ABSTRACT
Objectives: To summarize the impact of relapsed/refractory primary cutaneous T-cell lymphomas (CTCL) on quality of life (QoL) and the efficacy of available treatments in two systematic reviews (SRs).
Methods: Searches were performed on 16 January 2018 and 23 January 2018, respectively, in Medline, Medline in process, the Cochrane database, and EconLit. Studies reporting QoL outcomes in adults with CTCL or treatment efficacy in relapsed/refractory CTCL were included.
Results: Based on 15 QoL studies, CTCL symptoms/complications negatively affect patients’ physical, emotional, and social functioning. Skin problems pose considerable symptom burden, while advanced disease stage is associated with poorer QoL. CTCL negatively affects caregivers, primarily through family dynamics and relationships. The clinical efficacy SR included 72 publications covering 23 therapies. Overall response rate (ORR) ranged from 14% (belinostat) to 95% (total skin electron beam therapy). ORRs >50% were reported for several therapies including brentuximab vedotin (50–78%) and bexarotene (39–86%). Over half (13 of 23 therapies) had ORRs <30%. Median progression-free survival varied between treatments (3.5–116.4 months) and was >20 months for brentuximab vedotin and alemtuzumab.
Conclusion: CTCL negatively affects patients’ and caregivers’ QoL. A considerable proportion of patients have no response or no sustainable response to current treatments.
1. Introduction
Cutaneous T-cell lymphomas (CTCLs) are a group of rare non-Hodgkin lymphomas derived from T lymphocytes that infiltrate the skin [Citation1–Citation3], with incidence reported to range from 0.43 (Kuwait) to 0.69 (USA) per 100,000 person-years [Citation4–Citation8]. Characterized by heterogeneous clinical, immunologic, histologic, and molecular features, the pathology of CTCL is diverse [Citation1,Citation9], with mycosis fungoides (MF) and Sezary syndrome (SS) the most commonly diagnosed types of CTCL (55% and 5% of cases, respectively) [Citation3]. CTCL is usually diagnosed in patients between 50 and 70 years of age and is more common in men and people of black race than in women and people of white race [Citation4,Citation5,Citation10–Citation12]. A proportion of patients with CTCL are first diagnosed when their disease is at an advanced stage [Citation13]. Among those diagnosed with early-stage disease, approximately 24% progress to advanced-stage disease as a result of disease relapse or the development of resistance to treatment [Citation14,Citation15]. Once CTCL has progressed, life expectancy is substantially impacted and is estimated to be 3.2–9.1 years [Citation1].
CTCL has been reported to negatively affect multiple aspects of patients’ health-related quality of life (HRQoL), including emotional, social, and physical functioning [Citation16–Citation22]. In addition, CTCL can impact family life and relationships [Citation17,Citation19]. Both the physiological aspects and the psychological effects of CTCL contribute to reducing patient HRQoL and must be considered when seeking to optimize treatment [Citation16–Citation22]. Due to the heterogeneity of the disease and its clinical presentation, treatment is highly individualized and there exists no standard treatment plan [Citation9]. Current treatment options have a variety of mechanisms of action and range from skin-directed therapies in the early stage of the disease to systemic therapies in refractory disease [Citation1–Citation3,Citation9]. Conventional therapies include systemic chemotherapy with agents such as gemcitabine and temozolomide, pegylated- or non-pegylated anthracyclines (doxorubicin or daunorubicin), and treatment with interferon-α2b (IFN), a systemic treatment option for patients with refractory CTCL [Citation3,Citation9,Citation23–Citation28]. Therapies indicated for the treatment of CTCL by the American Food and Drug Administration include retinoids such as bexarotene, the class 1 histone deacetylase inhibitor romidepsin and vorinostat, and the recombinant fusion protein denileukin diftitox [Citation29–Citation32]. However, as a considerable proportion of patients do not respond to current and approved therapies, investigational agents continue to be assessed including targeted agents such as the antibody-drug conjugate alemtuzumab [Citation9,Citation33]. The extent and durability of response to current treatments is variable and measured inconsistently between studies. This review will describe the effects of relapsed/refractory CTCL on patient HRQoL and the clinical efficacy of current treatments.
2. Methods
The objective of this analysis was to assess published evidence on the impact of CTCL on HRQoL and to summarize published evidence on the efficacy (randomized controlled trial) and effectiveness (observational data) of treatments of interest in patients with relapsed/refractory CTCL. Two systematic reviews were therefore conducted: (i) identification of published evidence for the impact of CTCL on patients’ HRQoL (clinical burden SR) and (ii) identification of published evidence on the efficacy (randomized controlled trial) and effectiveness (observational data) of treatments in patients with relapsed/refractory CTCL (clinical efficacy SR). The reviews were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation34]. The SR conducted to collate information on the impact of CTCL on patient HRQoL was also used to identify studies that reported economic evaluations of CTCL, epidemiology, or the cost-of-illness. However, this manuscript focuses on the HRQoL data collated and the results for the other searches are not reported.
2.1. Search methods
Using the Ovid platform, the original electronic database searches were conducted on 16 December 2016 and 9 January 2017 for the clinical burden SR and the clinical efficacy SR, respectively, which were updated on 16 January 2018 and 23 January 2018, respectively.
The following electronic databases were searched: Embase, MEDLINE®, MEDLINE® In-Process & Other Non-Indexed Citations, MEDLINE® ePub Ahead of Print, MEDLINE® Daily, EconLit, and the Cochrane Library. Supplementary hand searches of conference proceedings such as the American Society of Hematology and the European Haematology Society (2013–2018), relevant HTA websites, and clinical trial registries were conducted. In addition, the reference lists of included studies, systematic literature reviews, and meta-analyses were reviewed to identify any further potentially relevant publications that were not identified during the electronic database searches. A full list of the sources included in the search is provided in Supplementary Table 1. Search terms included terms for free text and medical subject heading terms for the population, interventions, and study designs of interest. The clinical burden SR was conducted as part of a wider search process, and search terms relevant to epidemiology, economic evaluations, and cost-of-illness were also included. Search strings are provided in Supplementary Table 2.
2.2. Eligibility criteria
The key eligibility criteria for study inclusion in the manuscript are provided in Supplementary Table 3. Studies were included in the clinical burden SR if they reported disease-specific or generic HRQoL outcomes for adult patients (aged 18 years or older) with a known diagnosis of CTCL (early to late stages, IA–IVB). For the clinical efficacy SR, studies were included if they reported clinical data for the efficacy/effectiveness of current treatments in patients with relapsed and/or refractory CTCL, enrolled >20 patients and were published in 2007 or later. Prospective and retrospective study designs including randomized controlled trials of any design or phase, and observational studies were included.
2.3. Data collection and analysis
Citations identified through the searches were assessed by one reviewer (CM) based on title and abstract and verified by a second independent reviewer (SM). Full publications were obtained for all citations of interest and were assessed by one reviewer (CM) and verified by a second independent reviewer (SM). Any uncertainties were resolved through discussion between reviewers. Reasons for exclusion were documented for all excluded citations.
Relevant data from eligible publications were extracted into a pre-designed data extraction table by a reviewer (CM) and verified by a second reviewer (SM). Any disputes were resolved through discussion. Quality assessment of randomized controlled trials (RCTs) reported as full publications was conducted using the seven criteria checklist provided in section 4.6 of the NICE single technology assessment (STA) user guide [Citation35]. Quality assessment of included non-RCTs was conducted using the ‘Quality Assessment Tool for Quantitative Studies’ produced as part of the Effective Public Health Practice Project (EPHPP) [Citation36]. For every study each component was rated as strong, moderate, or weak, with the components used to assign an overall rating for the study.
3. Results
3.1. Study selection
A total of 14,465 citations were identified in the full search process. The flow of citations through the SR process for the clinical burden and the clinical efficacy SR is illustrated in .
Figure 1. Systematic review study flows. Left, clinical burden review. Right, clinical efficacy review.
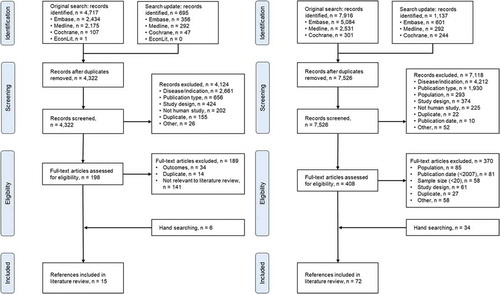
For the clinical burden SR, the combined electronic database searches identified 5412 citations. In total, 4322 citations were screened based on title and abstract after the removal of 1090 duplicates. Exclusion of 4124 citations at this stage resulted in 198 remaining citations for which full publications were obtained and assessed. After, further 189 citations were excluded based on the full-text review, 9 publications were eligible for inclusion. Hand-searching identified an additional 6 relevant publications, resulting in 15 publications which met the predefined inclusion criteria for the HRQoL review [Citation17–Citation19,Citation21,Citation22,Citation37–Citation46].
For the clinical efficacy SR, the combined electronic database searches identified 9053 citations, of which 7526 were screened based on title and abstract after removal of 1527 duplicates. Exclusion of 7118 citations at this stage based on inclusion criteria resulted in 408 remaining citations for which full publications were obtained and assessed. Further 370 citations were excluded based on the full-text review, including 81 published prior to 2007 and 58 which reported data for <20 patients. Hand-searching identified an additional 34 publications. In total, 72 publications reporting on 59 unique studies were identified which met the predefined inclusion criteria for the clinical efficacy review [Citation25,Citation33,Citation40,Citation47–Citation115]. The majority of studies were conducted in North America or Europe.
3.2. Health-related quality of life
CTCL and its associated symptoms and complications affect multiple aspects of HRQoL with respect to physical, emotional, and social functioning. In the identified studies, a variety of methods were used to assess QoL including semi-structured interviews and self-administered questionnaires such as the 12-item General Health Questionnaire (GHQ-12), 36-Item Short-Form Health Survey (SF-36), European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30), Functional Assessment of Cancer Therapy – General (FACT-G), pruritus visual analogue scale (VASitch), Revised Illness Perception Questionnaire (IPQ-R), and Skindex-29. The HRQoL scales used in the included studies were non-CTCL-specific, highlighting the paucity of disease-specific, validated questionnaires to assess QoL in patients with CTCL and their caregivers.
3.2.1. Symptoms, physical and functional impact
A total of 12 publications reported data on the symptoms, physical, and functional impact of CTCL [Citation17–Citation19,Citation21,Citation22,Citation37–Citation43]. Studies for which HRQoL outcomes were reported are presented in . Itch, hair loss, and embarrassing skin problems were frequently reported symptoms in patients with CTCL [Citation17]. In one study, skin lesions were described as oozing and infected, intensely dry and exfoliating, thin, and sensitive [Citation17]. Discomfort, cracking and bleeding skin, particularly on the hands and feet, were also reported in this study [Citation17]. Pruritus was also reported to be a common and severe symptom in patients with CTCL [Citation17,Citation19,Citation37,Citation38]. Talpur et al., 2016 reported that in patients with MF the highest (most impactful) mean Skindex-29 scores at baseline were reported for pain, fatigue, sleep disruption, distress, and enjoyment of life [Citation39]. Prince et al., 2017 [Citation40] reported that CTCL is associated with considerable symptom burden and assessed the effect of brentuximab vedotin on QoL in CTCL patients. Using Skindex-29, the study demonstrated significantly greater symptom reduction in the brentuximab vedotin arm compared with the physician’s choice (methotrexate or bexarotene) arm (mean maximum reduction of −27.96 vs −8.62, p < 0.001). Mean change from baseline of FACT-G total scores was similar between the two treatment arms and no substantial differences in QoL were observed on EQ-5D US time trade-off (TTO), UK TTO, or visual analogue scores.
Table 1. Studies assessing the impact of cutaneous T-cell lymphomas on HRQoL in patients with relapsed and/or refractory disease.
The function was severely affected in patients with CTCL as a result of the substantial negative impact of physical symptoms [Citation17,Citation19]. Symptoms limited patient travel, participation in sports and hobbies, and in some cases resulted in patients reducing their working hours or retiring as the disease advanced [Citation17]. Late-stage CTCL was associated with worse patient-reported QoL compared with early-stage disease [Citation38,Citation41–Citation43].
3.2.2. Mental, emotional, and social
Nine studies were collated which assessed the effect of CTCL on the mental, emotional, and social well-being of patients () [Citation17–Citation19,Citation21,Citation22,Citation39,Citation41–Citation43]. The burden of physical symptoms impacted patients’ psychological and social well-being [Citation16–Citation19,Citation21,Citation39]. Patients were concerned with disease progression, the seriousness of disease, the impact on appearance, employment, and dying [Citation16,Citation17]. Demierre et al., 2006 reported that greater than 25% of respondents (n = 63) who completed a questionnaire used to gain patients’ perspectives on the psychosocial impact of CTCL were not satisfied with how they were coping with their disease [Citation19].
CTCL substantially affected patients’ close relationships with their partner and family members and negatively impacted on the sexual aspect of some patients’ relationships [Citation17,Citation19]. Overheating and discomfort contributed to patients no longer sharing a bed with their partner with pruritus causing problems with sleeping patterns [Citation17]. Furthermore, patients were self-conscious due to the visibility of the condition, particularly when affecting exposed areas such as the face, hands, or legs [Citation17].
Psychological and social well-being were more affected at later stages of disease [Citation16,Citation41,Citation42], and patients with early-stage disease (stage IB) had higher FACT-G scores (better HRQoL) in emotional and social subscales at baseline, compared with patients with more advanced disease [Citation43]. Compared with other skin diseases, Sampogna et al., 2009 reported that the Skindex-29 emotions mean score in patients with MF was similar to that of patients with vitiligo, and lower (better HRQoL) than in arthropathic psoriasis [Citation42].
3.2.3. Disease perception
One study reporting on patients’ perspective of their disease was identified [Citation44]. The Revised Illness Perception Questionnaire (IPQ-R) was used to evaluate 22 patients with different variants of CTCL on two consecutive visits to a hospital-based primary cutaneous lymphoma ward [Citation44]. At baseline, participants experienced CTCL as long-lasting (timeline acute/chronic: 19.0 ± 2.8, range 5–25), with little variation in perception depending on disease course (stable disease or timely variation of disease severity) (timeline cyclical: 10.7 ± 3.4, range 4–20). With respect to control, patients had poor belief in personal control (10.8 ± 3.4, range 4–20), but a stronger belief in treatment control (13.1 ± 3.4, range 4–20). Moderate disease understanding with respect to illness coherence was demonstrated (11.4 ± 3.6, range 5–25) and patients had a moderately negative emotional response to their illness (15.0 ± 4.8, range 5–25).
3.2.4. Demand on caregivers
Two publications were identified which reported information on the burden/negative impact of CTCL on the QoL of caregivers [Citation45,Citation46]. Caregivers experienced multiple demands including providing medical and physical care, psychological support, completing household duties, and driving [Citation45]. Financial difficulties relating to travel expenses and reduced working hours (patient and caregiver) and restricted physical intimacy were also reported to affect caregiver QoL [Citation45]. CTCL was reported to impact on caregivers coping resources including family or health-care provider support with isolation and loneliness reported when no support network was in place [Citation45]. Skin symptoms, as opposed to other medical issues, contributed to the majority of caregiver burden [Citation46]. With increasing disease severity caregivers were reported to be increasingly bothered by itching and scratching behaviors [Citation46].
3.3. Current treatments
Twenty-three individual therapies for CTCL were identified in the SR; the number of studies extracted for each therapy ranged from 1 to 8. Full details of the included studies and patient populations are provided in Supplementary Table 4. Clinical data for patient survival and response to current therapies, categorized as conventional therapies, approved therapies, and investigational and off label therapies, were collated and are provided in full in Supplementary Table 5.
Where reported, all patients had recurrent or refractory disease following ≥1 therapy for CTCL prior to the therapy for which data were extracted. The majority of trials were single-arm investigations with patient cohorts ranging in size from 21 [Citation47–Citation49] to 216 [Citation50] patients, with 30/58 studies enrolling ≤50 patients (publications meeting the inclusion criteria but reporting data on patient populations of <20 patients were excluded). Four of the studies included, including the two largest (N = 377 [Citation51] and N = 372 [Citation52]), were RCTs comparing CTCL therapies. Three RCTs were open-label trials comparing multiple therapies (interferon and methotrexate vs interferon and retinoids; brentuximab vedotin vs methotrexate or bexarotene; mogamulizumab vs vorinostat) [Citation51–Citation53], and one was a double-blind, placebo-controlled trial of denileukin diftitox [Citation54]. Clinical data for patient survival and response to current therapies, categorized as conventional therapies, approved therapies, and investigational and off label therapies, were collated. Details of the quality assessment are reported in the supplementary material.
3.4. Durability of response to treatments
The durability of response to current treatments was assessed using a range of outcomes across studies. Overall response rate (ORR), complete response rate (CRR), progression-free survival (PFS), and duration of response (DOR) to treatment were the most commonly reported outcomes.
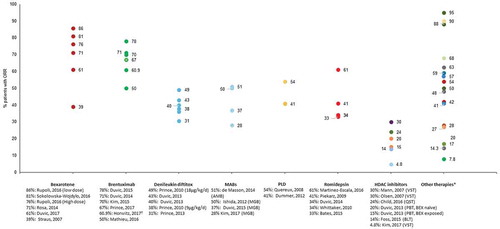
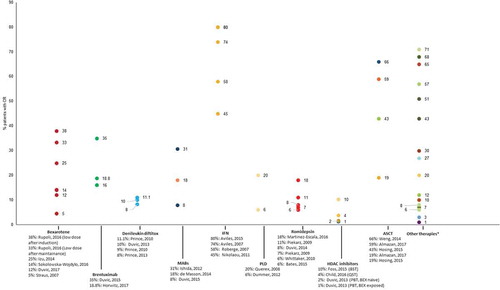
ORR was reported for all therapies in at least one publication, with the exception of IFN/MTX, IFN/retinoids, total skin electron irradiation (TSEI)/IFN and PUVA (). Across therapies, ORR ranged from 14% for belinostat [Citation55] to 95% for total skin electron beam therapy (TSEB) [Citation56]. The novel therapies allogeneic stem cell transplant and alemtuzumab were associated with ORRs >50% as were the conventional and approved therapies mogamulizumab, bexarotene, brentuximab vedotin, pegylated liposomal doxorubicin, romidepsin, gemcitabine, PUVA/IFN, and TSEB. The majority of other therapies were associated with ORRs of <30%. Prince et al., 2017 reported an objective global response lasting at least 4 months (ORR4) as a clinical efficacy outcome [Citation53]. ORR4 captures both the proportion of patients achieving a response and response duration as a single measurement and was reported to be 56% for patients with CTCL following treatment with brentuximab vedotin and 13% for patients treated with physician’s choice of methotrexate or bexarotene. In an update to this study, the authors (Horwitz et al., 2017) reported ORR4 values of 60.9% and 7.8% for brentuximab vedotin and physician’s choice, respectively [Citation57].
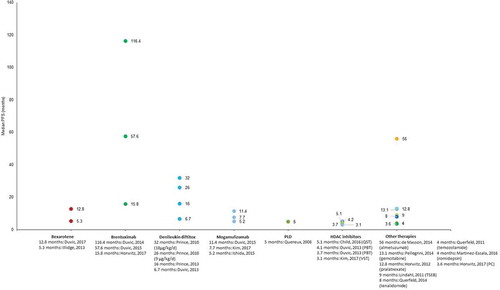
Figure 2. Overall response rates reported in patients treated for refractory cutaneous T-cell lymphomas.
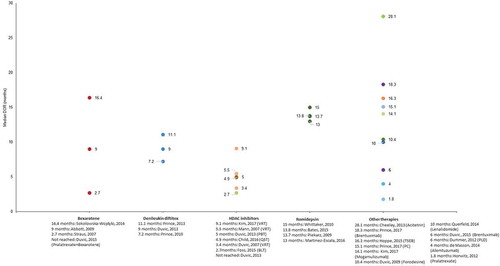
CRR ranged from 1.3% following treatment with panobinostat [Citation58] to 80% following treatment with IFN/retinoids [Citation51] (). TSEB, IFN, and PUVA were the only therapies for which CRR was >50%. A single study [Citation51] reported the exact time-point of assessment (i.e. CRR was reported at 6 months and 1 year). However, the median follow-up time was reported in 16 studies and ranged from 10.6 months [Citation59] to 155 months [Citation60].
Figure 3. Complete response rates reported in patients treated for refractory cutaneous T-cell lymphomas.
The length of PFS was reported for 14 different interventions and median PFS ranged from 3.1 months to 116.4 months across studies for vorinostat and brentuximab vedotin, respectively [Citation52,Citation61] (). A PFS of >20 months was reported only for the investigational therapies brentuximab vedotin (range: 15.8–116.4 months) [Citation57,Citation61,Citation62] and alemtuzumab (56 months) [Citation33]. Considerable variation was observed in PFS where it was reported in multiple studies for a single intervention. The greatest variation was for brentuximab vedotin for which PFS ranged from 15.8 months to 116.4 months [Citation57,Citation61]. Variation in PFS was also observed between studies following treatment with bexarotene combination therapies (range: 5.3–12.8 months) [Citation63,Citation64], denileukin diftitox (range: 6.7–32 months) [Citation54,Citation65], and mogamulizumab (range: 5.2–11.4 months) [Citation66,Citation67]. Time to tumor progression was reported for six different CTCL therapies (supplementary material): <5 months for belinostat and alemtuzumab [Citation33,Citation55]; 5–8 months for romidepsin, vorinostat, and pegylated liposomal doxorubicin [Citation59,Citation68–Citation72]; and 11 months for bexarotene [Citation73]. Median OS was reported for 12 different therapies and ranged from 13.7 to 72 months following treatment with mogamulizumab or ECP, respectively (Supplementary material) [Citation67,Citation74].
Median DOR was reported for 15 different therapies and ranged from 1.8 months for pralatrexate [Citation75] to 28.9 months for pralatrexate plus bexarotene [Citation63] (). A median DOR >12 months was reported for bexarotene, romidepsin, TSEB, and acitretin. There was considerable variability in DOR between studies of bexarotene (range: 2.7–16.4 months) [Citation47,Citation76]. The median DOR reported following treatment with acitretin was 28.1 months [Citation77]. For the HDAC inhibitors, vorinostat, belinostat, panobinostat, and quisinostat as well as the novel therapies brentuximab vedotin, alemtuzumab, and pralatrexate, the median DOR was ≤6 months.
4. Discussion
This SR was undertaken to assess the effect of CTCL on patients’ HRQoL and the durability of clinical response to current treatments. The results highlight that CTCL influences multiple aspects of patient HRQoL, with a negative effect on emotion, symptom, and function domains. A range of generic HRQoL tools were used to assess the effects of CTCL on patient HRQoL. Despite being non-disease-specific, these tools identified consistent key problem areas including physical symptoms and psychological and social impacts. Embarrassing skin problems, including pruritus, were a considerable symptom burden which contributed to reduced patient QoL [Citation17]. Poorer patient QoL was associated with more advanced disease stage [Citation38,Citation41–Citation43]. Caregivers were also reported to be negatively affected by CTCL, primarily through the effect on family dynamics and relationships [Citation45,Citation46]. The effect of CTCL on HRQoL has implications for clinical practice, as it highlights areas in which patient and caregiver support is required. Identification of the symptoms contributing to reduced HRQoL may enable the development of therapeutic strategies that specifically address these symptoms and improve patient HRQoL as well as manage disease progression. It will, therefore, be necessary in future to standardize the assessment of HRQoL in CTCL via the development of CTCL-specific tools that capture the multidimensional effects of CTCL on QoL.
Clinical efficacy data were collated for a range of skin-directed, systemic, and stem-cell therapies currently used in the treatment of relapsed/refractory CTCL. ORR was the most consistently reported clinical outcome and the highest ORR was reported in patients treated with conventional TSEB therapy (range: 50–95%) [Citation56,Citation78,Citation79]. Of the pharmacological interventions, the highest ORR was observed following treatment with the approved therapy bexarotene (range: 39–86%) [Citation48,Citation76]. For the remaining pharmacological therapies, a very broad range of ORRs were reported (range: 14–78%) [Citation55,Citation62]. The durability of response to current treatments (PFS, DOR) was reported less frequently than ORR and varied considerably between treatments and studies assessing the same treatment. The greatest DOR was reported for acitretin (median DOR: 28.1 months) [Citation77], with the highest PFS seen with brentuximab vedotin (median PFS 15.8, 57.6, and 116.4 months in three studies) [Citation57,Citation61,Citation62]; median PFS with alemtuzumab was 56 months [Citation33]. The median DOR was ≤28 months for other approved therapies [Citation47,Citation68–Citation71,Citation73,Citation76,Citation77,Citation80–Citation82], ≤17 months for conventional therapies [Citation59,Citation78] and <12 months for investigational therapies [Citation61–Citation63,Citation75,Citation83,Citation84]. Median PFS was ≤13 months for other investigational, approved, and conventional therapies [Citation25,Citation63,Citation64,Citation75,Citation85,Citation86]. These results are in line with those reported by Zinzani et al., 2016, in an SR of current and future treatments for relapsed/refractory CTCL [Citation9].
The SR was conducted using predefined eligibility criteria and conformed to PRISMA guidelines. However, there were some limitations. The searches for clinical efficacy data were restricted to identify only those studies published after the year 2007 and which enrolled at least 20 patients. As a result, a number of therapies, e.g. isotretinoin and nivolumab, which are currently used in the treatment of patients with relapsed and/or refractory CTCL was excluded from analysis. For the therapies which were included, there was a disparity between treatments in the number of available studies which assessed their clinical efficacy. In addition, studies lacked standardized inclusion criteria and standardized clinical efficacy, or HRQoL outcomes, allowing only qualitative analysis. There were differences between studies in the disease stage and the subtype of the patients included, the prior treatments to which patients had been exposed and the number of prior treatments. In addition, there was considerable variation in the number of patients enrolled in different studies (range: 11–486 patients). The majority of included studies were open label, and the absence of double-blinding may be considered a limitation as this increases the risk of performance bias [Citation116]. Treatment and study comparisons were also limited by inconsistencies in the time-point for assessment of clinical efficacy outcomes and this was not reported in all studies. As CTCL is a heterogeneous disease, it would be ideal to consider each type of CTCL separately, both in terms of QoL and treatment efficacy. In spite of the large number of studies, the data did not allow such a detailed analysis. With the majority of studies having been conducted in North America or Europe, sub-analyses by location or ethnicity were also not possible.
Prince et al., 2017 [Citation53,Citation57] reported ORR4 data for patients treated with brentuximab vedotin, an outcome which captures both the proportion of patients achieving a response and response duration as a single measurement. However, not all studies reported patient follow-up time or the time-point for assessment of response and it was not possible to draw any firm conclusions regarding the durability of response rates with increasing treatment/follow-up duration. Though not routinely used in the assessment of the efficacy of treatments for CTCL, ORR4 may present a useful tool enabling treatment comparisons for emerging targeted therapies.
Since completion of this SR, an additional analysis from the MAVORIC study was published, reporting that patient-reported outcomes as measured by Skindex-29 and FACT-G demonstrated a significant QoL benefit in MF/SS patients treated with mogamulizumab compared with vorinostat (p < 0.05) [Citation117].
5. Conclusion
CTCL and its associated symptoms have been reported to significantly negatively affect multiple aspects of patients’ HRQoL. The novel, targeted treatments, such as belinostat, have been demonstrated to reduce the distressing skin problems, including pruritus, reported to be a considerable symptom burden contributing to reduced patient QoL [Citation17,Citation55]. Skin symptom assessment with Skindex-29 demonstrated significantly greater reductions in symptom burden with brentuximab vedotin compared with physician’s choice. In addition, ORR of >50% has been reported for therapies such as bexarotene, brentuximab vedotin, and alemtuzumab, highlighting the beneficial therapeutic effects achievable. However, although high response rates can be achieved with certain currently available treatments, a considerable proportion of patients do not respond to or do not achieve a sustainable response to therapy. Therefore, there remains an unmet need for treatments with improved efficacy which also address the HRQoL burden, especially for patients with aggressive relapsed disease. Although progress has been made toward the standardization of response criteria in CTCL, the use of various efficacy outcomes and their evaluation remains inconsistent. The routine use of clearly defined efficacy outcomes in clinical trials is required to enable robust comparisons of therapeutic efficacy.
Author contributions
All authors contributed to the conception, design, and drafting of the article; revised it critically for important intellectual content; and approved the final version to be submitted. MD: all of the above (except drafting); SM: conception and design; CM: conception, design, and drafting the article; EZ: conception, design, and approved final version; AG: conception, design, and approved final version.
Declaration of interest
M Dalal is employed by Takeda and owns stocks in the aforementioned; S Mitchell is a former employee of DRG Abacus who was commissioned by Takeda to conduct the systematic review; C McCloskey is a current employee of DRG Abacus who was commissioned by Takeda to conduct the systematic review. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (267.4 KB)Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Rodd AL, Ververis K, Karagiannis TC. Current and emerging therapeutics for cutaneous T-cell lymphoma: histone deacetylase inhibitors. Lymphoma. 2012, [290685]. doi:10.1155/2012/290685
- Bagherani N, Smoller BR. An overview of cutaneous T cell lymphomas. F1000Res. 2016 Jul 28;5. pii: F1000 Faculty Rev-1882. doi: 10.12688/f1000research.8829.1. eCollection 2016. Review. PubMed PMID: 27540476; PubMed Central PMCID: PMC4965697.
- Trautinger F, Eder J, Assaf C, et al. European organisation for research and treatment of cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome - update 2017. Eur J Cancer. 2017;77:57–74.
- Alsaleh QA, Nanda A, Al-Ajmi H, et al. Clinicoepidemiological features of mycosis fungoides in Kuwait, 1991–2006. Int J Dermatol. 2010;49(12):1393–1398.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007 Jul;143(7):854–859.
- Del Guzzo C, Levin A, Dana A, et al. The incidence of cutaneous T-Cell lymphoma in the veteran population. J Invest Dermatol. 2016;136(5):S24.
- Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013 Nov;149(11):1295–1299.
- Litvinov IV, Tetzlaff MT, Rahme E, et al. Identification of geographic clustering and regions spared by cutaneous T-cell lymphoma in Texas using 2 distinct cancer registries. Cancer. 2015 Jun 15;121(12):1993–2003.
- Zinzani PL, Bonthapally V, Huebner D, et al. Panoptic clinical review of the current and future treatment of relapsed/refractory T-cell lymphomas: cutaneous T-cell lymphomas. Crit Rev Oncol Hematol. 2016;99:228–240.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009 May 21;113(21):5064–5073.
- Imam MH, Shenoy PJ, Flowers CR, et al. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma. 2013 Apr;54(4):752–759.
- Weinstock MA, Gardstein B. Twenty-year trends in the reported incidence of mycosis fungoides and associated mortality. Am J Public Health. 1999 Aug;89(8):1240–1244.
- Arulogun SO, Prince HM, Ng J, et al. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008 Oct 15;112(8):3082–3087.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014 Feb;70(2):223.e1-17; quiz 240–2.
- Hanel W, Briski R, Ross CW, et al. A retrospective comparative outcome analysis following systemic therapy in mycosis fungoides and Sezary syndrome. Am J Hematol. 2016 Dec;91(12):E491–e495.
- Beynon T, Radcliffe E, Child F, et al. What are the supportive and palliative care needs of patients with cutaneous T-cell lymphoma and their caregivers? A systematic review of the evidence. Br J Dermatol. 2014 Mar;170(3):599–608.
- Beynon T, Selman L, Radcliffe E, et al. We had to change to single beds because I itch in the night’: a qualitative study of the experiences, attitudes and approaches to coping of patients with cutaneous T-cell lymphoma. Br J Dermatol. 2015 Jul;173(1):83–92.
- Bouwhuis SA, Gonzalez-Arriaza HL, McEvoy MT, et al. Physical and mental health survey of 20 patients with Sezary syndrome using the medical outcomes study 36-item short-form health survey. J Eur Acad Dermatol Venereol. 2003 Nov;17(6):724–725.
- Demierre MF, Gan S, Jones J, et al. Significant impact of cutaneous T-cell lymphoma on patients’ quality of life: results of a 2005 national cutaneous lymphoma foundation survey. Cancer. 2006 Nov 15;107(10):2504–2511.
- Salehi M, Azimi Z, Fatemi F, et al. Incidence rate of mycosis fungoides in Isfahan (Iran). J Dermatol. 2010 Aug;37(8):703–707.
- Talpur R, Wieser I, Wang C, et al. Evaluation of health related questionnaire among mycosis fungoides and lymphomatoid papulosis. Blood. 2015;126(23):5603.
- Porkert S, Lehner-Baumgartner E, Knobler R, et al. J-04. Assessment of QoL, illness perception, and illness behavior in 92 patients with primary cutaneous lymphoma. Presented at the 3rd World Congress of Cutaneous Lymphomas, New York, NY; 2016 Oct 26–28.
- Chmielowska E, Studzinski M, Giebel S, et al. Follow-up of patients with mycosis fungoides after interferon alpha2b treatment failure. Postepy Dermatol Alergol. 2015 Apr;32(2):67–72.
- Olsen EA. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther. 2003;16(4):311–321.
- Straus DJ, Duvic M, Horwitz SM, et al. Final results of phase II trial of doxorubicin HCl liposome injection followed by bexarotene in advanced cutaneous T-cell lymphoma. Ann Oncol. 2014 Jan;25(1):206–210.
- Assaf C, Becker JC, Beyer M, et al. Treatment of advanced cutaneous T-cell lymphomas with non-pegylated liposomal doxorubicin–consensus of the lymphoma group of the working group dermatologic oncology. J Dtsch Dermatol Ges. 2013 Apr;11(4):338–347.
- Wollina U, Dummer R, Brockmeyer NH, et al. Multicenter study of pegylated liposomal doxorubicin in patients with cutaneous T-cell lymphoma. Cancer. 2003 Sep 1;98(5):993–1001.
- Wollina U, Hohaus K, Schonlebe J, et al. Liposomal daunorubicin in tumor stage cutaneous T-cell lymphoma: report of three cases. J Cancer Res Clin Oncol. 2003 Jan;129(1):65–69.
- FDA. Vorinostat PI. [cited 2020 Jan 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021991s002lbl.pdf
- FDA. Romidepsin PI. [cited 2020 Jan 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022393lbl.pdf
- FDA. Denileukin diftitox PI. [cited 2020 Jan 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/103767s5094lbl.pdf
- FDA. Bexarotene PI. [cited 2020 Jan 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021055s010lbl.pdf
- de Masson A, Guitera P, Brice P, et al. Long-term efficacy and safety of alemtuzumab in advanced primary cutaneous T-cell lymphomas. Br J Dermatol. 2014 Mar;170(3):720–724.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 Aug 18;151(4):264–9, w64.
- National Institute for Health and Care Excellence. Single technology appraisal: user guide for company evidence submission template. 2015.
- Effective Public Health Practice Project. Quality assessment tool for quantitative studies. 2004.
- Vij A, Duvic M. Prevalence and severity of pruritus in cutaneous T cell lymphoma. Int J Dermatol. 2012 Aug;51(8):930–934.
- Wright A, Wijeratne A, Hung T, et al. Prevalence and severity of pruritus and quality of life in patients with cutaneous T-cell lymphoma. J Pain Symptom Manage. 2013 Jan;45(1):114–119.
- Talpur R, Wieser I, Wang C, et al. Evaluation of health related questionnaire among mycosis fungoides and lymphomatoid papulosis. J Am Acad Dermatol. 2016;74(5):AB117.
- Prince HM, Dummer R, Whittaker S, et al. Patient-reported outcomes and quality of life in patients with cutaneous T cell lymphoma: results from the phase 3 ALCANZA study [conference abstract]. Hematol Oncol. 2017 June;35(Supplement 2):247–248.
- Demierre MF, Tien A, Miller D. Health-related quality-of-life assessment in patients with cutaneous T-cell lymphoma. Arch Dermatol. 2005 Mar;141(3):325–330.
- Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma. Br J Dermatol. 2009 Apr;160(4):815–822.
- Demierre MF, Ferzli P, Miller D. Measuring HRQOL in patients with cutaneous T-cell lymphoma undergoing therapy with oral bexarotene and extracorporeal photopheresis. Arch Dermatol. 2007 May;143(5):659–661.
- Eder J, Kammerstatter M, Erhart F, et al. Illness perception in primary cutaneous T-cell lymphomas: what patients believe about their disease. Acta Derm Venereol. 2016 Mar;96(3):381–385.
- Selman LE, Beynon T, Radcliffe E, et al. ‘We’re all carrying a burden that we’re not sharing’: a qualitative study of the impact of cutaneous T-cell lymphoma on the family. Br J Dermatol. 2015 Jun;172(6):1581–1592.
- McCann S, Astley E, Huwe J, et al. Poster - caregiver burden and quality of life factors affecting caregivers caring for patients with cutaneous T-cell lymphoma. 2016 [cited 2017 Jun 09]. Available from: http://www.nursinglibrary.org/vhl/handle/10755/621226
- Sokolowska-Wojdylo M, Florek A, Zaucha JM, et al. Polish lymphoma research group experience with bexarotene in the treatment of cutaneous T-cell lymphoma. Am J Ther. 2016 May-Jun;23(3):e749–56.
- Rupoli S, Canafoglia L, Goteri G, et al. Results of a prospective phase II trial with oral low-dose bexarotene plus photochemotherapy (PUVA) in refractory and/or relapsed patients with mycosis fungoides. Eur J Dermatol. 2016 Jan-Feb;26(1):13–20.
- Rupoli S, Goteri G, Micucci G, et al. Time to next treatment analysis for early and advanced stages of mycosis fungoides/Sezary syndrome treated with bexarotene and puva in combination [conference abstract]. Haematologica. 2017 June;102(Supplement 2):466–467.
- Izu R, Yanguas I, Servitje O, et al. Experience with bexarotene in cutaneous T-cell lymphoma: a collaborative study of the Spanish group of cutaneous lymphoma. J Am Acad Dermatol. 2014;70(5):Suppl.1; AB123.
- Aviles A, Neri N, Fernandez-Diez J, et al. Interferon and low doses of methotrexate versus interferon and retinoids in the treatment of refractory/relapsed cutaneous T-cell lymphoma. Hematology. 2015 Oct;20(9):538–542.
- Kim YH, Bagot M, Pinter-Brown L, et al. Anti-CCR4 monoclonal antibody, mogamulizumab, demonstrates significant improvement in PFS compared to vorinostat in patients with previously treated cutaneous T-cell lymphoma (CTCL): results from the phase III MAVORIC study. Blood. 2017;130(Suppl 1):817.
- Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017 Jun 06;390(10094):555–566.
- Prince HM, Duvic M, Martin A, et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2010 Apr 10;28(11):1870–1877.
- Foss F, Advani R, Duvic M, et al. A phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015 Mar;168(6):811–819.
- Morris SL, McGovern M, Bayne S, et al. Results of a 5-week schedule of modern total skin electron beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013 Aug 01;86(5):936–941.
- Horwitz SM, Scarisbrick JJ, Dummer R, et al. Updated analyses of the international, open-label, randomized, phase 3 alcanza study: longer-term evidence for superiority of brentuximab vedotin versus methotrexate or bexarotene for CD30-positive cutaneous T-cell lymphoma (CTCL). Blood. 2017;130(Suppl 1):1509.
- Duvic M, Dummer R, Becker JC, et al. Panobinostat activity in both bexarotene-exposed and -naive patients with refractory cutaneous T-cell lymphoma: results of a phase II trial. Eur J Cancer. 2013 Jan;49(2):386–394.
- Dummer R, Quaglino P, Becker JC, et al. Prospective international multicenter phase II trial of intravenous pegylated liposomal doxorubicin monochemotherapy in patients with stage IIB, IVA, or IVB advanced mycosis fungoides: final results from EORTC 21012. J Clin Oncol. 2012 Nov 20;30(33):4091–4097.
- Aviles A, Nambo MJ, Neri N, et al. Interferon and low dose methotrexate improve outcome in refractory mycosis fungoides/Sezary syndrome. Cancer Biother Radiopharm. 2007 Dec;22(6):836–840.
- Duvic M. Phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphomas and lymphoproliferative disorders. Clin Adv Hematol Oncol. 2014 Feb;12(2 Suppl 6):10–11.
- Duvic M, Tetzlaff MT, Gangar P, et al. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J Clin Oncol. 2015 Nov 10;33(32):3759–3765.
- Duvic M, Kim YH, Zinzani PL, et al. Results from a phase I/II open-label, dose-finding study of pralatrexate and oral bexarotene in patients with relapsed/refractory cutaneous T-cell lymphoma. Clin Cancer Res. 2017 Jul 15;23(14):3552-3556. doi: 10.1158/1078-0432.CCR-16-2064. Epub 2017 Feb 6. PubMed PMID: 28167509; PubMed Central PMCID: PMC5511551.
- Illidge T, Chan C, Counsell N, et al. Phase II study of gemcitabine and bexarotene (GEMBEX) in the treatment of cutaneous T-cell lymphoma. Br J Cancer. 2013 Nov 12;109(10):2566–2573.
- Duvic M, Martin AG, Olsen EA, et al. Efficacy and safety of denileukin diftitox retreatment in patients with relapsed cutaneous T-cell lymphoma. Leuk Lymphoma. 2013 Mar;54(3):514–519.
- Duvic M, Pinter-Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015 Mar 19;125(12):1883–1889.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012 Mar 10;30(8):837–842.
- Foss F, Duvic M, Lerner A, et al. Clinical efficacy of romidepsin in tumor stage and folliculotropic mycosis fungoides. Clin Lymphoma Myeloma Leuk. 2016 Nov;16(11):637–643.
- Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010 Oct 10;28(29):4485–4491.
- Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007 Jan 01;109(1):31–39.
- Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007 Apr 15;13(8):2318–2322.
- Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007 Jul 20;25(21):3109–3115.
- Abbott RA, Whittaker SJ, Morris SL, et al. Bexarotene therapy for mycosis fungoides and Sezary syndrome. Br J Dermatol. 2009 Jun;160(6):1299–1307.
- Atilla E, Atilla PA, Bozdag SC, et al. Extracorporeal photochemotherapy in mycosis fungoides [Photochimiotherapie extracorporelle dans les mycoses fongiques.]. Transfus Clin Biol. 2017a November;24(4):454–457.
- Horwitz SM, Kim YH, Foss F, et al. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood. 2012 May 03;119(18):4115–4122.
- Straus DJ, Duvic M, Kuzel T, et al. Results of a phase II trial of oral bexarotene (Targretin) combined with interferon alfa-2b (Intron-A) for patients with cutaneous T-cell lymphoma. Cancer. 2007 May 01;109(9):1799–1803.
- Cheeley J, Sahn RE, DeLong LK, et al. Acitretin for the treatment of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2013 Feb;68(2):247–254.
- Hoppe RT, Harrison C, Tavallaee M, et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol. 2015 Feb;72(2):286–292.
- Lindahl LM, Kamstrup MR, Petersen PM, et al. Total skin electron beam therapy for cutaneous T-cell lymphoma: a nationwide cohort study from Denmark. Acta Oncologica. 2011 Nov 01;50(8):1199–1205.
- Bates SE, Eisch R, Ling A, et al. Romidepsin in peripheral and cutaneous T-cell lymphoma: mechanistic implications from clinical and correlative data. Br J Haematol. 2015 Jul;170(1):96–109.
- Duvic M, Kim YH, Rook AH, et al. Responses to romidepsin in patients with cutaneous T-cell lymphoma (CTCL) and prior treatment with systemic chemotherapy: subanalysis from the pivotal phase 2 study. Blood. 2014;124(21):4451.
- Kim EJ, Kim YH, Rook AH, et al. Clinically significant responses achieved with romidepsin across disease compartments in patients with cutaneous T-cell lymphoma. Leuk Lymphoma. 2015;56(10):2847–2854.
- Duvic M, Forero-Torres A, Foss, F, et al. Long-term treatment of CTCL with the oral PNP inhibitor, forodesine. J Clin Oncol. 2009;27:15_suppl, 8552-8552.
- Querfeld C, Rosen ST, Guitart J, et al. Results of an open-label multicenter phase 2 trial of lenalidomide monotherapy in refractory mycosis fungoides and Sezary syndrome. Blood. 2014 Feb 20;123(8):1159–1166.
- Martinez-Escala ME, Kuzel TM, Kaplan JB, et al. Durable responses with maintenance dose-sparing regimens of romidepsin in cutaneous T-cell lymphoma. JAMA Oncol. 2016 Jun 01;2(6):790–793.
- Quereux G, Marques S, Nguyen JM, et al. Prospective multicenter study of pegylated liposomal doxorubicin treatment in patients with advanced or refractory mycosis fungoides or Sezary syndrome. Arch Dermatol. 2008 Jun;144(6):727–733.
- Atilla E, Ataca P, Civriz Bozdag S, et al. Extracorporeal photopheresis for the treatment of mycosis fungoides. Blood. 2015;126(23):5062.
- Coronel-Perez IM, Carrizosa-Esquivel AM, Camacho-Martinez F. [Narrow band UVB therapy in early stage mycosis fungoides. A study of 23 patients]. Actas Dermosifiliogr. 2007 May;98(4):259–264.
- Jidar K, Ingen-Housz-Oro S, Beylot-Barry M, et al. Gemcitabine treatment in cutaneous T-cell lymphoma: a multicentre study of 23 cases. Br J Dermatol. 2009 Sep;161(3):660–663.
- Pellegrini C, Stefoni V, Casadei B, et al. Long-term outcome of patients with advanced-stage cutaneous T cell lymphoma treated with gemcitabine. Ann Hematol. 2014 Nov;93(11):1853–1857.
- Nikolaou V, Siakantaris MP, Vassilakopoulos TP, et al. PUVA plus interferon alpha2b in the treatment of advanced or refractory to PUVA early stage mycosis fungoides: a case series. J Eur Acad Dermatol Venereol. 2011 Mar;25(3):354–357.
- Roberge D, Muanza T, Blake G, et al. Does adjuvant alpha-interferon improve outcome when combined with total skin irradiation for mycosis fungoides? Br J Dermatol. 2007 Jan;156(1):57–61.
- Hernández Z, Peñate Y, Hernández-Machín B, et al. Treatment of stage Ia and Ib mycosis fungoides with psoralen UVA monotherapy: an observational study in tertiary hospitals in the Canary Islands. Int J Dermatol. 2014;53(11):1417–1422.
- Hoot JW, Wang L, Kho T, et al. The effect of phototherapy on progression to tumors in patients with patch and plaque stage of mycosis fungoides. J Dermatological Treat. 2018 May;29(3):272-276.
- Querfeld C, Rosen ST, Guitart J, et al. Multicenter phase II trial of temozolomide in mycosis fungoides/Sezary syndrome: correlation with O(6)-methylguanine-DNA methyltransferase and mismatch repair proteins. Clin Cancer Res. 2011 Sep 01;17(17):5748–5754.
- Hinds GA, Alhariri J, Klein RQ, et al. Treatment of mycosis fungoides with total skin electron beam: response and relapse by ethnicity and sex. Am J Clin Oncol. 2013 Oct;36(5):481–485.
- Kroeger K, Elsayad K, Moustakis C, et al. Low-dose total skin electron beam therapy for cutaneous lymphoma: minimal risk of acute toxicities. Strahlenther Onkol. 2017 Dec;193(12):1024–1030.
- Prince HM, Martin AG, Olsen EA, et al. Denileukin diftitox for the treatment of CD25 low-expression mycosis fungoides and Sezary syndrome. Leuk Lymphoma. 2013 Jan;54(1):69–75.
- Duvic M, Geskin L, Prince HM. Duration of response in cutaneous T-cell lymphoma patients treated with denileukin diftitox: results from 3 phase III studies. Clin Lymphoma Myeloma Leuk. 2013 Aug;13(4):377–384.
- Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009 Nov 10;27(32):5410–5417.
- Kim YH, Tavallaee M, Rozati S, et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides or Sezarysyndrome: final results show significant clinical activity and suggest correlation with CD30 expression. Blood. 2014;124(21):804.
- Kim YH, Tavallaee M, Sundram U, et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and Sezary syndrome with variable CD30 expression level: a multi-institution collaborative project. J Clin Oncol. 2015 Nov 10;33(32):3750–3758.
- Dalal MR, Whittaker S, Horwitz S, et al. Phase 3 ALCANZA study of brentuximab vedotin or physician’s choice of methotrexate or bexarotene in CD30-positive cutaneous T-cell lymphoma: number needed to treat analysis. P637. European Hematology Association Annual Congress. June 22–25, 2017; Madrid, Spain.
- Mathieu S, Ram-Wolff C, Quéreux G, et al. The French experience of treatment of cutaneous T cell lymphoma with brentuximab vedotin: a series of 32 cases. 3rd world congress of cutaneous lymphoma, New York, NY; 2016. Abstract no: P-04. 2016.
- Dummer R, Duvic M, Scarisbrick J, et al. Final results of a multicenter phase II study of the purine nucleoside phosphorylase (PNP) inhibitor forodesine in patients with advanced cutaneous T-cell lymphomas (CTCL) (mycosis fungoides and Sezary syndrome). Ann Oncol. 2014 Sep;25(9):1807–1812.
- Anlong L, Girardi M, Parker T, et al. Pralatrexate in cutaneous T cell lymphoma: retrospective experience with and without leucovorin. 3rd world congress of cutaneous lymphoma, New York, NY; 2016. Abstract no: N-03. 2016.
- Child F, Ortiz-Romero PL, Alvarez R, et al. Phase II multicentre trial of oral quisinostat, a histone deacetylase inhibitor, in patients with previously treated stage IB-IVA mycosis fungoides/Sezary syndrome. Br J Dermatol. 2016 Jul;175(1):80–88.
- Almazan T, Armstrong R, Kim YH, et al. 380 Determination of molecular remission in patients with mycosis fungoides (MF) and Sezary Syndrome (SS) following allogeneic transplant using high throughput sequencing (HTS). J Invest Dermatol. 2017;137(5):S65.
- de Masson A, Beylot-Barry M, Bouaziz JD, et al. Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: a study from the French society of bone marrow transplantation and French study group on cutaneous lymphomas. Haematologica. 2014 Mar;99(3):527–534.
- Duarte RF, Canals C, Onida F, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2010 Oct 10;28(29):4492–4499.
- Hosing C, Bassett R, Dabaja B, et al. Allogeneic stem-cell transplantation in patients with cutaneous lymphoma: updated results from a single institution. Ann Oncol. 2015 Dec;26(12):2490–2495.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014 Nov;49(11):1360–1365.
- Mehta-Shah N, Teja S, Tao Y, et al. Successful treatment of mature T-cell lymphoma with allogeneic stem cell transplantation: the largest multicenter retrospective analysis. Blood. 2017;130(Suppl 1):4597.
- Menter T, Medani H, Olavarria E, et al. Pathology findings in patients with cutaneous T-cell lymphomas treated with allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2016 Mar 01;180(6):904–908.
- Weng W-K, Armstrong R, Arai S, et al. Non-myeloablative allogeneic transplantation resulting in clinical and molecular remission with low non-relapse mortality (NRM) in patients with advanced stage mycosis fungoides (MF) and Sézary syndrome (SS). Blood. 2014;124(21):2544.
- Turner RM, Spiegelhalter DJ, Smith GC, et al. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc. 2009 Jan;172(1):21–47.
- Porcu P, Hudgens S, Quaglino P, et al. Quality of life in cutaneous T-cell lymphoma subjects treated with anti-CCR4 monoclonal antibody mogamulizumab versus vorinostat: results from the phase 3 MAVORIC trial. J Clin Oncol. 2018 May 20;36(15_suppl):7577.