Edited by Dr Sandro Barni, Director of Oncology Department, Medical Oncology Unit, ASST Bergamo Ovest Treviglio (BG). The present supplement has been prepared in collaboration with Dr. Germano Tarantino, Scientific Director, Pharmanutra SpA.
Anemia is a very frequent symptom associated with a high number of diseases as the most frequent laboratoristic sign, complicating them as a side effect.
In onco-hematology, anemia plays a pivotal role in altering the quality of life, since it is one of the principal components of fatigue, and in delaying or interfering with oncologic treatments, worsening clinical course and prognosis.
This year, we have pointed out our attention on the use of preemptive iron to avoid futile transfusions, on the use of iron in all internal medicine branches disease (e.g. gastroenterology), and on the approach to iron supplementations in hard-to-treat forms of anemia such as anemia due to molecular agents used in oncology, and functional anemia due to chronic disease, best depicted by cancer-related anemia.
Sucrosomial® Iron represents a relatively new but still unique preparation of ferric pyrophosphate conveyed through a phospholipid and sucrose esters of fatty acids matrix, that appears useful in all that conditions associated with chronic inflammation or iron deficiency in, and not only, onco-hematology diseases. Gastroenterology and nephrology specialists, for example, can now benefit from this new formulation, and onco-hematologists can safely replace older iron tablets, usually associated with bothersome gastrointestinal adverse events, with Sucrosomial® Iron, with similar efficacy of IV iron, potentially associated with acute side effects and ambulatory rooms accesses.
Some experts in internal medicine, hematology, and oncology subspecialties have discussed important setting where Sucrosomial® Iron could be widespread used. A preclinical model of pharmacokinetic and liver toxicity of Sucrosomial® Iron was presented by Italian scientists. Celiac disease, a chronic condition with reduced iron absorption, and bariatric surgery, a branch of abdominal surgery specialties, represent new platforms where Sucrosomial® Iron could be implemented. Inflammatory bowel diseases are further chronic conditions where anemia of inflammation is the rule. Guillermo Bastida showed us the role of Sucrosomial® Iron in patients intolerant to other oral iron formulation. Ideally, this means that all branches of internal medicine could be the arena for this new oral iron formulation for the de novo user or for those shifting from other types of iron salts.
The metabolism of iron in cancer patients was well depicted by Paolo Pedrazzoli, who described iron parameters in oncology, a condition where increased iron storage and reduced iron saturation determine the so-called functional anemia. A new frontier of iron anemia in cancer patients is due to the use of molecular agents, targeting specific pathways and critical for tumor growth. Some of these targets (e.g. cKIT, flt-3, and mTOR) can influence erythropoiesis in cancer patients leading to frequent cases of low-moderate grades of anemia during treatment. The management of such type of anemia is controversial because erythropoiesis-stimulating agents are not labeled for this indication. Fausto Petrelli has presented new literature data reviewing the frequency of anemia with targeted therapy in more than 90 clinical trials. The experience with Sucrosomial® Iron in preexisting low-grade anemia before the start of chemotherapy is surprising and intriguing. Will it be the way we will manage these cases of anemia with new oncologic drugs? Finally, Giulio Giordano presented a randomized study comparing high dose of Sucrosomial® Iron to IV iron in hematologic patients. Last, but not least, Garcia Erce explained how a transfusion-sparing protocol could be implemented in our countries from a transfusional service point of view. Saving blood means saving lives, costs, and toxicity of allogeneic blood transfusions.
The magisterial lecture, held by Mercè Cladellas, has underlined the negative effects of anemia in cardiology and cardiac surgery and how the cardiology field could benefit from an effective iron supplementation in terms of prognosis, outcome, and quality of life. Due to the emerging importance of a correct iron supplementation in cardiac patient, this aspect should be studied further and included in the next Iron Anemia Course.
The new frontiers of treating anemia in internal medicine deserve today an appropriate international audience, well performed in this 4th Mediterranean Multidisciplinary Course on Iron Anemia held in Madrid on 29th and 30th April 2016, of which we report official congressional acts. The 4th Mediterranean Multidisciplinary Course on Iron Anemia represented a significant opportunity to share different opinions and to convey various clinical experiences, mainly about the recent evidences on Sucrosomial® Iron in treating iron deficiency anemia.
At the end of the course, I hope, we have added some more pieces of information to the knowledge learned from the last Anemia Course held in Rome in 2015. New lessons learned today in Madrid are still of paramount importance. We can now, likely, appreciate a new product, such as Sucrosomial® Iron, for managing hard-to-treat cases of chronically ill patients who require a treatment for anemia and that would mean treating their fatigue, anorexia, mood depression, malaise, which are all signs of an impaired quality of life. We have learned some new insights about some causes of anemia and, in particular, we would like to thank Antonello Pietrangelo for the magisterial lecture he offered in the auditorium. Finally, I hope this meeting will teach us that taking care of (preemptive) management of anemia with iron products will reduce the chance for our patients to receive futile transfusions, potentially dangerous for most subjects, so expensive in terms of resource consuming, cost, and side effects.
Declaration of interest
S Barni and G Tarantino have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
ABSTRACTS
In vitro and ex vivo models to study Sucrosomial® Iron pharmacokinetics
Ylenia Zambito
Department of Pharmacy, University of Pisa, Pisa, Italy
Introduction
Iron deficiency is one of the most widespread nutritional deficiencies. Oral therapy for iron deficiency is mainly based on immediate-release formulations of ferrous iron. To overcome gastrointestinal epithelial barriers, there is a need for new absorption enhancers. Recent data indicate that sucrose esters can enhance drug permeability through both transcellular and paracellular routes. Several in vitro and ex vivo systems have been proposed to assess iron bioavailability. In order to understand Sucrosomial® Iron absorption mechanism, we have performed absorption study using Caco-2 cell model and a permeation study using ex vivo model.
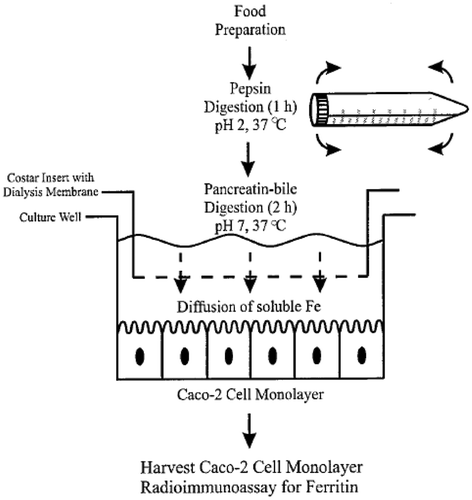
In fact, an experimental protocol that conjugates the simulation techniques of the gastrointestinal digestion with the iron internalizing phase by the Caco-2 cells has been set up [Citation1]. Such a protocol appears very useful and predictive of the in vivo behavior so far, as it takes into account the phase of Fe3+ dissolution in the gastrointestine and the subsequent permeation across the epithelium. Indeed, it cannot be taken for granted that the latter phase is slower than the former and hence that it is the rate-determining one. It is known that at pH values higher than 3, the Fe3+ ion tends to form Fe(OH)3, the water solubility of which is practically null. In fact, the Fe3+ bioavailability is very poor just because of its poor solubility at the physiologic pH of the intestine. For this reason, the organism has developed an efficient transport system [Citation2]. The first barrier iron encounters is the apical membrane of the duodenal enterocyte that is a specialized absorbent cell of the intestinal epithelium involved in the iron transport. Iron is initially solubilized through reduction of Fe3+ to Fe2+. This is then carried to the cell interior by a transport process mediated by the carrier DMT1. Subsequently, iron is transferred to the basolateral side of the enterocyte, where it may either be stored via binding to ferritin or cross the membrane and reach the systemic circulation. Quite hypothetically another type of Fe3+ transport across the intestinal epithelium could involve the cellular endocytosis of a Fe3+ carrier, e.g. nano- or microparticulate. From here, the importance is understood of the pharmaceutical formulation which should directly carry the ferric ion in the intestine and promote its absorption. The formulation should not release the Fe3+ before reaching the general circulation that is the site of action. Therefore, to evaluate the ability of the carrier to transport Fe3+ across the epithelium, an ex vivo model based on excised rat intestine could be more predictive. The present work compares the data obtained by the two models, namely that based on the Caco-2 cells monolayer and that based on the excised rat intestine, to understand the mechanism of the permeation of iron and to compare the bioavailability of the innovative oral iron formulation based on Sucrosomial® Iron (SiderAL®) with different iron formulations.
Methods
To determine iron uptake by Caco-2 cell monolayer, a previously reported method was used [Citation1]. Tested samples are listed in .
Table 1. Samples tested.
Each sample contained a different quantity of iron; therefore, in order to make homogeneous comparison between formulations, in each experiment an equivalent dose of 200 µg of iron was used. The experimental protocol used is shown in . Briefly, Caco-2 cells were seeded at a density of 105 cells/well in 12-well plates, maintained in cell culture medium and used in the iron uptake experiments at 14 days post-seeding. Immediately before the intestinal digestion period, the growth medium was removed and a sterilized insert ring was inserted creating the two-chamber system. A 1.5-mL aliquot of the intestinal digest was pipetted into the upper chamber and the insert ring and digest were removed. After 24-h incubation, cells were harvested for analysis. Each treatment was performed in duplicate for each replication of the experiment. Caco-2 cell ferritin content was measured through an enzyme-linked immunosorbent assay (ELISA) kit (USCN Life Science, USA) following the manufacturer’s instructions.
For permeation studies, the intestinal mucosa was excised from non-fasting male Wistar rats weighing 250–300 g. Rats were killed and the first 20 cm of jejunum was immediately removed. The excised intestine was cut into strips of 1.5 cm, rinsed free of luminal contents, and mounted in Ussing-type chambers (0.78 cm2 exposed surface area) without stripping off the underlying muscle layer. One milliliter of phosphate buffer pH 6.8, 0.13 M, made isotonic by the addition of sodium chloride (PBS 6.8), was added to the apical side and 3 mL of a phosphate buffer solution pH 7.4, 0.13 M, isotonic (PB 7.4), was added to the basolateral side (acceptor medium). In order to ensure oxygenation and agitation, a mixture of 95% O2 and 5% CO2 was bubbled through each compartment. The Ussing chambers were then placed in a water bath at 37°C. After a 20-min equilibration period, the medium in each apical compartment was replaced by 1 mL of pre-thermostated sample dispersion, (corresponding to 200 µg of iron). The apical to basolateral transport of Fe3+ was investigated. At 30-min intervals for a total of 240 min, 1 mL sample was withdrawn from the acceptor compartment and substituted with an equal amount of fresh pre-thermostated medium. The permeated Fe3+ amount was determined by a previously described method [Citation3].
Results and discussion
Data on iron uptake by the Caco-2 cell monolayer are shown in , where it can be seen that Caco-2 cell monolayers treated with SRM were able to significantly increase ferritin expression about 7.4 times than those treated with ferrous sulfate (FS) ().
Figure 2. Caco-2 cell ferritin formation as measured 24 h after the start of the intestinal digestion period. *p<0.001, as compared to FS (one-way ANOVA followed by Dunnett’s multiple comparison test).
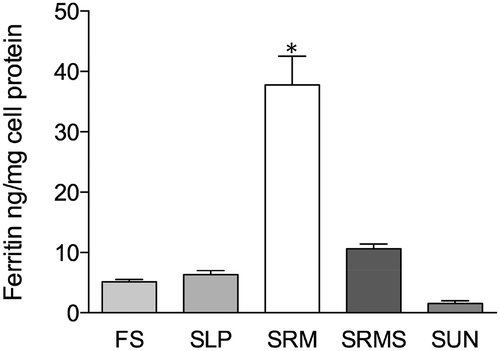
Among various ex vivo intestinal epithelium models, the excised rat jejunum model was chosen for the permeability studies because its tight junctions are similar, in tightness and number, to those of the human jejunum. The sample SRM showed a higher effectiveness in enhancing Fe3+ permeability across the excised rat intestine than samples SLP, SRMS, and SUN.
Conclusions
The data described in this work shows that Sucrosomial® Iron formulation promotes Sucrosomial® Iron absorption across the intestinal epithelium.
References
- Glahn RP, Lee OA, Yeung A, et al. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J Nutr. 1998;128:1555–1561.
- Srai SK, Bomford A, McArdle HJ. Iron transport across cell membranes: molecular understanding of duodenal and placental iron uptake. Best Prac Res Clin Haematol. 2002;15:243–259.
- Shyla B, Bhaskar CV, Nagendrappa G. Iron(III) oxidized nucleophilic coupling of catechol with o-tolidine/p-toluidine followed by 1,10-phenanthroline as new and sensitivity improved spectrophotometric methods for iron present in chemicals, pharmaceutical, edible green leaves, nuts and lake water samples. Spectrochimica Acta Part A, Molecular and Biomolecular Spectroscopy. 2012;86:152–158.
Sucrosomial® Iron supplementation in anemic patients with celiac disease not tolerating oral ferrous sulfate
Luca Elli
Center for the Prevention and Diagnosis of Coeliac Disease, Gastroenterology and Endoscopy, Unit Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
Celiac disease (CD) is a common autoimmune disease of the small bowel. Its presentation is extremely heterogenous with a broad range of manifestations with variable severity ranging from asymptomatic individuals to severe malnourishment or infertility [Citation1]. The worldwide prevalence of CD is difficult to estimate and ranges from 1% to 3% of the general population in Europe and the USA [Citation2–Citation6]. CD may present at any stage of the life; clinical pictures are defined as: classical, particularly common among children, characterized by malabsorption (diarrhea, statorrhea, lack of appetite, growth retardation, and deficiencies in fat soluble vitamins, iron, calcium, and folic acid) and atypical, more common during adolescence and adulthood and presenting with laboratory abnormalities, irritable bowel syndrome, osteopenia, fertility problems, and iron deficiency anemia (IDA) [Citation7]. Both genetic and environmental factors are involved in the development of CD. The disease is triggered and maintained in genetically susceptible individuals, by an immunological response following the ingestion of wheat gluten and similar alcohol soluble proteins (prolamines) [Citation8,Citation9]. All patients express the human leukocyte antigen (HLA) type II DQ2 and/or HLA-DQ8 haplotypes [Citation12]. Gluten peptides, after being deamidated by the transglutaminase 2 (TG2), bind HLA-DQ2 and HLA-DQ8. The consequent result is a destructive intestinal CD4+ T cell response [Citation8]. After their activation, the CD4+ T cells produce cytokines, which support an inflammatory cascade with an intestinal inflammation. All this mechanism results in the alteration of the intestinal mucosa, characterized by villous atrophy, crypt hyperplasia, and infiltration of inflammatory cells [Citation10]. CD is currently diagnosed with IgA anti-tissue transglutaminase (tTG) serology tests, followed by confirmatory biopsies of the duodenal bulb and second part classified by the Marsh classification [Citation11]. CD frequently presents IDA, which usually reverts with a gluten-free diet (GFD). However, some patients present persistent IDA despite their clinical responsiveness to GFD. The contributions of malabsorption, inflammation, or genetics to CD-associated IDA remain unclear, although response to iron supplementation could be improved by the use of a tolerated iron or by the use of an iron formulation increasing the intestinal absorption [Citation12].
Experimental section
Sucrosomial® Iron (Sideral® Forte), a preparation of ferric pyrophosphate conveyed within a phospholipid membrane associated with ascorbic acid, is a new-generation oral iron which shows a high gastrointestinal absorption and high bioavailability with a low incidence of side effects due to lack of any direct contact with intestinal mucosa. In comparison with the other standard oral iron preparations, Sucrosomial® Iron seems to be a promising new strategy of iron replacement in CD patients. The ongoing study treated with Sucrosomial® Iron treated CD patients with IDA and a previous therapeutic failure using sulfate iron tablets. It was a prospective open study. All patients were following a strict GFD for at least 12 months with normalization of anti-transglutaminase IgA. Moreover, consecutive enrolled patients were previously investigated by means of duodenal histology to look for possible correlations with atrophy degrees (Marsh scale). Clinical and demographic data were recorded, and symptomatic response to iron tablets was evaluated through 10-cm long visual analog scales (VASs). Evaluated symptoms were: diarrhoea, constipation, epigastric, and abdominal pain, stool consistency satisfaction. A specific VAS evaluated general well-being. Other causes of IDA were investigated when clinically indicated. Associated vitamin or folic acid deficiencies were looked for and excluded. Primary outcome of the study was Hb levels; secondary outcomes were the levels of symptomatic VASs.
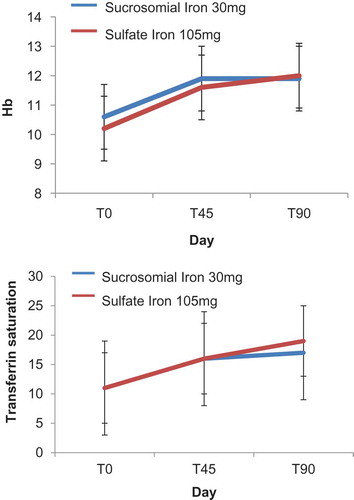
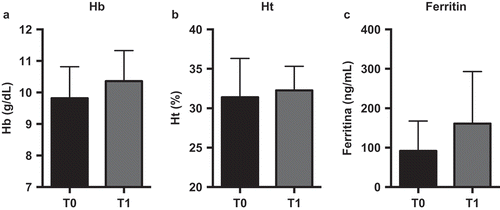
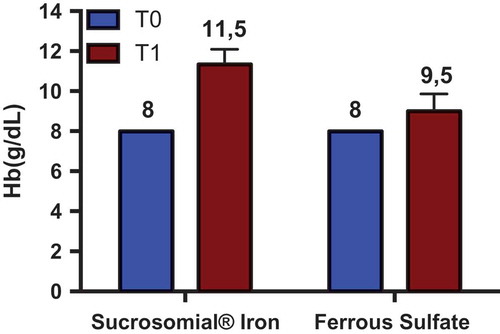
Nowadays, 34 treated CD patients have been enrolled (33 females, 42 ± 9 years of age). In total, 18 (53%) subjects reported previous side effects with sulfate iron (all females, 44 ± 10 years of age) and thus received Sucrosomial® Iron (Sideral® Forte 30 mg); 16 (1 male, 41 ± 8 years of age) were administered with sulfate iron tablets (ferrograd 105 mg) once a day for 90 days. Patients were followed after 45 and 90 days from the beginning of the supplementation. No statistical differences were present between the two groups about demographic, clinical characteristics, smoking habits, and number of used tampons. At enrolment, Hb was 10 ± 1 g/dL, Ht 34% ± 3%, MCV 74 ± 5 fl, iron 46 ± 27 g/dL, transferrin saturation 11% ± 7%, ferritn 12 ± 14 ng/mL. Both therapies induced a statistically significant increase of Hb 12 ± 1 and 12.3 ± 1 in patients administered with Sucrosomial® Iron and sulfate iron, respectively. No variation of VAS was evidenced between patients assuming sulfate of Sucrosomial® Iron. Only well-being VAS significantly increased, independently by the type of supplementation from 4 ± 2 to 6 ± 2.
Among CD patients, 12 (33%) maintained villous atrophy in spite of strict GFD (Marsh 3a, 3b or 3c); 6 assumed sulfate iron and 6 sucrosimial iron. Also, in this case, both the therapies significantly increased the Hb levels and other iron status parameter. Also, the levels of Hb after treatment did not differ between patients with or without duodenal atrophy.
In conclusion, the presented preliminary findings indicate that Sucrosomial® Iron is effective and well tolerated in CD patients with a previous failure of sulfate iron.
References
- Bai JC, Fried M, Corazza GR, et al. World gastroenterology organisation global guidelines on celiac disease. J Clin Gastroenterol. 2013;47:121–126.
- Catassi C, Fabiani E, Ratsch IM, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr. 1996;412:29–35.
- Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42:530–538.
- Catassi C, Ratsch IM, Fabiani E, et al. Coeliac disease in the year 2000: exploring the iceberg. Lancet. 1994;343:200–203.
- Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188.
- Ferguson A. Coeliac disease research and clinical practice: maintaining momentum into the twenty-first century. Bailliere’s Clin Gastroenterol. 1995;9:395–412.
- Ludvigsson JF, Leffler DA, Bai JC, et al. The oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52.
- Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–242.
- Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–1933.
- Marsh MN. The small intestine: mechanisms of local immunity and gluten sensitivity. Clin Sci. 1981;61:497–503.
- Rubio-Tapia A, Hill ID, Kelly CP, et al., American College of Gastroenterology. Acg clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–676; quiz 677.
- Zamani F, Mohamadnejad M, Shakeri R, et al. Gluten sensitive enteropathy in patients with iron deficiency anemia of unknown origin. World J Gastroenterol. 2008;14:7381–7385.
Response to Sucrosomial® oral Iron supplementation in patients underwent bariatric surgery
Andreea Ciudin
Endocrinology and Nutrition Department, Vall d´Hebron University Hospital, Barcelona, Spain
Introduction
In the postoperative period, all the techniques of bariatric surgery induce a significant reduction in the intake or absorption of most of the nutrients. Therefore, bariatric surgery can be associated with a risk of nutritional deficiency, which increases over the years. One of the nutrients whose absorption is affected in a significant way is iron, and the women of childbearing age are the most vulnerable. The daily recommendation of iron intake in adults is 8 mg a day for men and women more than 50 years, and 18 mg per day for women of age less than 50 years. The incidence of iron deficiency and anemia after bariatric surgery is variable according to the type of surgery and the time after the surgery. For instance, after a Roux-en-Y gastric bypass (RYGB), iron deficiency anemia is diagnosed between 10% and 40% of all the cases. Bariatric surgery techniques produce changes of the digestive tract and dietary habits that favor the iron deficiency. These changes are: (a) intolerance to red meat, leading to a decrease in the intake; (b) decreased gastric acid secretion by resection of the proximal stomach, with the consequent relative deficit of parietal cells and iron absorption deficiency; (c) exclusion of the duodenum, which is the main place of molecular iron and heme iron absorption. Most of the patients, especially women of childbearing age, require oral iron supplementation after the bariatric surgery.
Additionally, a non-negligible percentage of women, who previously underwent bariatric surgery, require parenteral iron therapy supplementation until the establishment of the menopause. This fact is due in part to lower pre-surgery iron deposits in obese patients, a phenomenon which also translates into a great variability in the time of occurrence of the deficit (from months to years). Also, most of the obese women who undergo bariatric surgery restore the menstrual period in almost 40% of the cases after a 4,6% weight loss. In many cases, despite taking high oral doses of conventional iron supplementation, often causing gastrointestinal intolerance, they fail to achieve optimal levels, requiring therefore chronic parenteral therapy.
In our series of patients (1100 patients who underwent bariatric surgery since the year 2000 until today), about 120 women of childbearing age, require parenteral treatment with intravenous iron every 3 months, chronically, until the menopause is installed. The administration of intravenous iron, in the day hospital, takes between 2 and 3 hours, for 2 consecutive days, every 3 months, which represents an important distortion of work and daily life of the patients.
Sucrosomial® oral Iron may represent a treatment alternative in these cases. Due to its new technology, based on the contact of sucrosome with intestinal cell membrane, the ‘usual mechanisms’ of intestinal absorption of the iron are not so important (such as gastric acid secretion, duodenum). Therefore, the bioavailability of Sucrosomial® Iron rises to 3.5 times compared with the conventional iron. Sucrosomial® Iron also has been proven to be effective and well tolerated, compared with oral supplements of conventional iron in pregnancy, newborn, infant, chronic kidney disease, and inflammatory bowel disease. This form of oral iron has demonstrated its effectiveness even against conventional iron in patients with intravenous therapy.
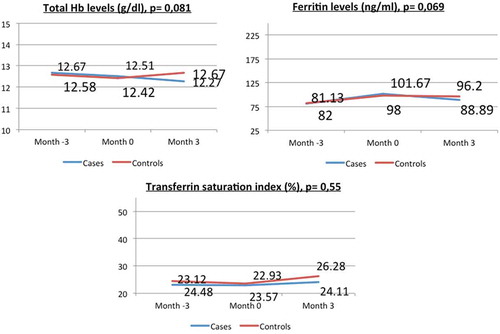
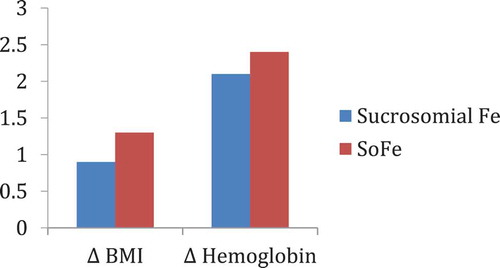
To our knowledge, to date, there is no study using Sucrosomial® oral Iron in patients undergoing bariatric surgery. In order to shed light on this issue, we have designed a single-center, open, prospective, interventional trial, including 40 women of childbearing age, who previously underwent RYGB, and currently require chronic intravenous iron therapy. The subjects were divided into two parallel groups: 20 cases and 20 controls matched by age, previous level of hemoglobin (Hb), years after surgery, and percentage of weight lost. The 20 cases were discontinued from the parenteral iron treatment and were treated with oral Sucrosomial® Iron 28mg/day for three months. The 20 controls continued with 300mg iron sucrose endovenously every 3 months. Total hemoglobin (Hb), ferritin, and transferrin saturation index (TSI) were determined before and after 3 months of treatment in both groups, as well as tolerability.
Results
No significant differences were seen between the levels of Hb (12.67 ± 1.06 g/dL vs 12.267 ± 1,35 g/dL, p = 0.081), ferritin (101.67 ng/dL vs. 88.89 nd/dL, p = 0.069), and TSI (24.11% vs. 26.28%, p = 0.55) before and after the 3 months of treatment with Sucrosomial® Iron. We did not find any adverse effect during this period in the treatment group. Compared to the control group, the final Hb levels were similar (Hb 12.267 ± 1.35 g/dL and 12.1 ± 1.74 g/dL, respectively, p = 0.09).
Conclusion
Our study suggests that oral Sucrosomial® Iron might represent an alternative therapy in patients who require parenteral treatment with iron after bariatric surgery.
Final remark: Sucrosomial® Iron might be an alternative treatment in patients with severe iron deficiency after bariatric surgery, which currently requires parenteral iron therapy due to intolerance to existing oral products or therapeutic failure. At the same time, it might help to reduce healthcare costs and improve the quality of life of these patients.
Efficacy and tolerability of Sucrosomial® Iron supplementation in IBD patients with iron deficiency anemia and intolerance to iron oral salts
Guillermo Bastida Paz
Unidad de Enfermedad Inflamatoria Intestinal, Hospital Universitario y politécnico La Fe, Valencia, Spain
Anemia is the most frequent systemic complication in inflammatory bowel disease (IBD) [Citation1–Citation2], most of all in patients with Crohn’s disease (CD) [Citation3]. Its prevalence is quite variable in the different studies, according to its evaluation criteria and the types of patients included [Citation4,Citation5]. A systematic review of the literature describes prevalence of anemia in IBD of 16% in outpatients with IBD and of up to 70% in inpatients. If we take into account that the main cause of anemia is IBD itself, we understand that the cause of the anemia in the majority of the patients is mixed, with an iron deficiency through continuous gastrointestinal losses associated to the anemia of chronic illnesses [Citation6]. In addition, we must not forget that there are other causes of anemia such as the deficiency of vitamin B12 or of folic acid, malnutrition, the malabsorption or the ingesting of several medications (e.g. salazopyrine or thiopurines). The importance of anemia, even in non-anemic iron deficiency, resides in the serious disruption of quality of life that it implies. Actually, the presence of anemia can condition a disruption of quality of life similar to that of advanced cancer patients [Citation2].
This is a very important aspect, since not long ago the presence of anemia was only considered a marker of the activity of IBD, on occasion going unnoticed, in such a way that it was assumed that a certain anemia represented a normal discovery in IBD patients and, as such, did not require treatment. Now, it is well known that iron deficiency anemia should be treated with iron as soon as it is detected. Likewise, the therapeutic objective of the treatment should be to completely cure the anemia and the iron deficiency, and not only to partially raise the hemoglobin or ferritin numbers.
The treatment of the anemia should be started once the cause in known. In the case of treating an iron deficiency anemia, iron should be administered at the same time as the medication to control the inflammation.
Intravenous iron is very quick and efficient both for curing anemia and for maintaining the iron deposits in IBD patients [Citation6]. Because of its cost, and because of the need for it to be administered in a hospital, current guidelines [Citation1] recommend the administration of intravenous formulas for those patients in which hemoglobin numbers are lower than 10 g/dL, in those who tolerate iron orally or in those who require erythropoiesis-stimulating agents [Citation1]. The majority of the patients is going to have mild anemia and will be treated with oral iron [Citation7]. Oral iron is an efficient, simple, cheap, and safe treatment. The efficiency of oral iron varies between different studies. For example, in a study carried out in Spain, oral iron managed to normalize hemoglobin numbers in more than 85% of patients. In this study, oral iron was well tolerated, without assessing the activity of IBD and with just 5% withdrawal through intolerance. The patients that did not tolerate or did not cure their anemia with oral iron received intravenous iron with excellent results [Citation7].
However, these excellent results were not reproduced in all studies and undoubtedly oral iron presents clear and significant limitations [Citation8]. One of its greatest limitations resides in the fact that a considerable percentage of IBD patients show poor tolerance to treatment with oral iron. In fact, on many occasions it can cause diarrhea, constipation, or abdominal pain, on the one hand hindering the performance (and with this real efficiency) and on the other it can mislead us with an outbreak. In fact, a systematic review describes that the appearance of secondary effects lead to the suspension of oral iron in up to 21% of patients with CD [Citation4]. This percentage can reach a significant magnitude, for example, Lugg et al. describe the failure of oral iron in controlling anemia in two out of every three individuals with IBD, due in part to the secondary effects, which appear in more than half of its patients [Citation9]. These secondary effects could be related to experimental data and suggest that the iron that is not absorbed could be toxic to intestinal mucous and even precipitate outbreaks of IBD [Citation10].
For all of these reasons, we understand that a new oral iron formula, with better tolerability, would be of great interest due to the higher frequency of anemia and iron deficiency of patients with IDB as well as the adverse effects associated to classic iron formulas with ferrous salts (ferrous sulfate, ferrous gluconate, ferrous fumarate, and ferrous lactate). In this regard, a recent formula, Sucrosomial® Iron, could have some advantages. This is a Sucrosomial® ferrous pyrophosphate, which contains 30 mg of iron that is covered in sucrosoma. The sucrosoma is formed by a liposome, a spherical structure of a phospholipidic nature similar to those cell membranes of the human body, which are immersed in a matrix of sucrose esters of fatty acids that allow it to cross the gastric acid barrier, reaching the small intestine unscathed and avoiding the pro-oxidant effect of other iron salts. Once there, the sucrosoma and the iron that it contains are integrally absorbed through the intestinal M cells, without the need for specific transporters [Citation11]. The M cells, due to their low lysozyme content, can transport antigens with almost no enzymatic degradation and allow their liberation without modifying the lymphatic system. This differential absorption of the Sucrosomial® Iron gives it greater availability. Therefore, the protection of the sucrosoma avoids the appearance of the classic secondary effects of the treatment with other iron salts allowing the microelement to overcome unscathed the gastric environment to be directly absorbed into the intestine and directly liberated into the liver [Citation12].
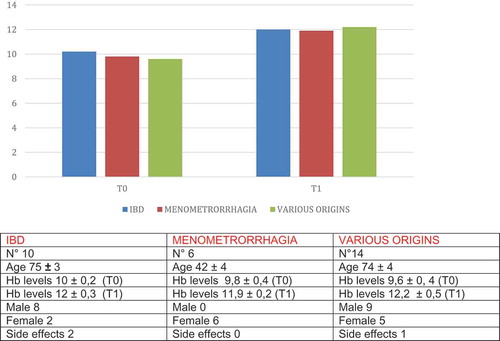
An observational and multicentric study is currently being carried out to evaluate the tolerability of oral Sucrosomial® Iron (dietary supplement Fisiogen Ferro Forte®) in the collision of the iron deficit in patients with IBD previously intolerant to the salts normally used in oral iron. The intermediate analysis has been made with the data gathered until September 2015. At this time, there were 39 valid patients, all with prior poor toleration of oral iron. Of them, 22 (64.1%) of the patients showed CD, 16 (73%) in remission at the beginning of the study. In total, 17 (35.9%) showed ulcerative colitis (UC), 8 (67%) were in remission. The average age of the patients was 42 years, the lower and upper limits were 21 and 62 years.
In total, 79.5% of the patients showed symptoms of iron deficiency anemia. Asthenia was the most frequent symptom among the patients, present in all patients, followed by difficulty in concentrating and irritability in 48.4% and 45.2%, respectively.
The patients were treated with a daily dose of Sucrosomial® Iron over 3 months. The majority of the patients (90.5%) performed well. The tolerability of Sucrosomial® Iron, the primary objective of the study in this population of patients intolerant to other oral iron formulas was good. During the study, the general well-being of the patient was recorded. Almost all patients, 100% and 92%, had a good or slightly lower than normal opinion of the 4 and 12 weeks, respectively. Additionally, the percentage of the patients with gastrointestinal symptoms after taking the Sucrosomial® Iron at 4 and 12 weeks (abdominal pain, constipation, loss of appetite, nausea, vomiting, change of color to the feces, or the presence of a metallic taste) did not increase over the monitoring.
Although the percentage of the adverse effects gathered was high (31 patients of 38 showed a total of 125 adverse occurrences over the course of the study), two-thirds of them, 66.4%, were of low intensity and only 6.4% were serious. The most frequent secondary effects were a change in color of the feces (63.2%), abdominal pain (44.7%), diarrhea (34.2%), constipation (23.7%), and a metallic taste (21%), although only 42.7% were found to relate to the case. In fact, in 93.6% of the occurrences, there was no change with regard to taking Sucrosomial® Iron, 0.8% temporarily interrupted and 5.6% permanently.
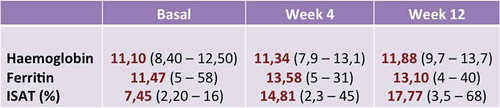
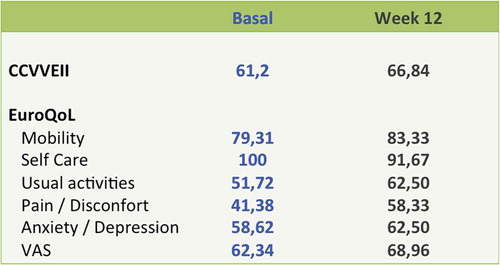
In relation to the efficiency, one-third of the patients normalized the iron-deficient anemia data in 12 weeks. Hemoglobin values increased at 12 weeks from the average of 11.1 g/dL to 11.8 g/dL (p = 0.0023) and those of ferritin from 11.47 mcg/L to 13.1 mcg/L ().
The recovery of the data on iron deficiency was accompanied by an improvement in the questionnaires on quality of life. The average rating in the questionnaire on quality of life of IBD (CCVVEII-9) improved from 61.2 points to 66.8 points on the final visit. The generic questionnaire on the quality of life, the EuroQoL, improved in all dimensions (mobility, daily activities, pain/discomfort, and anxiety/depression) except in personal care ().
Therefore, Sucrosomial® Iron is well tolerated in patients with IBD with prior intolerance to oral iron, and should be considered as an alternative for the treatment of iron deficiency anemia in those patients who do not tolerate classic prepared doses of oral iron.
References
- Dignass AU, Gasche C, Bettenworth D, et al.; European Crohn’s and Colitis Organisation [ECCO]. European consensus on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211–222.
- Gasche C. Anemia in IBD: the overlooked villain. Inflamm Bowel Dis. 2000;6:142–150.
- Bergamaschi G, di Sabatino SA, Albertini A, et al. Prevalence and pathogeneis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica. 2010;95:199–205.
- Kulnigg S, Gasche C. Systematic review: managing anemia in Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1507–1523.
- Gomollón F, Gisbert JP. Current management of iron deficiency anemia in inflammatory bowel diseases: a practical guide. Drugs. 2013;73:1761–1770.
- Rizvi S, Schoen RE. Supplementation with oral vs. intravenous iron for anemia with IBD or gastrointestinal bleeding: is oral iron getting a bad rap? Am J Gastroenterol. 2011;106:1872–1879.
- Gisbert JP, Bermejo F, Pajares R, et al. Oral and intravenous iron treatment in inflammatory bowel disease: haematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15:1485–1491.
- Bermejo F, García S. Iron deficiency anemia in inflammatory bowel disease. Enferm Inflam Intest Dia. 2015;14:11–20.
- Lugg S, Beal F, Nightingale P, et al. Iron treatment and inflammatory bowel disease: what happens in real practice? J Crohns Colitis. 2014;8:876–880.
- Carrier J, Aghdassi E, Platt I, et al. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment Pharmacol Ther. 2001;15:1989–1999.
- Harokopakis E, Hajishengallis G, Michalek SM. Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun. 1998;66:4299–4304.
- Torchilin VP. Lipid-core micelles for targeted drug delivery. Curr Drug Deliv. 2005;2:319–327.
Iron parameters in cancer patients and prediction of hemoglobin response
Paolo Pedrazzoli, Simona Secondino, Sara Delfanti, Giovanni Rosti
SC Oncologia, Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy
Anemia is common in cancer patients, and is associated with significant decrease in quality of life (QOL) and a negative impact on prognosis. The etiology of cancer-related anemia is multifactorial, but in most cases it is a consequence of the chronic disease process associated with malignancy.
Alleviating anemia with erythropoiesis-stimulating agents (ESAs) improves energy, activity, and overall QOL, particularly among patients with mild-to-moderate anemia, and facilitate patient coping with active treatments. However, research suggests that anemia is still under-recognized and undertreated. This may be partly due to the limitations of current ESA therapy, which includes a large percentage of patients who do not respond to this treatment, the need for frequent dosing, and the relatively slow time to response. Adequate patient selection for treatment with ESA or other drugs/procedure is of pivotal importance in this setting.
Dysregulations of iron metabolism causing iron deficiency represent a major cause of anemia of chronic diseases (ACDs). Also, data from the dialysis and cancer populations has clearly shown that an important factor which seriously limits response to ESA is functional iron deficiency (FID), which is an imbalance between iron needs in the erythropoietic marrow and iron supply. FID may be either preexisting or occurring during ESA therapy, when red cells are produced at a rate that outstrips labile iron availability. As a consequence, iron supplementation may still be required to achieve or maintain an optimal response to ESA. In anemic cancer patients, iron deficiency has to be investigated by dosing transferrin saturation, a parameter that is modestly influenced by inflammation. Ferritin, in contrast, belongs to the group of acute-phase proteins and often does not reflect iron stores in cancer, due to its interdependence with inflammatory reactions. The iron regulatory peptide, hepcidin, is the key factor underlining the occurrence of iron dysregulation in the ACDs, including cancer. Hepcidin is upregulated in ACD, resulting in the inhibition of iron transport across cell membranes, which decreases the accessibility of storage iron and gastrointestinal absorption of dietary iron, leading to an increased frequency of iron-restricted erythropoiesis, especially during therapy with ESA. Hepcidin disregulation may well represent the mechanism by which oral iron supplementation has been reported ineffective in cancer patients.
Prospective trials published over the last decade demonstrate that anemic patients with cancer undergoing chemotherapy and receiving ESA respond better, without additional toxicity, when parenteral iron is administered. Such benefit is more relevant when FID is present at baseline but appears to be independent of baseline iron variables in one large study. This issue is clinically relevant because appropriate iron supplementation, apart from allowing more patients to benefit from ESA therapy, may represent a strategy to improve the cost effectiveness of ESA in oncology, as it has occurred in nephrology. However, the use of iron supplementation during treatment with ESA is not rigorously pursued in anemic patients with cancer as it has in chronic kidney disease. This underuse is likely to be related to: (i) the false perception that cancer patients do not have decreased iron stores (as measured by serum ferritin) and therefore thought not to require iron supplementation during ESA therapy, (ii) the often misinterpreted incidence and clinical nature of serious adverse events of intravenous iron, (iii) the lack of studies demonstrating the efficacy of traditional oral iron agents to favor response to ESA.
Novel iron preparations capable of increasing iron absorption and bioavailability, including those carried by a phospholipid plus sucrester membrane, may well facilitate a more widespread use of iron supplementation in cancer anemia. A randomized study is currently ongoing aimed to define the potential benefit of Sucrosomial® Iron in improving response to ESA in anemic cancer patients.
References
- Cella D, Dobrez D, Glaspy J. Control of cancer-related anemia with erythropoietic agents: a review of evidence for improved quality of life and clinical outcomes. Ann Oncol. 2003;14:511–519.
- Cella D, Stone AA. Health-related quality of life measurement in oncology: advances and opportunities. Am Psychol. 2015;70:175–185.
- Sankaran VG, Weiss MJ. Anemia: progress in molecular mechanisms and therapies. Nat Med. 2015;21:221–230.
- Tessitore N, Solero GP, Lippi G, et al. The role of iron status markers in predicting response to intravenous iron in hemodialysis patients on maintenance erythropoietin. Nephrol Dial Transplant. 2001;16:1416–1423.
- Pedrazzoli P, Farris A, Del Prete S, et al. Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alfa. J Clin Oncol. 2008;25:1619–1625.
- Ludwig H, Müldür E, Endler G, et al. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013;24:1886–1892.
- Auerbach M. Intravenous iron in chemotherapy-induced anemia. Am J Hematol 2014;89:1153.
- Petrelli F, Borgonovo K, Cabiddu M, et al. Addition of iron to erythropoiesis-stimulating agents in cancer patients: a meta-analysis of randomized trials. J Cancer Res Clin Oncol. 2012;138:179–187.
- Pedrazzoli P, Rosti G, Secondino S, et al. Iron supplementation and erythropoiesis-stimulatory agents in the treatment of cancer anemia. Cancer. 2009;115:1169–1173.
- Yuan L, Geng L, Ge L, et al. Effect of iron liposomes on anemia of inflammation. Int J Pharm. 2013;454:82–89.
Anemia with molecular-targeted therapies used for solid tumors: an updated pooled analysis of literature and new perspectives with sucrosomal iron
Fausto Petrelli and Sandro Barni
Oncology Unit, ASST Bergamo Ovest, Treviglio, Italy
Introduction
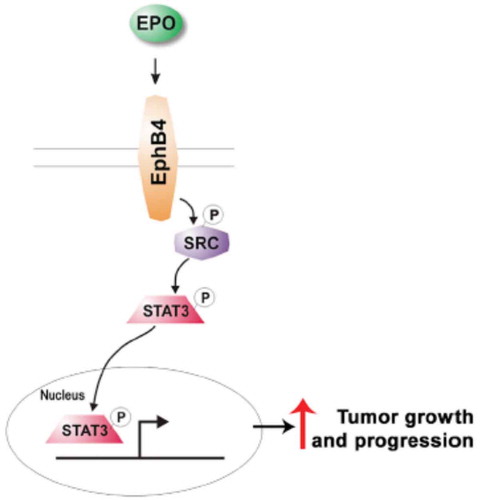
Anemia is a common manifestation of cancer patients and is usually associated with advanced disease, malnutrition, and poor prognosis. It is one of the reasons for fatigue, delay/reduction and change in dose intensity of cancer treatments, poor activity of radiation therapy due to reduced oxygen effect, increase use of blood transfusions, and finally of rise of financial burden in oncology setting. Rapid correction of hemoglobin (Hb) levels is necessary for patients’ well-beings, and needs iron plus or minus erythropoiesis-stimulating agents (ESAs) and eventually, if they do not permit to correct Hb, red blood cells (RBCs) transfusions. Usually, ESAs associated with parenteral iron are appropriate to treat moderate (grade (G2)) anemia, conversely more severe and symptomatic grade of anemia (G3–4) needs prompt transfusions. ESAs significantly reduced the use of RBC transfusions (relative risk (RR) 0.65, 95% CI 0.62 to 0.68) according to a 2012 Cochrane meta-analysis [Citation1]. Nowadays, in chemotherapy-related form of anemia, parenteral iron has showed a better and rapid response of ESAs agents according to a meta-analysis of randomized trials [Citation2]. Iron should be given during ESA therapy, if necessary, in order to maintain a transferrin saturation of ≥20% and a serum ferritin level of ≥100 ng/mL. The reason of failure of oral iron formulation in cancer anemic patients seems related to the hepcidin protein, an acute-phase protein produced primarily by the liver. Several observation suggests that hepcidin, and perhaps other regulatory molecules produced in the liver [Citation3–Citation5], plays a major role as a negative regulator of intestinal iron absorption and iron release from macrophages [Citation6–Citation7], by interacting with, and inactivating, the iron export protein ferroportin [Citation8]. Molecular-targeted agents as monoclonal antibodies (e.g. anti-HER2, epidermal growth factor receptor (EGFR), or vascular endothelial growth factor (VEGF) agents) or small molecules (multi-target) tyrosine kinase inhibitors (e.g. sunitinib or sorafenib) are able to interfere with specific pathway leading to a reduced/altered erythropoiesis as reflected by high rate of G1-2 anemia in clinical trials. However, the use of EPO with new molecular agents is not currently labeled, and its safety with anti-angiogenetic therapies is debatable, due to a possible growth stimulation of EPO mediated by EPO receptors (EPO-R). In this setting, not functional EPO-R but EphB4 protein, working as an EPO-R promoting tumor growth and progression via Stat3 signaling, was recently discovered by Pradeep and colleagues (). In particular, abundance of EphB4 receptor on human breast and ovarian cancer tissues predicted poor survival, in particular, when EPO was associated [Citation9,Citation10]. As for now, data with EPO in patients treated with targeted agents for solid tumors were ever being conducted, and the treatment of these emergent forms of anemia is unknown.
Etiology and frequency of anemia with molecular agents
The causative role of anemia with molecular agents is partially unknown, but the general opinion is that they can interfere with myelo- and erythropoiesis in bone marrow. If other etiologies cannot be excluded (microangiopathic hemolytic anemia or macrocytic anemia associated with sunitinib therapy [Citation11–Citation17]), the main reason seems related to the interference with the FLT-3 pathway. Fms-like tyrosine kinase 3 (known as FLT-3 or CD 135) is a cytokine receptor that belongs to the receptor tyrosine kinase class III. CD135 is the receptor for the cytokine Flt3 ligand (FLT3L). It is expressed on the surface of many hematopoietic progenitor cells. Signaling of FLT3 is necessary for the proper development of hematopoietic stem cells and progenitor cells. An old study in rabbit showed that hematopoietic recovery occurred after total body irradiation if protected by FLT-3 ligand, and suggests a radioprotective clinical potential of FLT3 receptor [Citation18].
Analyzing data from n = 92 prospective series or phase II–III trials that included common labeled targeted agents used as single agent, for the treatment of common solid malignancies, an high rate of all grades and G1–2 anemia events were found (Petrelli personal communication). The risk was overall by 32% (all grades) and by 22% for G1–2 events. Risk is particularly relevant with mTOR inhibitors (e.g. everolimus) and multitarget tyrosine kinase inhibitors (e.g. sunitinib), where the risk of treatment-related anemia were 31.7% and 38.6% ( and ). Conversely, risk with new immunotherapies is low (<10%).
Risk was largely associated with agents targeting multiple pathways as VEGFR, RET, cKIT, FLT-3, CSF-1R that likely interfere with myelo- and erythropoiesis as previously described above. The hypothesis of targeting FLT-3 and its downstream pathway is confirmed by the different toxicity profiles of sunitinib and pazopanib, the last targeting VEGFR1,2,3, PDGFR and c-KIT but not FLT-3, an exquisite target of sunitinib. The value of Hb, fall down the 2 weeks after the cycle of sunitinib (4-weeks-on and 2-weeks-off schedule), conversely, the anemia levels during pazopanib therapy remained quite steady during treatment, and largely above the lower normal limit in the COMPARZ study [Citation19].
Therapy considerations
Treatment of anemia with targeted agents is commonly not conventional. In fact, ESAs are not labeled for this indication and blood transfusions are needed only for lower Hb levels (G3–4 anemia or symptomatic anemia). However, a preemptive strategy can be suggested due to high risk of fatigue observed with these agents that could worsen anemia symptoms [Citation20]. Published guidelines regarding management of anemia with sunitinib suggest that G3/4 anemia usually does not require relevant dose modification; however, because of concern about the potential toxicities and angiogenesis triggering, the use of ESAs should be cautioned [Citation21]. Baseline evaluation of nutritional status, iron balance, B12, and folate deficiency should be performed. Chronic bleeding must be promptly recognized and treated (e.g. with palliative radiotherapy for example), and treatment did not start in presence of risk of bleeding. Seldom a blood transfusion is needed (but possibly avoided if not necessary) before starting treatment, and iron supplementation can be offered. In particular, Sucrosomial® Iron is an attractive way of iron administration in cancer patients. Pyrophosphate Sucrosomial® Iron (Sideral forte ®) is composed of protected iron and vitamin C, useful in case of deficiency or increased requirements. The iron, included is uniquely coated using a liposomal technology that allows the molecule to pass through the stomach, avoiding any gastrointestinal irritation, to be directly absorbed through the lining of the digestive tract. In particular, Sucrosomial® Iron has been showed to be effective in a similar way to intravenous (IV) iron when used in association with epoetin alfa in patients with refractory anemia. In particular, a clinical, feasibility study in cancer patients treated with molecular agents seems to be urgently needed in medical oncology to verify safety and efficacy in anemic patients with metastatic tumors.
Conclusions and future perspectives
In conclusion, mild anemia is a common event in patients treated with targeted therapies for solid tumors (up to 40% of patients showing anemia adverse event mainly of low grade). Early treatment of this hematological toxicity is of paramount importance due to deterioration of quality of life; increase of fatigue and cost saving with transfusion prevention. Due to lack of a standardized treatment, not labeling of ESAs agents and largely unknown cause of anemia, the treatment is a challenge. Use of the sucrosomial iron in patients treated with tyrosine kinase inhibitors could be an appealing way to treat anemia associated with these agents that could rise up to 50% with sunitinib. A prospective observational study was launched in 2014 at Oncology Unit of Treviglio Hospital with Sideral forte® (one tablet daily for 3 months) for patients with mild (G1: Hb level 10–12 g/dL) anemia before starting chemotherapy for solid tumors. The first series of patients treated (n = 10) showed a good tolerability and no fall of Hb below 10 g/dL after 12 weeks (Petrelli, personal communication).
In the absence of proper guidelines, preemptive use of iron, schedule changing (e.g. with sunitinib for example), correction of Hb level before starting with treatment, and short-dose interruption/reduction of these drugs should be implemented to treat hematological toxicities associated with these treatments.
References
- Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2012 Dec 12;12:CD003407.
- Petrelli F, Borgonovo K, Cabiddu M, et al. Addition of iron to erythropoiesis-stimulating agents in cancer patients: a meta-analysis of randomized trials. J Cancer Res Clin Oncol. 2012 Feb;138(2):179–187.
- Lin L, Valore EV, Nemeth E, et al. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007 Sep 15;110(6):2182–2189.
- Muckenthaler M, Roy CN, Custodio AO, et al. Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat Genet. 2003 May;34(1):102–107.
- Meynard D, Babitt JL, Lin HY. The liver: conductor of systemic iron balance. Blood. 2014 Jan 9;123(2):168–176.
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003 Aug 1;102(3):783–788.
- Frazer DM, Inglis HR, Wilkins SJ, et al. Delayed hepcidin response explains the lag period in iron absorption following a stimulus to increase erythropoiesis. Gut. 2004 Oct;53(10):1509–1515.
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004 Dec 17;306(5704):2090–2093.
- Pradeep S, Huang J, Mora EM, et al. Erythropoietin stimulates tumor growth via EphB4. Cancer Cell. 2015 Nov 9;28(5):610–622.
- Patterson SD, Rossi JM, Paweletz KL, et al. Functional EpoR pathway utilization is not detected in primary tumor cells isolated from human breast, non-small cell lung, colorectal, and ovarian tumor tissues. PLoS One. 2015 Mar 25;10(3):e0122149.
- Talebi TN, Stefanovic A, Merchan J, et al. Sunitinib-induced microangiopathic hemolytic anemia with fatal outcome. Am J Ther. 2012 Jul;19(4):e143–e145.
- Bevacizumab + sunitinib: microangiopathic haemolytic anemia. A serious drug interaction between 2 cancer drugs. Prescrire Int. 2009 Aug;18(102):165.
- Price J, Shaarbaf R, Wood L. Sunitinib causes macrocytosis in patients with advanced renal cell carcinoma. Curr Oncol. 2010 Apr;17(2):30–3.
- Rini BI, Choueiri TK, Elson P, et al. Sunitinib-induced macrocytosis in patients with metastatic renal cell carcinoma. Cancer. 2008 Sep 15;113(6):1309–1314.
- Jain R, Mathew P, Wood CG, et al. Sunitinib-induced acute hemolysis without hypertension: a case report. Clin Genitourin Cancer. 2008 Sep;6(2):122–123.
- Schallier D, Trullemans F, Fontaine C, et al. Tyrosine kinase inhibitor-induced macrocytosis. Anticancer Res. 2009 Dec;29(12):5225–5228.
- Billemont B, Izzedine H, Rixe O. Macrocytosis due to treatment with sunitinib. N Engl J Med. 2007 Sep 27;357(13):1351–1352; author reply 1352.
- Gratwohl A, John L, Baldomero H, et al. FLT-3 ligand provides hematopoietic protection from total body irradiation in rabbits. Blood. 1998 Aug 1;92(3):765–769.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013 Aug 22;369(8):722–731.
- Santoni M, Conti A, Massari F, et al. Treatment-related fatigue with sorafenib, sunitinib and pazopanib in patients with advanced solid tumors: an up-to-date review and meta-analysis of clinical trials. Int J Cancer. 2015 Jan 1;136(1):1–10.
- Kollmannsberger C, Bjarnason G, Burnett P, et al. Sunitinib in metastatic renal cell carcinoma: recommendations for management of noncardiovascular toxicities. Oncologist. 2011;16(5):543–553.
- Funakoshi T, Latif A, Galsky MD. Risk of hematologic toxicities in cancer patients treated with sunitinib: a systematic review and meta-analysis. Cancer Treat Rev. 2013 Nov;39(7):818–830.
Patient blood management. Strategies and protocols to improve hemoglobin levels
José Antonio García Erce
Investigador Instituto Aragonés de Ciencias de la Salud, Coordinador Grupo de Trabajo de la Sociedad Española de Transfusión Sanguínea (SETS) “Hemoterapia Basada en el Sentido Común”, Servicio de Transfusión. Servicio de Hematología y Hemoterapia, Hospital San Jorge, Salud, GIEMSA-AWGE, IdiPAZ 49, Spain
Introduction
Preoperative anemia or suboptimal hemoglobin (Hb) and perioperative allogeneic blood transfusion (ABT) are both identifiable and preventable surgical risks. There is an increasing evidence that preoperative anemia is associated with increased patient morbidity and mortality following surgery. Patient blood management (PBM) is a multimodal approach to address this issue. PBM is based on three pillars of medical care: the detection and treatment of this perioperative anemia; the reduction of perioperative blood loss; and harnessing and optimizing the patient-specific physiological reserve of anemia, including restrictive Hb transfusion triggers and autologous transfusion. All those different measures included in these three pillars can be applied in three moments: before, during, or after the surgery or invasive procedure. All these complementary strategies must be part of a multimodal program focus on the major actor: our patient.
The objective of this lecture is briefly reviewing the efficacy, safety, and recommendations of one of the PBM pillar: the stimulation of erythropoiesis and iron treatment. Standard operating procedures, multimodal strategies, and protocols are needed to improve the Hb perioperative levels to avoid (or to reduce to minimum) ABT and to achieve the best clinical outcome. Iron therapy is one of the successful clues of these programs.
Myths, legends, and facts of blood transfusion
The phrase ‘one blood donation saves three lives’ has been frequently repeated, but in light of a wealth cumulative of evidence blood transfusion may no longer be the first choice today [Citation1]. Blood transfusion has become a routine medical response despite cheaper and safer alternatives in some settings. It has become apparent that the risks associated with ABT may not be outweighed by the potential benefits in many patients who are routinely transfused. [Citation2]. This classical ‘liberal’ use of blood has not been based upon scientific evaluation of benefits neither weight its real risks. Preoperative anemia is still frequently ignored, with indiscriminate allogeneic blood transfusion used as a ‘quick fix,’ ignoring that ABT is a transitory measure. Blood is a precious ‘gift’ – from healthy, voluntary, altruist, and generous donor, but it is not free of risk neither free of increasing costs – with an ever limiting supply due to the aging population. Ethical code invites us to ‘prescribe regimens for the good of my patients according to my ability and my judgment and will do no harm or injustice to them’ [Citation1].
Transfuse or not transfuse is not the unique question. Decisions to transfuse should be based on assessment of an individual patient including their underlying cause of anemia, tolerance, speed, and treatment availability. Our objective must improve the patient’s conditions to reduce the transfusion needs. Iron deficiency anemia must be one of our therapeutic targets. We must also treat the underlying cause, not only the figure (Hb level at the CBC). According to the American Association Blood Banks (AABB) recommendations, please ‘Focus on Your Patient, Not the Transfusion’ (). [Citation3]
We must not transfuse red blood cells for iron deficiency without hemodynamic instability. Preoperative patients with iron deficiency and patients with chronic iron deficiency without hemodynamic instability (even with low Hb levels) should be treated with iron and not with transfusion. There is high quality evidence that demonstrates a lack of benefit and, in some cases, harm to patients transfused to achieve an arbitrary transfusion threshold. If necessary, transfuse only the minimum number of units required instead of a liberal transfusion strategy. This is first of the pillars of PBM.
Anemia. Is it normal?
The prevalence of preoperative anemia may be high among surgical patients, depending on the patients’ comorbidities, gender, age, and the underlying pathology for which they require surgery [Citation4]. This preoperative anemia is quite common (20–50% depending on conditions) and is an independent risk factor for morbidity and mortality [Citation5,Citation6]. The Hb level is an independent transfusional risk factor and there is a dose-dependent relationship between postoperative complications and the ABT [Citation4,Citation5,Citation6,Citation7]. Iron deficit has been described as a nosocomial infection factor. The procedures in orthopedic and trauma surgery, vascular, cardiac, or oncological surgery can cause significant blood loss and provoke a postoperative acute anemia, or aggravate previous preoperative anemia, which will increase the requirements of ABT. There is increasing evidence that preoperative anemia is associated with increased patient morbidity and mortality following surgery. Also, ABTs are associated with a worse prognosis, including all-cause mortality, cancer-related mortality, and cancer recurrence – mainly in gastric, colon, and prostate – at 1 and 5 years [Citation5].
In a recent study [Citation6], a total of 2,225,054 total joint arthroplasties (TJAs) cases were identified from 1993 till 2011. One-stage bilateral TJA (OR, 3.30), anemia due to chronic blood loss (OR, 2.69), deficiency anemia (OR, 2.59), and Charlson comorbidity index (OR, 1.24) were independent predictors of ABT (p < 0.001). RBC transfusion was an independent predictor of in-hospital mortality (OR, 1.54).
Postoperative anemia is more frequent (till 90%) and must be corrected, which does not necessarily imply that shall be done by the administration of ABT (only if clinically is needed: ‘restrictive transfusion criteria’).
Patient blood management (PBM)
These clinical, financial, and logistical disadvantages of ABT have promoted the development of generically known as PBM multidisciplinary and multimodal programs whose aim is to reduce or eliminate the need for ABT and improve clinical outcome [Citation1–Citation7]. PBM has been the evolution of the old ‘Blood Saving Program’ [Citation1,Citation2,Citation7].
These programs are supported by the application of four groups of perioperative measures: (1) use of ‘restrictive’ transfusion criteria (administer the minimum effective dose guided by clinical signs or symptoms); (2) stimulation of erythropoiesis (diagnosing and treating the perioperative anemia); (3) reducing bleeding (improving the hemostasis and avoiding the hyperfibrinolysis); and (4) autologous blood transfusion [Citation7].
PBM is an evidence-based approach to optimizing the care of patients who could or might need transfusion. A focus on improved patient outcomes and economic and operational pressures are leading key industry thinkers to examine appropriate blood usage with new interest. Hospitals are eager to improve patient safety and clinical outcomes, while also reducing the need for allogeneic blood components. PBM programs can achieve these goals by reducing variation in transfusion practice and managing patients with no transfusion – and, if appropriate, transfusion – treatment modalities. [Citation7]
Assessment and management of preoperative patients involve maximizing Hb levels to prevent anemia and optimizing coagulation function to limit bleeding. Starting with the primary care physician, the health care team supporting medical and presurgical patients should focus efforts on determining whether there is a reason to suspect any medical conditions that might predispose the patient to transfusion [Citation7–Citation8]. Among the various strategies utilized in PBM, perhaps the most important is the timely detection and management of anemia [Citation8].
As any leading organization in the education of health care professionals about blood management and utilization review, the scientific societies related with ABT (like the American Association of Blood Banks) must offer resources that address the various aspects of PBM, helping members achieve their goals of optimizing patient outcomes, preventing unnecessary blood usage and auditing physician compliance with established criteria for transfusion [Citation3,Citation6,Citation7].
The PBM coordination requires support from hospital administrators (facilitating organization), health authorities (reallocating funding and providing regulations), and medical societies (offering advice to health authorities and developing clinical guidelines). Continuing medical education should be offered to health professionals in order to refresh and update knowledge on different perioperative strategies within each pillar committing to the program [Citation8].
Perioperative stimulation of erythropoiesis
Perioperative stimulation of erythropoiesis is the second fundamental pillar of PBM program. Normal erythropoiesis needs a healthy bone marrow with an adequate supply of various nutrients (iron, vitamins C, B1, B6, B12, D, and folic acid), and hormones (erythropoietin, thyroid hormones, and steroids). In the absence of information on other hematinics, only the possible benefit of oral and IV iron administration to reduce transfusion rate has been studied.
Diagnosis and treatment of perioperative anemia
In patients scheduled for any major surgery, should investigate the presence of preoperative anemia at least 30 days before surgery, for differential diagnosis and appropriate therapy, if needed (GRADE 1C) [Citation8,Citation11]. Faced with an unexpected anemia, an elective surgical procedure should be postponed until it has been properly classified and treated.
Usually, the presence of anemia is diagnosed if Hb level is under 13 g/dL in men or under 12 g/dL in women. Perhaps, we need a different definition in surgical patients and a higher Hb level objective. Women have a lower tidal volume than men, while blood loss in these surgical procedures is similar for both genders. We must optimize their red cell mass.
Therefore, for women scheduled for major surgery, like arthroplasties, ‘anemia definition’ should be at least the same as for male patients; i.e. Hb <13 g/dL. Some authors invite to change the term ‘preoperative anemia’ by ‘suboptimal level of preoperative Hb’ when it is <13 g/dL. Consequently, the aim of preoperative treatment should be to optimize to reach a level of Hb ≥13 g/dL (closer to 14 g/dL) and minimize the risk of transfusion without increasing the risk of thrombosis.
In the case of postoperative anemia, the goal of treatment is to achieve the safe Hb levels that avoid any transfusion and the correction of anemia in the shortest time to facilitate functional recovery and enhance the quality of life. In this period, one should pay attention to drug interactions that may cause or worsen anemia.
Another important aspect, and frequently overlooked, is the diagnosis of hematinics deficiencies without anemia (iron deficit, B12 vitamin, or folate deficiency), since its correction is crucial to optimize preoperative Hb levels, especially in case of patients under treatment with recombinant erythropoietin (rHU-EPO), and to ensure and accelerate the recovery of postoperative anemia (Grade 1C) [Citation7,Citation11].
Iron therapy indications
Some studies have shown that for patients presenting with iron deficiency and iron deficiency anemia, administration of oral iron (ferrous salts 100–200 mg/day for 4–6 weeks) improves presurgical Hb levels, reduces transfusion rates and, in some cases, shortens the time spent in hospital [Citation3,Citation4,Citation8,Citation12–Citation14].
If there is poor absorption or poor tolerance of oral iron or an accelerated response to treatment is required, preoperative IV iron supplementation, starting 3–4 weeks prior to the scheduled procedure, increases Hb levels and/or corrects anemia and reduces ABT requirements [Citation1,Citation3,Citation4,Citation8]. The intramuscular route for iron administration is not more recommended [Citation8]
As for patients presenting with slight anemia (Hb between 10 and 13 g/dL), but without iron deficiency and/or with clinical or laboratory signs of inflammation, preoperative administration of rHU-EPO has been proven to increase effective and safety Hb levels and reduce the rate of ABT [Citation7–Citation10]. The minimum effective dose of ESA for this indication is presently unknown, but it has been shown that most patients attain the target Hb level with only one or two doses [Citation7–Citation10]. All patients under treatment with rHU-EPO must receive an adequate source of iron (and vitamins) to ensure a nice response and avoid reactive thrombocytosis.
Evidence and recommendations
The European Society of Anaesthesiology (ESA) Guidelines recommends treating iron deficiency by administration of oral or IV iron (GRADE 1B) [Citation11], but this recommendation must be tempered by the severity of the anemia, the type of surgery, and the time available to treat it. Faced with a preoperative iron deficiency anemia, whenever possible and the necessary time, consider the use of oral iron for its low cost and easy administration (Grade 2B) () [Citation11].
The update of Seville’s Document [Citation10] suggests the preoperative administration of oral iron to improve preoperative Hb levels and/or reduce transfusion rate (Grade 2B). In anemic colon cancer patients, the preoperative administration of oral iron (ferrous salts), starting 14–30 days prior surgery, improved the level of Hb and decreased ABT [Citation12,Citation13]. In patients scheduled for total knee or hip arthroplasty, the administration of oral iron, together with a restrictive transfusion protocol, improved Hb levels, reduced transfusion rates and, in some cases, the length of hospital stay [Citation14].
However, sometimes, either by malabsorption, contraindication, poor tolerance, or short availability before surgery, has fully justified the use of IV iron instead of classical oral iron, with which the medullary response and repletion deposits will be faster (1–2 weeks) (Grade 2B) () [Citation11]. In anemic patients scheduled for surgery, the administration of IV iron increases the Hb levels, the anemia was mostly corrected and reduced ABT needs.
Commentary
Although the orthodox view is that early preoperative anemia assessment (at least 1 month before surgery) [Citation15], classification, and management is preferred, data from more pragmatic approaches suggest that anemia treatment (mainly with iron) should always be attempted in any major surgical procedures, as any time may be a good time for patients to benefit from it.
The PBM multimodal programs must be well defined, adapted to the means of each hospital, the characteristics of our patients, and the experience of the health professionals involved. This is the right way to reach the objective of conducting major surgical procedures without the use of ABT, without complications and reducing costs.
Table 1. American Association of Blood Banks (patient blood management awareness week (November 2–6, 2015)).
Table 2. Preoperative correction of anemia of ‘Management of severe perioperative bleeding ESA Guidelines.’
References
- Thomson A, Farmer S, Hofmann A, et al. Patient blood management- a new paradigm for transfusion medicine? ISBT Sci Ser. 2009;4:423–435.
- Shander A, Javidroozi M, Perelman S, et al. From bloodless surgery to patient blood management. Mt Sinai J Med. 2012;79(1):56–65.
- Spahn DR. Anemia and patient blood management in hip and knee surgery. A systematic review of the literature. Anesthesiology. 2010;113:482–495.
- Patient Blood Management. [ cited 2015 Dec]. Available from: http://www.aabb.org/pbm/Documents/pbm-whitepaper-form-website.html.
- Li L, Zhu D, Chen X, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcome for patients undergoing gastric carcinoma surgery: a meta-analysis. Medicine (Baltimore). 2015;94(39):e1574.
- Rasouli MR, Maltenfort MG, Erkocak OF, et al. Blood management after total joint arthroplasty in the United States: 19-year trend analysis. Transfusion. 2016;56(5):1112–1120.
- Canillas F, Gómez-Ramírez S, García-Erce JA, et al. “Patient blood management” in orthopaedic surgery. Rev Esp Cir Ortop Traumatol. 2015;59(3):137–149.
- Muñoz M, Gómez-Ramírez S, Kozek-Langeneker S, et al. ‘Fit to fly’: overcoming barriers to preoperative haemoglobin optimization in surgical patients. Br J Anaesth. 2015;115(1):15–24.
- Muñoz M, Gómez-Ramírez S, García-Erce JA. Implementing patient blood management in major orthopaedic procedures: orthodoxy or pragmatism? Blood Transfus. 2014;12(2):146–149.
- Leal-Noval SR, Muñoz M, Asuero M, et al.; Spanish Expert Panel on Alternatives to Allogeneic Blood Transfusion. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11(4):585–610.
- Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013 Jun;30(6):270–382.
- Lidder PG, Sanders G, Whitehead E, et al. Pre-operative oral iron supplementation reduces blood transfusion in colorectal surgery - a prospective, randomised, controlled trial. Ann R Coll Surg Engl. 2007;89:418–421.
- Okuyama M, Ikeda K, Shibata T, et al. Preoperative iron supplementation and intraoperative transfusion during colorectal cancer surgery. Surg Today. 2005;35:36–40.
- Cuenca J, García-Erce JA, Martínez F, et al. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int J Surg. 2007;5(2):89–94.
- Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22.
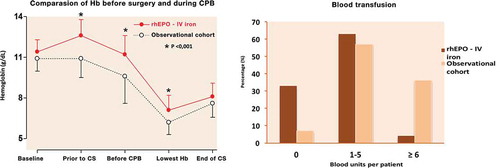
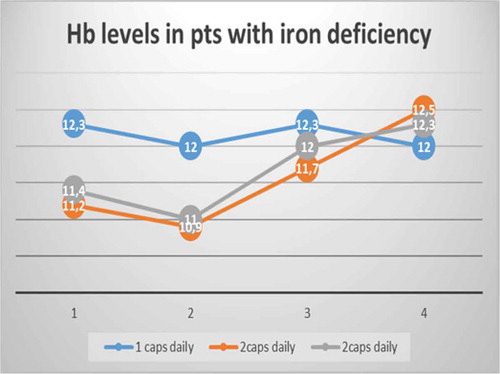
Oral high-dose Sucrosomial® Iron vs intravenous iron in sideropenic anemia intolerant/refractory to iron sulfate. Multicentric randomized study
Giulio Giordano
Regional Hospital ‘A. Cardarelli’, ASREM, Campobasso, Italy
Background
In iron deficiency anemia, support with intravenous iron allows a faster anemia correction and a faster ferritin increase than iron sulfate [Citation1–Citation4]. Frequently, iron sulfate and intravenous iron generate adverse events as hypotension, urticarial reactions, shock, epigastralgia, constipation, or diarrhea [Citation5–Citation11]. High doses of oral iron frequently are poorly tolerated because of adverse events.
Aim
Aim of this study is to verify if high doses of oral Sucrosomial® Iron are safe, cost effective, and well tolerated as standard doses of intravenous ferrigluconate in patients with iron deficiency anemia intolerant/refractory to iron sulfate.
Patients and methods
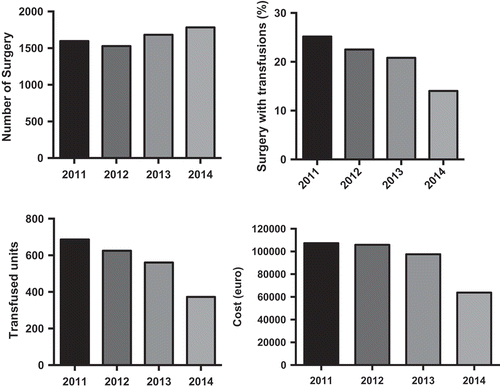
We considered two groups of patients (randomized 1:1) with iron deficiency anemia without other relevant comorbidities. In group A, M/F was 2/3, 15 patients had hemorrhagic gastritis, 8 hemorrhagic enteric bleeding angiodysplasia, 22 hypermenorrhea, median level of hemoglobin (Hb) was 8.5 g/dL (R 6.5–10), median ferritin level was 5 ng/mL (R 3–21), with a normal level of C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR), and received sucrosomial iron 30 mg 4 tablet/day. In group B, M/F was 2/3, 18 patients had hemorrhagic gastritis, 6 hemorrhagic enteric bleeding angiodysplasia, 21 hypermenorrhea, median level of Hb was 8.2 g/dL (R 7.5–9.5), median ferritin level was 7 ng/mL (R 2–19), with a normal level of CRP or ESR, and received IV sodium ferrigluconate 62.5 mg IV in normal saline 100 mL in 3 h/day. The median treatment costs in each group were calculated considering the monthly global treatment cost for each patient in the treatment period. This provided an estimate of the costs, independent of the precise cost of the drug, but tied to the final outcome (efficacy) of the therapeutic strategy used during the observation period.
Results
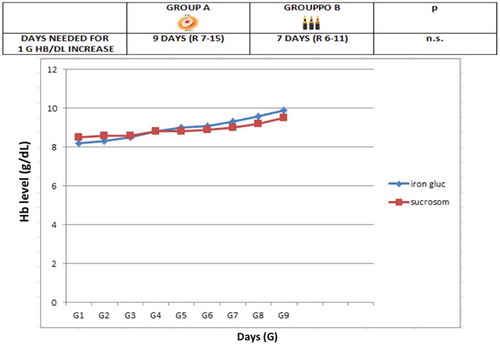
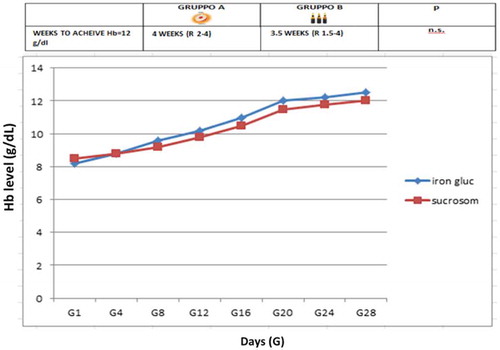
In group A, 1 g Hb increase was observed after a median of 9 days (R 7–15) (), a target Hb level of 12 g/dL was achieved in a median time of 4 weeks (R 2–5) () with a median cost of €120/month (R 95–180), 12 (26%) patients showed adverse events (7 epigastralgia, 5 diarrhea). In group B, 1 g Hb increase was observed after a median of 7 days (R 6–11) (), a target Hb level of12 g/dL was achieved in a median time of 3 weeks (R 1.5–4) () with a median cost of €300/month (R 250–380), 10 (22%) patients showed adverse events (2 hypotension, 2 urticaria and headache).
Conclusion
Oral high dose Sucrosomial® Iron support is safe, fast, well tolerated, and cost effective as intravenous iron in sideropenic anemia. This study needs confirmation on a larger cohort of patients.
References
- Crosby WH. The rationale for treating iron deficiency anemia. Arch Intern Med. 1984;144:471.
- Alleyne M, Horne MK, Miller JL. Individualized treatment for iron-deficiency anemia in adults. Am J Med. 2008;121:943.
- DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(3):1324.
- Kruske SG, Ruben AR, Brewster DR. An iron treatment trial in an aboriginal community: improving non-adherence. J Paediatr Child Health. 1999;35:153.
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383.
- Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29:291.
- Rimon E, Kagansky N, Kagansky M, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005;118:1142.
- Werner E, Kaltwasser JP, Ihm P. [Oral iron treatment: intestinal absorption and the influence of a meal (author’s transl)]. Dtsch Med Wochenschr. 1977;102:1061.
- Boggs DR. Fate of a ferrous sulfate prescription. Am J Med. 1987;82:124.
- Faich G, Strobos J. Sodium ferric gluconate complex in sucrose: safer intravenous iron therapy than iron dextrans. Am J Kidney Dis. 1999;33:46448.
- Miller HJ, Hu J, Valentine JK, et al. Efficacy and tolerability of intravenous ferric gluconate in the treatment of iron deficiency anemia in patients without kidney disease. Arch Intern Med. 2007;167:1327.
Anemia in pre and post cardiac surgery
Mercè Cladellas
Head of Section Cardiology Service, Hospital del Mar and IMIM investigator, Barcelona, Spain
Introduction
Anemia is a relatively common finding in patients with cardiac disease, affecting a third of patients undergoing elective cardiac surgery (CS). Currently, the reasons for this prevalence are not clear, may be related to age, comorbidities, previous treatment, or the cardiovascular disease process itself. However, observational studies show a strong association between preoperative anemia and worse outcomes following CS (Ranucci, Zindrou, Boening, Williams Elmistakaway, Cladellas). In addition, these patients receive a higher number of blood transfusions during surgery compared to non-anemic patients (Habbit, van Straten). These findings underline the difficulty of distinguishing between whether increasing postoperative morbidity and mortality is secondary to anemia or to the adverse effects of one’s own blood transfusions. Consequently, despite these limitations, diagnosis and management of preoperative anemia represent an opportunity to optimize patients before CS, thereby reducing blood transfusions and potentially improving postoperative outcomes.
Differences in CS: on-pump or off-pump approach
Traditionally, CS is performed with cardiac arrest (on-pump) coupled with cardiopulmonary bypass (CPB), also known as a heart-lung machine. This machine temporarily performs the function of the heart and lungs during surgery. The use of this circuit is associated with coagulation disorders, activation of inflammatory cells by contact of the bloodstream with foreign surfaces, and alterations in tissue perfusion. In order to maintain optimal tissue perfusion during CPB, it is necessary to provide a large volume of fluid in the system (about 1.5 liters of crystalloid or colloid solutions) that facilitates a decrease in blood viscosity and an improvement in microcirculatory flow. Although a certain degree of hemodilutional anemia is well tolerated, excessive hemodilution may compromise systemic oxygen delivery and could induce ischemic organ injury. In this context, a transfusion of red blood cells (RBCs) is necessary, although it is not without risks (Ferraris). In spite of many studies to determine the optimal degree of hemodilution during CPB, currently it remains uncertain. In a cohort of 5000 cardiac surgical patients, Habib et al. divided patients into five groups according to the lowest hematocrit (Hc) during CPB. They showed that the degree of anemia during CPB is associated with preoperative Hb () and the number of RBC transfusions was higher when the nadir of Hb on CPB decreased. Furthermore, they reported that postoperative morbidity and mortality as well as resource utilization increased significantly as Hc values decreased (). This study advises maintaining Hc over 22% on CPB in order to improve postoperative outcomes.
An alternative to conventional surgery in patients with ischemic heart disease is CS off-pump. Coronary revascularization with this technique is performed on the beating heart with the use of stabilizing devices. It has been developed to decrease perioperative complications related to the use of CPB. A multicenter study conducted in 79 hospitals in 19 countries that included 4752 patients, found that the need for RBC was 50.7% in coronary artery bypass grafting off-pump versus 63.3% in conventional surgery (Lamy A).
Preoperative anemia as a risk factor for early operative mortality
Despite the absence of randomized trials, the association between anemia and postoperative adverse outcomes is widely accepted. This relationship is poorly understood and the cause of this anemia is probably multifactorial: (a) low Hb concentrations may compromise perioperative oxygen delivery leading to tissue hypoxia and organ injury; (b) the treatment of perioperative anemia is RBC transfusions and this is associated with additional increases in risk. Ferraris et al., in a large sample of surgical patients, demonstrated that transfusion of a single unit of RBC increased the risk of mortality and serious postoperative complications compared to patients who did not receive transfusion (propensity matched, mortality: 5.2% vs. 6.1%, p = 0.005, respectively); (c) preoperative anemia may be a marker of comorbid conditions. shows an increase in preoperative comorbidities when preoperative Hb decreases.
On the other hand, postoperative mortality and morbidity is higher in valve replacement than isolated coronary revascularization as shown in the risk score models (EuroScore, STS). Moreover, mitral valve replacement or concomitant coronary revascularization increases the risk of early mortality in relation to aortic valve replacement. This fact together with the population included in studies (age, preoperative comorbidities) can account for the variability in results among researchers who are attempting to demonstrate this association (). However, it is reasonable to suggest that preoperative assessment and treatment of preoperative anemia should improve outcomes in these patients.
Table 1. Preoperative comorbidities according to the degree of anemia (Habib et al.).
Table 2. Association between preoperative anemia and outcomes after cardiac surgery.
Treatment of preoperative anemia
In patients with cardiovascular disease, iron deficiency is common either due to depletion of whole-body iron stores (absolute iron deficiency (AID)) or because of restricted availability of iron for erythrogenesis (functional iron deficiency (FID)). The latter is a state in which there is insufficient iron incorporation into the hematopoietic tissue despite adequate iron stores. It is common in chronic inflammatory disorders, where iron uptake from the gut, as well as the release of iron from macrophages, is inhibited.
Although preoperative anemia can readily be identified, diagnosis of anemia due to functional iron deficiency is a subject under debate. A recent study showed that the cause of anemia prior to CS was FID in 49% and AID only in 7% (Hung), unlike another study that showed an increase in AID anemia (12 of 25 anemic patients) (Piednoir). In this context, preoperative anemia could respond to intravenous iron administration or injection of recombinant erythropoiesis-stimulating agents (rhEPO) along with combinations thereof. Although the use of iron therapy by itself for preoperative anemia can increase preoperative Hb, currently the effect of this treatment in CS is unknown.
Preoperative treatment with rhEPO in anemic patients scheduled for CS is associated with a reduction in preoperative anemia (Alghamdi, Muñoz). However, there was a great variability in total rhEPO doses, time of administration, as well as outcomes (). In our experience, in anemic patients undergoing valve replacement, treatment with rhEPO and intravenous (IV) iron which started 4 weeks prior to surgery increased preoperative Hb and decreased of RBC transfusions (). This resulted in shorter hospital stays and better postoperative survival in the group in comparison to the group without treatment (Cladellas). However, the risk of thromboembolic events and hypertension in this therapy has been described, but in CS these side effects have not been observed (Cladellas, Weltert, Yoo). Currently, guidelines of several societies support the use of rhEPO combined with iron in patients with a high risk for postoperative anemia (Class IIa, level B) (Leal-Noval, Brown).
Treatment of postoperative anemia
Postoperative recovery from anemia after CS is delayed by the decrease in endogenous production of erythropoietin due to inflammation response to CPB, and perioperative renal ischemia may also contribute to this reduced endogenous production. In this regard, treatment with intravenous iron can be more effective than oral iron to restore postoperative anemia. One randomized controlled trial by Johansson et al. evaluated the effect of preoperative administration of IV iron isomaltoside in non-anemic patients undergoing CS. At 4 weeks after CS, 92% of patients in the placebo group remained anemic as opposed to 61% of the group treated with iron isomaltoside (p > 0.05). In contrast, the other clinical trials did not identify a significant difference in subsequent Hb levels using IV iron, with or without rhEPO (Garrido-Martín, Madi-Jebara). However, doses of iron administered (<500 mg) in these trials were lower than in the study by Johansson et al. On the other hand, preliminary studies indicate that new oral iron formulations such as oral liposomal iron in the context of inflammation seem to offer advantages over conventional oral iron supplements (Pisani), although more studies are required.
Table 3. Treatment with rhEPO-iron according to different researchers.
Conclusions
Preoperative anemia in CS
Is associated with an increase in both postoperative mortality and morbidity.
It is a powerful predictor of the need for blood transfusions.
Preoperative treatment with iron and EPO can decrease the need for blood transfusions and improve postoperative survival. However, randomized clinical trials are necessary in the diagnosis and treatment of preoperative anemia.
Postoperative anemia
Intravenous iron therapy has been shown to be beneficial in improving postoperative anemia. However, as in case of preoperative anemia, randomized clinical trials are needed to assess the effectiveness of this treatment.
References
- Ranucci M, Di Dedda U, Castelvecchio S et al. Impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg. 2012;94:1134–1141.
- Zindrou D, Taylor KM, Bagger JP. Preoperative haemoglobin concentration and mortality rate after coronary artery bypass surgery. Lancet. 2002;359:1747–1748.
- Boening A, Boedeker RH, Scheibelhut C, et al. Anemia before coronary artery bypass surgery as additional risk factor increases the perioperative risk. Ann Thorac Surg. 2011;92:805–810.
- Williams ML, He X, Rankin JS, et al. Preoperative hematocrit is a powerful predictor of adverse outcomes in coronary artery bypass graft surgery: a report from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2013;96:1628–1634.
- Elmistekawy E, Rubens F, Hudson C, et al. Preoperative anemia is a risk factor for mortality and morbidity following aortic valve surgery. Eur J Cardiothorac Surg. 2013;44:1051–1055.
- Cladellas M, Bruguera J, Comín J, et al. Is pre-operative anemia a risk marker for in-hospital mortality and morbidity after valve replacement? Eur Heart J. 2006;27:1093–1099.
- Habib RH, Zacharias A, Schwann TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–1450.
- van Straten AH, Hamad MA, van Zundert AJ, et al. Preoperative hemoglobin level as a predictor of survival after coronary artery bypass grafting: a comparison with the matched general population. Circulation. 2009;120:118–125.
- Ferraris VA, Davenport DL, Saha SP, et al. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012 Jan;147(1):49–55.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497.
- Carrascal Y, Maroto L, Rey J, et al. Impact of preoperative anemia on cardiac surgery in octogenarians. Interact Cardiovasc Thorac Surg. 2010;10(2):249–255.
- Muñoz M, Gómez-Ramírez S, Martín-Montañez E, et al. Perioperative intravenous iron: an upfront therapy for treating anemia and reducing transfusion requirements. Nutr Hosp. 2012;27:1817–1836.
- Karkouti K, Wijeysundera DN, Beattie WS, Reducing Bleeding in Cardiac Surgery (RBC) Investigators. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117(4):478–484.
- Bell ML, Grunwald GK, Baltz JH, et al. Does preoperative hemoglobin independently predict short-term outcomes after coronary artery bypass graft surgery? Ann Thorac Surg. 2008 Nov;86(5):1415–1423.
- Matsuda S, Fukui T, Shimizu J, et al. Associations between preoperative anemia and outcomes after off-pump coronary artery bypass grafting. Ann Thorac Surg. 2013 Mar;95(3):854–860.
- Paparella D, Guida P, Scrascia G, et al. On-pump versus off-pump coronary artery bypass surgery in patients with preoperative anemia. J Thorac Cardiovasc Surg. 2015 Apr;149(4):1018–1026.e1.
- Riera M, Ibáñez J, Molina M, et al. Preoperative anemia in coronary surgery: a risk factor?. Med Intensiva. 2009 Nov;33(8):370–376.
- Kim CJ, Connell H, McGeorge AD, et al. Prevalence of preoperative anaemia in patients having first-time cardiac surgery and its impact on clinical outcome. A retrospective observational study. Perfusion. 2015 May;30(4):277–283.
- Kulier A, Levin J, Moser R, et al. Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007 Jul 31;116(5):471–479.
- van Straten AH, Külcü K, Özdemir HI, et al. Preoperative hemoglobin level as a predictor of mortality after aortic valve replacement. J Cardiothorac Vasc Anesth. 2013 Aug;27(4):716–722.
- Hung M, Ortmann E, Besser M, et al. A prospective observational cohort study to identify the causes of anemia and association with outcome in cardiac surgical patients. Heart. 2015 Jan;101(2):107–112.
- Piednoir P, Allou N, Driss F, et al. Preoperative iron deficiency increases transfusion requirements and fatigue in cardiac surgery patients: a prospective observational study. Eur J Anaesthesiol 2011;11:796–801.
- Alghamdi AA, Albanna MJ, Guru V, et al. Does the use of erythropoietin reduce the risk of exposure to allogeneic blood transfusion in cardiac surgery? A systematic review and meta-analysis. J Card Surg. 2006;21:320–326.
- Kyo S, Omoto R, Hirashima K, et al. Effect of human recombinant erythropoietin on reduction of homologous blood transfusion in open-heart surgery. A Japanese multicenter study. Circulation 1992;86(supplII):413.
- Watanabe Y, Fuse K, Naruse Y, et al. Subcutaneous use of erythropoietin in heart surgery. Ann Thorac Surg.1992 Sep;54(3):479–483; discussion 483–4.
- Kulier AH, Gombotz H, Fuchs G, et al. Subcutaneous recombinant human erythropoietin and autologous blood donation before coronary artery bypass surgery. Anesth Analg. 1993 Jan;76(1):102–106.
- Schmoeckel M, Nollert G, Mempel M, et al. Effects of recombinant human erythropoietin on autologous blood donation before open heart surgery. Thorac Cardiovasc Surg. 1993 Dec;41(6):364–368.
- Hayashi J, Kumon K, Takanashi S, et al. Subcutaneous administration of recombinant human erythropoietin before cardiac surgery: a double-blind, multicenter trial in Japan. Transfusion. 1994 Feb;34(2):142–146.
- Gombotz H. Subcutaneous epoetin alfa as an adjunct to autologous blood donation before elective coronary artery bypass graft surgery. Semin Hematol. 1996 Apr;33(2 Suppl 2):69–70; discussion 71–72.
- D'Ambra MN, Gray RJ, Hillman R, et al. Effect of recombinant human erythropoietin on transfusion risk in coronary bypass patients. Ann Thorac Surg. 1997 Dec;64(6):1686–1693.
- Sowade O, Warnke H, Scigalla P, et al. Avoidance of allogeneic blood transfusions by treatment with epoetin beta (recombinant human erythropoietin) in patients undergoing open-heart surgery. Blood. 1997 Jan 15;89(2):411–418.
- Kiyama H, Ohshima N, Imazeki T, et al. Autologous blood donation with recombinant human erythropoietin in anemic patients. Ann Thorac Surg. 1999 Nov;68(5):1652–1656.
- Weltert L, D'Alessandro S, Nardella S, et al. Preoperative very short-term, high-dose erythropoietin administration diminishes blood transfusion rate in off-pump coronary artery bypass: a randomized blind controlled study. J Thorac Cardiovasc Surg. 2010;139:621–626.
- Yoo YC, Shim JK, Kim JC, et al. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. 2011;115:929–937.
- Cladellas M, Farré N, Comín-Colet J, et al. Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement. Am J Cardiol. 2012; 110:1021–1026.
- Leal-Noval SR, Muñoz M, Asuero M, et al. The 2013 Seville Consensus Document on alternatives to allogenic blood transfusion. An update on the Seville Document. Rev Esp Anestesiol Reanim. 2013;60:263.e1–263.e25.
- Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ann Thorac Surg. 2011;91:944–982.
- Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang. 2015;109:257–266.
- Madi-Jebara SN, Sleilaty GS, Achouh PE, e t al. Postoperative intravenous iron used alone or in combination with low-dose erythropoietin is not effective for correction of anemia after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:59–63.
- Garrido-Martín P, Nassar-Mansur MI, de la Llana-Ducrós R, et al. The effect of intravenous and oral iron administration on perioperative anemia and transfusion requirements in patients undergoing elective cardiac surgery: a randomized clinical trial. Interact Cardiovasc Thorac Surg. 2012;15:1013–1018.
- Pisani A, Riccio E, Sabbatini M, et al. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: a randomized trial. Nephrol Dial Transplant 2015;30:645–652.
Poster Discussion
Effect of oral Sucrosomial® Iron in CKD patients with anemia
Ioannis Griveasa,b,c
aNephrology Department, 417 Veterans Army Administration Hospital (NIMTS), Athens, Greece; bPrivate Dialysis Unit “Nefroiatriki”, Athens, Greece; cPrivate Renal Clinic “Athens-nephrology”, Athens, Greece
Background: Anemia is a common manifestation in patients with chronic kidney disease (CKD) and is linked with iron deficiency. The optimum route of administration of iron is controversial in this group of patients, since oral administration is easier, safer, and less expensive, but may be linked to gastrointestinal side effects and suboptimal iron absorption. Sucrosomial® Iron is a new iron formulation in a phospholipid membrane with reported high bioavailability, low incidence of side effects and satisfactory tolerability.
Objectives: The purpose of this study was to investigate the efficacy and tolerability of oral Sucrosomial® Iron in CKD patients with anemia.
Methods: A total of 10 patients with CKD stage 3–5 (eGFR <60 mils/min, range: 12–48) and anemia (Hb<12 g/dL, ferritin<200 ng/mL) were enrolled in our study. During the 6-month study period, all of the patients had stable renal function, did not need to be transfused or admitted to the hospital for any reason and received oral Sucrosomial® Iron (Sideral®) once daily. Hematological profile and renal function were recorded at the beginning of the study, 3 months later and at the end of the study protocol. The primary efficacy end points of the study included the change in Hb values from baseline to end of treatment. Adverse effects and compliance data were reported from the day of initial treatment to the end of treatment. Data were analyzed using t-test (SPSS).
Results: Hemoglobin levels were 9.82 ± 2 g/dL at the beginning of the study and ended to be 10.36 ± 0.97 g/dL, which represented a 5.5% increase (p = NS) (). At the same time, Hct levels increased from 31.4% ± 4.92% at the beginning of the protocol to 32.28% ± 3.05% at the end (increase 3.12%, p = NS) (). Ferritin levels, which are one index of iron stores also increased from 91.9 ± 75.74 mcg/L to 129.28 ± 177.05 mcg/L (increase 40.67%, p = NS) (). Oral Sucrosomial® Iron was well tolerated and no significant adverse effects were recorded.
Conclusions: Oral Sucrosomial® Iron seems to be a safe and efficacious alternative in managing CKD patients with anemia. Despite the small amount of patients in our study protocol, the low rate of adverse events with Sucrosomial® Iron and its practicality suggest that this formulation has all the potential to be the first step to correct anemia in stable CKD patients. Further, larger studies are needed to investigate Sucrosomial® Iron effects in complicated CKD patients and help scientific community to reach solid conclusions.
Efficacy of Sucrosomial® Iron (Sideral® Forte) in the treatment of anemia in patients affected by systemic sclerosis
Simone Parisi, Maria Bruzzone, Marco Scarati, Marta Priora, Clara Lisa Peroni, Enrico Fusaro
Rheumatology Unit, Azienda Ospedaliero Universitaria Città della Salute e della Scienza, Torino, Italy
Background: Systemic Sclerosis (SSc) is a chronic autoimmune disease characterized by progressive collagen production with accumulation in skin and internal organs, and endothelium dysfunction. Anemia is one of the most common hematological issues in these types of patients. The etiology is multifactorial: especially chronic inflammatory status and malnutrition. Indeed, gastrointestinal involvement commonly affects patients with SSc (50–88%) and is quite heterogeneous varying from asymptomatic disease to significant dysmotility causing complications like malabsorption, weight loss, and severe malnutrition. Sucrosome has an anti-inflammatory effect and transports its content, beyond gastric and enteric wall, directly in the bloodstream. Moreover, the sucrose esters, at nontoxic concentrations, are able to significantly reduce the resistance of the gastrointestinal barrier by increasing the permeability.
Objectives: The aim of this study is to evaluate the efficacy of Sucrosomial® Iron than iron sulfate treatment in the correction of anemia in patients affected by SSc.
Methods: We examined 43 patients affected by SSc according to ACR Criteria 2013, divided into Group I and Group II.
Group I: 21 patients (10 SSc diffuse, 11 SSc limited) median age 54.2 years ± SD 11.80, Hb 10.2 g/dL ± SD 1.70, with a median ferritin level of 130 ng/mL ± SD 72.30, saturation of iron binding capacity <20%, ESR 26 mm/h ± SD 19.20, CRP 5.50 mg/I ± SD 4.20, received Sucrosomial® Iron 60 mg/day (Sideral® Forte 2cps/day), for 12 weeks.
Group II: 22 patients (12 SSc diffuse, 10 SSc limited) median age 51.6 years ± SD 12.70, Hb 10.7 g/dL ± SD 1.50, with a median ferritin level of 110 ng/mL ± SD 64.3, saturation of iron binding capacity <20%, ESR 23 mm/h ± SD 16.90, CRP 5.0 mg/L ± SD 3.90, received iron sulfate 330 mg/day, for 12 weeks.
Results: After 12 weeks of treatment, the Group I showed an improvement of hemoglobin level up to 13.4 g/dL ± SD 1.60, a median ferritin level of 240 ng/mL ± SD 60.10. ESR and CRP decreased to 9.6 mm/h ± SD 10.80 and 3.50 mg/L ± SD 2.10, respectively (). On the other hand, the Group II showed a median hemoglobin level of 11.9 g/dL SD ± 1.40, a median ferritin level of 150 ng/dL ± SD 60.3, ESR 19 mm/h ± SD 13.60, PCR 4.2 mg/L ± SD 2.10 (). In Group II, two patients showed nausea, two constipation, and one diarrhea.
Conclusions: Sucrosomial® Iron (Sideral® Forte) seems to be more effective and well tolerated than iron sulfate for correction of anemia in SSc patients who have both chronic inflammation and gastrointestinal malabsorption issues.
Table A. Group I (21 patients): Sucrosomial® Iron treatment; Group II (22 patients) sulfate iron treatment.
Sucrosomial® Iron versus ferrous sulfate for anemia in patients undergoing peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
María Barragansa, Miguel Camblorb, Cristina Cuerdaa, Irene Bretóna, Marta Motillaa, Luisa Carrascala, Cristina Velascoa, Isabel Higueraa, Wenceslao Vasquezb, Luis Gonzalez-Bayónb, José Luis García-Sabridob and Pilar Garcíaa
aUnit of Clinical Nutrition and Dietetics, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain, bGeneral Surgery Department, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain
Background: Iron deficiency anemia is common after cytoreductive surgery with intraperitoneal hyperthermic chemotherapy because it is an aggressive surgical therapeutic process. Recovery typically takes several weeks or months. Conventional oral treatment with ferrous salts gets reverse slowly. The evidence base for this practice is limited.
Objectives: We have evaluated the effectiveness, tolerance, and safety of Sucrosomial® Iron treatment compared to standard ferrous salt treatment in iron deficiency anemic patients with peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy surgery.
Methods: Fifteen patients with an hemoglobin level <11 g/dL, after treatment by cytoreductive surgery with intraperitoneal hyperthermic chemotherapy, were randomized to receive either a 3-month course of standard 80 mg daily of ferrous sulfate therapy or Sucrosomial® Iron (Fisiogen Ferro Forte®, ferric pyrophosphate in sucrosomes, Ultrafer®) with the following composition: 30 mg of elemental iron and Vitamin C 64 mg. Hematologic and simple anthropometry parameters were measured before and after 3 months of surgery. Statistical analysis was performed by a non parametric Mann–Whitney test.
Results: Median characteristics of both groups are shown in and . The most frequent bowel resections were ileocecectomy and there was only a total gastrectomy. Primary tumors were appendicular (6), colon (4), ovary (4), and gastric (1) (). The mean rise in hemoglobin levels, 1 month after discharge from the hospital, was 2.1 g/dL in the Sucrosomial® Iron group, compared with 2.4 g/dL in the ferrous sulfate group (p = NS). The mean rise in body mass index was 0.9 kg/m2 and 1.3 kg/m2 in the two groups, respectively (). There was no significant difference between the two groups with regard to neither the hematologic parameters nor the peritoneal cancer index (PCI). Two patients from both groups reported mild adverse effects of the medication (diarrhea, discoloration of stools, and constipation).
Conclusions: Sucrosomial® Iron therapy is safe, well tolerated, and effective at least as a standard ferrous salt therapy in patients undergoing cytoreductive surgery with intraperitoneal hyperthermic chemotherapy. The improvement in hemoglobin was similar to standard therapy despite having a lower weight gain. This study needs confirmation on a larger cohort of patients.
Table 1. Patients’ characteristics.
Table 2. Baseline values.
A cost-effective implementation of preoperative protocol with Sucrosomial® Iron supplementation
M. Scardinoa, F. Martorellia, E. Perab and G. Tarantinob
aHip and Prosthetic Orthopedics OU, Humanitas research hospital, Rozzano, Italy; bPharmanutra SpA, Pisa, Italy
Background: The condition of anemia in patients undergoing surgery occurs in almost 90% of patients. The prevention of postoperative anemia is pivotal regardless of the type of surgery, as low Hb values correlate to increased length of hospital stay, greater use of blood transfusions, and a higher mortality. Indeed, it was demonstrated that patients with anemia undergoing surgery have a 42% higher risk of death and 35% more risk of severe complications post surgery compared to non-anemic patients. It is therefore essential for the patient undergoing surgery to maintain hemoglobin levels greater than 13 g/dL. Patients supplemented with iron, undergoing knee replacement, showed a lower transfusion rate (5.8%) compared to control group (32%). Sucrosomial® Iron (Sideral® Forte) is a unique iron formulation that provides for the protection of the iron within a phospholipid bilayer enveloped in a layer of sucrester and it shows greater bioavailability and tolerability compared to other iron formulations. Several studies on Sucrosomial® Iron confirmed the high tolerability and the improvement of the patient’s anemic condition in terms of serum iron, hemoglobin, and ferritin. Moreover, thanks to its high bioavailability, it was also demonstrated equivalence, in terms of increase in Hb values between the oral Sucrosomial® Iron and intravenous iron therapy, with results confirmed in both cancer and nephropathic patients. Given the risks and possible complications of intravenous iron administration and the limits of oral iron therapy, the Sucrosomial® Iron represents an effective and safe alternative to use in patients scheduled for surgery.
Objective: To evaluate the efficacy of Sucrosomial® Iron in patients undergoing prosthetic hip surgery in terms of hemoglobin recovery, length of hospital stay, and blood transfusion required.
Methods: All patient (6589) undergoing prosthetic hip surgery between 2011 and 2014 were retrospectively analyzed. Since 2011, a standard preoperative protocol was followed and in 2014 it was implemented with the introduction of iron supplementation performed as following. During the preoperative visit, 21 days before the scheduled surgery, full blood exams were performed, which included PCR and iron parameters such as ferritin. If ferritin levels were <100 mcg/L and Hb levels <14 g/dL for male and 13 g/dL for female, Sucrosomial® Iron (Sideral® Forte, 1 cps/day for 30 days) supplementation was prescribed to the patient. Similarly, patients were also supplemented with Sucrosomial® Iron if their ferritin levels were >100 mcg/L, but Hb levels were <14 g/dL for male and 13 g/dL for female and PCR levels were elevated.
Results: The implementation of the standard preoperative protocol with the addition of Sucrosomial® Iron supplementation showed a reduction in the length of hospital stay from an average of 15 days in 2011 to 10 days in 2014, thanks to a faster recovery in the hemoglobin levels during the postoperative period. Moreover, the amount of blood transfusions significantly decreased from 686 units in 2011 to 373 units in 2014. The estimated saving for the shorter length of hospitalization could be calculated in 2000 euro/patient, which it does not take into consideration the increase of efficiency in the use of hospital beds, which also increases the diagnosis-related groups (DRGs).
Conclusion: Sucrosomial® Iron supplementation was able to improve the preoperative protocol allowing a shorter hospital stay and lower blood transfusions. Thus, Sucrosomial® Iron supplementation is not only able to produce a faster hemoglobin recovery after surgery, but it is able to decrease surgery-related cost.
Oral Sucrosomial® Iron (Sideral® Forte) supplementation in patients with advanced prostate cancer and bone metastasis treated with 223 radium dichloride
Fabio Monari, Alessio Giuseppe Morganti, Giovanni Frezza, Valeria Dionisi, Stefano Giusto Picchi and Elisa Lodi Rizzini
Radioterapia Metabolica, Azienda ospedaliera – Universitaria di Bologna Policlinico Sant’Orsola Malpighi, Bologna, Italy
Background: Anemia is a common condition in oncology. It can be caused by the tumor or by cancer treatments. In particular, anemia in men with advanced prostate cancer and bone metastasis may be caused by several factors, including androgen deprivation, nutritional deficits such as vitamin B12 and/or folic acid and/or iron deprivation, bone marrow infiltration, myelosuppression secondary to chemotherapy or radiotherapy, blood loss and, in particular, hematuria caused by internal growth of the tumor into the urethra or into the bladder wall, and the chronic inflammatory state. Castration, considering both orchiectomy and androgen deprivation, is a well-documented cause of anemia, being testosterone required to produce erythropoietin in the kidney [Citation1–Citation2]. Anemia due by bone marrow infiltration, defined leukoerythroblastic anemia, has been well documented in various solid tumors with impaired hematopoiesis: in a study by Shamdas and colleagues, it was shown that 28.6% of men with metastatic prostate cancer were found to have leukoerythroblastic anemia [Citation3].
The production of inflammatory cytokines (in particular, interleukin-1 and tumor necrosis factor) by prostate cancer can decrease erythropoietin production leading to anemia of chronic disease. Patients with bone metastasis due by castration-resistant prostate cancer (CRPC), without visceral involvement, can be treated, as palliative care, with 6 monthly injections of an alpha emitter, Radium223DiChloride, which shows as common adverse events anemia, thrombocytopenia, and neutropenia [Citation4]. For this reason, patients with hemoglobin (Hb) level <10 g/dL must stop treatment [Citation5], which causes worsening of therapy effect [Citation6]. The development of anemia in patients with advanced prostate cancer and bone metastasis is gradual and progressive, and it is generally associated with adverse prognosis: its typical symptoms such as drowsiness and fatigue can require supportive therapy. Furthermore, anemia can compromise the delivery of sufficient amounts of oxygen to cells, including tumor cells: this hypoxic condition can worsen the results of radiotherapy and chemotherapy, because low tissue oxygenation is associated with a reduced sensitivity of tumors to radiation and some forms of chemotherapy, contributing to cancer progression and reduced survival [Citation7]. Oral Sucrosomial® Iron (Sideral® Forte) is an oral formulation with good bioavailability and tolerability and may improve anemia, quality of life, efficacy radiotherapy, and chemotherapy treatment [Citation8-Citation9]. Early treatment of mild-grade anemia could prevent a severe decrease in Hb level requiring blood transfusion or erythropoiesis-stimulating agents (ESAs).
Objectives: The aim of the study was to analyze the efficacy of the oral Sucrosomial® Iron supplementation (30 mg/day – Sideral® Forte) in patients with advanced CRPC and bone metastasis treated with Radium223DiChloride: therefore, we compared the different response between a group of patients receiving oral Sucrosomial® Iron supplementation and a group not receiving it, in terms of quality of life improvement and decreased fatigue in correlation with Hb increase and with Radium233DiChloride treatment compliance.
Methods: From November 2014 to January 2016, 30 patients with CRPC prostate bone metastasis cancer received monthly intravenous injections of Radium233 DiChloride. Twenty three patients presented anemia at the beginning of the treatment (Hb level <13 g/dL); 15 of those patients had Hb level between 10 g/dL and 12 g/dL, while 8 patients had 12 g/dL<Hb<13 g/dL. Sucrosomial® oral Iron was presented to 15 patients with Hb level between 10 g/dL and 12 g/dL. This patient population was divided in 3 groups: five patients refused to take the medication (Group A), seven patients have regularly taken the prescribed medication (Group B), three patients stopped taking the drug after 2 cycles of radiopharmaceutical treatment (Group C).
Results: After treatment with Sucrosomial® oral Iron, Group B had an increment of 1 g/dL of Hb level, with significant improvement of fatigue and quality of life. Instead, Group C after an early mean increase of 0.3 g/dL of Hb level in the first 2 months of treatment with Sucrosomial® oral Iron, showed a subsequent progressive decrease of Hb level (mean decrease: 1 g/dL), similarly to the five patients of Group A (mean decrease 1.35 g/dL). Furthermore, some of these patients (three for Group A and one for Group C) showed severe decrease of Hb level (<10 g/dL), and therefore they had to stop Radium223DiChloride treatment, while all seven patients of Group B, showing Hb level >10 g/dL, completed the 6 cycles of radiopharmaceutical treatment.
Conclusions: Patients with advanced prostate cancer and bone metastasis treated with monthly intravenous injections of Radium233DiChloride may require iron implementation. Sucrosomial® oral Iron (Sideral® Forte) can be used as supportive therapy to reduce fatigue and improve quality of life. It is well tolerated and increases Hb level maintaining it above the minimum level (Hb >10 g/dL), allowing better treatment compliance. We could consider its prophylactic use to prevent transfusion/ESAs in patients with preexisting mild anemia and to improve compliance at treatment with monthly intravenous injections of Radium233 DiChloride. Furthermore, it also may increase tissue oxygenation improving tumors sensitivity to radiation and some forms of chemotherapy. However, these data need confirmation on a larger cohort of patients.
References
- Esper P, Redman BG. Supportive care, pain management, and quality of life in advanced prostate cancer. Urol Clin North Am. 1999; 26:375–389.
- Fonseca R, Rajkumar SV, White WL, et al. Anemia after orchiectomy. Am J Hematol. 1998;59:230–233.
- Shamdas GJ, Ahmann FR, Matzner MB, Ritchie JM. Leukoerythroblastic anemia in metastatic prostate cancer: clinical and prognostic significance in patients with hormone-refractory disease. Cancer. 1993;71:3594–3600.
- Harrison MR, Wong TZ, Armstrong AJ, et al. Radium-223 chloride: a potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Manag Res. 2013;5:1–14.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223.
- Roque IFM, Martinez-Zapata MJ, Scott-Brown M, et al. Radioisotopes for metastatic bone pain. Cochrane Database Syst Rev. 2011;7:CD003347.
- Calabrich A, Katz A. Management of anemia in cancer patients. Future Oncol. 2011;7(4):507–517.
- Vaupel P, Dunst J, Engert A, et al. Effects of recombinant human erythropoietin (rHuEPO) on tumor control in patients with cancer-induced anemia. Onkologie. 2005;28:216–221.
- Zhao KL, Liu G, Jiang GL, et al. Association of haemoglobin level with morbidity and mortality of patients with locally advanced oesophageal carcinoma undergoing radiotherapy – a secondary analysis of three consecutive clinical Phase III trials. Clin Oncol (R Coll Radiol). 2006;18:621–627.
Sucrosomial® Iron and radiotherapy in the neoadjuvant treatment of rectal cancers. Good news for patients?
Domenico Barzaghi, Olga Cristiano, Massimo Elmo and Cesare Guida
Radiation Therapy Department, AO SG Moscati, Avellino, Italy
Background: Short-course neoadjuvant rectal radiation therapy (RT) (5 Gy x 5 days a week) has been recently improved by using dose-escalating radiotherapy treatment through simultaneous integrated boost (SIB) approach, and/or sequential chemotherapy (CT) and delayed surgery after 6–8 weeks from the end of RT. These improvements allowed to reach yCR and tumor downstaging comparable to conventional long-course CT–RT and established a new clinical practice in debilitated patients, who are in a sub-occlusive state or not controlled bleeding, or are not suitable for CT or surgery. Radiobiological studies suggest that the level of Hb affects the tumor oxygenation with an impact on the effectiveness of the treatment. Therefore, anemia correction is a fundamental step for the possibility of good response to RT.
Objectives: In this study, we evaluated the efficacy of Sucrosomial® Iron (Sideral® Forte) compared to intravenous (IV) iron, conventionally administered to treat anemia in cancer patients, to increase the level of Hb in patients (pts) with symptomatic bleeding, subjected to RT.
Methods: From January 2012 to December 2014, 23 pts affected by advanced rectal tumor (III stage, high risk for positive circumferential margin, lymph nodes +, and/or located very low (<5 cm from anal verge), and/or ECOG ≥2) were subjected to short-course intensity-modulated radiation therapy (IMRT), using dose escalation with SIB. The gross tumor volume, GTVT, was outlined by TC scan in the prone position and bellyboard, after deformable fusion with MRI of the pelvis (±mdc) e/o PET/CT. The care plan of SIB with doses of 5.5 Gy/fx at CTVT (GTVT + GTVN + mesorectum) e 5Gy/fx at CTVN (lymphatic chains), planned at Xio, (ICRU 62/83) was delivered with LINAC Siemens Oncor Impression Plus, in no more than 7 days (5 fx/w). In total, 12 pts were treated with neoadjuvant therapy, and in 4 of these patients’ RT was followed by 2 cycles of CT (capecitabine 1650 mg/mq). Surgical treatment was performed after 32–45 days. A total of 11 pts were not eligible for surgery due to medical contraindications and were treated with exclusive radiotherapy (RT) with SIB alone. In total, 15 pts were symptomatic for iron deficiency anemia with a median hemoglobin (Hb) level of 8 g/dL (range 9.2–7.8 g/dL), median ferritin level of 100 ng/mL, and median transferrin saturation level of 25%. Seven of these pts showed symptomatic bleeding. Out of these 15 pts, 11 pts were treated with oral Sucrosomial® Iron 30 mg/day (SF) and 4 pts were treated with IV iron and folic acid. These pts were compared, in terms of Hb and RT responses, with similar anemic pts treated with FH in conventional doses (100 mg/day) and RT. The iron treatment, with SF or FH, was continued from 7 days to 7 days after the end of RT. No pts reported side effects during treatment with SF or needed to interrupt RT. Hb concentration, even in pts with bleeding (n = 7), at the end of the treatment with SF (n = 11) showed a significant increase (11.4 ± 1.1 vs. 11.6 g/dL ± 0.8 g/dL), which was even higher compared to pts (n = 4) treated with intravenous iron (). Acute gastrointestinal toxicity (CTCAE IV) = 3 (diarrhea/proctitis) was detected in 2 pts. No GU, hematological, or cutaneous toxicity >2 was registered in the group treated with SF. Conversely, in 3 patients treated with FH, it was observed the transient discomfort at the intravenous injection site.
Results: All patients were assessed with a mean follow-up of 12 months. All patients achieved the remission of symptoms. In all 11 pts treated with exclusive RT, clinical and instrumental (MRI), partial remissions were observed at 9 months. The surgical specimens of the other 12 pts, that underwent surgery, showed complete pathological response in 9 pts (ypT0 with TRG 1–2 – according to surgical specimens): in all 4 pts treated with CT, SIB and dose escalation, and in 5 out of 8, treated exclusive SIB. A partial response (TRG 2-3) was reported in the remaining three. All patients treated with exclusive RT and SIB were in clinical remission (colonoscopy – rmn) at mean follow-up of 18 months (months 6–28). The use of oral Sucrosomial® Iron has not increased the patient’s discomfort, especially gastrointestinal, which is usual with other iron oral supplementation.
Conclusions: The short-course RT in rectal cancer is able to give comparable results to conventional treatment in terms of yCR and tumor downstaging. This experience has demonstrated the efficacy, tolerability, and higher ease of administration, versus other iron supplements in pts with symptomatic bleeding. The correction of Hb levels may be easily achieved with oral administration of Sucrosomial® Iron, without causing increase in GI adverse effects. The oral supplementation of iron salt previously used often induced nausea, vomiting, epigastric discomfort, sensation of heaviness, and poor gastrointestinal tolerability. Data shows that oral iron administration, compared to the intravenous iron therapy, is better tolerated and pts are more compliant to oral prescription and showed a significant increase in terms of Hb level.
Table 1. Levels of hemoglobin in the two treated groups before and after the treatments.
POSTERS
Important key role of Sucrosomial® Iron therapy in hematological disorders: a case study
M. Sagristania, A. Nasutib and F. Sessab
aHematology Unit 53, Sant’Agnello, Napoli, Italy; bHematology, P.O. San Leonardo, Castellammare di Stabia, Napoli, Italy
Background: Anemia is one of the most common disorders in hematological diseases. The etiology is multifactorial and includes dyserythropoiesis, blood loss, hemolysis, bone marrow infiltration, etc. Most of the drugs taken by these patients may impair normal erythropoiesis and produce anemia requiring supportive therapy such as blood transfusions or the use of erythropoiesis-stimulating agents (ESA). Iron supplementation is essential for the treatment of anemia in these patients. Sucrosomial® Iron (Sideral® Forte) is a preparation of ferric pyrophosphate carried within a phospholipid bilayer membrane. Compared to other oral formulations, it is well absorbed from the gut and demonstrates high bioavailability, high gastrointestinal absorption, and a low incidence of side effects. Thus, it appears to be a promising new strategy for iron therapy. Sideral® Forte is useful to improve anemia and prevent further reduction in the hemoglobin (Hb) levels that require blood transfusions, improving also the response to cytostatic therapy and the patient compliance.
Objectives: The primary aim was to evaluate the increase in Hb level from baseline to demonstrate that oral Sucrosomial® Iron therapy in patients affected by myeloproliferative disorders, and mild anemic status is effective in avoiding blood transfusions and improving quality of life (QoL). Iron status, compliance, and adverse effects were also evaluated.
Methods: A patient affected by essential thrombocythemia undergoing hydroxyurea treatment was examined. The blood exams showed mild sideropenic anemia without blood loss (Hb ≤10 g/dL, ferritin ≤100 ng/mL, transferrin saturation ≤25%). The patients received oral Sucrosomial® Iron, 60 mg/day for 3 months. The patient was monitored during the treatment and the follow-up was of 1 month.
Results: After 8 weeks of oral administration of Sideral® Forte (2 capsule/day), an increase of 2 g/dL in the Hb level was observed, without a reduction in the dose of hydroxyurea. The patient continued chemotherapy without blood transfusion support and showed a decrease in the fatigue level and an improvement in the QoL.
Conclusions: Our study showed that oral Sucrosomial® Iron is safe and able to correct anemia in a mild anemic patient undergoing chemotherapy for hematological disorder; it represents a valid support therapy, highly effective in improving the response and compliance of the patient. In this study, the patients with essential thrombocythemia receiving oral Sucrosomial® Iron for symptomatic anemia had clinically and statistically significant improvements in Hb levels and QoL.
Effectiveness of Sucrosomial® Iron in ambulatory patients
F. Simula
ASL RM 1 Ospedale S. Filippo, Roma, Italy
Background: Iron deficiency anemia is certainly the most common problem in hematological ambulatory practice. Distribution between M/F is 1:4; in feminine population its presentation is typically in age 35–54 and in premenopausal condition (almost 60% of all cases of iron loss/deficiency anemia).
Objectives: The objective of this presentation is to demonstrate the effectiveness of treatments with Sucrosomial® Iron in almost all cases.
Methods: We used an observational study, comparing IV iron treatments and oral treatments on a population of 307 patients affected by iron deficiency anemia. Of these, 264 were women aged 16–85 (mean 43).
The hemoglobin range varied between 5.6–10.4 g/dL (mean 8.4), ferritin 1–10 ng/mL. No patient received RBC transfusion. Some patients were treated initially with IV therapy and subsequently shifted to oral treatment; the goal was to achieve a value of Hb up to 12 g/dL and ferritin up to 20 ng/mL.
Results: Almost all patients had beneficial effects by the treatment, depending on the causes of anemia and comorbidities. Only 12 of them did not have any advantage from treatments with Sucrosomial® Iron; in these cases, we faced particular diseases, like gastric antral vascular ectasia (GAVE), Menetrier’s disease, and severe condition of HHT (Rendu–Osler Disease). For these patients, only IV therapy was effective, but the recovery always had a short persistence. Most of the patients had to continue oral therapy, due to the original reason of iron loss (mostly gynecological problems).
Conclusions: In past times, martial therapy with ferrous sulfate was the only efficient available treatment, but with a very high percentage of dropouts due to adverse effects. Thus, IV therapy was the alternative, but it may present severe side effects too. Furthermore, IV therapy is only practicable in the presence of doctors, well-trained nursing; it needs material and has long infusion time. Thus, it is really expensive for health systems and for society/insurances due to loss of working days. Our observation showed how oral supplementation with Sucrosomial® Iron is actually effective, safe, and preferred by patients to avoid IV therapy favoring QOL and, last but not least, it has a very low cost for public health.
Efficacy and tolerability of Sucrosomial® Iron in elderly (≥75 years) patients with solid tumors treated with anticancer agents: a retrospective analysis.
Francesco Grillonea, Simona Gualtierib, Monica Venturab, Natascia Costantinob, Pierfrancesco Tassonea,b and Pierosandro Tagliaferria,b
aAzienda Ospedaliera, Universitaria “Mater Domini”, Catanzaro, Italy; bDipartimento di Medicina Sperimentale e Clinica, Università Magna Grecia, Catanzaro, Italy
Background: Iron deficient anemia is the most frequent cancer-associated hematologic event, in which patient-, disease-, and treatment-related causal factors have been recognized. Due to blood loss or malabsorption, anemia is also commonly observed in symptomatic geriatric patients. Specific treatments, such as iron supplementation, ESA, and others are low effective and often induce toxicity in very frail patients.
Objectives: The aim of this retrospective analysis is to assess efficacy and tolerability of oral Sucrosomial® Iron in ≥75-year-old cancer patients, who underwent treatments including conventional chemotherapy, targeted agents, hormonal- and/or biological-therapy.
Methods: From 2008 to 2015, we retrospectively identified 30 consecutive patients (median aged 79 years) with mild anemia according to WHO criteria (<13 g/dL in men and <12 g/dL in women), all treated with anticancer agents for solid tumors. Patients were supplemented only with oral Sucrosomial® Iron (Sideral® Forte 30 mg/day) for at least 2 months. Efficacy was evaluated as median hemoglobin (mHb) level reached after 2 months of iron supplementation and improvement in symptoms related to anemia. Tolerability was evaluated according common toxicity criteria (CTC v4.03).
Results: Basal mHb was of 11.2 g/dL and after 2 months of iron supplement, mHb was 11.3 g/dL. 16 patients had an increase in mHb level of 0.5 g/dL, while 14 patients had a decrease in mHb of 0.4 g/dL. 3 patients had an increase of hemoglobin, which reached the range of normality. 3 patients had a decrease of hemoglobin ≤10 g/dL and 2 started ESA. 9 of the 16 patients with an increase in hemoglobin level showed improvement in anemia-related symptoms, especially fatigue. The iron supplement was well tolerated. Only 3 patients reported gastrointestinal symptoms and only 1 patient discontinued iron for grade II diarrhea.
Conclusions: Oral Sucrosomial® iron supplement is an effective and well-tolerated therapy for iron deficiency anemia in elderly cancer patients. It appears to prevent the risks and reduce the costs of ESA treatment.
High dose of oral Sucrosomial® Iron in postpartum period: a case report
A. Di Bartolomeia, P. Peruginib, M. B. Rondinellia and L. Pierellia
aUOC SIMT Azienda Ospedaliera San Camillo Forlanini, Roma, Italy; bUOC Ostetricia e Ginecologia Azienda Ospedaliera San Camillo Forlanini, Roma, Italy
Background: Iron deficiency anemia (IDA), defined by the WHO, as hemoglobin (Hb) less than 12 g/dL, is the most common cause of anemia during pregnancy and postpartum period. The problem of anemia in both prepartum and postpartum is far more prevalent in developing countries than in the Western societies. The conditions for mother and child in the postpartum, nursing, and lactation period should be as favorable as possible. During pregnancy, women have a great requirement of iron. The amount of iron required for a pregnant woman is 680 mg. Oral iron therapy is currently the treatment of choice for the majority of patients. In fact, this therapy is the first-line therapy for IDA. The causes of postpartum anemia are prepartum anemia, increased iron needs, combined with acute bleeding anemia, due to blood losses during delivery. Normal peripartum blood losses are approximately 300 mL, but hemorrhage >500 mL occurs in 5–6% of women. The peripartum bleeding is the most important etiology of IDA. During pregnancy, the prevalence of anemia is 24%, but after delivery the prevalence of anemia increases up to 50%. Usually, this is a combination of preexisting IDA and hemorrhagic anemia.
Objectives: From March 2015, we started an interdisciplinary hospital pathway, in collaboration with obstetrics, gynecologists, and hematologists to evaluate the administration of oral Sucrosomial® Iron after the hematological baseline examination. The aim of this study was to verify the effectiveness of high doses of Sucrosomial® Iron during pregnancy for the treatment of postpartum anemia.
Methods: As part of this interdisciplinary pathway, we present one of the most interesting cases in this clinical setting. We presented a case report regarding a 37-year-old woman, with alpha thalassemia trait, that received Sideral® Forte (Sucrosomial® Iron) 1 cps/day and folic acid 5 mg/day for the entire pregnancy. At enrollment, the patient had a very low level of Hb and therefore we prescribed her Sideral ®Forte, 2 cps/day for first 2 months. Before delivery her level of Hb was 10.4 g/dL, while after delivery the level of Hb was 7.7 g/dL for intercurrence of acute bleeding.
Results: The level of Hb was increased to 9.6 g/dL after 8 days, 10.6 g/dL after 30 days from delivery, and 11.7 g/dL after 50 days. The patient did not show adverse events. Moreover, the patient presented a significant increase in Hb level, even if she is afflicted with a genetic hemoglobinopathy, showing a good response and a high compliance to the treatment.
Conclusions: High dose of Sucrosomial® Iron is safe, well tolerated, and well absorbed from the gut. It demonstrates high bioavailability without any side effects. This study needs confirmation on a large cohort of patients; in fact, we are evaluating the data of this study to confirm these results. National health authorities should establish guidelines to prevent iron deficiency in pregnancy and postpartum in order to facilitate a prosperous future for both mothers and children in a continuing globalized world.
Retrospective evaluation of iron deficiency patients in a Northwestern Italy anemia ambulatory: experience with Sucrosomial® Iron
Andrea Bellodia, Elisa Molinaria, Carlo Genovaa, Lisette Del Corsoa, Serena Favorinia, Nicholas Bardia, Sabrina Beltraminia, Maria Canepaa, Paola Manginib, Francesco Puppoa, Riccardo Ghioa and Eleonora Arboscelloa
aIRCCS Azienda Ospedaliera Universitaria San Martino IST, Genova, Italy; bDistretto 13, ASL, Liguria, Italy
Background: The burden of iron deficiency anemia (IDA) is high, often being correlated with relevant symptoms and potential worsening of preexisting comorbidities.
Objectives: Here, we describe our real-life experience with Sucrosomial® Iron (SI), in order to test its safety and efficacy in IDA.
Methods: We retrospectively examined 91 patients (pts) treated with SI for IDA related to different etiologies, including gastrointestinal (GI), gynecological (Gyn), malabsorption (Mal), and not determined (ND). Three groups were identified according to first-line treatment: Group A included pts treated with oral SI 30 mg/day; pts in Group B were treated with intravenous iron (IV) (ferric carboxymaltose 500 mg or equivalent dose of ferrous gluconate) followed by maintenance with SI 30 mg/day; pts in Group C were treated with red blood cells transfusions plus IV iron and then with SI 30 mg/day as maintenance. Data for hemoglobin (Hb) and serum ferritin (Ft) were separately collected at baseline (bl) and at two follow-up visits (T1 and T2, defined according to clinical practice and tailored on the single case). Group C was excluded from the statistical analysis due to its small size. Data were collected from clinical records and analyzed using XLSTAT (Version 2015.6.01.23953).
Results: The groups were characterized as follows:
In Group A, bl median Hb was 10.3 g/dL, while Ft was 7.5 µg/L. At T1 (median: 74 days), both median Hb and Ft were increased compared to bl, resulting 11.9 g/dL (p < 0.0001) and 14.5 µg/L (p < 0.05), respectively. At T2 (median: 190 days), median Hb was significantly increased, compared to bl, to 11.60 g/dL (p < 0.0001), while median Ft increase was not significant (27.5 µg/L; p = 0.614). In Group B, bl median Hb and Ft were 8.8 g/dL and 5 µg/L. At T1 analysis (median: 39 days), a significant improvement of both median Hb and Ft was reported, their values being 11.35 g/dL (p < 0.0001) and 77.5 µg/L (p < 0.0001), respectively. At T2 (median: 202 days), median Hb and Ft were increased to 12.2 g/dL (p < 0.0001) and 27 µg/L (p < 0.01), respectively. In Group C, bl median Hb was 7.6 g/dL and Ft was 8 µg/L. At T1 (median: 22 days), median Hb and Ft were 10.7 g/dL and 72.5 µg/L, respectively. At T2 (median: 210 days), median Hb was 12.45 g/dL, while median Ft was 17.5 µg/L. In Groups A and C, no adverse events were recorded, while in Group B three pts (3.3%) experienced G1 dyspepsia with IS and four pts (4.4%) developed G2 cutaneous allergy on IV ferrous gluconate.
Conclusions: Although the study population was heterogeneous and characterized by limited numbers, SI proved to be safe in a real-life clinical setting, with good efficacy in increasing Hb levels; this finding was consistent even in the case of pts with relevant iron deficiency who often require frequent intravenous iron supplementations.
Effect of oral Sucrosomial® Iron supplementation in an elderly anemic patient treated with neoadjuvant chemoradiotherapy for locally advanced rectal cancer with insidious bleeding at the time of diagnosis: a case report
Carmelo Tuscanoa, Anna Maria Marchionea, Raffaella Carmine Spagnaa, Salvatore Costarellab, Said Al Sayyada and Giuseppe Scenic
aRadiation Oncology Department, AO “Bianchi-Melacrino-Morelli”, Reggio Calabria, Italy; bMedical Physics Department, AO “Bianchi-Melacrino-Morelli”, Reggio Calabria, Italy; cGeneral Surgery Department, AO “Bianchi-Melacrino-Morelli”, Reggio Calabria, Italy
Introduction: Tumor hypoxia has been linked to tumor progression, development of treatment resistance, and thus poor prognosis. Since anemia is a major factor causing tumor hypoxia, the association between blood hemoglobin (Hb) level and tumor oxygenation status has been a field of continuous clinical research [Citation1]. Locally advanced rectal cancer patients with a clinical indication for a neoadjuvant treatment with radiotherapy or radiochemotherapy often present at the time of the diagnosis insidious trans-rectal bleeding with consequent development of normochromic-normocytic anemia. In this report, we describe the effect of oral Sucrosomial® Iron supplementation in improving Hb levels in an old patient treated with neoadjuvant radiotherapy and an oral fluoropyrimidine for a locally advanced low rectal cancer.
Case report: A 72-year-old man with a long history of cigarette smoking and with high intake of red meat dietary habits was referred from his general practitioner for insidious rectal bleeding. After a preliminary clinical evaluation, he underwent a colonoscopy in which it was reported a friable, white-colored lesion located 6 cm into the anal sphincter. The histologic exam confirmed a moderately differentiated rectal adenocarcinoma. A baseline evaluation demonstrated an Hb level of 8.6 g/dL. Mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) were normal suggesting an anemia induced by neoplastic bleeding. Staging was assessed by pelvic MRI in which it was observed a locally advanced rectal cancer (cT3 N1) and a thoracic-abdominal contrast enhanced CT scan in which distant metastases were not diagnosed. To prevent worsening of his condition by fecal transit, it was decided to implant a diverting colostomy. The clinicians started an oral supplementation with Sucrosomal® Iron (30 mg/twice a day) 7 days before starting chemoradiotherapy (50.4 Gy associated with 825 mg/m2 b.i.d. of capecitabine) with a single dose of epoetin alpha (40000 U) administered on the first day of the integrated treatment and a maintaining dose of 10000 U/week for the first 3 weeks. The patient was followed up for the treatment period and 1 month after drug withdrawal. The Hb value at the end of treatment was significantly higher (12.4 g/dL). In this patient, blood transfusion was not needed. Surgeons practiced an anal sparing total mesorectal excision 8 weeks after the end of the chemoradiation treatment and pathologic findings showed a significant downstaging lesion (ypT1Nx).
Discussions: Correct and prompt management of anemic status in patients with locally advanced cancer with insidious bleeding is of paramount importance to improve their quality of life and to improve the treatment outcomes. Recent studies, including large retrospective analyses, have demonstrated the dramatic adverse impact of anemia upon locoregional tumor control and survival [Citation2]. This case report underlines the efficacy of oral Sucrosomial® Iron supplementation in improving the anemic status in conjunction with low doses of epoetin alpha. As an important clinical observation, we have also noticed a reduction in the small bowel irritative symptoms, suggesting an active role of the Hb oxygenation function in the repair of the mucosal cells damaged by irradiation.
References
- Vaupel P, Mayer A, Höckel M. Impact of hemoglobin levels on tumor oxygenation: the higher, the better? Strahlenther Onkol Organ Dtsch Röntgenges Al febbraio. 2006;182(2):63–71.
- Kumar P. Impact of anemia in patients with head and neck cancer. Oncologist. 2000;5 Suppl 2:13–18.
The burden of iron deficiency in modern hematology clinics
Monia Marchetti
Oncology Unit, Hospital Cardinal Massaia, Asti, Italy
Background: The prevalence of iron deficiency in Western countries is high in specific patients, such as the elderly and fertile women. Iron deficiency is mostly managed by general practitioners; therefore, hematologists are expected to deal with a selected patient population. However, diagnostic and therapy pathways for the management of iron deficiency cannot be implemented without assessing the local specific epidemiology and clinical patterns.
Objectives: To assess the characteristics of iron-deficient patients managed by hematology clinics at a community hospital in Northern Italy.
Methods: A chart review was conducted on consecutive patients visited from 1st January 2016 to 22nd January 2016 at Cardinal Massaia Hospital, Asti, Northern Italy. Patients receiving iron or erythropoietin prescriptions were included and analyzed.
Results: Out of 344 overall visits, 41 different patients were prescribed iron therapy and 32 ones erythroid-stimulating agents (ESA). Overall 13% of the visited patients were or had been on iron therapy and 10% on ESA therapy. Median age of patients considered for iron therapy was 74 (range 29–92) and two-third of the patients were female, including a large portion of non-fertile women and only one pregnant woman. A relevant portion of patients had IRC (12%), cirrhosis (10%), or severe dysmetabolic syndrome (10%). Two patients had known gastrointestinal angiodysplasia and one patient was reported to have colon cancer in the workup. Six patients were on anticoagulant or antiplatelet therapy. Documented chronic inflammation was reported for 5 patients (12%). Half of the patients managed with iron therapy had a blood cancer either actively treated or not: one patient had both severe bowel angiodysplasia and chronic myelomonocytic anemia.
Median baseline hemoglobin of assessed patients was 10.4 g/dL. Serum transferrin receptor was requested in only 3 cases (7%) and total iron-binding capacity was not assessed in one-quarter of patients. Twelve out of 23 evaluable patients (52%) achieved hemoglobin response to treatment, mostly with intravenous iron (8 patients) followed by oral iron. Sucrosomal® Iron was prescribed to 9 out of 28 patients receiving oral iron. Cumulative doses of intravenous iron ranged from 375 to 1312 mg. Carboxymaltose iron was prescribed to one patient, while all the others receive iron gluconate: no infusion reactions were reported in the time frame we analyzed. Lack of response to iron therapy was considered to be due to progression of the blood cancer, persistent inflammation, persistent bleeding, requirement of ESA, or supplementation with cyanocobalamin or folates. Most of non-responders, however, achieved a stable hemoglobin value. Serum ion level assessment 2 hours after administration of oral iron was not requested in any of the reported patients. Unexpectedly, two patients with documented MPD and thrombocytosis also reported iron deficiency: platelet count was significantly reduced after oral iron supplementation.
Conclusions: Iron deficiency is a very frequent problem in general hematology clinics; however, hematologists are rarely requested to manage iron deficiency of fertile women. Hematologists usually deal with complex patients showing multiple causes of anemia and with a low chance of response to iron therapy; therefore, an extended assessment of iron deficiency and an intensive treatment plan (including cyanocobalamin and folates) should be implemented upfront, also for the prevention of relative iron deficiency in ESA-treated patients. A locally standardized diagnostic and therapeutic pathway should be implemented to allow response assessment and optimization of resource consumption: specific clinical scenarios should be addressed.
Oral Sucrosomial® Iron supplementation in patients affected by Hodgkin lymphoma with mild anemia before chemotherapy: an observational study
A. Romano, C. Conticello, G. Motta, A. L. Caruso, A. Chiarenza, A. Figuera, E. Schinocca, M. L. Consoli, M. Parisi, V. Calafiore and F. Di Raimondo
Division of Hematology, Ospedale Ferrarotto, Catania, Italy
Background: Anemia is a presenting symptom in approximately 40% of patients with Hodgkin’s lymphoma (HL). It is more frequently observed in advanced stages and is usually associated with B symptoms such as fever, night sweats, and weight loss. In general, the anemia is normochromic and normocytic and is usually mild, with hemoglobin (Hb) levels between 10 g/dL and 12 g/dL, associated to the inflammatory status typical of the disease at diagnosis.
Oral Sucrosomial® Iron (Sideral® Forte) is an oral formulation with good bioavailability and tolerability, and may improve anemia similarly to intravenous iron. Treating early mild-grade anemia could prevent a fall in Hb levels and, thus, avoid the use of erythropoiesis-stimulating agents (ESAs).
Objectives: A retrospective observational study was designed to evaluate iron parameters before and after chemotherapy using supplementation of oral Sucrosomial® Iron in anemic HL patients in advanced stage.
Methods: Our analysis included 25 patients (median age, 35 years; range 26–44 years; 15 females and 10 males) diagnosed with HL between August 2014 and January 2015 and observed at the Institute of Hematology of the University of Catania. All patients were staged 2B or higher, according to Ann Arbor classification, none of them showed bone marrow infiltration. A continued treatment with oral Sucrosomial® Iron (Sideral® Forte) 30 mg/day was performed for the whole period of ABVD chemotherapy administration and iron parameters were tested after the first 2 months and at the end of the planned treatment. All parameters of iron metabolism were determined in the central laboratory of our division.
Results: At baseline, medium Hb level was 10.2 g/dL. The median serum iron and total iron-binding capacity in HL patients were 35 μg/dL (range 9–183 μg/dL) and 244 μg/dL (range 154–366 μg/dL), respectively, and were lower than the normal range (40–150 μg/dL for iron and 250–425 μg/dL for total iron-binding capacity). Ferritin levels showed a wide variation with a median of 90 ng/mL and a range between 7 and 7500 ng/mL. Five female patients had iron deficiency anemia, with microcytosis and ferritin levels <12 ng/mL.
After 2 months, no patients stopped Sucrosomial® Iron supplementation that was well tolerated. Hb increased up to the medium Hb level of 11.3 g/dL. At the end of treatment, medium Hb level was 12.8 g/dL. The median serum iron and total iron-binding capacity in HL patients increased up to 95 μg/dL (range 60–483 μg/dL, p = 0.03) and 264 μg/dL (range 154–318 μg/dL, p = 0.03). No patient was transfused.
Conclusions: In our setting of young advanced-stage HL patients, supplementation of oral Sucrosomial® Iron was well tolerated and maintained Hb above levels requiring further supportive therapy.
Significant anemia treatment in pregnancy with Sucrosomial® Iron: case study.
Fabrizio Niglioa, Carolina Senatorea, Elena Moria, Elena Caltrana, Ilenia Giuntib and Piero Pallaa
aBlood Bank, Livorno, Italy; bPsychiatrist, Pisa, Italy
Background: A young woman with thalassemia (minor) trait in the first trimester showed significantly reduced hemoglobin (Hb) and hematocrit (Ht) values (Hb 8.6 g/dL and Ht 26.6%), ferritin values of 118 ng/dL, and serum iron of 135 mcg/dL, associated with signs of inflammation (PCR 0.96 mg/dL and erythrocyte sedimentation rate 25 mm/h).
Objectives: To restore Hb values such as to avoid the risk of transfusion during delivery or in the postpartum, and to reduce the inflammatory state exploiting the potential of Sucrosomial® Iron.
Methods: The study included a clinical work-up of anemia by assessing various parameters: Hb electrophoresis, CBC, serum iron, ferritin, transferrin, abdomen ultrasound, VES, PCR. After clinical work-up of GI tract, and in the presence of a high ferritin value associated with clear signs of inflammation, it was decided, in accordance with the patient, to begin an oral iron supplementation using Sucrosomial® Iron, considering the importance of the anti-inflammatory action of this particular iron formulation in these situations. The treatment included 1 capsule per day for 30 days followed, given the results, by 2 capsules per day for the last 2 months of gestation. The patient had a psychological support for therapy management. During the postpartum period, the treatment was continued with 1 capsule per day for additional 60 days (Hb 11.7).
Results: The values of Hb and Ht remained stable for the first period and then gradually increased until the day of delivery (Hb 9.7 g/dL). During the postpartum, there was an Hb drop to acceptable values (Hb 7.2 g/dL), which recovered well with the continuation of the oral therapy (2 months), reaching an Hb value of 11.7 g/dL (). When the Hb value reached 11.7 g/dL, ferritin values of 210 ng/dL, serum iron of 140 µg/dL, PCR 0.10 mg/dL, and erythrocyte sedimentation rate 07 mm/h, the treatment was suspended and periodic checks of hematochemical values were performed. No side effects were recorded during the treatment. The childbirth, completed without problems, was carried out with planned caesarean section.
Conclusions: Good results can be obtained with oral iron therapy using iron molecules easily assimilated with demonstrated anti-inflammatory activity (Sucrosomial® Iron) in subjects with normal ferritin in the presence of inflammation. Well structured iron therapy allows to normalize the inflammatory state, restoring the normal iron homeostasis through decreasing the mechanism that blocks the use of iron systemically. The advantage in the use of Sucrosomial® Iron was to avoid the transfusion in a 30-year-old woman, while maintaining acceptable Hb values during pregnancy and delivery, and then to determine the normalization of Hb and Ht values in postpartum.
Effectiveness and compliance of oral Sucrosomial® Iron (Sideral® Forte) in asymptomatic inflammatory bowel disease
Giuseppe Scarpulla, Serena Garufi, Gioacchina La Ferrera, Michele Manganaro, Giuseppe Scalisi and Salvatore Cammilleri
Gastroenterology, “M. Raimondi” Hospital, San Cataldo, Italy
Background: Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic, relapsing, immunologically mediated disorders that are collectively referred to as inflammatory bowel diseases (IBD). Epidemiologic observations indicate that there are strong environmental influences on IBD; however, the etiologies of these diseases remain an enigma. Among the most frequent complications and/or extraintestinal manifestations of IBD, there is anemia that can be found already in the asymptomatic stage of IBD. Iron deficiency is the most common cause of anemia in CD and ulcerative colitis UC patients, due to impaired duodenal iron absorption capacity of the mucosa, which creates a negative iron imbalance, often seen in IBD; another cause of iron deficiency anemia (IDA) can be gastrointestinal bleeding. While our understanding of IBD has grown over the past decades, the prevalence of IBD-associated anemia has changed little: one-third of IBD patients still have hemoglobin levels below 12 g/dL. The World Health Organization (WHO) estimates that the prevalence of IDA in patients with IBD is 27% for CD and 21% for UC, respectively, and more than half of the anemic patients (57%) were found to be iron deficient. Other authors report a prevalence of anemia in IBD patients ranging between 62% and 73.3%. Not only anemia, but also iron deficiency may impact the quality of life of IBD patients. Chronic fatigue, a frequent IBD symptom itself, is commonly caused by anemia and may debilitate patients as much as abdominal pain or diarrhea. The treatment of IDA in IBD patients, based on traditional oral iron such as iron sulfate, is limited by poor absorption, intolerance, and induction of oxidative stress at the site of bowel inflammation. In this prospective, a new strategy was tested to correct IDA, in particular, an innovative Sucrosomial® oral Iron (Sideral® Forte) was used in patients affected by asymptomatic IBD (CD and UC).
Objectives: To determine the effectiveness of Sucrosomial® oral Iron treatment in IBD patients affected by mild anemia. Moreover, the compliance to treatment and side effects was evaluated.
Methods: In total, 10 IBD (6 UC and 4 CD) patients with mild anemia (Hb 10.5 ± 0.7 g/dL) were retrospectively studied. The median age was 34 years (18–50-year old). The % transferrin saturation (TSAT) values were borderline with the standard reference value (%TSAT ≤20%) and the median value of ferritin was slightly lower than the standard levels considering the patients’ gender (ferritin ≤200 ng/L). These patients received Sucrosomial® Iron 30 mg (Sideral® Forte), 1 capsule orally/day for 8 weeks. After the treatment, Hb values, compliance, and side effect were evaluated.
Results: An average increase of 1.8 g/dL Hb after 8 weeks of treatment was reported, without side effects in all patients who completed the Sucrosomial® Iron treatment without abdominal pain or discoloration of feces.
Conclusions: This study points out the tolerability and efficacy of Sucrosomial® oral Iron therapy. Indeed, in a cohort of 10 patients with mild IBD-related anemia, the administration of Sucrosomial® Iron for 8 weeks increases the Hb values significantly. For this reason, Sucrosomial® Iron supplementation can be considered an efficacious and tolerated alternative for the treatment of mild anemia in IBD patients. The effectiveness of this therapeutic approach is also associated with good compliance to treatment since Sucrosomial® Iron appears safer and tolerated than iron sulfate treatment.
References
- Rogler G, Vavricka S. Anemia in inflammatory bowel disease: an under-estimated problem? Front Med (Lausanne). 2015 Jan 19;1:58.
- Sakata T, Niwa Y, Goto H, et al. Asymptomatic inflammatory bowel disease with special reference to ulcerative colitis in apparently healthy persons. Am J Gastroenterol. 2001 Mar;96(3):735–739.
- Gasche C, Lomer MCE, Cavill I, et al. Iron, anemia, and inflammatory bowel diseases. Gut. 2004 Aug;53(8):1190–1197.
Sucrosomial® Iron is effective in correcting inflammatory bowel disease anemia and is more tolerable than sulfate iron
Maurizio Romano
Gastroenterology Unit, Hospital San Giovanni di Dio e Ruggi d’ Aragona, Salerno, Italy
Background: Iron deficiency anemia is very frequent in inflammatory bowel disease (ulcerative colitis and Crohn’s disease) due to causes such as chronic gastrointestinal bleeding, enteric malabsorption, and impaired iron utilization. Anemia in IBD is often hardly manageable, in terms of treatment efficacy and patient compliance to traditional ferrous salts. Sucrosomial® Iron has proved capable to transport iron directly into the blood bypassing gastric and enteric mucosa by alternative absorption.
Objectives: To evaluate the efficacy of Sucrosomial® Iron in restoring hemoglobin levels and the tolerance comparable to iron sulfate.
Methods: 12 patients were enrolled and divided in two groups:
Group A: patients, 3 males (2 ileocolic Crohn’s disease and 1 left colitis) and 3 females (2 with left UC and 1 with ileal Crohn’s Disease), mean age 35 years (R 27–45), Hb at T0 = 8 g/dL, mean ferritin level of 5 ng/mL (normal interval 10–150 ng/mL) and normal levels of vitamin B12 and folates, were supplemented with two capsules of Sideral® forte (60 mg of Sucrosomial® Iron) a day for 2 months.
Group B: 6 patients, 4 males (2 left colitis, 2 ileal Crohn’s disease) and 2 females (1 ileocolic Crohn’s disease, 1 left colitis), mean age 38 years (R 26–50), mean hemoglobin levels at T0 = 8 g/dL, mean ferritin level of 6 ng/mL and normal levels of vitamin B12 and folates, were supplemented with two oral sulfate iron tablets corresponding to 210 mg of elemental iron, a day for 2 months.
Both groups performed complete blood count at the beginning and at the end of the study; tolerability was assessed by questionnaire on the presence/absence of dyspeptic symptoms, abdominal pain, and bowel habits at the beginning, during, and at the end of the survey period. Pain extent was measured in same time intervals with visual analog scale (VAS). At the beginning, the disease activity, assessed with MAYO SCORE for ulcerative colitis (score 6–10) and CDAI for Crohn’s disease (mean score 300), was moderate. All recruited patients were treated with mesalazine and steroids. No one was on immunosuppressors or treated with TNF antagonists.
Results: After treatment, Group A patients showed mean hemoglobin levels of 11.5 (R 10.5–12) and mean ferritin levels of 15 ng/mL. The most frequent side effects were of dyspeptic type with epigastric fullness sensation and nausea in two and one case, respectively. Moreover, reported as mild, but non-interfering with every day activity. Group A were compliant to Sucrosomial® iron treatment. After the treatment, Group B showed mean hemoglobin levels of 9.5 g/dL (R 8–9.5) and mean ferritin levels of 9 ng/mL. Epigastric pain appeared in four patients, one of whom had to stop therapy. Epigastric fullness sensation and nausea appeared in three patients and diarrhea in other two patients.
Conclusions: Sucrosomial® Iron has proved to be more effective than iron sulfate in correcting anemia. IBD patients are usually more frequently resistant to oral therapy and they show low compliance. Sucrosomial® Iron showed excellent compliance and treatment adherence.
Clinical experience with oral Sucrosomial® Iron in a severe anemic patient with chronic kidney disease
Ioannis Griveasa,b,c
aNephrology Department, 417 Veterans Army Administration Hospital (NIMTS), Athens, Greece; bPrivate Dialysis Unit “Nefroiatriki”, Athens, Greece; cPrivate Renal Clinic “Athens-nephrology”, Athens, Greece
Background: Iron deficiency is common in patients with chronic kidney disease (CKD). Factors predisposing to the above fact include, among others, increased blood losses, decreased duodenal iron absorption, or impaired iron release from tissue stores. Gastrointestinal (GI) causes of increased blood losses are quite common and in many cases difficult to manipulate due to the location of GI tract that bleeds.
Objectives: We present the case of a woman with CKD and recurrent GI bleeding with severe and refractory anemia that against all odds remained stable receiving oral Sucrosomial® Iron.
Case history: An 83-year-old woman with CKD (eGFR: 35 mils/min) presented with severe anemia (Hb 8.1 g/dL, Hct 25%) and low levels of ferritin (10 mcg/L) despite long stand therapy with oral ferrous iron preparations. Further investigation of anemia and intention to treat attitude guided her to hospital admission. During her stay, she underwent GI endoscopy that revealed gastritis, tubular adenoma of rectum, polypectomy at stomach dome had taken place along with cautery of angiodysplasia in the 2nd fate of duodenum. Patient was transfused and discharged from the hospital with Hct 30.5% and Hb 9.7 g/dL. 3 months later the above patient had Ηct 28.7%, Hb 9.5 g/dL with ferritin levels of 177 mcg/L and started oral Sucrosomial® Iron treatment.
Results: For the next following months, patient has Hct of 28%, Hb of 9.4 g/dL after 3 months with oral Sucrosomial® Iron therapy, and Hct of 29.3%, Hb of 9.8 g/dL after 6 months with oral Sucrosomia® iron therapy, with ferritin levels 144 mcg/L. During the above period, our patient did not receive IV Fe infusions, erythropoietin (EPO) or transfusions. Using oral iron treatment, she showed good gastric tolerability. Her clinical condition remained stable and no side effects were reported. She felt well by herself and did not need to be admitted to the hospital again.
Conclusions: For those of us who have experience in treating old CKD patients with anemia and GI angiodysplasia, it is clear that holding the patients’ anemia without admissions to hospital, transfusions, or EPO administration is a major accomplishment. Angiodysplasia may cause slow-releasing bleeding in a permanent basis. We showed that using oral Sucrosomial® Iron in a CKD patient with severe anemia that had also other contributing factors as causes of that anemia may be an effective and reliable option.
Interleukin-6 on iron functional deficiency in anemic hemodialysis patients. Role of the neutrophils
Miguel Uriol Riveraa, Sonia Jiménez Mendozaa,bJuan Robles Bauzáb, Josep Miguel Bauçab, Angel Garcia Alvarezc, Sheila Cabello Pelegrina, Juan Rey Valerianoa and Manuel Luque Ramírezd
aDepartment of Nephrology, Son Espases University Hospital, Palma de Mallorca, Spain; bDepartment of Clinical Analyses, Son Espases University Hospital, Palma de Mallorca, Spain; cDepartment of Pharmacy, Son Espases University Hospital, Palma de Mallorca, Spain; dDepartment of Endocrinology and Nutrition, Ramón y Cajal University Hospital, Madrid, Spain
Background: Iron functional deficiency is characterized by the presence of adequate iron stores, as defined by conventional criteria, but with insufficient iron mobilization to adequately support the erythropoietic process. Newer assays for inflammation biomarkers may allow a more targeted management of the anemia in these conditions. Neutrophils are involved in iron homeostasis, however, their influence on iron metabolism in hemodialysis patients (HDPs) is not well known.
Objectives: To evaluate the association between the changes in plasma IL-6 levels and iron metabolism, hematologic parameters and iron requirements in anemic HDP. The association between changes in neutrophils (as percentage), in IL-6, and iron markers were also assessed.
Methods: Data were obtained from the MIR-EPO study (EudraCT: 2009-015511-40). Erythropoiesis-stimulating agent (ESA) and intravenous iron (100 mg of iron sucrose) supplements were administered in order to maintain hemoglobin (Hb) between 10.5 and 12 g/dL and transferrin saturation (TSAT) ≥20%. IL-6 plasma levels were measured after the ESA titration period (month 0–month 3) in two time points (month 3 and month 6 (evaluation period)). Correlations were used to explore relationships between the changes in IL-6, iron, and hematologic parameters during the evaluation period. Changes in ESA and iron were also assessed. Assays: IL-6: Quantikine® ELISA Human IL-6.
Results: A total of 28 patients were evaluated. Intact parathyroid hormone (iPTH), ESA, and iron supplements did not change during the study. The median IL-6 levels (p25-p75) were similar during the evaluation period (month 3: 8.14 (3.82–13.24) pg/mL vs. month 6: 7.88 (4.01–13.66) pg/mL, p = 0.98). The changes in IL-6 correlated negatively with the changes on serum iron (Pearson r: −0.38, p = 0.04), TSAT (Spearman r: −0.40, p = 0.03), and positively with the changes in iron supplements (Spearman r: 0.52, p < 0.01). No association was found between the changes on IL-6 and the changes on iron stores, hematologic parameters, and with ESA doses. The changes in neutrophil percentages correlated positively with the change in IL-6 (Spearman r: 0.41, p = 0.03) and negatively with serum iron (Pearson r: −0.68, p < 0.01) and TSAT (Spearman r: −0.69, p < 0.01).
Conclusions: IL-6 is associated with iron for the erythropoiesis process as well as iron requirements. However, IL-6 does not seem to be associated with iron stores in our HDP. Neutrophils showed an important correlation with serum iron and TSAT; this influence could be mediated by IL-6.
Sucrosomial® Iron therapy in dialysis
C Brunati and G Colussi
Department of Nephrology, Niguarda Hospital, Milan, Italy
Background: Patients on dialysis treatment (HDP) are commonly iron deficient (ID) with an average loss of 1–2 g of iron per year. The presence of an ID is the most common cause for resistance to erythropoietin therapy (ESA). Due to the presence of a chronic disease, an absolute iron deficiency is difficult to diagnose in HDP. It is likely to be present when the serum ferritin (SF) is less than 200 ng/mL. However, HDP with SF above these levels (approximately 500 ng/mL) could respond to iron. Intravenous (IV) iron is more efficacious than conventional oral iron treatment due to limited adherence caused by gastrointestinal adverse effects and/or for a reduce absorption. On the other hand, IV iron can be associated with anaphylactic reactions. Moreover, IV is difficult to prescribe in HDP on a home-based dialysis treatment or in patients (pts) in decentralized dialysis units without the presence of a doctor. Sucrosomial® iron (SI), a new drug with a good tolerance and good absorption, could be an interesting alternative therapy in these pts.
Objective: To evaluate the use of Sucrosomial® Iron in dialysis pts.
Methods: From December 2015 to March 2016, 7 HDP were treated with SI for at least 45 days: 2 patients with 1 capsule (cps) a day while 5 pts with 2 cps a day. Three out of seven pts had a presumable iron deficiency (SF 112.11 ng/mL and 196 ng/mL), while 4 pts had SF between 200 and 500 ng/mL.
Results: An increase in Hb levels were observed only in 2 pts with ID and treated with 2 cps daily. In all deficient pts no modifications of SF levels or EPO dose were observed. In pts with higher SF levels, Hb levels were 11.7±1.4 g/dL before therapy and 11.3±0.9 g/dL after therapy; SF were 398±75 ng/mL before therapy and 348±132 ng/mL after therapy suggesting a low absorption. However, in a pts treated with 1 cps daily, a little decrease of EPO dose could be observed (from 1128 UI/gHb to750 UI/gHb)
Conclusions: Therapy with 2 cps daily of SI could be an alternative therapy in HDP with iron deficiency.
Efficacy, tolerance, and adherence to treatment with Sucrosomial® Iron in patients with chronic kidney disease stages 3–4 and iron deficiency
M. Dolores Arenas, Alba Cristina Herrera, Andrea Chacón and Elizabeth Alzate
Servicio de Nefrología, Vithas Hospital Internacional Perpetuo, Alicante, Spain
Background: Iron deficiency is a common cause of anemia in non-dialysis chronic kidney disease (ND-CKD) patients. In these patients, oral iron preparations are less efficacious and poorly tolerated due to non-absorbed iron-mediated gastrointestinal (GI) side effects. Sucrosomial® Iron, a new-generation oral iron with high GI absorption and bioavailability and a low incidence of side effects, seems to be a promising new strategy of iron replacement.
Objective: To evaluate the efficacy, tolerance, and adherence to treatment with Sucrosomial® Iron in CKD stages 3–4 patients with iron deficiency associated with GI tract diseases.
Method: Open-label, single-arm, single-center, prospective, 6-month study, in 24 patients with ND-CKD and iron deficiency treated with Sucrosomial® Iron (Fisiogen Ferro Forte®: 30 mg/day). All patients included in the study had been treated previously, for at least 3 months with other oral iron preparations and had shown lack of response or bad tolerance. Two patients left the study since they requested to be treated with medication financed by the National Health Insurance. Therapeutic adherence was estimated by the SMAQ questionnaire. Values are expressed as means (SD: standard deviation) or percentages.
Results: Patients mean age: 73.4 (SD 8.19) years; 66.6% were men; mean glomerular filtration rate (MDRD IV): 38.1 (SD 12.1) mL/min. All patients showed a statistically significant rise in Hb levels, ferritin concentration, and transferrin saturation since the first evaluation of the study (3 months), which increased at 6 months (). The adherence to Sucrosomial® Iron was significantly higher as compared with previous oral iron preparations (94.4% vs. 33.3%, respectively; p < 0.05). Sucrosomial® Iron was well tolerated and no patients referred side effects.
Conclusions: Sucrosomial® Iron is an efficacious, well tolerated, and with an excellent therapeutic adherence treatment option for patients with ND-CKD.
Table 1. Values of the variables tested at baseline and at two time points.
Erythropoietin (EPO) plus oral Sucrosomial® Iron versus EPO alone for the treatment of severe anemia in no end-stage chronic kidney disease
Francesco Equitani
Department of Transfusion Medicine and Hematotherapy, S. Maria Goretti General Hospital, Latina, Italy
Background: Anemia is frequently diagnosed in patients (pts) with no end-stage chronic kidney disease (CKD) due to the severe endogenous deficiency in the synthesis of erythropoietin and the inflammatory status. Anemia significantly affects quality of life (QoL) and daily living abilities particularly in elderly pts. Subcutaneous (s.c.) and/or intravenous (i.v.) erythropoietin is the gold standard treatment for anemia in CKD.
Objectives: To evaluate efficacy of 3-month s.c. EPO treatment plus oral Sucrosomial® Iron vs. EPO alone on anemia in CKD pts with severe anemia (primary endpoint) and on QoL (secondary endpoint). Compliance to safety and tolerability of oral Sucrosomial® Iron was also evaluated.
Methods: Retrospective analysis of 16 pts, with no end-stage CKD and severe anemia.
Eight pts (5 M/3 F) were treated with s.c. alpha erythropoietin, 40,000 IU a week, plus oral Sucrosomial® iron 30 mg b.i.d. for 12 weeks (EPO + Iron Group). Their median age was 73.3 y (range 59–94) and median hemoglobin (Hb) level was 8.6 g/dL (range 6.2–10.1). They presented anemia in association with asthenia (8 pts), fatigue (6 pts), headache (2 pts), inability to ordinary activities (7 pts), tachycardia (6 pts), and mild dyspnea (2 pts). The median value in transferrin saturation (TSAT) and ferritin was 24% (range 17–44%) and 12 ng/mL (range 2–61 ng/mL), respectively. No pts were diagnosed as suffering for ongoing bleeding disorders, even mild.
Eight pts (4 M/4 F) were treated by 12-week period s.c. alpha erythropoietin alone, 40,000 IU a week with no oral iron supplementation (EPO Group). The median age was 77.2 y (range 66–92 y), they showed a median hemoglobin level 8.9 g/dL (range 7.3–10.8 g/dL) anemia in association with asthenia (8 pts), fatigue (8 pts), headache (5 pts), inability to ordinary activities (7 pts), tachycardia (5 pts), and mild dyspnea (1 pts). The median value in TSAT and ferritin was 28% (range 14–49%) and 21 ng/mL (range 9–112 ng/mL), respectively; no pts were diagnosed as suffering from ongoing bleeding disorders.
ECOG PS was ≥1 in all cases of both groups. After 12 weeks of treatment, clinical and laboratory parameters were reevaluated.
Results: No side effects, no need for stopping treatment, or admission to the emergency unit was reported during the 12-week treatment. In the EPO + Iron Group, there was the amelioration of all the hematological parameters (hemoglobin increased to a median level of 12.6 g/dL (range 9.8–13.7 g/dL), TSAT 39% (range 29–52%), and ferritin 68 ng/mL (range 27–118 ng/mL) plus the improvement of QoL as shown by an ECOG PS ≤1 in 7 patients out of 8. One patient, recently diagnosed with a stroke, claimed persistence of fatigue and not recovering daily ability, thus his score remained ≥1. In the EPO Group, hematological parameters improved significantly (hemoglobin median level 11.4 g/dL (range 9.0–12.2 g/dL), TSAT 19% (range 7–42%), ferritin 21 ng/mL (range 4–52 ng/mL). Fatigue persisted in 3 pts and inability to ordinary activities in 3 pts as well. ECOG PS was ≤1 (5 pts) and ≥1 (3 pts).
Conclusions: The findings of our retrospective analysis further document that addition of Sucrosomial® Iron to the 12-week standard regimen with s.c. erythropoietin is effective to improve hematological parameters but, more importantly, the QoL with no side effects and excellent tolerability in elderly anemic patients with no end-stage CKD.
Effects of oral Sucrosomial® Iron (Sideral® Forte) on inflammatory markers and endothelial dysfunction in CKD patients: preliminary data.
D. Altieri, G. Geraci, G. Mule, C. Guido, M. G. Vario and S. Cottone
Nephrology Department, University of Palermo, Palermo, Italy
Background: Several studies demonstrated that administration of iron reduced the activation of inflammatory systemic state as well as ameliorated the vascular endothelial dysfunction in anemic nephropathic patient. However, no studies exist nowadays about the relationship between oral Sucrosomial® Iron (Sideral® Forte) on inflammatory markers in patients with chronic kidney disease stages III–IV.
Objectives: The aim of our study was to assess the effect of oral Sucrosomial® Iron (Sideral® Forte) on erythrocyte sedimentation rate (ESR) in patients with stable CKD on conservative treatment.
Methods: We enrolled 67 patients: 37 with standard therapy plus oral Sucrosomial® Iron (Sideral® Forte) (group 1) and 30 with only standard therapy (group 2). All patients had hemoglobin levels between 8 and 10 g/dL.
Results: Mean values of ESR were significantly lower in the group 1 (32±9 mm/h) compared with group 2 (48±12 mm/h; p = 0.036). ESR values were lower both in patients with stage III (n = 28; p = 0.040) and stage IV CKD (n = 39; p = 0.024), and differences remained significant after age adjustment (p = 0.042 in overall study population). At multivariate analysis, the relationship between ESR and treatment group lost significance.
Conclusions: Our results show that oral Sucrosomial® Iron (Sideral® Forte), in anemic CKD patients stages III–IV, was associated to a reduced activation of the inflammatory state, as assessed by reduction of ESR, although this is a preliminary study and further studies are needed to confirm these results.
Oral Sucrosomial® Iron in peritoneal dialysis patients
V. Duarte, A. Montoya, Y. Molina, M. Fulquet, V. Esteve, M. Pou, A. Saurina, F. Moreno, I. Tapia and M. Ramirez de Arellano
Nephrology department, Hospital de Terrassa, Consorci Sanitari Terrassa, Terrassa, Spain
Background: The main cause of anemia in chronic kidney disease is a lack of erythropoietin, but iron deficiency, hemolysis, and low response to erythropoietin (inflammation) also contribute. Patients who require treatment with erythropoietin also have functional and absolute iron deficiency. KDOQI guidelines recommend intravenous iron in hemodialysis, but there are no specifications for pre-dialysis and peritoneal dialysis. We should minimize punctures in these patients to reduce vascular damage for future vascular access. Oral Sucrosomial® Iron can preserve vascular access, improve quality of life, and save time in peritoneal dialysis patients.
Objectives: To evaluate oral Sucrosomial® Iron administration in maintaining transferrin saturation (>20%) and ferritin within recommended parameters (100–500 mg/dL). To analyze gastrointestinal tolerance, safety, and satisfaction of oral treatment.
Methods: Peritoneal dialysis patients who require intravenous iron (n = 19/24) were assessed. We excluded 3 patients with digestive bleeding, 1 active malignancy, 1 coronary event, 1 peritonitis. Due to multiple oral medications, 4 patients refused oral iron. Thus, nine patients were included. We controlled anemia parameters before and after administration of Fisiogen Forte® for 12 weeks. We evaluated gastrointestinal symptoms and quality of life through GSRS and QIGLI test, respectively, before and after the treatment.
Results: All patients maintained transferrin saturation levels unchanged. Ferritin levels decreased significantly (p = 0.02) but in within therapeutic range. No changes in hemoglobin or erythropoietin doses were observed. All patients finished treatment without serious adverse events; one patient referred dark feces and a second one referred constipation. No significant changes in gastrointestinal symptoms and quality of life tests were seen. Visual analogue scale for Satisfaction (0–10) result showed a median value of 7.8.
Conclusions: Oral Sucrosomial® Iron treatment is safe and efficacious in maintaining transferrin saturation levels in peritoneal dialysis patients. Although ferritin levels decreased, they remained within therapeutic range. No gastrointestinal adverse effects were reported.
Efficacy of rEPO with Sucrosomial® Iron supplementation in anemia of different causes and causes of loss of response: a case study
Massimo Catarini, Mario Ferranti, Lucia Calcabrini and Roberto Catalini
U.O. Medicine, Macerata, Italy
Background: In the elderly population, anemia is more frequent with chronic kidney disease (CKD) [Citation1] often associated to iron deficiency also in non-hemodialysis CKD patients [Citation2]. Myelodisplastic syndromes are more frequent in this setting and often are the cause of EPO therapy failure [Citation3]. In the present case, Sucrosomial® Iron appeared more effective than iron sucrose and more useful in different scenarios, also in the presence of previous not successful iron treatment. The Sucrosomial® Iron supplementation is useful in iron-restricted erythropoiesis or iron deficiency because it is able to bypass the inhibited intestinal iron adsorption and/or reticuloendothelial sequestration of iron resulting in a decrease in iron availability for bone marrow.
Objectives: We reported a case of anemia in CKD and the evolution into myelodysplastic syndrome, where EPO and Sucrosomial® Iron treatment were used.
Methods: An 88-year-old Caucasian woman was referred to our institution for severe and symptomatic anemia (Hb = 8 g/dL) in August 2014. Medical history comprised moderate chronic renal failure (uremia 49 mg, creatinine 1.79 mg/dL, creatinine clearance 35 mL/min), hypothyroidism secondary to Hashymoto disease, idiopathic hypertension treated with beta blocker (carvediolol) and valsartan. PS ECOG 2, multiparametric geriatric evaluation: ADL 6, CIRS.G 2, and no geriatric syndrome.
Complete blood counts showed microcytic anemia (MCV 77 fl, Hb 8 g/dL, R 1%, iron = 47 mcg/dL, ferritin = 47 mcg/L, and transferrin saturation = 14%, transferrin = 270 mg/dL, erythropoietin = 150 UI/µl, normal platelets = 198,000 mm3, and leukocytes = 4.7 × 109/L, neutrophils 3.2 X 109/L). There were no signs of hemolytic anemia since reticulocyte counts, LDH, haptoglobulin, and bilirubin levels were within normal range. The patient received two allogeneic blood transfusions and nephrologist prescribed darbopoietin 60 mcg weekly and a tablet of iron gluconate 62.5 mg every day. After 2 months, the Hb value was 8.7 g/dL and iron treatment was suspended due to diarrhea and intestinal pain. Thus, Sucrosomial® Iron (Sideral®) 30 mg twice a day was prescribed to the patient.
Results: After 2 months of Sucrosomial® Iron treatment, Hb level were raised to 10.5 g/dL and no gastrointestinal symptoms were reported. For 1 year, the patient continued Sucrosomial® Iron therapy maintaining a stable Hb (11 g/dL), until August 2015 when she showed fever and anemia (Hb = 7 g/dL) and she was referred back to our institution. Complete blood counts showed macrocytic anemia: MCV = 100 fL, platelets = 80,000 mm3, and leucocytes = 2000 mm3 (neutrophils 50%). Blood smears showed 1% blasts, erythrocytes macrocytosis and dysgranulopoiesis, and vitamin B12 and folates within normal ranges. Therefore, a bone marrow trephine biopsy was performed, which showed the presence of erythroid dysplasia, abnormal maturation of myeloid cells with 5% blasts, no cytogenetic abnormalities, no fibrosis, no iron deposition. We diagnosed the patient with MDS RCCD with IPSS low, int 1, serum creatinine of 2 mg/dl, EPO of 200 UI/µL and iron parameters showed serum ferritin of 150 mcg/L and reduced transferrin saturation (16%), transferrin of 200 mg/dL and no signs of hemolytic anemia. The patient was transfusion dependent with a 4 PRBC needed after diagnosis and at the same time she started EPO treatment (rEPO alfa (eprex) 40,000 UI weekly), and Sucrosomial® Iron 30 mg twice a day. After 4 months of treatment, Hb was raised to 10 g/dl, MCV was 97 fl and she was not transfusion dependent.
The latest control performed on the 10 March 2016 showed Hb of 10.8 g/dL, MCV of 98 fl, ferritin of 189 mcg/l, TSAT of 17%, no blast in peripheral blood smears, ECOG = 1, CIRS.G of 2, no transfusion dependence.
Conclusions: This case report brought further evidences to the importance of providing an adequate iron intake to support efficacious hematopoiesis independently from the cause of anemia, CKD, MDS, and showed the superiority of Sucrosomial® Iron confirming its low side effects. ESAs require iron for stimulating effective erythropoiesis and it has been estimated that 1 gram of iron is needed to raise the hemoglobin level from 8 to 11–12 g/dL. Therefore, in MDS patients with no iron overload or reduced iron deposit is important to remember that iron supplementation may improve or trigger hematologic responses in those subjects with no previous benefit from ESAs [Citation2]. Furthermore, the loss of ESA response associated with iron supplementation must lead to research the causes, which may be related to a defect in iron absorption, functional iron deficiency ESA, and no ESA-related and other marrow diseases.
References
- Zumbremman B, Babbit JL. The iron cicle in chronic kidney disease from genetics and experimental models to CKD patients. Neprol Dial Transplant. 2014;29:263–273.
- Camaschella C. Iron deficiency; new insights into diagnosis and treatment. Hematology. 2015;2015:8–13.
- Fraenkel P. Understanding anemia of chronic disease. Hematology. 2015;2015:14–18.
Oral Sucrosomial® Iron (Sideral® Forte) is effective and well tolerated in elderly patients affected by iron deficiency anemia of various origins
A. Nasutia, M. Sagristanib and F. Sessaa
aEmatologis, P.O. San Leonardo, Castellammare di Stabia, Napoli, Italy; bEmatologia Disretto 53, Sant’Agnello, Napoli, Italy
Background: Iron deficiency anemia is a common manifestation of elderly patients and is usually associated to malnutrition or poor diet, intake of gastrolesive drugs, and gastrointestinal disease-like diverticulitis or ulcers. These patients usually show alteration in blood parameter values, such as low levels of serum ferritin, high total iron binding capacity, high levels of serum transferrin receptor, and low transferrin saturation (TSAT).
In past years, the first-line treatment has been based on oral ferrous iron such as ferrous sulfate, ferrous gluconate, and ferrous fumarate. Those oral iron salts have been the mainstay of oral iron supplementation because they are inexpensive, effective at restoring iron balance, and do not require IV access. However, absorption of oral iron salts is inadequate, and the poor tolerance results in reduced adherence to therapy. In elderly patients, their use may be limited by the manifestation of many side effects such as nausea, diarrhea, constipation, abdominal pain, or heartburn. Gastrointestinal side effects often induce these patients to a low therapeutic adherence and therapeutic failure.
The introduction of Sucrosomial® Iron (Sideral® Forte), a newer oral iron formulation, with elevated gastrointestinal absorption and higher bioavailability, has reduced gastrointestinal side effects and improved compliance. Sucrosomial® Iron is composed by pyrophosphate iron protected within a phospholipid bilayer (sucrosome). The iron coated using the Sucrosomial® technology passes intact through the acid environment of the stomach, and it is absorbed through alternative pathways arriving intact to the liver, where the sucrosome is degraded to release iron. The trivalent ferric pyrophosphate form has a high affinity to the newly liver-synthesized transferrin, which is responsible for transporting the body.
Objectives: The objective was to evaluate the median increase of the hemoglobin (Hb g/dL) at 8 week in the most frequent diseases that cause anemia. A second objective was to evaluate, through an interview, any side effects due to iron therapy.
Methods: A retrospective analysis was performed in 30 patients (median age 70 years) affected by iron deficiency anemia (Hb ≤10 g/dL) due to different causes. Sideral® Forte is the only iron formulation that uses innovative technology to avoid common side effects and absorption issue related to iron therapy ensuring effectiveness similar to the intravenous iron. This innovative technology significantly increases the absorption of iron and due to its high absorption is able to grant smaller amount of iron per capsule, about 1/3 of the content of other oral iron preparations. Sucrosomial® Iron (Sideral® Forte cps) was administered twice a day for 2 months. After at least 2 months, patients were assessed for blood parameters and side effects.
Results: The patients accepted Sucrosomial® Iron without the report of side effects and they were adherent to therapy. Only three of them reported mild nausea and constipation. Blood exams showed an increase in the hemoglobin values with a median increase of Hb levels of 2.2 g/dL ± 0.4, reducing transfusion rates and avoiding hospitalization.
Conclusions: The Sucrosomial® Iron is safe, well tolerated, and effective in elderly patients, producing a great improvement in the anemia condition without side effects. These results are important for the improvement of the quality of life in these patients.
Reduced insulin need in patients with type 2 diabetes mellitus (T2DM) with iron deficiency anemia treated with Sucrosomial® Iron versus intravenous sodium ferrigluconate. Multicentric prospective study
Giulio Giordanoa, Fabio D’Amicob, Antonio Commatteob, Albino Parenteb, Luca Lucianob, Roberto Fratangelob, Sabrina Lavoretanob, Rosanna Giglia, Giovanna Niroa, Marilù Magria, Antonietta Liciancia, Luigi Di Marzioa, Bruno Carabellesea, Giuseppe Berardic, Donata Berardid, Divina Traficantee, Liberato Di Lulloe, Graziamaria Corbib and Maurizio Gasperib
aRegional Hospital “A. Cardarelli”, ASREM, Campobasso, Italy; bFaculty of Medicine “V. Tiberio”, Molise Unversity, Campobasso, Italy; cGeneral Medicine, Campobasso, Italy; dFaculty of Medicine, “La Sapienza” University, Rome, Italy; eGeneral Hospital “F. Veneziale”, ASREM, Isernia, Italy
Background: About one out of four diabetic patients show iron deficiency anemia. IV sodium ferrigluconate is frequently used as iron deficiency anemia therapy. The Sucrosomial® Iron is a new compound used in this type of therapy, made of iron-containing liposomal-like structure enveloped in sucrose esters of fatty acids layer. Sodium ferrigluconate increases the amount of free radicals. The increase of free radicals increases inflammation. Inflammation reduces insulin sensitivity in diabetic patients.
Objectives: To assess whether the use of Sucrosomial® Iron involves less use of insulin in patients with T2DM with iron deficiency anemia.
Methods: This study is a multicentric randomized study. We considered 40 T2DM patients with iron deficiency anemia, with TIBC saturation <10% and hemoglobin <10 g/dL, without documented infections, tumors, or autoimmune diseases. All patients received diabetic diet. They received lispro insulin TID + glargine insulin once daily.
In group A, 20 patients, M/F = 12/8, median age 75 years (R65–82), median blood glucose 230 mg/dL (R170–350), median CRP at onset 10 mm/Ih (R2–22), were treated with sodium ferrigluconate 62.5 mg in 250 cc NS ic IV in 4 hours/day for 12 days. In group A, 10 patients had anemia by gastrointestinal hemorrhage, 5 by atrophic gastritis, 5 by insufficient intake.
In group B, 20 patients, M/F = 11/9, median age 78 years (R67–83), median blood glucose 220 mg/dL (R180–380), median CRP at onset 12 mm/Ih (R2–20), were treated with Sucrosomial® Iron 60 mg orally per day for 30 days. In group B, 12 patients had anemia by gastrointestinal hemorrhage, 4 by atrophic gastritis, 4 by insufficient intake.
Differences between the two groups were not statistically significant. Statistical analysis was done with Fisher exact test and with Chi Square test.
Results: In group A, at day 6 of iron support, the median values of CRP were 38 mm/Ih (R4–127), with 5 documented infections (urinary 3, lung 1, skin 1); only 8 patients achieved blood glucose values ≤140 mg/dL with a median total lispro insulin dose of 42 U (R25–60) and glargine insulin dose of 22 U (R10–28).
In group B, at day 6 of iron support, the median values of CRP were 12 mm/Ih (R2–12), with 2 documented infections (urinary 2); 15 patients achieved blood glucose values ≤140 mg/dL with a median total lispro insulin dose of 20 U (R12–23) and glargine insulin dose of 20 U (R10–22).
Conclusions: In diabetic patients with iron deficiency anemia supported with Sucrosomial® Iron, the median lispro insulin need appears to be lower than that of the patients supported with IV sodium ferrigluconate. This study needs confirmation on a larger cohort of patients.
Sucrosomial® Iron is safe and cost effective in HCV patients with type II diabetes and anemia due to esophageal or gastric bleeding
Giulio Giordanoa, Fabio D’Amicob, Antonio Commatteob, Albino Parenteb, Luca Lucianob, Roberto Fratangelob, Sabrina Lavoretanob, Rosanna Giglia, Giovanna Niroa, Marilù Magria, Antonietta Liciancia, Luigi Di Marzioa, Bruno Carabellesea, Giuseppe Berardic, Donata Berardid, Divina Traficantee, Liberato Di Lulloe, Graziamaria Corbib and Maurizio Gasperib
aRegional Hospital “A. Cardarelli”, ASREM, Campobasso, Italy; bFaculty of Medicine “V. Tiberio”, Molise University, Campobasso, Italy; cGeneral Medicine, Campobasso, Italy; dFaculty of Medicine, “La Sapienza” University, Rome, Italy; eGeneral Hospital “F. Veneziale”, ASREM, Isernia, Italy
Background: HCV patients frequently show anemia due to esophageal or gastric bleeding and type 2 diabetes. Iron support reduces degree of anemia. Iron support might cause hepatic function worsening or hepatocellular cancer onset. Non-transferrin-bound iron may sustain inflammation and increase insulin resistance.
Objectives: Aim of this study is to verify if Sucrosomial® oral Iron support versus ferric gluconate IV iron support versus transfusional support is safe and effective in patients with HCV treatment with iron deficiency anemia.
Methods: 35 patients with HCV-related anemia for esophageal varices and gastric bleeding with a median Hb level of 8 g/dL (R8–9.5 g/dL), were treated 15 with Sucrosomial® Iron 30 mg 1 tablet TID for 3 months (group A), 10 with IV ferric gluconate 62.5 mg/day for 15 days (group B) and 10 with 1 blood transfusion/day (group C) until Hb increase level of 1 g/dL was reached. Median Hb and glucose level in group A were 8 g/dL and 140 mg/Dl, respectively, in group B 9.5 g/dL and 130 mg/dL, in group C were 7 g/dL and 160 mg/dL. All patients received an abdomen echography to detect hepatocellular carcinoma (HCC) at 1, 3, 6 months, respectively.
Results: Patients in group A gained 1 g/dL Hb after 1 month (R 3–6 weeks), with a median blood glucose level of 130 mg/dL (R120–230) and a median cost of 30€/month (R 20–80), patients in group B gained 1 g/dL in 7 days (R 6–13 days), with a median blood glucose level of 310 mg/dL (R190–430) and a median cost of 1240€/month (R 830–2800), patients in group C gained 1 g/dL in 1 day (R 2–4 days), with a median blood glucose level of 210 mg/dL (R160–330) and a median cost of 400€/month (R 350–950). Only 1 patient in group B and 1 patient in group C developed HCC at 6 months. Worsening of liver function blood test was observed only in group C.
Conclusions: Sucrosomial® Iron is safe and cost effective in HCV patients with type II diabetes and anemia due to esophageal or gastric bleeding.
Efficacy of oral Sucrosomial® Iron in puerperium anemia
S. Berardi, L. Foltran, I. Pascoli, A. Pepe, M. G. Salmeri and E. Busato
Department of Obstetrics and Gynecology, Ca’ Foncello Hospital, Treviso, Italy
Background: Iron therapy implements iron deposits, treating and preventing anemia during pregnancy and puerperium. The martial supplementation is usually administered orally as ferrous sulfate capsules. The tolerability of oral ferrous sulfate is characterized by frequent gastrointestinal side effects. The intravenous formulations are used in case of severe anemia (Hb<9 g/dL) and is frequently characterized by hypersensitivity reactions or signs of intolerance. Furthermore, extravasation of the drug at the injection site may produce skin irritation and dark skin coloration of potentially long duration at the injection site. Recently, pharmacology proposed Sucrosomial® vesicles as carriers of bioactive molecules: Sucrosomial® Iron. The chemical characteristics of the Sucrosomial® Iron complex ensure a high absorption and improved gastrointestinal tolerance.
Objectives: To evaluate the effectiveness and the side effects in the use of oral Sucrosomial® Iron in patients with anemia after vaginal and cesarean delivery. The primary end point was the evaluation of the hemoglobin increase at the end of the therapy and secondary end point was the evaluation of gastrointestinal side effects (gastralgia, diarrhea, abdominal pain, dark stools) or systemic side effects (headache, feeling of heat, allergic reactions) during treatment.
Methods: All the patients, at the time of admission, had normal blood count and good performance status without hematologic disorders. All the patients with iron deficiency anemia with hemoglobin ≤10 g/dL the first day after vaginal and cesarean delivery and with blood loss at the time of the delivery >500 cc were included in the study. All patients were treated with Sucrosomial® Iron 2 cps twice a day for 1 week for a total of 4 cps in a day. Blood count 3 days and 6 days after delivery were assessed.
Results: In our obstetric division, there were 148 deliveries between 11 February 2016 and 04 March 2016. Eight patients had blood loss >500 cc and presented anemia 1 day after delivery; these patients were treated with Sucrosomial® Iron 2 cps twice a day for 1 week. The mean value of hemoglobin at the time of the admission was 12.05 g/dL. The mean value of blood loss at the time of the delivery was 858 cc (Range: 700–1600 cc). The mean hemoglobin increase 3 days after delivery was 0.25 g/dL. After 1 week, the mean hemoglobin increase was 1.50 g/dL. No patient had gastrointestinal side effects (gastralgia, diarrhea, abdominal pain, dark stools) or systemic side effects (headache, feeling of heat, allergic reactions, inflammation in the site of infection) during treatment.
Conclusions: After a week treatment, anemic patients increased their level of hemoglobin without adverse effects. The administration of oral Sucrosomial® Iron gave excellent results. Further studies should be conducted to confirm these preliminary results.
Up-front Sucrosomial® Iron supplementation in patients with preexisting G1 anemia before planned chemotherapy: a prospective observational study
Sandro Barni, Fausto Petrelli, Andrea Coinu, Mary Cabiddu, Mara Ghilardi, Karen Borgonovo and Veronica Lonati
Oncology Unit, ASST Bergamo Ovest, Treviglio, Italy
Background: Anemia is a common manifestation of neoplastic disease associated to bad prognosis, symptoms as fatigue and can require supportive therapy as blood transfusions or use of erythropoiesis-stimulating agents (ESAs). Oral Sucrosomial® Iron (Sideral® Forte) is an oral formulation with good bioavailability and tolerability, and may improve anemia similarly to intravenous iron, with or without ESAs. Preemptive treatment of mild anemia (Grade [G]1 severity), could prevent a fall of hemoglobin (Hb) level below concentrations that could require the use of ESAs or transfusions.
Objectives: We started a prospective observational study in mild anemic cancer patients treated with oral Sucrosomial® Iron (30 mg/day), with the primary aim to evaluate iron parameters during treatment and the rate of patients with Hb drop below 10 g/dL.
Methods: Patients with any solid tumors and planned to start chemotherapy, with baseline G1 anemia (Hb level 10–12 g/dL and transferrin saturation (TSAT) 15–50% before chemotherapy) were included. Patients already ongoing any chemo or radiotherapy treatments were included. Any use of ESA agents or other iron formulations were not permitted. Treatment continued for 3 months. Iron parameters were checked at 6 and 12 weeks. The planned accrual is 80 patients.
Results: Up to January 2016, 16 patients were enrolled (n = 9 and n = 7 in advanced and adjuvant setting, respectively). In n = 3 cases, treatment lasted <12 weeks. Among them, 1 patient stopped Sucrosomial® Iron for chemotherapy-related vomiting after few days. Concomitant chemotherapy was platinum based in 60%, fluorouracil or anthracycline based in the remaining 40%. At baseline, medium Hb level was 11.1 g/dL. After 6 and 12 weeks (in n = 14 full evaluable patients), medium Hb levels were 10.95 and 10.96 g/dL, respectively. Two patients had G3 nausea and G2 vomiting + G2 diarrhea unrelated to the studied drug. Two patients had G2 nausea related to concurrent (highly emetogenic) chemotherapy. TSAT increased from 13.45% to 20.4% and 20.8% at 6 and 12 weeks, respectively. No patient was transfused or received ESAs.
Conclusions: In a series of 16 patients with G1 anemia when starting cytotoxic therapy, assumption of Sucrosomial® Iron for 3 months maintained Hb above level that requires supportive therapy and may potentially worsen cancer-related symptoms. Its use could be considered as a prophylactic measure to prevent transfusions/ESAs in cancer patients treated with chemotherapy and preexisting mild anemia.
Anemia in chronic kidney disease patients: comparison between Sucrosomial® Iron and ferrous sulfate
C. Cuzzola, A. Mancini and V. Giancaspro
UOC Nephrology and Dialysis at P.O. Venere, Bari, Italy
Background: One of the most frequent complications in patients with chronic kidney disease is anemia, which may be due to many causes. Iron deficiency is the main cause of anemia and, therefore, its correction is essential before undertaking any other type of therapy. In fact, the possible subsequent treatment with EPO may not determine improvement if martial correction has not been carried out properly. Ferrous sulfate is the most widely used oral iron formulation and despite its effectiveness, it often determines discontinuation of therapy due to the high incidence of gastrointestinal side effects. The use of Sucrosomial® Iron (Sideral® Forte) represents, thanks to its high absorption and lower incidence of side effects, a therapeutic opportunity.
Objectives: To assess response to therapy and the side effects produced from the use of Sucrosomial® Iron in patients who have had to discontinue therapy due to intolerance to ferrous sulfate.
Methods: Patients with chronic kidney disease intolerant to ferrous sulfate, who presented serious side effects (abdominal pain, constipation, diarrhea, and other GI disorders) were selected. After 1 month from the discontinuation of ferrous sulfate treatment, 35 patients equally distributed by sex were supplemented with Sucrosomial® Iron. All iron parameters (hemoglobin, serum iron, transferrin, ferritin, and TSAT) were tested at the beginning of the study (T0) and after 3 months (T1).
Results: At baseline (T0), the average Hb level was 9.3 g/dL, which increased to 11.1 g/dL after 3 months of treatment (T1). Serum iron increased from 42.3 mcg/dL (T0) to 102.3 mcg/dL (T1), transferrin increased from 280.3 mg/dL (T0) to 399.8 mg/dL (T1) and TSAT was 11.2% at T0 and 8.9% at T1. After 3 months of treatment, a survey on the presence of side effects and/or discontinuation of therapy was performed. No patient had to interrupt the treatment and/or presented side effects.
Conclusions: Oral Sucrosomial® Iron (Sideral® Forte) is an effective and valid treatment to correct iron deficiency anemia with no side effects. Therefore, it is an important alternative to other oral iron formulation.
Anemia occurrence in antiangiogenetic-treated patients: a series update and association with other prognostic factors
Maurizio Destroa, Giuseppina P. Dogninia, Fausto Petrellib and Sandro Barnib
aMedical Department, ASST Bergamo Ovest, Treviglio, Italy; bOncology Department, ASST Bergamo Ovest, Treviglio, Italy
Background: Antiangiogenetic drugs (AAG) are currently approved for the treatment of a number of cancers. Their use has been associated with a new toxicity profile, comprising hypertension (HTN), whose development may be related with a better prognosis. Anemia represent as well a potential side effect whose incidence ranges between ‘very common’ (>1/10) and ‘common’ (≥1/100 to 1/10).
Objectives: Aims of this paper are: (1) to update a previous series analysis evaluating the incidence of anemia in AAG-treated patients (pts) at Treviglio-Caravaggio Hospital (Italy) between March 2012 and March 2016; (2) to explore a possible relationship between anemia and HTN development with its consequent prognostic impact. Anemia was defined and graded (G) according to the NIH Common Terminology Criteria for Adverse Events, and HTN according to the ESH/ESC guidelines. Those patients (pts) receiving a concomitant chemotherapy regimen were excluded from the study. Oral iron administration was started in pts developing anemia.
Results: 56 consecutive AAG-treated pts were evaluated (median age = 67 years, range = 48–84 years; male/female = 39/17). Overall, 65 AAG treatments were considered since 9 pts received 2 or more AAG lines. Twenty-seven pts were excluded from the study because of concomitant chemotherapy. The remaining 38 were treated with: sorafenib (N = 14), sunitinib (N = 15), axitinib (N = 3), pazopanib (N = 2), and regorafenib (N = 4). Cancer sites were: kidney (N = 18), liver (N = 10), colon (N = 4), kidney + colon (N = 5), liver + colon (N = 1). Before starting, AAG anemia was already present in 14 pts (=37%; G1 in 13 and G2 in 1), and after AAG introduction a further decrease of Hb was observed in 5 (G1 in 4 and G2 in 1). Among the remaining 21 evaluable pts (data lacking in 3) in whom basal Hb was normal, a decrease ≥1 g/dL after AAG starting was documented in 6. The overall incidence of anemia was therefore 29% (G2 = 5%, G3–4 = 0%) and oral iron was administered in all but one patient (with G2 anemia, receiving red blood cells infusion). When exploring a possible association between anemia and HTN development, no statistically significant relation did emerge (p = 0.08).
Conclusions: Our updated data confirms literature reports and suggests that (1) anemia does not represent a ‘limiting’ adverse event for AAG-treated pts; (2) it is not associated with HTN; (3) it is generally of low grade so that iron-based therapy should be considered as a proper approach.
Sucrosomial® Iron and aortic stiffness in cirrhotic patients
Paola Vallerioa, Miriam Stucchia,b, Domenico Siricoa,b, Andrea Buonoa,b, Ivana Rinaldia, Matteo Palazzinab, Cristina Giannattasioa,b
aCardiology IV, “A.De Gasperis” Department, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy; bHealth Science Department, Milano-Bicocca University, Milan, Italy
Background: The fall of hemoglobin (Hb) below the normal level (anemia) is encountered quite frequently in clinical practice; it embraces almost all internal medical specialties and is associated with several chronic disease conditions. Anemia in patients with chronic liver diseases is multifactorial: nutritional deficits such as vitamin B12, folic acid, or iron; myelosuppression secondary to inflammation and blood loss. It is important to recall that transferrin-bound iron is the primary iron source for erythropoiesis. Iron may be obtained by absorption of dietary iron and/or mobilization of macrophages and liver iron stores. Therefore, as daily iron absorption (1–2 mg) just balances daily loss, internal turnover of iron is essential to meet the bone marrow requirements for erythropoiesis. Oral Sucrosomial® Iron formulation, which has an absorption mechanism different to iron salts, may represent an efficacious alternative to IV iron. Sucrosomial® Iron has a described anti-inflammatory effect and transports its content, beyond gastric and enteric wall, directly into the bloodstream.
Methods: 8 patients with HCV-related cirrhosis (CHILD A), 6 men and 2 women, median age 42±8 years, received Cardiosideral® 2 cps/day (corresponding to 60 mg of Sucrosomial® Iron) orally for 1 month. All patients were normotensive (mean 112/75 mmHg) and none had history of cardiovascular disease. Hemodynamic, non-invasive arterial investigation (Pulse Wave velocity (cfPWV), Augmentation Index (AIx), and central blood pressure) and iron metabolism status were performed before starting therapy and after 1 month.
Results: All patients showed a significant increase in all blood parameters tested already after 1 month of iron supplementation: Hb went from 9.4 (T0) to 10.1 (T1) g/dL, serum iron from 28.0 (T0) to 51.0 (T1) mg/dL, ferritin from 10.0 (T0) to 36.0 (T1) ng/mL, and TSAT from 19 (T0) to 24 (T1)%. No significant variation of both peripheral, central blood pressure, and AIx were observed, while we documented a decrease of cfPWV between T0 (9.7±2.5 m/sec) to T1 (9±2.3 m/sec).
Conclusions: Sucrosomial® Iron is effective in replenishing iron storage in cirrhotic patients, and despite the use of a high dose it is well tolerated. The reduction in aortic stiffness could be secondary to early improvement of hyperdynamic status and linked to the increase in Hb level. Further studies are necessary to investigate possible pleiotropic effects on cardiovascular system due to anti-inflammatory potential of this kind of treatment.