang, C., Li, A., Jin, C., Chen, Y., Wang, H., & Zhang, X. (2018). Interactive brain activity: Review and progress on EEG-based hyperscanning in social interactions. Frontiers in Psychology, 9, 1862. https://doi.org/10.3389/fpsyg.2018.01862
- Li, Y., Wu, S., Shi, W., Tong, S., Zhang, Y., & Guo, X. (2021). Enhanced inter-brain connectivity between children and adults during cooperation: A dual EEG study. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), 6289–6292.
- Louwerse, M. M., Dale, R., Bard, E. G., & Jeuniaux, P. (2012). Behavior matching in multimodal communication is synchronized. Cognitive Science, 36(8), 1404–1426. https://doi.org/10.1111/j.1551-6709.2012.01269.x
- Luo, Q., Mitchell, D., Cheng, X., Mondillo, K., Mccaffrey, D., Holroyd, T., Carver, F., Coppola, R., & Blair, J. (2009). Visual awareness, emotion, and gamma band synchronization. Cerebral Cortex, 19(8), 1896–1904. https://doi.org/10.1093/cercor/bhn216
- Lu, K., Qiao, X., & Hao, N. (2019). Praising or keeping silent on partner’s ideas: Leading brainstorming in particular ways. Neuropsychologia, 124, 19–30. https://doi.org/10.1016/j.neuropsychologia.2019.01.004
- Matsumoto, D. (1992). American-Japanese cultural differences in the recognition of universal facial expressions. Journal of Cross-Cultural Psychology, 23(1), 72–84. https://doi.org/10.1177/0022022192231005
- Ménoret, M., Varnet, L., Fargier, R., Cheylus, A., Curie, A., Portes, V., Nazir, T. A., & Paulignan, Y. (2014). Neural correlates of non-verbal social interactions: A dual-EEG study. Neuropsychologia, 55, 85–97. https://doi.org/10.1016/j.neuropsychologia.2013.10.001
- Miller, J. G., Vrtička, P., Cui, X., Shrestha, S., Hosseini, S. H., Baker, J. M., & Reiss, A. L. (2019). Inter-brain synchrony in mother-child dyads during cooperation: An fNIRS hyperscanning study. Neuropsychologia, 124, 117–124. https://doi.org/10.1016/j.neuropsychologia.2018.12.021
- Morris, A. S., Silk, J. S., Steinberg, L., Myers, S. S., & Robinson, L. R. (2007). The role of the family context in the development of emotion regulation. Social Development, 16(2), 361–388. https://doi.org/10.1111/j.1467-9507.2007.00389.x
- Mu, Y., Cerritos, C., & Khan, F. (2018). Neural mechanisms underlying interpersonal coordination: A review of hyperscanning research. Social and Personality Psychology Compass, 12(11), 12421. https://doi.org/10.1111/spc3.12421
- Mu, Y., Han, S., & Gelfand, M. J. (2017). The role of gamma interbrain synchrony in social coordination when humans face territorial threats. Social Cognitive and Affective Neuroscience, 12(10), 1614–1623. https://doi.org/10.1093/scan/nsx093
- Nguyen, T., Schleihauf, H., Kayhan, E., Matthes, D., Vrtička, P., & Hoehl, S. (2020). The effects of interaction quality on neural synchrony during mother-child problem solving. Cortex, 124, 235–249. https://doi.org/10.1016/j.cortex.2019.11.020
- Nguyen, T., Schleihauf, H., Kayhan, E., Matthes, D., Vrtička, P., & Hoehl, S. (2021). Neural synchrony in mother–child conversation: Exploring the role of conversation patterns. Social Cognitive and Affective Neuroscience, 16(1–2), 93–102. https://doi.org/10.1093/scan/nsaa079
- Norton, E. S., Manning, B. L., Harriott, E. M., Nikolaeva, J. I., Nyabingi, O. S., Fredian, K. M., & Wakschlag, L. S. (2022). Social EEG: A novel neurodevelopmental approach to studying brain‐behavior links and brain‐to‐brain synchrony during naturalistic toddler–parent interactions. Developmental Psychobiology, 64(3), 22240. https://doi.org/10.1002/dev.22240
- Perrin, F., Pernier, J., Bertrand, O., & Echallier, J. F. (1989). Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology, 72(2), 184–187. https://doi.org/10.1016/0013-46948990180-6
- Pion-Tonachini, L., Kreutz Delgado, K., & Makeig, S. (2019). Iclabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage, 198, 181–197. https://doi.org/10.1016/j.neuroimage.2019.05.026
- Reindl, V., Gerloff, C., Scharke, W., & Konrad, K. (2018). Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by Fnirs-based hyperscanning. NeuroImage, 178, 493–502. https://doi.org/10.1016/j.neuroimage.2018.05.060
- Safdar, S., Friedlmeier, W., Matsumoto, D., Yoo, S. H., Kwantes, C. T., Kakai, H., & Shigemasu, E. (2009). Variations of emotional display rules within and across cultures: A comparison between Canada, USA, and Japan. Canadian Journal of Behavioural Science/Revue Canadienne des Sciences du Comportement, 41(1), 1. https://doi.org/10.1037/a0014387
- Santamaria, L., Noreika, V., Georgieva, S., Clackson, K., Wass, S., & Leong, V. (2020). Emotional valence modulates the topology of the parent-infant inter-brain network. NeuroImage, 207, 116341. https://doi.org/10.1016/j.neuroimage.2019.116341
- Sinha, N., Maszczyk, T., Wanxuan, Z., Tan, J., & Dauwels, J. (2016). EEG hyperscanning study of inter-brain synchrony during cooperative and competitive interaction. 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Budapest, Hungary, 004813–004818.
- Sun, J., Hong, X., & Tong, S. (2012). Phase synchronization analysis of EEG signals: An evaluation based on surrogate tests. IEEE Transactions on Biomedical Engineering, 59(8), 2254–2263. https://doi.org/10.1109/TBME.2012.2199490
- Szymanski, C., Pesquita, A., Brennan, A. A., Perdikis, D., Enns, J. T., Brick, T. R., Müller, V., & Lindenberger, U. (2017). Teams on the same wavelength perform better: Inter-brain phase synchronization constitutes a neural substrate for social facilitation. Neuroimage, 152, 425–436. https://doi.org/10.1016/j.neuroimage.2017.03.013
- Turk, E., Vroomen, J., Fonken, Y., Levy, J., & Heuvel, M. I. (2022). In sync with your child: The potential of parent–child electroencephalography in developmental research. Developmental Psychobiology, 64(3), 22221. https://doi.org/10.1002/dev.22221
- Vijver, I., & Cohen, M. X. (2019). Electrophysiological phase synchrony in distributed brain networks as a promising tool in the study of cognition. In New methods in cognitive psychology (pp. 214–244). Routledge.
- Yang, K., Tong, L., Shu, J., Zhuang, N., Yan, B., & Zeng, Y. (2020). High gamma band EEG closely related to emotion: Evidence from functional network. Frontiers in Human Neuroscience, 14, 89. https://doi.org/10.3389/fnhum.2020.00089
- Zhang, L., Li, Z., Zhang, F., Gu, R., Peng, W., & Hu, L. (2020). Demystifying signal processing techniques to extract task-related EEG responses for psychologists. Brain Science Advances, 6(3), 171–188. https://doi.org/10.26599/BSA.2020.9050018
- Zhu, L., Lotte, F., Cui, G., Li, J., Zhou, C., & Cichocki, A. (2018). Neural mechanisms of social emotion perception: An EEG hyper-scanning study. 2018 International Conference on Cyberworlds (CW), Singapore, 199–206.
