1. Introduction
Effective radiation therapy requires respect for the therapeutic ratio, ensuring that coverage of tumor areas does not outweigh potential short- and long-term toxicities from irradiating nearby healthy tissues. This principle is particularly evidenced in the evolution of lymphoma management. Historically, Hodgkin lymphoma was treated with definitive radiotherapy using high doses and large fields, but such cures came at the price of long-term toxicity, particularly in the mediastinum wherein the tumor is situated near the heart, lungs, and, for female patients, the breasts. With the introduction of chemotherapy, radiotherapy field size has shrunk from extended-field to involved-field to present day involved-site radiotherapy. With the therapeutic ratio ever present, the desire to reduce treatment (particularly in diseases with excellent cancer outcomes) remains a prevalent theme in mediastinal lymphoma management, as recently evidenced by the publication of HD 16 [Citation1]. With these recent results, radiotherapy remains an essential tool available within the oncology armamentarium. Proton therapy, rather than conventional x-ray-based radiation therapy, produces a dosimetric benefit due to its ability to deposit radiation at a target and minimize distal dose deposition. This physical phenomenon means that patients receive lesser doses to healthy tissues.
While radiotherapy has been used in cancer treatment for approximately a century, proton therapy is a relatively new modality with increased use in the last decade. Yet, approximately 15 years ago, proton therapy was hypothesized to provide benefits to patients with mediastinal lymphomas [Citation2]. That early review served as a harbinger for the clinical and dosimetric studies that soon followed. In 2009, investigators at the University of Florida published their first dosimetric comparison in patients with lymphoma, comparing plans using conventional radiotherapy, intensity-modulated radiotherapy (IMRT), and 3-dimensional proton radiotherapy [Citation3]. Their dosimetric evaluation of 9 patients across 3 modalities demonstrated significant reductions to breast, lung, and integral body doses. This study led to a slew of similar comparison studies that also illustrated dose reductions to the heart, lung, breast, thyroid, and esophagus [Citation4–Citation6]. These reductions reflect possible targets for further improvement of the therapeutic ratio. A review published by the Particle Therapy Cooperative Group (PTCOG) Lymphoma Subcommittee provided an in-depth evaluation of how dose reductions may lead to reduced toxicities such as valvular disease, coronary heart disease (CHD), lung cancer, pulmonary toxicity, breast cancer, hypothyroidism, thyroid cancer, and esophagitis [Citation7].
Paralleling this deluge of dosimetric investigations for mediastinal lymphoma were watershed publications that better elucidated the relationship between heart dose and subsequent major cardiovascular events (MCEs) [Citation8]. These retrospective works demonstrated a significant association between average heart dose and subsequent MCEs, including CHD and congestive heart disease. Importantly, in survivors of Hodgkin lymphoma, the association between mean heart dose and CHD demonstrated results portending a 2.5-fold increased risk of CHD for patients receiving a mean heart dose of 20 Gy from mediastinal radiotherapy [Citation9].
Clinically, it is important to note that outcomes with proton therapy have demonstrated similar oncologic control to published photon radiation control rates. A multi-institutional experience of 138 patients with Hodgkin lymphoma treated with consolidative proton therapy following chemotherapy, some as early as 2008, demonstrated similar early relapse-free survival rates to reported photon treatments with no early grade 3 radiation-related toxicities [Citation10]. Another study of young adult and pediatric patients demonstrated very low rates of pneumonitis [Citation11], less than those reported with IMRT [Citation12].
2. Consensus recommendations
As dosimetric studies have demonstrated an improved therapeutic ratio with proton therapy, the International Lymphoma Radiation Oncology Group (ILROG) has published guidelines to better characterize adults who may benefit the most from this therapy. While the aforementioned publications summarize and highlight the expected benefits, we await long-term evidence of clinical benefits (with median follow-up exceeding 10 years). Nevertheless, The ILROG expert panel identified three groups of adult patients with lymphoma for whom proton therapy would most substantially benefit (1): ‘patients with mediastinal disease that spans below the origin of the left main stem coronary artery and is anterior to, posterior to, or on the left side of the heart; (2) young female patients for whom proton therapy can reduce breast dose and risk for secondary breast cancer; and (3) heavily pretreated patients who are at higher risk for radiation-related toxicity to the bone marrow, heart, and lungs’ [Citation13]. As we explore heart dose and cardiotoxicity, an evidence-based review provided by PTCOG has identified dose reductions that would be associated with a reduced incidence of second malignancies, pneumonitis, hypothyroidism, and esophagitis [Citation7].
3. Cardiac risk determination as proton therapy advances
With proton therapy’s conformality, the association between average cardiac dose and subsequent risk of CHD may not be accurately depicted. Earlier studies demonstrating the heart dose-cardiotoxicity relationship relied on treatments from the 1960s to 1980s that, as mentioned earlier, employed large field sizes for which dose homogeneity across the heart would have been more uniform. Owing to the reduction in field sizes as well as increased conformality, the utility of average heart dose as a predictor for CHD may no longer hold steady [Citation14].
Newer studies have started to investigate the relationship between doses to cardiac substructures and specific cardiotoxicities, such as valvular doses associated with valvular disease and left ventricle doses with congestive heart failure [Citation8,Citation15]. Investigators at the University of Florida demonstrated that proton therapy reduces mean doses to all heart chambers; the aortic, mitral and triscupid valves; and the left anterior descending, left circumflex, and right circumflex coronary arteries [Citation16]. However, when the association between mean heart dose and average heart dose to the individual cardiac substructures was recently evaluated at the same institution, clear correlations were not seen for proton therapy [Citation14]. In fact, no strong correlations (r > 0.6) were seen between proton therapy and any substructure, and the only moderate correlation between mean heart dose with proton therapy and average cardiac substructure dose was in the left ventricle.
As we await long-term clinical data to clarify the prediction models describing cardiac and substructure dose and outcome, efforts to determine cardiovascular biomarkers are underway [Citation17]. In a cohort including lymphoma patients, it was shown that heart dose was significantly associated with an increase in serum levels of placental growth factor (PIGF) and growth differentiation factor 15 (GDF-15) [Citation18]. While these markers reflect both vascular function and inflammation and oxidative stress, respectively, we similarly await data to validate that these associations (and radiographic studies involving echo and magnetic resonance imaging) can, in fact, be predictive of long-term late effects.
4. Childhood populations
Of those with mediastinal lymphoma, pediatric patients are likely to benefit the most from proton therapy. Updates from the Childhood Cancer Survivor Study continue to underscore the importance of dose and volume reduction to reducing associated cardiac disease rates [Citation19]. Apart from cardioprotection, protons deliver less integral dose (dose to the entire body) and such reductions can lead to decreased secondary cancers, like sarcomas. Cohort studies have demonstrated that as lymphoma management has reduced the radiotherapy dose, the risk of secondary neoplasms has also been reduced [Citation20]. Logically then, similar focal and precise treatments (with reduced healthy tissue irradiation) may lead to reduced secondary cancers.
5. Patient selection and challenges
Regarding histologic subtypes of mediastinal lymphoma best treated with proton therapy, the modality has been employed in Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, and diffuse large B-cell lymphoma [Citation21]. In the absence of published experiences, one would posit a similar benefit in those with mediastinal Gray zone lymphoma. Others for whom proton therapy may be most beneficial include patients with relapsed or refractory disease. Indeed, in any patient who receives a significant amount of treatment and for whom the therapeutic ratio is highly sensitive, the reductions in cardiac and pulmonary dose achievable with proton therapy are all the more meaningful [Citation22]. Despite these dosimetric benefits, proton therapy presents a few challenges. While phase II dosimetric evidence exists, there is no prospective level I evidence demonstrating the clinical superiority of protons, which, in turn, influences insurance coverage, limiting access to those patients for whom proton therapy may be most beneficial. Similarly, given the cost of facility construction, proton therapy centers represent fewer than 1% of radiotherapy of facilities, limiting access to those patients residing near the few existing centers, which are overwhelmingly located in populated urban areas. Finally, mediastinal lymphoma represents a diverse spectrum of disease presentations; therefore, individual anatomic distribution of disease portends the greatest potential benefit.
6. Conclusion
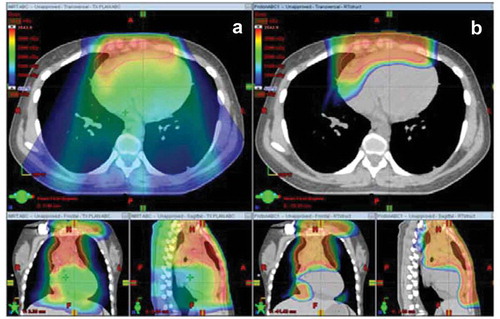
With an approximate decade of clinical history, proton therapy is a relatively new tool available for the treatment of mediastinal lymphomas, and its potential to significantly improve the therapeutic ratio is immense. The management of lymphoma is storied in its evolution – from its large high-dose fields to dose-reduced tailored targets – with its balance of excellent cancer control and reduction of late effects as its North Star. Compared with conventional radiotherapy, proton therapy significantly reduces doses to critical organs in the mediastinum, as shown in . In light of trials that continue to emphasize radiotherapy’s role in curative lymphoma treatment, proton therapy provides an avenue toward continuing to utilize radiotherapy while sparing healthy tissues – a particular boon for pediatric patients with mediastinal lymphoma. Future directions will continue to define the mechanics of radiotherapy’s effects on organs like the heart and provide the long-term clinical data for this nascent technology. We excitedly await the clinical outcomes.
Figure 1. Colorwash dose distribution for a patient with bulky anterior mediastinal disease that draped over the heart. Panel A demonstrates the dose distribution with free-breathing intensity-modulated radiotherapy and panel B demonstrates free-breathing proton therapy. Reprinted from Hoppe BS, Mendenhall NP, Louis D, Li Z, Flampouri S. Comparing Breath Hold and Free Breathing during Intensity-Modulated Radiation Therapy and Proton Therapy in Patients with Mediastinal Hodgkin Lymphoma. Int J Part Ther. 2017 Spring;3(4):492-496. doi: 10.14338/IJPT-17-00012. Epub 2017 Jul 11. PubMed PMID: 31772999; PubMed Central PMCID: PMC6871555.
Declaration of interest
B Hoppe is a Scientific consultant for Merck & Co., Inc, and Bristol-Myers Squibb; RB Mailhot Has received an IBA travel grant unrelated to this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Fuchs M, Goergen H, Kobe C, et al. Positron emission tomography-guided treatment in early-stage favorable hodgkin lymphoma: final results of the international, randomized phase III HD16 trial by the german hodgkin study group. J Clin Oncol. 2019 Nov 1;37(31):2835–2845.
- Bjork-Eriksson T, Bjelkengren G, Glimelius B. The potentials of proton beam radiation therapy in malignant lymphoma, thymoma and sarcoma. Acta Oncol. 2005;44(8):913–917.
- Chera BS, Rodriguez C, Morris CG, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II hodgkin’s lymphoma patients: conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009 Nov 15;75(4):1173–1180.
- Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for stage IA-IIIB mediastinal hodgkin lymphoma: preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys. 2012 May 1;83(1):260–267.
- Maraldo MV, Brodin NP, Aznar MC, et al. Estimated risk of cardiovascular disease and secondary cancers with modern highly conformal radiotherapy for early-stage mediastinal hodgkin lymphoma. Ann Oncol. 2013 Aug;24(8):2113–2118.
- Cella L, Conson M, Pressello MC, et al. Hodgkin’s lymphoma emerging radiation treatment techniques: trade-offs between late radio-induced toxicities and secondary malignant neoplasms. Radiat Oncol. 2013 Jan 30;8:22.
- Tseng YD, Cutter DJ, Plastaras JP, et al. Evidence-based review on the use of proton therapy in lymphoma from the Particle Therapy Cooperative Group (PTCOG) lymphoma subcommittee. Int J Radiat Oncol Biol Phys. 2017 Nov 15;99(4):825–842.
- van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017 Apr 20;129(16):2257–2265.
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of hodgkin lymphoma. J Clin Oncol. 2016 Jan 20;34(3):235–243.
- Hoppe BS, Hill-Kayser CE, Tseng YD, et al. Consolidative proton therapy after chemotherapy for patients with hodgkin lymphoma. Ann Oncol. 2017 Sep 1;28(9):2179–2184.
- Nanda R, Flampouri S, Mendenhall NP, et al. Pulmonary toxicity following proton therapy for thoracic lymphoma. Int J Radiat Oncol Biol Phys. 2017 Oct 1;99(2):494–497.
- Pinnix CC, Huo J, Milgrom SA, et al. Using benchmarked lung radiation dose constraints to predict pneumonitis risk: developing a nomogram for patients with mediastinal lymphoma. Adv Radiat Oncol. 2018 Jul-Sep;3(3):372–381.
- Dabaja BS, Hoppe BS, Plastaras JP, et al. Proton therapy for adults with mediastinal lymphomas: the international lymphoma radiation oncology group guidelines. Blood. 2018 Oct 18;132(16):1635–1646.
- Hoppe BS, Bates JE, Mendenhall NP, et al. The meaningless meaning of mean heart dose in mediastinal lymphoma in the modern radiotherapy era. Pract Radiat Oncol. 2019 Oct 2. pii: S1879-8500(19)30279-6.
- Cutter DJ, Schaapveld M, Darby SC, et al. Risk of valvular heart disease after treatment for hodgkin lymphoma. J Natl Cancer Inst. 2015 Apr;107(4). doi: 10.1093/jnci/djv008
- Hoppe BS, Flampouri S, Su Z, et al. Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012 Oct 1;84(2):449–455.
- Guzhva L, Mendenhall NP, Morris CG, et al. Evaluating cardiac biomarkers after chemotherapy and proton therapy for mediastinal hodgkin lymphoma. Int J Part Ther. 2017;4(2):35–38.
- Demissei BG, Freedman G, Feigenberg SJ, et al. Early changes in cardiovascular biomarkers with contemporary thoracic radiation therapy for breast cancer, lung cancer, and lymphoma. Int J Radiat Oncol Biol Phys. 2019 Mar 15;103(4):851–860.
- Bates JE, Howell RM, Liu Q, et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the childhood cancer survivor study. J Clin Oncol. 2019 May 1;37(13):1090–1101.
- Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970–2015. JAMA. 2017 Feb 28;317(8):814–824.
- Sachsman S, Flampouri S, Li Z, et al. Proton therapy in the management of non-hodgkin lymphoma. Leuk Lymphoma. 2015;56(9):2608–2612.
- Figura NB, Flampouri S, Hopper K, et al. Consolidative proton therapy following high-dose chemotherapy and autologous stem cell transplant in an adolescent with relapsed hodgkin lymphoma. J Adolesc Young Adult Oncol. 2011 Jun;1(2):103–106.
