ABSTRACT
Introduction: Twin-twin transfusion syndrome (TTTS) is a devastating complication of monochorionic twin pregnancy and remains a major challenge for worldwide fetal medicine specialists. In TTTS, intertwin transfusion through vascular anastomoses in the shared placenta leads to severe hemodynamic imbalance. This review summarizes the current knowledge of TTTS.
Areas covered: The most recent insights concerning the management of TTTS, as well as fetal and neonatal complications are described. Relevant articles were selected based on a Pubmed search using the keywords below. Understanding of the underlying pathophysiology has improved greatly as a result of placental injection studies. Advancements in antenatal management have led to increased perinatal survival and a decreased incidence of neonatal complications, including brain injury and neurodevelopmental impairment.
Expert opinion: Further opportunities for improvement comprise technological innovations in laser procedures and the prevention of preterm rupture of membranes with subsequent prematurity. A noninvasive treatment such as high-intensity focused ultrasound (HIFU) seems to hold promise for the future treatment of TTTS. Fetal MRI studies are important to improve our understanding of fetal brain injury and should relate their findings to long-term neurodevelopment. International collaboration and centralization of care are of paramount importance to ensure the best care for our patients.
1. Introduction
Twins are at increased risk of perinatal death and long-term neurologic morbidity, and this risk is highest for monochorionic (MC) twins [Citation1–Citation3]. A large part of the increased risk for MC twins is attributable to twin-twin transfusion syndrome (TTTS). TTTS is one of the most lethal conditions in fetal medicine and remains a major challenge for obstetricians and neonatologists across the world [Citation4,Citation5]. The implementation of strict protocols for the management of MC twin pregnancies have increased the opportunity for early diagnosis and timely management of TTTS [Citation6,Citation7]. The management of this devastating disease has advanced considerably since the introduction of fetoscopic laser surgery in the 1990s, leading to increased perinatal survival and more favorable neonatal and long-term outcomes [Citation8,Citation9].
TTTS results from an unbalanced blood flow through placental vascular anastomoses connecting the two fetal circulations. These vascular anastomoses in the shared placenta are present in virtually all monochorionic (MC) twin pregnancies, but only in about 10% lead to TTTS. The net transfusion of blood is at the expense of the so-called donor twin, who becomes hypovolemic and anemic, whereas the recipient twin becomes hypervolemic and polycythemic. This leads to the quick development of a significant discordance in amniotic fluid volume between the twins, described as twin polyhydramnios-oligohydramnios sequence (TOPS). Hormonal dysregulation has been implicated to play a role in the further development of the syndrome [Citation10,Citation11]. TOPS can be detected with prenatal ultrasound and is the hallmark of TTTS diagnosis. TTTS is staged using the criteria of Quintero, ranging from stage I disease characterized by TOPS with the donor’s bladder still visible, to stage V in which there is fetal demise of one or both twins [Citation12,Citation13]. The current review aims to summarize the knowledge gained in the last decade, its main focus being on pathophysiology, antenatal management, fetal and neonatal brain injury as well as long-term neurodevelopment after TTTS.
1.1. Literature search
Relevant papers were selected based on a Pubmed search of articles published after 2009, using combinations of the following search terms: twin-twin transfusion, laser, outcome, brain injury and neurodevelopment. Relevant literature references of the selected articles were identified and used for historic perspective in a few cases.
2. Placental injection studies
Although the exact mechanism by which TTTS develops is still poorly understood, placental injection studies using colored dye have much improved our knowledge of MC placentas in the past decade [Citation14–Citation16]. Vascular anastomoses in MC placentas are either arterio-arterial (AA), arterio-venous (AV) or veno-venous (VV) in nature. Injection studies were able to show a relationship between the type, number and size of placental anastomoses and the risk of developing TTTS and other MC pregnancy complications. TTTS placentas have significantly fewer AA anastomoses compared to uncomplicated MC placentas [Citation15,Citation17]. AA anastomoses allow for bidirectional flow of blood, compensating for any imbalance in intertwin blood volume caused by AV anastomoses, hereby reducing, but not eliminating, the risk of TTTS.
Placental injection studies have played an important role in the discovery of another form of intertwin transfusion in MC pregnancy termed twin anemia-polycythemia sequence (TAPS) [Citation18]. Like TTTS, TAPS results from unbalanced blood flow through placental anastomoses. However, because TAPS is characterized by the presence of only very small anastomoses, transfusion in TAPS is much slower, allowing time for hemodynamic compensatory mechanisms to take effect and thus preventing the development of hypovolemia in the donor and hypervolemia in the recipient. Therefore, the essential difference between TTTS and TAPS is the absence of oligohydramnios/polyhydramnios in TAPS [Citation19,Citation20]. TAPS can be diagnosed antenatally by measurement of the middle cerebral artery peak systolic velocity (MCA-PSV) in both twins. In a recent study, it was shown that the difference in MCA-PSV between the donor and recipient is the most accurate predictor of postnatal TAPS with high sensitivity (83%) and specificity (100%). Based on these findings, a new antenatal classification system was proposed using a delta MCA-PSV of > 0.5 multiples of the median (MoM) as criterion for Stage 1 TAPS. Postnatally, TAPS is present when the intertwin hemoglobin difference is > 8 g/dL, combined with either a reticulocyte count ratio > 1.7 or the presence of only very small (< 1 mm) placental anastomoses [Citation21]. TAPS can occur spontaneously (3-5% of MC pregnancies) or after laser for TTTS [Citation22].
3. Antenatal management of TTTS
Before the development of fetoscopic laser surgery, antenatal treatment in TTTS was mainly based on serial amnioreduction to reduce polyhydramnios and the consequent risk of preterm delivery [Citation4]. However, short and long-term outcome after serial amnioreduction were poor. Perinatal survival rates usually did not exceed 50% and the risk of neurodevelopmental injury in survivors was high, up to 25% [Citation23–Citation26].
3.1. Fetoscopic laser surgery
Fetoscopic laser surgery is now considered the best available treatment for advanced stages (Quintero stage ≥ 2) TTTS [Citation24,Citation27–Citation30]. The technique using a fetoscopic laser to disrupt blood flow through the vascular communications was developed in the 1980s and it soon became clear that this therapy would become a ‘game changer’ in the field of TTTS [Citation31]. In contrast to serial amnioreduction, laser surgery is the only causative treatment, aiming to stop the intertwin fetal transfusion process. The first laser procedures were performed under general anesthesia and required laparotomy and hysterotomy for introduction of the endoscope, followed by laser photocoagulation. Since then, many developments have taken place. Laser technique has evolved from so-called ‘non-selective’ ablation of all vessels at the chorionic surface close to the intertwin membrane, to the selective technique in which the goal is to only coagulate anastomotic vessels at the vascular equator [Citation29,Citation32–Citation35]. In TTTS, the vascular equator is at some distance from the intertwin membrane due to the discordant amniotic fluid volumes between the twins. Laser surgery in TTTS is now minimally invasive, usually with local or regional anesthesia and percutaneous fetoscopy under continuous ultrasound guidance, thereby minimizing the risk of maternal complications [Citation36]. Whether laser treatment is the best option for stage I TTTS is uncertain, as different groups have reported different results [Citation37]. A multicenter RCT of stage I disease has recently been carried out and the results will hopefully answer this important question.
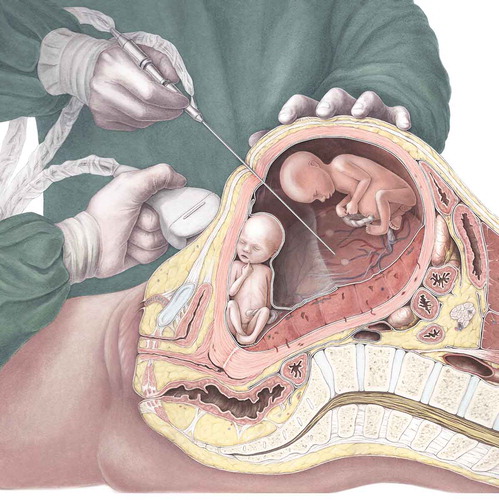
illustrates how a laser procedure is performed in a case of TTTS.
Figure 1. Realistic depiction of a modern-time laser procedure under ultrasound guidance. Courtesy of Amanda Gautier.
3.1.1. Sequential selective laser surgery
In 2007, Quintero and colleagues described a laser technique in which they choose to target the anastomoses in a specific order based on the physiological assumption that further hemodynamic shifts during the laser procedure might contribute to post-laser fetal demise, especially of the donor. They called this technique the sequential selective laser photocoagulation of communicating vessels and reported positive results in terms of post-laser fetal demise and dual survival rates [Citation38]. A systematic review in 2015 concluded that although data from three cohort studies suggest that dual survival was higher (75% versus 52%) and fetal demise rates were lower after sequential selective laser as compared to standard selective laser surgery, these results must be interpreted with caution, as no randomized controlled trials (RCT) were done and the three included studies had a high risk of bias due to methodological limitations [Citation39].
3.1.2. Solomon technique
Placental injection studies after laser therapy have revealed that residual anastomoses are present in over 30% of placentas [Citation40,Citation41]. Residual anastomoses can cause post-laser TAPS or recurrence of TTTS and these complications are linked to adverse fetal, neonatal and long-term outcomes [Citation42,Citation43]. To minimize the risk of residual anastomoses, a new laser technique was developed, designed to coagulate the entire vascular equator. This technique was termed the ‘Solomon technique’ after the biblical story of King Solomon who, in order to determine which one of two women was its real mother, suggested to cut a baby in half, after which the true mother begged that the child would be spared and committed to the care of her rival. Obviously, the Solomon technique does not entail splitting a baby in half. It is a modification of the selective laser technique, in which a line is drawn with the laser from one edge of the placenta to the other following the vascular equator, connecting the anastomoses that were first coagulated using the selective technique. The effect is a functional ‘dichorionization’ of the placenta. The Solomon trial, an RCT comparing the standard selective laser technique to the Solomon technique, showed a significant reduction in post-laser TAPS and recurrence of TTTS from 16% and 7% with the selective technique to 3% and 1% with the Solomon technique, respectively [Citation44]. A secondary analysis showed that the Solomon technique does indeed reduce the risk of residual anastomoses, but they still occur, even when the procedure is recorded to be complete by the fetal therapist. Therefore, Slaghekke and her colleagues conclude that careful follow-up of the pregnancy remains essential also after Solomon laser [Citation41].
3.2. Survival
In 2015, Akkermans and colleagues systematically reviewed all published reports on survival after fetoscopic laser surgery for TTTS over 25 years and found that perinatal survival improved significantly during this time. The mean survival of both twins increased from 31% in reports dating from 1990 to 1995, to 62% in reports published between 2011 and 2014. Survival of at least one twin increased from 70 to 88% [Citation8]. This study also shows a significant improvement in double survival with the more recently developed sequential selective (64%) and Solomon laser technique (71%) compared to the older techniques. The authors argue that improved survival is likely to be multifactorial and could be the result of evolution in laser technique, a learning curve effect for fetal surgeons, and improvements in referral as well as neonatal care.
3.3. Complications of fetoscopic laser surgery
The most important complications after laser surgery are fetal demise and preterm prelabor rupture of the membranes (PPROM) [Citation36,Citation45]. Fetal demise rates are often not reported separately but comprise some combination of fetal demise in utero, miscarriage and selective feticide. In studies that do report the rate of fetal demise after laser, it occurs in 13 to 33% of cases. Some series report higher rates of fetal loss for donors than recipients [Citation46–Citation49]. Although gestational age at birth for TTTS pregnancies has become significantly higher since the introduction of laser therapy, most series report mean gestational ages at birth to be around 32 weeks [Citation9]. The fact that TTTS infants are still born prematurely is mostly due to PPROM after laser surgery. Iatrogenic PPROM occurs in up to 30% of cases [Citation50,Citation51]. Maternal complications of laser surgery are also not consistently reported, but appear to occur in about 5% of cases and include abdominal pain after leakage of amniotic fluid into the peritoneal cavity, chorioamnionitis, bleeding, pulmonary edema and placental abruption [Citation45].
4. Neonatal outcome
As the majority of TTTS survivors are still born prematurely, TTTS neonates are at risk for morbidity associated with prematurity, including respiratory disease, necrotizing enterocolitis, retinopathy of prematurity and cerebral injury [Citation52,Citation53]. Additionally, specific TTTS-related neonatal complications include cardiovascular morbidity, renal failure and hematologic disorders [Citation53–Citation55]. The risk of congenital heart disease is increased 12-fold in TTTS survivors compared to singletons and is attributed to severe fetal hemodynamic instability, leading to altered cardiac development [Citation56]. Although fetal functional and/or structural cardiac abnormalities improve after successful laser surgery, they can persist beyond birth and sometimes require intervention. Cardiovascular complications are primarily seen in the recipient twin and include hypertension, cardiomyopathy, right ventricular outflow tract obstruction (which affects about 4% of recipients at birth) and persistent pulmonary hypertension of the newborn [Citation56,Citation57]. However, donors may be at increased risk for aortic coarctation, possibly due to reduced flow caused by hypovolemia [Citation58].
The most important and dreaded complication in TTTS survivors is cerebral injury, as it may have profound and lifelong impact on these infants.
4.1. Cerebral injury
Brain injury is a feared complication of MC pregnancies in general and of TTTS in particular. It is caused by the presence of vascular anastomoses and intertwin transfusion. The risk is highest for single survivors after intrauterine demise of their co-twin, which can cause severe hypovolemia and anemia due to the acute transfer of blood from the surviving fetus into their dying or dead sibling through patent vascular connections [Citation59–Citation61]. Laser surgery performed before fetal demise is protective of cerebral injury, provided that the surgery is complete and no anastomoses were missed. The incidence of cerebral injury in TTTS has dropped considerably since the introduction of laser therapy and reported ranges are now between 2 and 18% [Citation24,Citation25,Citation27,Citation62–Citation66]. In our own center, the incidence has decreased since the start of our laser surgery program in 2000 from 14% in the first five years, to 6% in the most recently studied cohort treated between 2011 and 2014 [Citation48,Citation67]. Various reasons have been suggested to explain the decrease in brain injury, including improvement in laser technique and a learning curve effect, both associated with a decrease in residual anastomoses. In a large cohort of 1023 TTTS pregnancies, postoperative TAPS and recurrence of TTTS after laser, both known to be caused by residual anastomoses, were associated with an increased risk of cerebral injury [Citation16,Citation42].
Several types of cerebral injury have been described in the literature, including (cystic) periventricular leukomalacia (PVL), intraventricular hemorrhage (IVH), posthemorrhagic ventricular dilatation, cerebral atrophy and arterial ischemic stroke. Fetal and neonatal MRI studies have shed more light on brain abnormalities detected in TTTS in the last decade. Several studies have reported additional findings of MRI compared to ultrasonography alone, including polymicrogyria and other migrational disorders, sinovenous thrombosis, and more subtle and/or diffuse white matter injury [Citation42,Citation64,Citation68–Citation71]. Donors and recipients are equally affected by cerebral injury, although the occurrence of cerebral arterial stroke seems to be a specific risk for recipients [Citation42,Citation62,Citation72]. The range of cerebral injury reported by different groups is quite wide. This is due to varying definitions of cerebral injury, differences in the timing and frequency of imaging, and the fact that some centers do not routinely perform cranial imaging in all TTTS survivors.
Prematurity is still more the rule than the exception in TTTS pregnancies, making survivors also prone to postnatally acquired cerebral damage related to (extreme) prematurity, especially IVH and PVL. Prematurity and low birth weight (with birth weight being strongly correlated with gestational age) have in fact been proven to be the most important risk factors for severe cerebral injuries in survivors of TTTS [Citation62,Citation66]. Reduction of severe prematurity in TTTS could hypothetically be achieved by reduction of the risk of PPROM and intrauterine infection through further technical improvements in fetoscopic surgery (for example by developing smaller fetoscopic instruments). Besides postnatal brain injury caused by prematurity, antenatally acquired cerebral lesions are more common in the context of TTTS compared to dichorionic twins, presumably because of the severe hemodynamic disturbances during pregnancy [Citation62]. When antenatal in origin, cerebral injury in donors is thought to be mainly caused by impaired cerebral perfusion as the result of hypovolemia and intertwin shifts of blood, leading to hypoxic-ischemic insults. Polycythemia and hyperviscosity with subsequent vascular sludging is the presumed mechanism for cerebral injury in recipients.
Given the remaining risk of cerebral injury for TTTS survivors, routine standardized antenatal and postnatal cerebral imaging protocols are strongly recommended to accurately evaluate origin, timing and type of damage. MRI may play a larger role in determining cerebral injury in the future. Increased awareness of the increased risk by neonatologists and pediatricians may improve neonatal and pediatric care for these children. The clinical relevance of neuroimaging findings should be determined using long-term neurodevelopmental outcome data of all TTTS survivors until at least school age.
5. Long-term outcome
5.1. Definition and incidence
The ultimate goal of fetal therapy should be survival without neurodevelopmental impairment (NDI). Especially now that survival rates and short-term outcome have greatly improved, long-term follow-up of survivors is essential to evaluate whether this goal is achieved [Citation46,Citation73]. Severe NDI in most studies is defined as at least one of the following: cerebral palsy (CP), severe motor and/or cognitive developmental delay, bilateral blindness, or deafness requiring amplification with hearing aids. Determining NDI requires a follow-up regimen that includes a physical and neurological examination, as well as cognitive and motor developmental assessments. Psychomotor development is ideally evaluated using standardized tests, such as the Bayley Scales for Infant and Toddler Development. However, several studies rely on the parent interview-based Ages and Stages Questionnaire (ASQ) for the evaluation of NDI. The ASQ has been shown to be a good screening tool for identifying infants who are severely delayed at 24 months of age and require neurological follow‐up or intervention, but cannot give the detailed information provided by face-to-face developmental tests performed by trained professionals [Citation74].
When combining studies that have clearly reported rates of NDI and/or CP after laser surgery at ≥ 2 years of age, the incidence of severe NDI in TTTS after laser surgery is 9% (range between 3 and 18%) and CP is reported at an average of 5% (range 2-12%). The results of these studies are summarized in .
Table 1. Studies of neurodevelopmental impairment and cerebral palsy in TTTS treated with laser.
The incidences of severe NDI and CP have decreased over the last two decades. Different factors may explain this improvement, including the development of stringent fetal monitoring protocols for MC pregnancies, learning curve effect of the laser procedure, increased awareness and improved neonatal care strategies for TTTS survivors [Citation7,Citation82].
5.2. Risk factors
Risk factors for long-term NDI identified in follow-up studies include advanced gestational age at laser surgery, Quintero stage, low gestational age at birth, low birth weight and severe cerebral injury [Citation48,Citation63,Citation83–Citation85]. The negative impact of advanced gestational age at laser and higher Quintero stage suggests that increasing disease severity may lead to increased long-term morbidity. Low gestational age and birth weight are well-recognized risk factors for developmental impairment in the general population as well: severe NDI is frequently found in children born preterm and is inversely associated with gestational age and birth weight.
5.3. Mild neurodevelopmental impairment
Long-term follow-up studies after TTTS have used widely varying methods with regard to inclusion criteria, timing of assessments, the definition of NDI and the used outcome measures and controls, making it difficult to compare results. Significant loss to follow-up rates are common amongst long-term follow-up studies and have the potential to cause bias, most likely causing an underestimation of developmental impairment [Citation86,Citation87]. In the majority of studies children were assessed at the age of two years, adjusted for prematurity, when major developmental impairment that requires intervention can be distinguished. However, neurodevelopmental assessment during early childhood can only moderately predict longer-term developmental outcome, especially for cognitive ability and academic performance. Certain developmental problems such as learning difficulties, behavioral problems and autism spectrum disorder are not always detected until school age, when children become more socially and academically challenged. To understand the clinical relevance of milder forms of impairment diagnosed in early childhood, follow-up until at least school age is essential. Data on mild neurodevelopmental impairment are limited and inconsistent. However, because of the decreasing trend in the rate of severe NDI, attention is shifting toward more subtle problems, including mild CP and neurocognitive impairments. Minor impairments can have a significant impact on the care and educational requirements of children and are reported in up to 30% of TTTS survivors [Citation25,Citation47,Citation48,Citation88–Citation90]. In order to gain more information on the exact burden and severity of NDI after laser surgery for TTTS, we encourage international collaboration to obtain larger sample sizes and statistical power, using a standardized follow-up regimen including uniform and clearly defined criteria for long-term neurodevelopmental impairment [Citation91].
6. Conclusion
The management of TTTS has evolved significantly in the 21st century. Placental injection studies have improved our understanding of underlying placental pathophysiology. Fetoscopic laser coagulation of placental anastomoses is the primary treatment option and many advancements have been made over the past decade. Laser surgery using the Solomon technique has been proven to decrease the risk of post-laser TAPS and recurrence of TTTS. The advancements in antenatal management of TTTS have led to improved survival, higher gestational ages at birth, a lower incidence of neonatal brain injury and a decrease in severe long-term neurodevelopmental impairment.
7. Expert opinion
Although the outcome of TTTS has improved greatly over the past decades, opportunities for further progress certainly still exist. The ultimate goals in the field of TTTS include prompt diagnosis or even better, accurate prediction of development of the syndrome, followed by a preferably noninvasive treatment with minimal complications. This treatment should achieve quick normalization of hemodynamic imbalance, minimizing any damage to the fetuses, without increasing the risk of PPROM and prematurity. The final goal for every TTTS pregnancy is (near-) term birth of two healthy neonates with normal long-term cognitive and motor development.
There is still a lot of ground to cover before these goals can be achieved. High-intensity focused ultrasound (HIFU) is a noninvasive technique which can be used to ablate blood flow in placental vessels, and therefore holds promise for the future treatment of TTTS. Although some preliminary experience with HIFU has been reported in animals as well as human cases of twin reversed arterial perfusion sequence (TRAP), safety and efficacy in humans with TTTS remain to be established [Citation92,Citation93]. In the meantime, technological innovations may be able to further improve visibility and accessibility of the vascular equator during laser procedures. The further development of instrumentation aimed at minimizing the risk of PPROM after laser surgery is another important focus of research in future years, hopefully helping to prolong pregnancies further beyond 32 weeks’ gestation and thereby minimizing the risks associated with preterm birth. Also, as the severity of placental damage after laser surgery is associated with PPROM and earlier delivery, the further fine-tuning of laser technique using minimal energy but obtaining maximum effect can certainly contribute to even better perinatal outcome.
With concern to the neonatal outcome of TTTS survivors, fetal MRI studies have begun to shed more light on the timing and mechanisms of fetal brain injury in TTTS, but studies that link prenatal MRI findings to long-term neurodevelopment are still lacking. Knowledge on long-term development beyond the age of 2 years is also very limited, especially concerning potential risk factors for adverse outcome and mild neurodevelopmental impairment. Although follow-up programs are costly and hard to realize, TTTS remains a serious risk factor for NDI. In our opinion, the treatment of fetuses in utero comes with the responsibility of providing careful and long-term follow-up of survivors until at least school age, in order to ensure that they will receive the care they need, if they need it. Long-term follow-up studies are indispensable for the continuous evaluation of the outcome of fetal therapy in TTTS pregnancies, as well as for the facilitation of evidence-based counseling of future parents. The formation of a core outcome set by a group of international experts is an important step in the joining of forces to help TTTS fetuses survive without complications, by making it possible to compare and combine research results. As briefly discussed in our review, severe fetal hemodynamic changes in TTTS can interfere with cardiac development leading to both functional and structural cardiac abnormalities. Follow-up studies of these cardiac changes are still very limited, but are essential for determining the need for cardiac screening and long-term monitoring in TTTS survivors, as well as for the counseling of parents about the prognosis of their children.
Lastly, an important issue to address is the need for centralization of treatment for TTTS in high-output fetal therapy centers. Following the advancements of laser treatment, there is an increasing number of small centers starting up fetal therapy programs in different countries. However, centralization has proven to be vital for the quality of care in a highly technical procedure such as fetoscopic laser surgery [Citation94]. The best possible care can only be realized in highly specialized centers with dedicated and experienced multidisciplinary teams, ideally comprising fetal therapists, neonatologists and psychologists and in collaboration with twin parents’ organizations.
Article highlights
Twin-twin transfusion syndrome (TTTS) is caused by intertwin shifts of blood through placental anastomoses in monochorionic twin pregnancies
Fetoscopic laser surgery is the primary treatment for TTTS and has improved with the development of new techniques over the past decades
The Solomon technique aims to separate the twins’ circulations completely and has been shown to decrease the incidence of post-laser twin anemia-polycythemia sequence (TAPS) and recurrence of TTTS
Brain injury and long-term neurodevelopmental impairment remain major concerns after TTTS, but fortunately the incidences of both have decreased significantly over time
Centralization in high output fetal therapy centers is crucial to guarantee the best quality of care in rare diseases needing highly specialized interventions like TTTS and laser surgery
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lewi L, Van Schoubroeck D, Gratacos E, et al. Monochorionic diamniotic twins: complications and management options. Curr Opin Obstet Gynecol. 2003 Apr;15(2):177–194. PubMed PMID: 12634610; eng.
- Dube J, Dodds L, Armson BA. Does chorionicity or zygosity predict adverse perinatal outcomes in twins? Am J Obstet Gynecol. 2002 Mar;186(3):579–583. PubMed PMID: 11904627; eng.
- Pharoah PO. Risk of cerebral palsy in multiple pregnancies. Clin Perinatol. 2006 Jun;33(2):301–313. PubMed PMID: 16765726; eng.
- Glennon CL, Shemer SA, Palma-Dias R, et al. The history of treatment of twin-to-twin transfusion syndrome. Twin Res Human Genet. 2016 Jun;19(3):168–174. PubMed PMID: 27203604; eng.
- Weir PE, Ratten GJ, Beischer NA. Acute polyhydramnios–a complication of monozygous twin pregnancy. Br J Obstet Gynaecol. 1979 Nov;86(11):849–853. PubMed PMID: 389280; eng.
- Gratacos E, Ortiz JU, Martinez JM. A systematic approach to the differential diagnosis and management of the complications of monochorionic twin pregnancies. PubMed PMID: 23006773; eng. Fetal Diagn Ther. 2012;32(3):145–155.
- Sueters M, Oepkes D. Diagnosis of twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anaemia-polycythaemia sequence, and twin reversed arterial perfusion sequence. Best Pract Res Clin Obstetrics Gynaecol. 2014 Feb;28(2):215–226. PubMed PMID: 24433823; eng.
- Akkermans J, Peeters SH, Klumper FJ, et al. Twenty-five years of fetoscopic laser coagulation in twin-twin transfusion syndrome: a systematic review. Fetal Diagn Ther . 2015;38(4):241–253. PubMed PMID: 26278319; eng. .
- Hecher K, Gardiner HM, Diemert A, et al. Long-term outcomes for monochorionic twins after laser therapy in twin-to-twin transfusion syndrome. Lancet Child Adolesc Health. 2018 Jul;2(7):525–535. PubMed PMID: 30169324; eng.
- Galea P, Barigye O, Wee L, et al. The placenta contributes to activation of the renin angiotensin system in twin-twin transfusion syndrome. Placenta. 2008 Aug;29(8):734–742. PubMed PMID: 18558429; eng.
- Djaafri F, Stirnemann J, Mediouni I, et al. Twin-twin transfusion syndrome - what we have learned from clinical trials. Semin Fetal Neonatal Med. 2017 Dec;22(6):367–375. PubMed PMID: 29122542; eng.
- Quintero RA, Morales WJ, Allen MH, et al. Staging of twin-twin transfusion syndrome. J Perinatol. 1999 Dec;19(8 Pt 1):550–555. PubMed PMID: 10645517; eng.
- Taylor MJ, Govender L, Jolly M, et al. Validation of the Quintero staging system for twin-twin transfusion syndrome. Obstet Gynecol. 2002 Dec;100(6):1257–1265. PubMed PMID: 12468171; eng.
- Lopriore E, Slaghekke F, Middeldorp JM, et al. Accurate and simple evaluation of vascular anastomoses in monochorionic placenta using colored dye. J Vis Exp. 2011 Sep 5;(55):e3208. DOI:10.3791/3208. PubMed PMID: 21912373; PubMed Central PMCID: PMCPMC3230184. eng.
- Zhao DP, de Villiers SF, Slaghekke F, et al. Prevalence, size, number and localization of vascular anastomoses in monochorionic placentas. Placenta. 2013 Jul;34(7):589–593. PubMed PMID: 23639577; eng.
- Lewi L, Deprest J, Hecher K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. Am J Obstet Gynecol. 2013 Jan;208(1):19–30. PubMed PMID: 23103301; eng.
- Denbow ML, Cox P, Taylor M, et al. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol. 2000 Feb;182(2):417–426. PubMed PMID: 10694346; eng.
- Lopriore E, Middeldorp JM, Oepkes D, et al. Twin anemia-polycythemia sequence in two monochorionic twin pairs without oligo-polyhydramnios sequence. Placenta. 2007 Jan;28(1):47–51. PubMed PMID: 16516289; eng.
- Lopriore E, Deprest J, Slaghekke F, et al. Placental characteristics in monochorionic twins with and without twin anemia-polycythemia sequence. Obstet Gynecol. 2008 Oct;112(4):753–758. PubMed PMID: 18827116; eng.
- Slaghekke F, Kist WJ, Oepkes D, et al. TAPS and TOPS: two distinct forms of feto-fetal transfusion in monochorionic twins. Z Geburtshilfe Neonatol. 2009 Dec;213(6):248–254. PubMed PMID: 20099211; eng.
- Tollenaar LSA, Lopriore E, Middeldorp JM, et al. Improved prediction of twin anemia-polycythemia sequence by delta middle cerebral artery peak systolic velocity: new antenatal classification system. Ultrasound Obstetrics Gynecol. 2019 Jun;53(6):788–793. PubMed PMID: 30125414; PubMed Central PMCID: PMCPMC6593803. eng.
- Tollenaar LS, Slaghekke F, Middeldorp JM, et al. Twin anemia polycythemia sequence: current views on pathogenesis, diagnostic criteria, perinatal management, and outcome. Twin Res Human Genet. 2016 Jun;19(3):222–233. PubMed PMID: 27068715; eng.
- Salomon LJ, Ortqvist L, Aegerter P, et al. Long-term developmental follow-up of infants who participated in a randomized clinical trial of amniocentesis vs laser photocoagulation for the treatment of twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2010 Nov;203(5):444.e1-7. PubMed PMID: 21055511; eng.
- van Klink JM, Koopman HM, van Zwet EW, et al. Cerebral injury and neurodevelopmental impairment after amnioreduction versus laser surgery in twin-twin transfusion syndrome: a systematic review and meta-analysis. Fetal Diagn Ther. 2013;33(2):81–89. PubMed PMID: 22922370; eng.
- Lenclen R, Ciarlo G, Paupe A, et al. Neurodevelopmental outcome at 2 years in children born preterm treated by amnioreduction or fetoscopic laser surgery for twin-to-twin transfusion syndrome: comparison with dichorionic twins. Am J Obstet Gynecol. 2009 Sep;201(3):291.e1-5. PubMed PMID: 19608152; eng.
- Li X, Morokuma S, Fukushima K, et al. Prognosis and long-term neurodevelopmental outcome in conservatively treated twin-to-twin transfusion syndrome. BMC Pregnancy Childbirth. 2011 Apr 22;11:32. PubMed PMID: 21510908; PubMed Central PMCID: PMCPMC3125386. eng.
- Senat MV, Deprest J, Boulvain M, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004 Jul 8;351(2):136–144. PubMed PMID: 15238624; eng.
- Roberts D, Neilson JP, Kilby MD, et al. Interventions for the treatment of twin-twin transfusion syndrome. Cochrane Database Syst Rev. 2014 Jan 30;(1):Cd002073. DOI:10.1002/14651858.CD002073.pub3. PubMed PMID: 24482008; eng.
- Hecher K, Plath H, Bregenzer T, et al. Endoscopic laser surgery versus serial amniocenteses in the treatment of severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 1999 Mar;180(3 Pt 1):717–724. PubMed PMID: 10076153; eng.
- Rossi AC, D’Addario V. Survival outcomes of twin-twin transfusion syndrome stage I: a systematic review of literature. Am J Perinatol. 2013 Jan;30(1):5–10. PubMed PMID: 22836822; eng.
- De Lia JE, Cruikshank DP, Keye WR Jr. Fetoscopic neodymium: YAG laser occlusion of placental vessels in severe twin-twin transfusion syndrome. Obstet Gynecol. 1990 Jun;75(6):1046–1053. PubMed PMID: 2342732; eng.
- Quintero RA, Comas C, Bornick PW, et al. Selective versus non-selective laser photocoagulation of placental vessels in twin-to-twin transfusion syndrome. Ultrasound Obstetrics Gynecol. 2000 Sep;16(3):230–236. PubMed PMID: 11169288; eng.
- Quintero RA, Morales WJ, Mendoza G, et al. Selective photocoagulation of placental vessels in twin-twin transfusion syndrome: evolution of a surgical technique. Obstet Gynecol Surv. 1998 Dec;53(12 Suppl):S97–103. PubMed PMID: 9870237; eng.
- Ville Y, Hecher K, Ogg D, et al. Successful outcome after Nd: YAG laser separation of chorioangiopagus-twins under sonoendoscopic control. Ultrasound Obstetrics Gynecol. 1992 Nov 1;2(6):429–431. PubMed PMID: 12796919; eng.
- Ville Y, Hyett J, Hecher K, et al. Preliminary experience with endoscopic laser surgery for severe twin-twin transfusion syndrome. N Engl J Med. 1995 Jan 26;332(4):224–227. PubMed PMID: 7808488; eng.
- Yamamoto M, El Murr L, Robyr R, et al. Incidence and impact of perioperative complications in 175 fetoscopy-guided laser coagulations of chorionic plate anastomoses in fetofetal transfusion syndrome before 26 weeks of gestation. Am J Obstet Gynecol. 2005 Sep;193(3 Pt 2):1110–1116. PubMed PMID: 16157121; eng.
- Khalil A, Cooper E, Townsend R, et al. Evolution of stage 1 Twin-to-Twin Transfusion Syndrome (TTTS): systematic review and meta-analysis. Twin Res Human Genet. 2016 Jun;19(3):207–216. PubMed PMID: 27137946; eng.
- Quintero RA, Ishii K, Chmait RH, et al. Sequential selective laser photocoagulation of communicating vessels in twin-twin transfusion syndrome. J Matern Fetal Neonatal Med. 2007 Oct;20(10):763–768. PubMed PMID: 17763279; eng.
- Akkermans J, Peeters SH, Klumper FJ, et al. Is the sequential laser technique for twin-to-twin transfusion syndrome truly superior to the standard selective technique? A meta-analysis. Fetal Diagn Ther. 2015;37(4):251–258. PubMed PMID: 25139419; eng.
- Lopriore E, Middeldorp JM, Oepkes D, et al. Residual anastomoses after fetoscopic laser surgery in twin-to-twin transfusion syndrome: frequency, associated risks and outcome. Placenta. 2007 Feb-Mar;28(2–3):204–208. PubMed PMID: 16644009; eng.
- Slaghekke F, Lewi L, Middeldorp JM, et al. Residual anastomoses in twin-twin transfusion syndrome after laser: the Solomon randomized trial. Am J Obstet Gynecol. 2014 Sep;211(3):285.e1-7. PubMed PMID: 24813598; eng.
- Stirnemann J, Chalouhi G, Essaoui M, et al. Fetal brain imaging following laser surgery in twin-to-twin surgery. BJOG. 2018 Aug;125(9):1186–1191. PubMed PMID: 27348600; eng
- Lewi L, Jani J, Cannie M, et al. Intertwin anastomoses in monochorionic placentas after fetoscopic laser coagulation for twin-to-twin transfusion syndrome: is there more than meets the eye? Am J Obstet Gynecol. 2006 Mar;194(3):790–795. PubMed PMID: 16522414; eng.
- Slaghekke F, Lopriore E, Lewi L, et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: an open-label randomised controlled trial. Lancet. 2014 Jun 21;383(9935):2144–2151. PubMed PMID: 24613024; eng.
- Merz W, Tchatcheva K, Gembruch U, et al. Maternal complications of fetoscopic laser photocoagulation (FLP) for treatment of twin-twin transfusion syndrome (TTTS). J Perinat Med. 2010 Jul;38(4):439–443. PubMed PMID: 20184399; eng.
- van Klink JM, Koopman HM, van Zwet EW, et al. Improvement in neurodevelopmental outcome in survivors of twin-twin transfusion syndrome treated with laser surgery. Am J Obstet Gynecol. 2014 Jun;210(6):540.e1–7. PubMed PMID: 24412743; eng.
- Tosello B, Blanc J, Haumonte JB, et al. Short and medium-term outcomes of live-born twins after fetoscopic laser therapy for twin-twin transfusion syndrome. J Perinat Med. 2014 Jan;42(1):99–105. PubMed PMID: 24006317; eng.
- Spruijt MS, Lopriore E, Tan R, et al. Long-term neurodevelopmental outcome in twin-to-twin transfusion syndrome: is there still room for improvement? J Clin Med. 2019 Aug 15;8(8):1226. PubMed PMID: 31443258; PubMed Central PMCID: PMCPMC6723379.
- Chmait RH, Kontopoulos EV, Korst LM, et al. Stage-based outcomes of 682 consecutive cases of twin-twin transfusion syndrome treated with laser surgery: the USFetus experience. Am J Obstet Gynecol. 2011 May;204(5):393.e1–6. PubMed PMID: 21411051; eng.
- Beck V, Lewi P, Gucciardo L, et al. Preterm prelabor rupture of membranes and fetal survival after minimally invasive fetal surgery: a systematic review of the literature. Fetal Diagn Ther. 2012;31(1):1–9. PubMed PMID: 22104520; eng.
- Malshe A, Snowise S, Mann LK, et al. Preterm delivery after fetoscopic laser surgery for twin-twin transfusion syndrome: etiology and risk factors. Ultrasound Obstetrics Gynecol. 2017 May;49(5):612–616. PubMed PMID: 27222097; eng.
- Halvorsen CP, Ek S, Dellgren A, et al. Survival and neonatal outcome after fetoscopic guided laser occlusion (FLOC) of twin-to-twin transfusion syndrome (TTTS) in Sweden. J Perinat Med. 2012 Sep;40(5):533–538. PubMed PMID: 23104796; eng.
- Lopriore E, Oepkes D, Walther FJ. Neonatal morbidity in twin-twin transfusion syndrome. Early Hum Dev. 2011 Sep;87(9):595–599. PubMed PMID: 21784588; eng.
- Verbeek L, Slaghekke F, Sueters M, et al. Hematological disorders at birth in complicated monochorionic twins. Expert Rev Hematol. 2017 Jun;10(6):525–532. PubMed PMID: 28460542; eng.
- Wohlmuth C, Boudreaux D, Moise KJ Jr., et al. Cardiac pathophysiology in twin-twin transfusion syndrome: new insights into its evolution. Ultrasound Obstetrics Gynecol. 2018 Mar;51(3):341–348. PubMed PMID: 28370497; eng.
- Gijtenbeek M, Shirzada MR, Ten Harkel ADJ, et al. Congenital heart defects in monochorionic twins: a systematic review and meta-analysis. J Clin Med. 2019 Jun 24;8(6):902. PubMed PMID: 31238552; PubMed Central PMCID: PMCPMC6617007. eng.
- Manning N, Archer N. Cardiac manifestations of twin-to-twin transfusion syndrome. Twin Res Human Genet. 2016 Jun;19(3):246–254. PubMed PMID: 27087122; eng.
- van den Boom J, Battin M, Hornung T. Twin-twin transfusion syndrome, coarctation of the aorta and hypoplastic aortic arch: a case series report. J Paediatr Child Health. 2010 Mar;46(3):76–79. PubMed PMID: 20105260; eng.
- Hillman SC, Morris RK, Kilby MD. Co-twin prognosis after single fetal death: a systematic review and meta-analysis. Obstet Gynecol. 2011 Oct;118(4):928–940. PubMed PMID: 21934458; eng.
- van Klink JM, van Steenis A, Steggerda SJ, et al. Single fetal demise in monochorionic pregnancies: incidence and patterns of cerebral injury. Ultrasound Obstetrics Gynecol. 2015 Mar;45(3):294–300. PubMed PMID: 25377504; eng.
- Adegbite AL, Castille S, Ward S, et al. Prevalence of cranial scan abnormalities in preterm twins in relation to chorionicity and discordant birth weight. Eur J Obstet Gynecol Reprod Biol. 2005 Mar 1;119(1):47–55. PubMed PMID: 15734084.
- Spruijt M, Steggerda S, Rath M, et al. Cerebral injury in twin-twin transfusion syndrome treated with fetoscopic laser surgery. Obstet Gynecol. 2012 Jul;120(1):15–20. PubMed PMID: 22914388; eng.
- Chmait RH, Chon AH, Schrager SM, et al. Neonatal cerebral lesions predict 2-year neurodevelopmental impairment in children treated with laser surgery for twin-twin transfusion syndrome. J Matern Fetal Neonatal Med. 2019 Jan;32(1):80–84. PubMed PMID: 28835143; eng.
- Quarello E, Molho M, Ville Y. Incidence, mechanisms, and patterns of fetal cerebral lesions in twin-to-twin transfusion syndrome. J Matern Fetal Neonatal Med. 2007 Aug;20(8):589–597. PubMed PMID: 17674276; eng.
- Korsakissok M, Groussolles M, Dicky O, et al. Mortality, morbidity and 2-years neurodevelopmental prognosis of twin to twin transfusion syndrome after fetoscopic laser therapy: a prospective, 58 patients cohort study. J Gynecol Obstet Hum Reprod. 2018 Dec;47(10):555–560. PubMed PMID: 29698746; eng.
- Vanderbilt DL, Schrager SM, Llanes A, et al. Prevalence and risk factors of cerebral lesions in neonates after laser surgery for twin-twin transfusion syndrome. Am J Obstet Gynecol. 2012 Oct;207(4):320.e1-6. PubMed PMID: 23021698; PubMed Central PMCID: PMCPMC3462319. eng.
- Lopriore E, van Wezel-meijler G, Middeldorp JM, et al. Incidence, origin, and character of cerebral injury in twin-to-twin transfusion syndrome treated with fetoscopic laser surgery. Am J Obstet Gynecol. 2006 May;194(5):1215–1220. PubMed PMID: 16647903; eng.
- Ascherl R, Sorge I, Thome U, et al. Severe gyration and migration disorder in fetofetal transfusion syndrome: two case reports and a review of the literature on the neurological outcome of children with lesions on neuroimaging. Child’s Nerv Syst. 2018 Jan;34(1):155–163. 10.1007/s00381-017-3595-7. PubMed PMID: 28971247; eng.
- Merhar SL, Kline-Fath BM, Meinzen-Derr J, et al. Fetal and postnatal brain MRI in premature infants with twin-twin transfusion syndrome. J Perinatol. 2013 Feb;33(2):112–118. PubMed PMID: 22743408; eng.
- Griffiths PD, Sharrack S, Chan KL, et al. Fetal brain injury in survivors of twin pregnancies complicated by demise of one twin as assessed by in utero MR imaging. Prenat Diagn. 2015 Jun;35(6):583–591. PubMed PMID: 25688852; eng.
- Robinson A, Teoh M, Edwards A, et al. Fetal brain injury in complicated monochorionic pregnancies: diagnostic yield of prenatal MRI following surveillance ultrasound and influence on prognostic counselling. Prenat Diagn. 2017 Jun;37(6):611–627. PubMed PMID: 28444780; eng.
- Rossi AC, D’Addario V. Comparison of donor and recipient outcomes following laser therapy performed for twin-twin transfusion syndrome: a meta-analysis and review of literature. Am J Perinatol. 2009 Jan;26(1):27–32. PubMed PMID: 18841532; eng.
- Rossi AC, Vanderbilt D, Chmait RH. Neurodevelopmental outcomes after laser therapy for twin-twin transfusion syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2011 Nov;118(5):1145–1150. PubMed PMID: 22015883; eng.
- Gollenberg AL, Lynch CD, Jackson LW, et al. Concurrent validity of the parent-completed ages and stages questionnaires, 2nd ed. with the bayley scales of infant development II in a low-risk sample. Child Care Health Dev. 2010 Jul;36(4):485–490. PubMed PMID: 20030657; eng.
- Sutcliffe AG, Sebire NJ, Pigott AJ, et al. Outcome for children born after in utero laser ablation therapy for severe twin-to-twin transfusion syndrome. BJOG. 2001 Dec;108(12):1246–1250. PubMed PMID: 11843386.
- Graef C, Ellenrieder B, Hecher K, et al. Long-term neurodevelopmental outcome of 167 children after intrauterine laser treatment for severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 2006 Feb;194(2):303–308. PubMed PMID: 16458621.
- Gray PH, Poulsen L, Gilshenan K, et al. Neurodevelopmental outcome and risk factors for disability for twin-twin transfusion syndrome treated with laser surgery. Am J Obstet Gynecol. 2011 Feb;204(2):159 e1–6. PubMed PMID: 21047615.
- Graeve P, Banek C, Stegmann-Woessner G, et al. Neurodevelopmental outcome at 6 years of age after intrauterine laser therapy for twin-twin transfusion syndrome. Acta Paediatrica. 2012 Dec;101(12):1200–1205. PubMed PMID: 22946904; eng.
- McIntosh J, Meriki N, Joshi A, et al. Long term developmental outcomes of pre-school age children following laser surgery for twin-to-twin transfusion syndrome. Early Hum Dev. 2014 Dec;90(12):837–842. PubMed PMID: 25463829.
- Vanderbilt DL, Schrager SM, Llanes A, et al. Predictors of 2-year cognitive performance after laser surgery for twin-twin transfusion syndrome. Am J Obstet Gynecol. 2014 Oct;211(4):388 e1–7. PubMed PMID: 24681290; PubMed Central PMCID: PMCPMC4175130.
- Schou KV, Lando AV, Ekelund CK, et al. Long-term neurodevelopmental outcome of monochorionic twins after laser therapy or umbilical cord occlusion for twin-twin transfusion syndrome. Fetal Diagn Ther. 2019;46(1):20–27. PubMed PMID: 30149379; eng.
- Morris RK, Selman TJ, Harbidge A, et al. Fetoscopic laser coagulation for severe twin-to-twin transfusion syndrome: factors influencing perinatal outcome, learning curve of the procedure and lessons for new centres. BJOG. 2010 Oct;117(11):1350–1357. PubMed PMID: 20670301; eng.
- Lopriore E, Ortibus E, Acosta-Rojas R, et al. Risk factors for neurodevelopment impairment in twin-twin transfusion syndrome treated with fetoscopic laser surgery. Obstet Gynecol. 2009 Feb;113(2 Pt 1):361–366. PubMed PMID: 19155907.
- van Klink JM, Koopman HM, Rijken M, et al. Long-term neurodevelopmental outcome in survivors of twin-to-twin transfusion syndrome. Twin Res Human Genet. 2016 Jun;19(3):255–261. PubMed PMID: 27137794.
- Sananes N, Gabriele V, Weingertner AS, et al. Evaluation of long-term neurodevelopment in twin-twin transfusion syndrome after laser therapy. Prenat Diagn. 2016 Dec;36(12):1139–1145. PubMed PMID: 27764900.
- Aylward GP, Hatcher RP, Stripp B, et al. Who goes and who stays: subject loss in a multicenter, longitudinal follow-up study. J Dev Behav Pediatr. 1985 Feb;6(1):3–8. PubMed PMID: 3882762.
- Wolke D, Sohne B, Ohrt B, et al. Follow-up of preterm children: important to document dropouts. Lancet. 1995 Feb 18;345(8947):447. PubMed PMID: 7531802; eng.
- Banek CS, Hecher K, Hackeloer BJ, et al. Long-term neurodevelopmental outcome after intrauterine laser treatment for severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 2003 Apr;188(4):876–880. PubMed PMID: 12712079.
- Mullers SM, McAuliffe FM, Kent E, et al. Outcome following selective fetoscopic laser ablation for twin to twin transfusion syndrome: an 8 year national collaborative experience. Eur J Obstet Gynecol Reprod Biol. 2015 Aug;191:125–129. PubMed PMID: 26117441; eng.
- Campos D, Arias AV, Campos-Zanelli TM, et al. Twin-twin transfusion syndrome: neurodevelopment of infants treated with laser surgery. Arq Neuropsiquiatr. 2016 Apr;74(4):307–313. PubMed PMID: 27097004; eng.
- Perry H, Duffy JMN, Umadia O, et al. Outcome reporting across randomized trials and observational studies evaluating treatments for twin-twin transfusion syndrome: systematic review. Ultrasound Obstetrics Gynecol. 2018 Nov;52(5):577–585. PubMed PMID: 29607558; eng.
- Shaw CJ, Civale J, Botting KJ, et al. Noninvasive high-intensity focused ultrasound treatment of twin-twin transfusion syndrome: a preliminary in vivo study. Sci Transl Med. 2016 Jul 13;8(347):347ra95. PubMed PMID: 27412787; eng.
- Seo K, Ichizuka K, Okai T, et al. Treatment of twin-reversed arterial perfusion sequence using high-intensity focused ultrasound. Ultrasound Obstetrics Gynecol. 2019 Jul;54(1):128–134. PubMed PMID: 30136326; eng.
- Diehl W, Diemert A, Grasso D, et al. Fetoscopic laser coagulation in 1020 pregnancies with twin-twin transfusion syndrome demonstrates improvement in double-twin survival rate. Ultrasound Obstetrics Gynecol. 2017 Dec;50(6):728–735. PubMed PMID: 28477345; eng.
