ABSTRACT
Objectives: The benefits of intravenous (IV) iron greatly outweigh the risks, but IV iron formulations carry a small risk of hypersensitivity reactions (HSRs). The objective was to use standardized Medical Dictionary for Regulatory Activities queries (SMQs) to compare the safety of ferric derisomaltose/iron isomaltoside 1000 (FDI), iron sucrose (IS), and ferric carboxymaltose (FCM) using prospective trial data.
Methods: Prospective trials reporting the incidence of SMQ-coded serious or severe HSRs were identified in the literature. Four SMQs were used: narrow hypersensitivity terms (A), and broad terms pertaining to potential respiratory HSRs (B), skin HSRs (C), and cardiovascular HSRs (D). Bayesian inference, naïve pooling, and adjusted indirect approaches were employed to compare HSR incidence.
Results: Twenty one prospective trials including over 8,000 patients receiving FDI, FCM or IS were retrieved. Odds ratios of any serious or severe HSR (all groups) with FDI relative to FCM were 0.41, 0.39, and 0.45 according to the Bayesian, naïve and adjusted approaches, respectively.
Conclusions: The risk of serious or severe HSRs was lower with FDI relative to FCM and IS. Using data from prospective trials including over 8,000 patients coded using a well-defined standard (SMQs) enabled a robust comparison of HSR incidence between the iron formulations.
1. Introduction
Iron deficiency is the top-ranking cause of anemia worldwide and intravenous (IV) iron has been shown to be superior to oral iron in achieving a sustained hematological response in patients with iron deficiency anemia (IDA) of various etiologies, including inflammatory bowel disease, chronic heart failure, chronic kidney disease and hemodialysis, heavy uterine bleeding, pregnancy, and when administered in the perioperative period as a part of a patient blood management strategy [Citation1–Citation6].
Specific IV irons have historically been associated with unacceptably high anaphylaxis and anaphylactic-type reactions, but these concerns arose overwhelmingly from events occurring in patients treated with old products which are no longer available in Europe and the US [Citation7–Citation10]. Newer IV iron formulations have since been made available including ferric derisomaltose/iron isomaltoside 1000 (Monofer®; Pharmacosmos A/S, Holbaek, Denmark; FDI) and ferric carboxymaltose (Ferinject®/Injectafer®; Vifor France, Paris, France; FCM). These newer iron formulations are colloidal, consisting of iron (III) hydroxide complexed with different carbohydrates, and can be administered rapidly in high doses, differentiating them from other products such as iron sucrose and iron gluconate. Ferumoxytol (Feraheme®; AMAG Pharmaceuticals Inc., Waltham, MA, USA) was also approved by the US Food and Drug Administration (FDA) in 2009, although it can only be dosed up to 510 mg per infusion and does not have marketing authorization in the European Union.
High-quality head-to-head data comparing these higher-dose, rapid-infusion IV iron formulations are limited, with only two randomized controlled trials (RCTs) published to-date in general IDA populations [Citation11,Citation12]. Alternative methods of comparison have therefore recently been employed to establish the relative safety of the two formulations of high-dose IV iron. For instance, recent studies have relied on spontaneous reporting of adverse reactions and market share data in an attempt to compare hypersensitivity reaction (HSR) rates as recorded in pharmacovigilance (PV) databases [Citation13]. Such studies are fundamentally flawed, with the underreporting and differential reporting of spontaneous adverse reactions resulting in scientifically invalid and potentially misleading conclusions [Citation14]. Furthermore, the use of discrete data sources for the numerator and denominator in rate, risk, hazard, or odds derivations violates the foundational principles of quantitative epidemiology to the extent that it is almost guaranteed to result in erroneous outcomes.
PV databases serve a useful purpose, particularly in providing a centralized data set for the identification of safety signals, but are not appropriate for the derivation of estimates of the relative safety or efficacy of medical intervention; both fall low down in the hierarchy of medical evidence [Citation15,Citation16]. One key area of improvement in evaluating the safety of IV irons would therefore be to obtain data from studies with fewer intrinsic sources of bias. A second potential area of improvement would be the manner in which HSRs are classified; to that end, an analysis of IV iron hypersensitivity risk was published in 2016 based on standardized MedDRA queries (SMQs), with the aim of standardizing hypersensitivity adverse reaction reporting [Citation17].
The aim of the present study was to use more robust and less biased data sources and statistical techniques than previous PV- and market share-based analyses of HSR risk by utilizing standardized (SMQ-based) definitions of HSRs as captured in prospective clinical trials conducted in patients with IDA of multiple etiologies. The analysis focused specifically on the incidence of serious or severe HSRs in patients with IDA treated with FDI, FCM and IS.
2. Methods
2.1. Data collection
Published, prospective studies of FDI, FCM and IS were identified by targeted literature searches of PubMed and Google Scholar, with a view to identifying all prospective studies reporting serious or severe HSRs categorized by SMQs. Data on ferumoxytol were not included on the grounds that it does not have marketing authorization in the European Union, and that the largest ferumoxytol registration trial (comparing ferumoxytol with FCM) reported moderate-to-severe HSRs rather than serious or severe HSRs, the latter of which being the exclusive focus of the present analysis [Citation18]. The searches were designed to capture both RCTs and prospective non-comparative studies; the former to facilitate comparisons of safety using the highest quality evidence available and the latter, where insufficient RCT data were available, to increase the size of the sample population for the purposes of naïve comparison. These data were combined with the SMQ data published by the Center for Drug Evaluation and Research (CDER; a division of the US FDA), which included pooled data from two RCTs of FCM versus IS that reported serious or severe HSRs using SMQs [Citation17,Citation19].
In line with the US FDA CDER report and the 2016 Kalra and Bhandari publication, HSR SMQs were used to classify serious or severe hypersensitivity reactions into four categories covering: anaphylactic reactions (group A), respiratory reactions potentially related to hypersensitivity (group B), skin reactions potentially related to hypersensitivity (group C), and cardiovascular reactions potentially related to hypersensitivity (group D) () [Citation17]. In addition to the individual groups, combinations of groups B and C; B and D; B, C and D; and A, B, C, and D were also analyzed throughout.
Table 1. Summary of studies of ferric derisomaltose included in the Bayesian inference and naïve pooled analyses.
Given that reporting of HSRs using SMQs would only be available in a subset of studies, a variety of statistical techniques were planned to compare the rates across studies and between products: a Bayesian inference of proportions using an uninformative prior to capture uncertainty around the pooled treatment effect, a naïve pooled analysis, and an indirect treatment comparison (ITC) using the Bucher et al. approach. The Bayesian inference of proportions analysis was selected as the primary analysis based on the a priori understanding that HSR data categorized by SMQs would not be widely available and that the use of uninformative priors would factor a level of uncertainty into the analysis, informed by the challenges encountered in previous studies attempting to compare the safety of the IV iron formulations.
2.2. Bayesian analysis
Bayesian inference techniques were employed to conduct an analysis of the naïvely pooled serious or severe HSR data from each of the IV iron formulations in SMQ groups: A, B, C, D, B + C, B + D, B + C + D, and A + B + C + D. To capture the uncertainty around the relative safety of the IV iron formulations, flat, uninformative priors were employed, specifically based on beta distributions with shape and scale parameters set to 1.
The Bayesian analysis was then conducted using Just Another Gibbs Sampler (JAGS) from R, using the RJAGS package [Citation20]. Gelman–Rubin statistics and Gelman plots were employed to establish whether the chain had converged [Citation21]. The numbers of tuning and burn-in steps in the reference case analysis were set to 500 and 1,000, respectively, based on exploratory analyses showing stable results with these iteration counts. Plots of the posterior distributions were then generated, including a region of practical equivalent (ROPE) covering an odds ratio of 1 ± 10%. Uncertainty around the mean odds ratio was summarized using 95% highest posterior density intervals (HDI), namely, the interval containing 95% of the posterior distribution of odds ratios.
2.3. Frequentist naïve pooling
Serious or severe HSR data from all FDI studies were pooled and compared with similarly pooled data from the identified studies of FCM. Odds ratios were derived directly from the pooled event counts and patient exposure, while the significance of the results was estimated using Fisher's exact tests, and confidence intervals around the mean odds ratios were derived using the Clopper Pearson methodology [Citation22].
2.4. Random effects meta-analysis of ferric derisomaltose versus iron sucrose and indirect treatment comparison with ferric carboxymaltose
Serious or severe HSR data from RCTs of FDI versus IS were pooled using a random effects meta-analysis for each SMQ individually and for the aforementioned combinations of SMQ groups. An ITC of FDI and FCM was then conducted using the Bucher et al. methodology, relying on the random effects meta-analysis to inform the comparison of FDI and IS, and the naïve pooled odds ratios to inform the comparison of IS and FCM.
3. Results
The literature searches identified a total of 21 prospective studies of FDI, FCM, and IS for which SMQ-coded serious or severe HSR data were available, with a total enrollment of 8,599. Of the 21 prospective studies, 19 published studies of FDI for which HSR data categorized by SMQs were available from the manufacturer and two published studies of FCM versus IS for which SMQ-categorized HSR data were available from a 2013 report from the US FDA CDER [Citation11,Citation12,Citation23–Citation40]. The 19 studies of FDI () had a total enrollment of 3,922 in the FDI arms, who experienced a total of 23 serious or severe HSRs, corresponding to 0.59% or one event per 171 treatments. Of the 19 prospective studies of FDI identified, four were RCTs comparing FDI with IS [Citation29,Citation30,Citation35,Citation36], and two were RCTs comparing FDI with FCM [Citation11,Citation12]. Three of the included studies were extensions to other studies identified in the review; in these instances, the HSR data were obtained from the extension period only to avoid double counting [Citation24,Citation31,Citation33].
The only data source identified for which SMQ-coded HSR data were available for FCM and IS was a 2013 report from the US FDA CDER. The report included data that was pooled from two studies: REPAIR-IDA, conducted in patients with chronic renal failure, and VIT09031, a trial conducted in patients with IDA associated with a broad range of etiologies [Citation39,Citation40]. Results from both REPAIR-IDA and VIT09031 were reported in clinicaltrials.gov, including adverse events reported using Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 terminology, although the clinicaltrials.gov reporting was not sufficiently comprehensive to unequivocally assign events to each of the SMQ groups in either study. As such, the pooled data from both studies as published in the CDER report were utilized directly in the analyses. REPAIR-IDA and VIT09031 included a total of 1,775 patients treated with FCM, of whom 26 experienced at least one serious or severe HSR, corresponding to 1.5% or one event per 68 patients treated. The REPAIR-IDA and VIT09031 comparator arms included 1,503 patients treated with IS, of whom 24 experienced at least one serious or severe HSR, corresponding to 1.6% or one event per 63 patients treated.
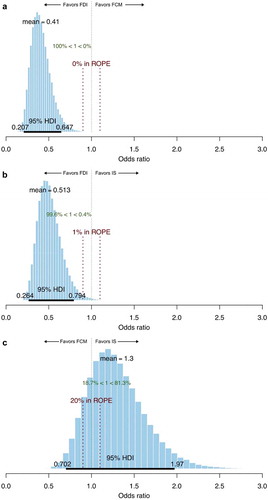
The Bayesian inference approach of comparing HSR incidence showed that the mean odds of experiencing any HSR in SMQ groups A + B + C + D were 59% lower with FDI relative to FCM (odds ratio 0.41; ) and 49% lower with FDI relative to IS (odds ratio 0.51; ). In the comparison of FDI and FCM, the 95% HDI spanned 0.21–0.64, and 0% of the posterior distribution fell within the ROPE, illustrating that the probability of ‘practical equivalence’ between FCM and FDI was extremely low and therefore that the likelihood of FDI being associated with lower risk of serious or severe HSRs than FCM was extremely high. In analyses of the individual SMQ groups, the group with the highest (i.e. least favorable to FDI) mean odds ratio comparing FDI with FCM was group B (Respiratory HSRs), in which the odds of an event were 45% lower with FDI than FCM, with 4% of the posterior distribution falling within the ROPE. In SMQ group A, which most closely matches the HSR definitions adopted by Ehlken et al., the Bayesian inference approach reported a 52% reduction in the odds (i.e. an odds ratio of 0.48) of an event with FDI versus FCM [Citation13].
Figure 1. Posterior distributions from a Bayesian comparison of the odds of hypersensitivity reactions in SMQ groups A + B + C + D with A) ferric derisomaltose versus ferric carboxymaltose, B) ferric derisomaltose versus iron sucrose, and C) ferric carboxymaltose versus iron sucrose.
Abbreviations: FCM, ferric carboxymaltose; FDI, ferric derisomaltose; HDI, highest posterior density interval; IS, iron sucrose; ROPE, region of practical equivalence.
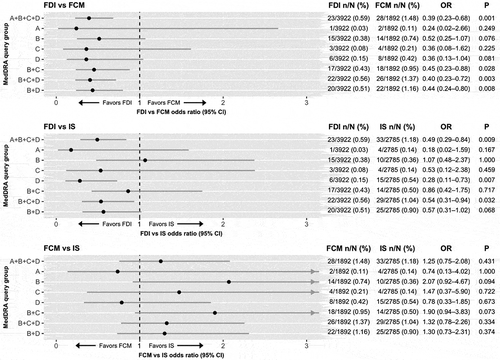
The naïve pooling approach, with the addition of odds ratios derived directly from the event counts, binomial confidence intervals derived using the Clopper Pearson methodology, and p values derived using Fisher's exact test showed that the odds of experiencing any HSR in SMQ groups A + B + C + D with FDI were 61% lower than with FCM (odds ratio 0.39, 95% confidence interval 0.23–0.68, p = 0.001; ), and 51% lower than with IS (odds ratio 0.49, 95% confidence interval 0.29–0.84, p = 0.009; ).
Figure 2. Naïve pooled analysis of the safety of ferric derisomaltose (FDI) versus ferric carboxymaltose (FCM), ferric derisomaltose versus iron sucrose (IS), and ferric carboxymaltose versus iron sucrose with odds ratios and confidence intervals derived using the Clopper Pearson method, and p values derived using the Fishers exact test.
Abbreviations: FCM, ferric carboxymaltose; FDI, ferric derisomaltose; IS, iron sucrose; MedDRA, Medical Dictionary for Regulatory Activities; OR, odds ratio.
The random effects meta-analysis of four RCTs of FDI versus IS reported an odds ratio of 0.56 (95% confidence interval 0.23–1.37) across SMQ groups A + B + C + D (). The ITC conducted by combining the results of the random effects meta-analysis of FDI versus IS with the pooled odds ratios from the naïve comparison of FCM with IS yielded an overall odds ratio of 0.45 (95% confidence interval: 0.16–1.25), corresponding to a 55% reduction in the odds of any HSR in SMQ groups A + B + C + D with FDI relative to FCM.
Table 2. Results of random effects meta-analyses comparing ferric derisomaltose with iron sucrose for each of the standardized MedDRA query groups for serious or severe hypersensitivity reactions.
A comparison of the findings of the three different approaches to comparing FDI with FCM, and FDI with IS showed a high level of agreement, particularly for the combined A + B + C + D SMQ group ( and ). The two approaches to comparing FCM with IS similarly showed a high level of agreement for the combined A + B + C + D SMQ group ().
Table 3. Summary of results from the three approaches to analyzing serious or severe hypersensitivity reaction data in ferric derisomaltose relative to ferric carboxymaltose.
Table 4. Summary of results from the three approaches to analyzing serious or severe hypersensitivity reaction data in ferric derisomaltose relative to iron sucrose.
Table 5. Summary of results from the two approaches to analyzing serious or severe hypersensitivity reaction data in ferric carboxymaltose relative to iron sucrose.
4. Discussion
The primary analysis using a Bayesian inference of proportions indicated that FDI would reduce the odds of experiencing serious or severe HSRs in SMQ groups A + B + C + D by 59% relative to FCM (mean odds ratio of 0.41), and by 49% relative to IS (mean odds ratio of 0.51). The analysis represents the most comprehensive effort to synthesize prospectively gathered evidence on the incidence of serious or severe HSRs in patients treated with modern IV iron formulations conducted to-date, including studies that collectively enrolled over 8,000 patients.
In addition to the Bayesian analysis, the present study also explored two frequentist statistical frameworks for the analysis: a naïve pooling of event counts with each of the three iron formulations, and an ITC. In the ITC, the comparison of FDI and IS was informed by a random effects meta-analysis of four trials, and the comparison of IS and FCM was informed by the same naïve pooling of HSR event counts based on data from the US FDA CDER report on FCM. Results from the three techniques were closely aligned with the Bayesian, ITC, and naïve pooling approaches yielding odds ratios of 0.41, 0.45 and 0.39, respectively, in favor of FDI. The measures of uncertainty across the three analysis techniques cannot be directly compared as they span Bayesian and frequentist statistical frameworks, but their implications for the purposes of driving clinical practice should be considered. The Bayesian inference of proportions approach showed that, based on 23 serious or severe HSR events occurring across a total enrollment of 3,922, and 28 serious or severe HSR events occurring in 1,892 patients treated with FCM, the mean odds ratio for HSR was 0.41 with FDI relative to FCM, with an HDI spanning from 0.20 to 0.64 and 0% of the posterior distribution falling above 1, reflecting an infinitesimally small probability that FCM would be associated with reduced odds of serious or severe HSRs relative to FDI. The frequentist ITC, in which the FDI versus IS comparison was driven by a random effects meta-analysis and the FCM versus IS comparison was driven by naïve pooling, yielded a similar mean estimated odds ratio of 0.45, with 95% confidence intervals spanning 0.16–1.25, which would not be considered statistically significant by a conventional p < 0.05 interpretation.
A clear strength of the present study is that it represents the largest collation of well-defined serious or severe HSR data from prospective trials of IV irons conducted to-date. Furthermore, there was a high level of agreement between the results generated using the most rigorous methodological approaches and those constrained by the lack of data availability which used less robust statistical techniques. For instance, the random effects meta-analysis of FDI versus IS based on four large-scale RCTs produced a mean pooled odds ratio of 0.56 for all serious or severe HSRs in SMQ groups A + B + C + D. The naïve pooled approach produced a corresponding estimate of 0.49, which is very closely aligned given the differences in data sources and the lack of random effects weighting in the pooled approach.
Despite the high levels of agreement between the various techniques, the primary limitation of the analysis was the loss of randomization arising from the naïve pooling of the SMQ data reported in the CDER report. REPAIR-IDA and VIT09031 were conducted in different trial populations and the crude pooling of data from both trials in the CDER report resulted in a loss of anchoring via a matched IS population [Citation39,Citation40]. This randomization loss provided the justification for capturing a high degree of uncertainty around the pooled odds ratios by using uninformative priors in a Bayesian framework in the primary analysis. Comparing the Bayesian approaches with the frequentist naïve pooling and ITC approaches showed that, especially for the SMQ groups with low event counts (e.g. SMQ group A), the contribution of the uninformative prior in the Bayesian resulted in much more conservative estimates of the mean odds ratios than the frequentist techniques. Regardless of the agreement between different statistical approaches, the issues around randomization loss should not be overlooked when interpreting the findings of the present analysis.
Two head-to-head studies of FDI and FCM have recently completed, which included HSR incidence as a pre-defined secondary endpoint. Based on a pooled analysis of data from the two head-to-head studies, serious or severe HSRs occurred in 0.8% in the FDI group and 1.7% in the FCM group, corresponding to a crude overall odds ratio of 0.47 [Citation41]. As the only available head-to-head data source comparing FDI with FCM, the agreement between the odds ratio of 0.47 from the pooled analysis, and the naïve pooled odds ratio in the present study of 0.45 is notable.
We acknowledge the challenges faced by previous investigators in attempting to establish the comparative safety of the current IV iron formulations in the absence of RCT data and further acknowledge the limitations of our own analysis arising from the prior pooling of the two studies of FCM and IS included in the CDER report. We would note, however, that we consider the objectives of the present analysis to represent the most robust approach to analyzing the comparative safety of IV iron formulations with regards to serious or severe HSR risk; namely the use of data from RCTs that use precise definitions of HSRs. The comparison between FDI and IS in the random effects meta-analysis managed to meet this high methodological standard based on four large-scale RCTs, but the comparison with FCM was confounded by the prior pooling of data from the trials comparing FCM with IS. Given this pooling, the Bayesian and naïve approaches to pooling were conducted by applying a lower requirement for the standard of evidence (namely the inclusion of non-comparative studies) in order to increase the sample sizes across all arms analyzed.
Despite the inclusion of non-comparative studies, the present analysis still represents a marked improvement on approaches that rely on combining data from PV and market share data, where agreement between events and exposure is extremely tenuous, doubly so when relying on proprietary market share data, which is not subject to any external scrutiny from peer reviewers, clinicians or marketing authorization holders [Citation13,Citation42]. Even in the case where exposure estimates could be derived from a source congruent with the event incidence data, previous analyses of HSR incidence after IV iron administration have also been confounded by highly selective use of preferred terms for HSRs and misaligned reporting periods, thereby conflating heightened post-launch vigilance and genuinely elevated incidence of HSRs [Citation13,Citation14,Citation43]. The present analysis is not affected by any of these issues, utilizing only prospective data to ensure agreement between exposure and event counts, and classifying all events using SMQs to ensure that HSRs were recorded consistently using validated and pre-specified definitions [Citation44]. Given the previously documented challenges in accurately assessing the safety of IV iron formulations, the use of SMQs for the recording and publication of HSR data should be adopted across future trials of IV iron and, given the rare nature of the serious or severe HSRs with modern IV iron treatments, ideally the SMQ-coded HSR data would originate from a large-scale head-to-head trial of the modern IV iron formulations.
5. Conclusions
The risk of serious or severe HSRs was lower with FDI relative to FCM and IS. Using safety data from more than 20 prospective trials including over 8,000 patients and coded using a well-defined standard (SMQs) enabled a robust comparison of HSR incidence between the three IV iron products.
We strongly recommend that future RCTs use the SMQ framework for the reporting of HSRs to facilitate future evidence synthesis efforts.
Author contributions
RFP and PB devised the analysis plan. RFP ran the analyses, generated the tables and figures, and drafted the manuscript. PB reviewed the manuscript for intellectual content. RFP then prepared the final version of the manuscript, which was reviewed by both RFP and PB prior to submission.
Declaration of interest
RFP is the director of Covalence Research Ltd, which received consultancy fees from Pharmacosmos A/S to conduct the statistical analyses and prepare the manuscript. PB has received lecturing and consultant fees from Vifor-Fresenius Medical care, Medice, and Pharmacosmos A/S. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Avni T, Bieber A, Steinmetz T, et al. Treatment of anemia in inflammatory bowel disease–systematic review and meta-analysis. PLoS One. 2013;8(12):e75540.
- Bonovas S, Fiorino G, Allocca M, et al. intravenous versus oral iron for the treatment of anemia in inflammatory bowel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(2):e2308.
- Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail. 2012;14(4):423–429.
- O’Lone EL, Hodson EM, Nistor I, et al. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2019;2:CD007857.
- Susantitaphong P, Alqahtani F, Jaber BL. Efficacy and safety of intravenous iron therapy for functional iron deficiency anemia in hemodialysis patients: a meta-analysis. Am J Nephrol. 2014;39(2):130–141.
- Reveiz L, Gyte GM, Cuervo LG, et al. Treatments for iron-deficiency anaemia in pregnancy. Cochrane Database Syst Rev. 2011;2011(10):CD003094.
- Chertow GM, Mason PD, Vaage-Nilsen O, et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21(2):378–382.
- Fletes R, Lazarus JM, Gage J, et al. Suspected iron dextran-related adverse drug events in hemodialysis patients. Am J Kidney Dis. 2001;37(4):743–749.
- Chertow GM, Mason PD, Vaage-Nilsen O, et al. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant. 2004;19(6):1571–1575.
- Wysowski DK, Swartz L, Borders-Hemphill BV, et al. Use of parenteral iron products and serious anaphylactic-type reactions. Am J Hematol. 2010;85:650–656.
- Pharmacosmos A/S. A trial comparing the incidence of hypophosphatemia in relation to treatment with iron isomaltoside and ferric carboxymaltose in subjects with iron deficiency anaemia (IDA-04). NCT03238911. [ cited 2019 Aug 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT03238911
- Pharmacosmos A/S. A Trial Comparing the Incidence of Hypophosphatemia in Relation to Treatment With Iron Isomaltoside and Ferric Carboxymaltose in Subjects With Iron Deficiency Anaemia (IDA-05). NCT03237065. [ cited 2019 Sep 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03237065
- Ehlken B, Nathell L, Gohlke A, et al. Evaluation of the reported rates of severe hypersensitivity reactions associated with ferric carboxymaltose and iron (III) isomaltoside 1000 in europe based on data from eudravigilance and VigiBase™ between 2014 and 2017. Drug Saf. 2019;42(3):463−71.
- Schaffalitzky de Muckadell P, Strom CC. Comment on ‘evaluation of the reported rates of severe hypersensitivity reactions associated with ferric carboxymaltose and iron(III) isomaltoside 1000 in Europe based on data from eudravigilance and vigibase™ between 2014 and 2017ʹ. Drug Saf. 2019;42(5):689–691.
- OCEBM Levels of Evidence Working Group. The oxford levels of evidence 2. Oxford Centre for Evidence-Based Medicine. [ cited 2019 Oct 14]. Available from: https://www.cebm.net/index.aspx?o=5653
- Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310.
- Kalra PA, Bhandari S. Safety of intravenous iron use in chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25(6):529–535.
- Adkinson NF, Strauss WE, Macdougall IC, et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: A randomized trial. Am J Hematol. 2018;93(5):683−90.
- US Food and Drug Administration. Center for drug evaluation and research. Application number: 203565Orig1s000. Medical Review. [ cited 2019 Oct 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203565Orig1s000MedR.pdf
- Plummer M. JAGS: a program for analysis of bayesian graphical models using Gibbs sampling. Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003), March 20–22, Vienna, Austria. ISSN 1609-395X.
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434–455.
- Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413.
- Gybel-Brask M, Seeberg J, Thomsen LL, et al. Intravenous iron isomaltoside improves hemoglobin concentration and iron stores in female iron-deficient blood donors: a randomized double-blind placebo-controlled clinical trial. Transfusion. 2018;58(4):974–981.
- Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang. 2015;109(3):257–266.
- Hildebrandt P, Bruun N, Nielsen O. et al. Effects of administration of iron isomaltoside 1000 in patients with chronic heart failure. A pilot study. Transfus Altern Transfus Med. 2010;11(4):131–137.
- Birgegård G, Henry D, Glaspy J, et al. A randomized noninferiority trial of intravenous iron isomaltoside versus oral iron sulfate in patients with nonmyeloid malignancies and anemia receiving chemotherapy: the PROFOUND trial. Pharmacotherapy. 2016;36(4):402–414.
- Wikström B, Bhandari S, Barany P, et al. Iron isomaltoside 1000: a new intravenous iron for treating iron deficiency in chronic kidney disease. J Nephrol. 2011;24(5):589–596.
- Kalra PA, Bhandari S, Saxena S, et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant. 2016;31(4):646–655.
- Bhandari S, Kalra PA, Kothari J, et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant. 2015;30(9):1577–1589.
- Pharmacosmos A/S. Iron isomaltoside and iron sucrose for the treatment of iron deficiency anemia in non-dialysis-dependent chronic kidney disease. NCT02940860. [ cited 2019 Oct 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT02940860
- Pharmacosmos A/S. An extension trial to assess the safety of re-dosing of iron isomaltoside (Monofer®) (FerWonExt). October 1, 2019 [cited 2019 Sept 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02962648
- Reinisch W, Staun M, Tandon RK, et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol. 2013;108(12):1877–1888.
- Reinisch W, Altorjay I, Zsigmond F, et al. A 1-year trial of repeated high-dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scand J Gastroenterol. 2015;50(10):1226–1233.
- Dahlerup JF, Jacobsen BA, van der Woude J, et al. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand J Gastroenterol. 2016;51(11):1332–1338.
- Derman R, Roman E, Modiano MR, et al. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol. 2017;92(3):286–291.
- Auerbach M, Henry D, Derman RJ, et al. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am J Hematol. 2019;94:1007–1014. Online publication ahead of print.
- Holm C, Thomsen LL, Norgaard A, et al. Intravenous iron isomaltoside 1000 administered by high single-dose infusions or standard medical care for the treatment of fatigue in women after postpartum haemorrhage: study protocol for a randomised controlled trial. Trials. 2015;16:5.
- Pharmacosmos A/S. Treatment of women after severe postpartum haemorrhage (PP-02). NCT01895205. [ cited 2019 September 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT01895205
- Onken JE, Bregman DB, Harrington RA, et al. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial. Nephrol Dial Transplant. 2014;29(4):833–842.
- Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54(2):306–315.
- Wolf M, Rubin J, Achebe M, et al. OR13-3 effects of iron isomaltoside versus ferric carboxymaltose on hormonal control of phosphate homeostasis: the PHOSPHARE-IDA04/05 randomized controlled trials. J Endocr Soc. 2019;3(Suppl 1): OR13–3.
- Nathell L, Gohlke A, Wohlfeil S. Reported severe hypersensitivity reactions after intravenous iron administration in the European Economic Area (EEA) before and after implementation of risk minimization measures. Drug Saf. 2019. Online publication ahead of print. DOI:10.1007/s40264-019-00868-5
- Weber JCP. Epidemiology of adverse reactions to nonsteroidal anti-inflammatory drugs. In: Rainsford KD, Velo GD, editors. Side-effects of anti-inflammatory drugs, advances in inflammation research. New York: Raven Press; 1984:1–7.
- Medical Dictionary for Regulatory Activities. Standardised MedDRA queries. [ cited 2019 October 29]. Available from: https://www.meddra.org/standardised-meddra-queries
Appendix
Appendix Standardized MedDRA query termsCitation17,Citation44
