ABSTRACT
Objective
To assess evidence on the safety and efficacy of ABVD (doxorubicin [Adriamycin®], bleomycin, vinblastine, and dacarbazine), BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), and A+AVD (brentuximab vedotin, with doxorubicin, vinblastine, and dacarbazine) for advanced-stage Hodgkin lymphoma (HL).
Methods
A systematic literature review (SLR) was conducted on 29 July 2016 (updated 26 July 2018) to identify randomized controlled trials (RCTs) and non-RCTs assessing the treatment of newly-diagnosed advanced-stage HL with ABVD and BEACOPP (and their variants), and A+AVD.
Results
The SLR identified 62 RCTs and 42 non-RCTs. Five-year overall survival rates for ABVD and BEACOPP were 60–97% and 84–99%, and 5-year progression-free survival rates were 58–81% and 83–96%, respectively. Both regimens were associated with tolerability issues and side effects. Discontinuation or dose reduction of bleomycin resulted in fewer adverse events, without significantly affecting efficacy. A head-to-head trial demonstrated improved efficacy for A+AVD vs ABVD, with an acceptable tolerability profile. No data from head-to-head trials comparing A+AVD with BEACOPP were available, and an indirect treatment comparison was not feasible.
Conclusion
New therapies, such as A+AVD, maintain the efficacy observed with current treatments, and may provide a more tolerable treatment option for patients with advanced-stage HL.
1. Introduction
Hodgkin lymphoma (HL) is categorized as either classical HL or nodular lymphocyte-predominant HL (NLPHL) (based on lymphoma cell morphology and phenotype, and the composition of the cellular infiltrate), which account for approximately 95% and 5% of cases, respectively [Citation1]. The Ann Arbor system categorizes HL into Stages I–IV; although not uniformly used, Stages I/II with no risk factors are considered limited stages, Stages I/II with at least one risk factor (such as large mediastinal mass, elevated erythrocyte sedimentation rate [ESR] or ≥4 nodal areas) are considered intermediate stages, and Stages III and IV are advanced [Citation2]. Stage IIB is considered advanced if associated with the risk factors of large mediastinal mass and/or extra-nodal disease [Citation2]. Extra-nodal involvement is less common in HL compared with non-Hodgkin lymphoma (NHL), with approximately 5% of HL occurring at extra-nodal sites [Citation3].
Current treatment of HL is considered a clinical success, with cure rates greater than 90% and 70% for early and advanced HL, respectively [Citation4]. The first successful combination therapy for the treatment of advanced HL was introduced in the 1960s and contained mustard, vincristine (Oncovin®), procarbazine, and prednisone (MOPP) [Citation5]. Today, the current standard of care, front-line treatments for advanced classical HL are the chemotherapy (CT) regimens ABVD (doxorubicin [Adriamycin®], bleomycin, vinblastine, and dacarbazine) and BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), often in combination with radiotherapy (RT) [Citation4]. The most commonly prescribed treatment in Asia, Latin America, the US and UK is ABVD, with BEACOPP more common in Germany and other parts of Europe [Citation6]. Use of treatment regimens may also depend on disease stage; ABVD is often regarded as standard of care for early-stage, while the BEACOPP escalated dose regimen (BEACOPPESC), has greater efficacy in advanced-staged HL, compared with other regimens, although this comes at the cost of greater treatment-related toxicity [Citation7].
While current front-line treatments have high survival rates, many patients experience short- and long-term health problems due to therapy-related side effects. Myelosuppression and associated cytopenias are the most common acute side effects, resulting in an increased risk of infection [Citation8]. Common serious long-term toxicities associated with HL treatment include secondary malignancies, sterility, RT-associated hypothyroidism, and cardiovascular disease [Citation8], and bleomycin in particular is associated with pulmonary toxicity [Citation9,Citation10].
Novel targeted agents have been developed for HL, including brentuximab vedotin (Adcetris®), an antibody–drug conjugate that targets CD30 [Citation11,Citation12]. In the US, approval for brentuximab vedotin, in combination with doxorubicin, vinblastine, and dacarbazine (A+AVD), has recently been expanded to include first-line treatment of Stage III or IV classical HL [Citation13]. In Europe in 2019, A+AVD was approved as a treatment for patients with previously untreated CD30+ Stage IV HL [Citation14].
The objective of this systematic literature review (SLR) was to determine the efficacy and safety of current treatments for newly-diagnosed advanced-stage HL. Particular focus was given to the front-line treatments ABVD, BEACOPP, and A+AVD. The objective was also to conduct a feasibility assessment for an indirect treatment comparison (ITC) between BEACOPP and any new targeted therapy-chemo combination approved.
2. Methods
The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation15].
2.1. Search methods
The original search was conducted on the 29 July 2016, and was updated on 26 July 2018, both using the following electronic databases via the OVID platform: MEDLINE®, Embase, and the Cochrane Library. The database searches were supplemented with hand-searches of relevant conference proceedings, previous health technology assessment (HTA) submissions, clinical trial registries, and reference lists of included studies. Search strategies and additional details are provided in the supplementary material.
Citations identified through the searches were assessed by a reviewer based on title and abstract using pre-defined eligibility criteria. Any uncertainties were resolved by discussion with a second reviewer. Full publications of potentially relevant citations were obtained and examined in full by two reviewers to identify publications eligible for inclusion in the systematic reviews. Disputes were resolved via discussion with a third party.
2.2. Eligibility criteria
Key eligibility criteria were recorded a priori in a review protocol (not registered) and are provided in the supplementary material (Supplementary Table 1). Eligibility criteria included newly diagnosed patients with advanced-stage HL (defined as Stage IIB, III, and IV), treated with CT regimens with/without RT. This manuscript focuses on data for ABVD and BEACOPP (and their variants), and A+AVD.
2.3. Quality assessment
Quality (risk of bias) assessment for the publications identified in the review was performed using the seven criteria checklist provided in Section 4.6 of the National Institute for Health and Care Excellence (NICE) single technology appraisal (STA) user guide [Citation16]. This approach is based on guidance provided by the Centre for Reviews and Disseminations for assessing the quality of studies included in systematic reviews [Citation17].
2.4. Feasibility assessment for treatment comparisons
The Phase III clinical trial of A+AVD (ECHELON-1) included an ABVD treatment arm, and therefore a published direct comparison of A+AVD and ABVD is available. BEACOPPESC is the standard of care BEACOPP regimen for patients <60 years of age with advanced stage HL in some European countries (BEACOPPESC has greater efficacy in advanced-staged HL compared with other therapies) [Citation2,Citation7]. In the absence of a head-to-head trial, an ITC is necessary in order to estimate the comparative efficacy and safety of A+AVD with BEACOPPESC. An assessment was conducted to determine the feasibility of a conventional ITC, which preserves randomization, using studies identified in the SLR. The population of interest included newly-diagnosed adult patients with advanced stage HL (Stage III or IV). All randomized controlled trials (RCTs) and non-randomized studies assessing BEACOPPESC (non-positron emission tomography [PET] guided therapies or PET-guided therapies) were considered for inclusion in the ITC. Study heterogeneity was assessed to confirm suitability of studies for pooling in an ITC.
3. Results
3.1. Systematic review – RCTs
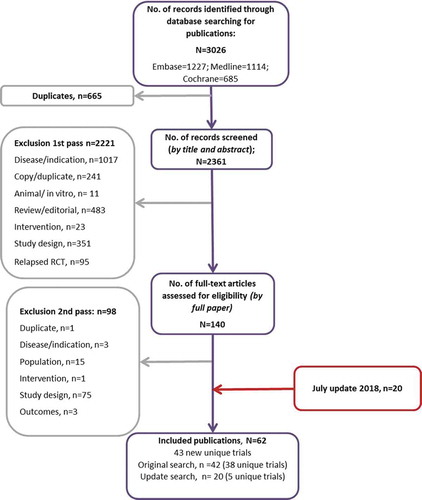
Across the original and update searches, 62 publications reporting on 43 RCTs were included [Citation18–79]. A PRISMA diagram for the RCT search is presented in , and identified treatment regimens are presented in . Study summaries are presented in the supplementary data (Supplementary Table 14).
Table 1. Treatment regimens in identified RCT and non-RCT/observational studies.
3.2. Systematic review – non-RCTs/observational studies
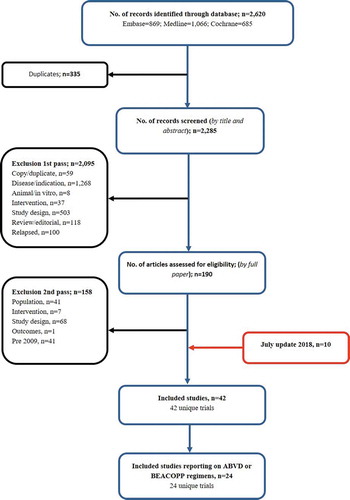
Across the original and update searches, 42 non-RCT/observational studies were identified [Citation80–121]. A PRISMA diagram for the non-RCT search is presented in , and identified treatment regimens are presented in . Study summaries are presented in the supplementary data (Supplementary Table 15).
3.3. Efficacy outcomes
The definition and results of efficacy outcomes (overall survival [OS], progression-free survival [PFS], relapse-free survival [RFS], and failure-free survival [FFS]) for the RCTs and non-RCTs identified in the SLR that included ABVD and/or BEACOPP treatment regimens are listed in the supplementary data (Supplementary Table 16 and Supplementary Table 17, respectively). Of the eight RCTs that compared ABVD with BEACOPP [Citation21,Citation26,Citation32,Citation35,Citation46,Citation59,Citation68,Citation78], none found significant differences between ABVD and BEACOPP with regard to OS rates (4-year, 5-year, and 7-year rates) [Citation21,Citation26,Citation46,Citation59,Citation68,Citation78,Citation122], although there was a significant difference in 10-year OS across three treatment arms (ABVD, BEACOPPBASE, and BEACOPPESC) in one study (p < 0.001) [Citation32]. Of the three studies comparing ABVD and BEACOPP that reported PFS [Citation26,Citation46,Citation78,Citation122], all had significantly better 5-year PFS rates after treatment with the BEACOPP regimens (BEACOPP4+4 and BEACOPP4+2), vs ABVD [Citation26,Citation46,Citation122] (p < 0.05). One non-RCT compared ABVD and BEACOPP, and reported that 2-year PFS was significantly higher for ABVD with BEACOPP than ABVD alone (p = 0.00015) [Citation114]. Among the included non-RCTs, patients treated with ABVD alone, with or without RT, had a 5-year OS rate ranging from 60% to 97% [Citation92,Citation119]. This was comparable with the RCT data, which had 5-year OS rates for ABVD ranging from 64% to 92% [Citation41,Citation46]. A follow-up study reported the 10-year OS for ABVD as 87% [Citation29].
Reported 5-year PFS in non-RCTs for standard ABVD ranged from 58% to 81% [Citation92,Citation119]. Among the non-RCTs evaluating different combinations of BEACOPPBASE/BEACOPPESC, the 5-year OS ranged from 84% to 97% [Citation80,Citation120] and the 10-year OS was 90% [Citation84]. This compares with data from RCTs in which 5-year OS ranged from 90% to 99% [Citation23,Citation46]. Follow-up studies found the 10-year OS rates were 80% and 86% with BEACOPPBASE and BEACOPPESC, respectively [Citation32]. Five-year PFS for BEACOPP for non-RCTs was 84% for BEACOPP2+6 [Citation87], and 87–96% with a variety of BEACOPP doses [Citation91,Citation120], compared with the 5-year PFS rates for RCTs, which ranged from 83% to 93% [Citation26,Citation46].
Several studies investigated Stanford V vs ABVD. Two studies found that ABVD was superior to Stanford V in terms of 5-year FFS, and 10-year FFS (78% vs 49%, and 75% vs 49%, respectively) [Citation29,Citation39]. However, one study found that 5-year FFS was similar between treatment groups (74% and 71% for ABVD and Stanford V, respectively) [Citation40]. Five-year PFS was also comparable between treatment groups in another study (76% and 74%, respectively) [Citation42].
3.3.1. Stage IV disease and extra-nodal involvement
To examine whether further advanced-stage HL had an impact on treatment efficacy, the review focused on studies reporting outcomes for the patient groups with Stage IV disease and extra-nodal involvement. One RCT involved patients exclusively with Stage IV disease, in which the complete remission rate with a treatment regimen of MOPP/ABVD was 88.9% [Citation24]. A further 10 RCTs [Citation22,Citation25,Citation33,Citation37,Citation40,Citation42,Citation48,Citation62,Citation64,Citation68] and three non-RCTs reported specific efficacy outcomes for patients with Stage IV and/or extra-nodal involvement [Citation81–Citation83].
Disease stage at the beginning of treatment influenced efficacy outcomes; several studies found lower efficacy in patients with Stage IV HL. In patients treated with either MOPP or an alternating MOPP/ABVD regimen, OS was significantly lower in Stage IV vs Stage III patients; 52% and 77%, respectively (p = 0.05) [Citation37]. With either MOPP, ABVD, or alternating MOPP/ABVD, FFS was significantly worse in patients with Stage IV vs Stage III disease [Citation25], and a significantly lower proportion of patients with Stage IV disease achieved complete response (54.0% vs 85.5%, respectively), although these results were not statistically significant (p = 0.1). Ten-year FFP and survival was lower in Stage IV vs Stage III disease in a treatment regimen involving ABVD and RT [Citation83]. A further study examining ABVD and BEACOPP treatment regimens found 3-year PFS was significantly lower in Stage IV vs Stage II and Stage III (79.6%, 90.0%, and 83.1%, respectively, p < 0.001) [Citation68]. However, in the HD18 trial, 3-year estimates for PFS and OS for patients treated with BEACOPPESC or R-BEACOPPESC remained largely unchanged when analysis was restricted to patients with Stage IV disease and compared with patients with Stage II–IV disease (OS: BEACOPPESC 95.0% vs 96.5%, R-BEACOPPESC 89.0% vs 94.4%; PFS BEACOPPESC 93.1% vs 91.4%, R-BEACOPPESC 89.9% vs 93.0%) [Citation62]. This suggests that intensified polychemotherapy may reduce or eliminate the prognostic impact of staging in HL.
Efficacy and response rates were also lower in patients with extra-nodal involvement. In patients treated with combined ABVD and MOPP, there were lower efficacy rates in patients with extra-nodal disease compared with nodal only for complete response (86% vs 90%, respectively), FFP (62% vs 70%, respectively), and RFS rates (73% vs 76%, respectively) [Citation48]. Similarly, in a study of patients receiving ABVD, MOPP, or MOPP alternating with ABVD, there were lower rates of FFS and OS with the presence of ≥2 extra-nodal sites, vs <2 (p < 0.001 and p = 0.03, respectively) with a treatment regimen involving MOPP and/or ABVD [Citation25]. The results from non-RCTs showed that, when using a treatment regimen involving ABVD, complete response was significantly lower in the patient group that had >1 extra-nodal site compared with ≤1 (60% vs 85.4%, respectively, p < 0.01) [Citation82].
Novel targeted therapies may afford benefits in patients with more advanced disease. The ECHELON-1 study suggests that patients with Stage IV disease and patients with involvement of >1 extra-nodal sites have improved outcomes with A+AVD vs a standard ABVD regimen [Citation64]. A subgroup analysis of this study demonstrated a greater treatment benefit of A+AVD vs ABVD in patients with Stage IV compared with Stage III HL; the hazard ratio for PFS was 0.92 (95% CI: 0.60, 1.42) and 0.71 (95% CI: 0.53, 0.96) for patients with Stage III and Stage IV HL, respectively [Citation64,Citation69,Citation71].
3.3.2. PET-guided treatment
Recently, PET response-adapted therapies have been assessed to improve efficacy and avoid toxicity. Four studies reported patients’ response to PET response-adapted therapy [Citation60,Citation61,Citation68,Citation73,Citation78].
Patients in the Phase III HD18 trial with a negative PET-2 scan received a reduction in BEACOPPESC from eight to four cycles without a loss of efficacy (as determined by PFS) [Citation60]. For patients with postive PET-2 findings following two cycles of BEACOPPESC, the addition of rituximab to standard BEACOPPESC treatment did not improve PFS [Citation61].
Omission of bleomycin from the ABVD regimen after negative PET-2 findings following two cycles of ABVD resulted in a lower incidence of pulmonary toxicity than with continued ABVD in Phase III RATHL trial, and did not significantly lower efficacy (PFS) [Citation68].
In the Phase III AHL 2011 LYSA trial, de-escalation of treatment from BEACOPPESC to ABVD in patients with a negative PET-2 scan following two cycles of BEACOPPESC did not impair efficacy (PFS). Treatment toxicity was significantly higher in the patients who received four additional cycles of BEACOPPESC following PET-2 scan compared with those de-escalated to ABVD (p < 0.002) [Citation73]. For patients with a positive PET-2 scan following two cycles of ABVD, the GITIL/FIL HD 0607 Phase II study demonstrated that early treatment intensification with BEACOPPESC is a feasible, safe, and effective therapeutic strategy in advanced HL [Citation78].
3.3.3. Patients aged ≥60 years
For patients with HL aged ≥60 years (median age 69 [range 62–88]) who were ineligible for frontline chemotherapy, a Phase II open-label study investigated brentuximab vedotin in combination with dacarbazine or bendamustine [Citation117]. The objective response rate (ORR) for brentuximab vedotin plus dacarbazine was 100% and the complete remission (CR) rate was 62%. Median PFS was 17.9 months. For brentuximab vedotin plus bendamustine, the ORR was 100.0% and the CR rate was 88%. Neither the median PFS or OS was reached. Despite disease activity, brentuximab vedotin plus bendamustine treatment arm was stopped prematurely due to an unacceptably high rate of serious adverse events (SAEs) (65%) and two deaths (10%). A separate study investigating treatment with brentuximab vedotin (before and after AVD) in patients aged ≥60 years of age. The 2-year survival rates were 80%, 84%, and 93%, for EFS, PFS, and OS, respectively [Citation123].
The HD9elderly RCT compared BEACOPPBASE and COPP-ABVD in elderly patients with HL (75 patients aged 66–75 years) and found that at 5 years there were no significant difference between treatment groups for CR, OS, and freedom from treatment failure [Citation21].
The E2496 RCT compared ABVD and Stanford V treatment regimens. In the elderly subgroup population (aged ≥60 years, n = 44), 5-year failure-free survival and 5-year OS, were lower in the older group compared with the younger patients (48% vs 74% [p = 0.002], and 58% vs 90% [p < 0.0001], respectively). In contrast, time to progression did not differ between age groups [Citation41].
3.4. Safety outcomes
3.4.1. Mortality
The mortality outcomes for ABVD and/or BEACOPP regimens are displayed in the supplementary data (Supplementary Table 18). Reported all-cause mortality ranged from 1–54% and 1.5–55% for ABVD- and BEACOPP-containing regimens, respectively [Citation21,Citation39,Citation46,Citation64,Citation68].
3.4.2. Pulmonary toxicity
In total, 19 RCTs and nine non-RCTs reported data associated with pulmonary toxicity (). Any-grade pulmonary toxicity ranged from 0% to 36% across both the RCT and non-RCT studies [Citation39,Citation76,Citation101]. Seven RCTs and four non-RCTs reported a causal relationship between bleomycin and pulmonary toxicities [Citation26,Citation30,Citation33,Citation38,Citation41,Citation42,Citation48,Citation68,Citation91,Citation93,Citation101]. Few studies reported the incidence of specific pulmonary toxicities, including interstitial lung disease, lung fibrosis, dyspnea, and pneumonitis. One RCT and one non-RCT reported data pertaining to interstitial lung disease [Citation64,Citation101], with any-grade interstitial lung disease reported by 4% of patients who received treatment with brentuximab vedotin + ABVD (A+ABVD) compared with 0% of patients treated with A+AVD [Citation101]. The incidence of lung fibrosis was reported in one RCT [Citation18]. In the RCT, a similar proportion of patients who received ABVD/MOPP and ABVD/OPP reported any-grade lung fibrosis (4% vs 5%, respectively) [Citation18].
Table 2. Proportion of patients with pulmonary toxicity with ABVD- and BEACOPP-containing treatment regimens.
Dyspnea was reported in two RCTs [Citation41,Citation68] and two non-RCTs [Citation101,Citation124]. In one non-RCT, patients receiving treatment with A+ABVD reported a higher incidence of any-grade dyspnea than those receiving A+AVD (33% vs 22%, respectively) [Citation101]. Patients receiving bleomycin also experienced a higher incidence of Grade 3/4 dyspnea in one RCT (ABVD vs Stanford V, 17% vs 0%, respectively) [Citation41] and one non-RCT (A+ABVD vs A+AVD, 12% vs 4%, respectively) [Citation101]. Pneumonitis was reported in two RCTs [Citation41,Citation68] and three non-RCTs [Citation84,Citation98,Citation101]. Any-grade pneumonitis ranged from 0% to 5% [Citation68,Citation84,Citation98,Citation101,Citation102,Citation124]. Grade 3/4 pneumonitis was reported by 8% of patients who received ABVD vs 0% who received Stanford V [Citation41].
3.4.3. Bleomycin discontinuation – impact on efficacy/safety
Four studies reported the impact of bleomycin on efficacy and safety outcomes; a reduction in the dose of bleomycin or discontinuation of bleomycin was associated with a decrease in the incidence of adverse events (AEs) such as pulmonary toxicity [Citation48,Citation64,Citation68,Citation91,Citation93].
Results from the RATHL study demonstrated that excluding bleomycin from the ABVD regimen reduced the incidence of pulmonary toxicity in patients with advanced HL without significant impact on the efficacy outcomes (absolute difference in the 3-year PFS rate [ABVD minus AVD]: 1.6%) [Citation68]. At a median follow-up of 41 months, the 3-year PFS and OS rates in the ABVD group were 85.7% and 97.2%, respectively, and the corresponding rates in the AVD group were 84.4% and 97.6%, respectively [Citation68].
Jalali et al., 2016 reported that in the ABVD regimen, bleomycin was the most commonly reduced or delayed intervention due to pulmonary toxicity. In the univariate analysis, significantly inferior 5-year OS rates were observed in patients with advanced disease with any dose change during treatment (either delay, reduction, or both) compared with those without any dose change during treatment (75% vs 93%, respectively, p < 0.01). However, no significant difference could be detected between the groups (with or without dose change during treatment) in the multivariate analysis [Citation93]. Haverkamp et al., 2015 evaluated the impact of dose reduction or delay due to drug-specific toxicity of bleomycin in patients with advanced HL receiving treatment with BEACOPP in the German Hodgkin Study Group HD12 and HD15 trials. Bleomycin discontinuation did not affect the overall efficacy of treatment. The authors reported that there were no significant differences in PFS or OS in patients receiving ≤4 or >4 cycles of bleomycin (5-year PFS difference: 1.7%; 5-year OS difference: 1.5%) [Citation91].
The ECHELON-1 trial compared A+AVD and ABVD regimens. A+AVD had improved efficacy compared with ABVD in patients with Stage III–IV HL; the 2-year modified PFS rate was significantly higher (82.1% vs 77.2%, respectively, p = 0.04), resulting in a 23% risk reduction in the A+AVD group [Citation64]. Furthermore, patients treated with A+AVD vs ABVD had a lower incidence of pulmonary toxicity; 2% vs 7%, respectively, with less than 1% of patients in the A+AVD group experiencing Grade 3 pulmonary events compared with 3% of patients in the ABVD group [Citation64].
3.4.4. Secondary malignancies
The incidence of secondary malignancies ranged from 0–15% and 0.61–14% for ABVD- and BEACOPP-containing treatments, respectively (Supplementary Table 19) [Citation21,Citation39,Citation59,Citation62]. Commonly reported secondary malignancies in the RCTs were the hematological malignancies NHL, acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). A study comparing BEACOPPBASE and COPP-ABVD in patients aged 66–75 years found that at a median follow-up of 80 months, 14% and 12% had secondary malignancies in each treatment group, respectively [Citation21].
Limited evidence on secondary malignancies was available from non-RCTs in advanced HL, with one study reporting three cases of large B-cell lymphoma with a BEACOPP treatment regimen [Citation84]. In the ABVD-containing treatment regimens, secondary malignancies included NHL, melanoma, colorectal cancer, lung cancer, breast cancer, second lymphoma, lymphoblastic leukemia, and carcinoma of the tongue [Citation83,Citation95]. Dann et al., 2017 reported that of 355 patients with early and advanced HL whose therapy was individualized based on initial prognostic factors and PET, 1.9% developed second malignancies during the study period (2006–2013), including secondary leukemia, lymphoblastic lymphoma, and breast cancer [Citation115].
3.4.5. Neutropenia
lists the incidence of all neutropenia, and specifically Grade 3/4 events. Incidence of total neutropenia ranged from 21% to 88% for ABVD [Citation25,Citation41,Citation46,Citation64,Citation68] and was reported in one study for BEACOPP (ABVD/BEACOPP regimen; 63.0%) [Citation68]. Grade 3/4 neutropenia incidence ranged from 8% for ABVD [Citation81] to 80% for A+ABVD [Citation101]. The proportion of BEACOPP-treated patients with Grade 3/4 neutropenia was 90%, 63%, and 67% for BEACOPP4+4, BEACOPP14, and BEACOPPESC, respectively [Citation68,Citation84]. In the ECHELON-1 trial comparing A+AVD and ABVD, the incidence of neutropenia was 58% and 45%, respectively, and febrile neutropenia was 19% and 8%, respectively [Citation64].
Table 3. Incidence of neutropenia in ABVD and BEACOPP treatment regimens in RCTs and non-RCTs.
Granulocyte-colony stimulating factor (G-CSF) can be administered to manage the risk of neutropenia [Citation125]. G-CSF use in the included studies varied from use as primary prophylaxis from Day 8 of treatment [Citation19], obligatory use for certain treatments such as BEACOPPESC [Citation84], and secondary prophylaxis reserved for patients with severe neutropenia [Citation39]. In the ECHELON-1 trial that compared A+AVD with ABVD, a protocol amendment was done after 75% of patients were enrolled and the patients enrolled thereafter in the A+AVD group received prophylactic G-CSF. It was observed that patients in the A+AVD group who received prophylactic G-CSF had a lower incidence of febrile neutropenia compared with those who did not (11% vs 21%, respectively) [Citation64]. Adverse events associated with G-CSF use were not identified in this review.
3.4.6. Peripheral neuropathy
The incidence of reported peripheral neuropathy in ABVD and BEACOPP treatment regimens is reported in . Rates for all-grade peripheral neuropathy ranged from 1% to 76% after treatment with standard ABVD and CHOPE/ABVD, respectively [Citation25,Citation95]. Two studies reported peripheral neuropathy associated with BEACOPP (6% and 23% for Grades 1–2 and Grade 3, respectively) [Citation84,Citation87]. One study reported all-grade peripheral neuropathy associated with BrECAPP and BrECADD; 32% and 35%, respectively [Citation76]. A trial comparing A+AVD with ABVD found that the incidence of all-grade peripheral neuropathy was 26.0% and 13.0%, respectively.
Table 4. Incidence of peripheral neuropathy in ABVD and BEACOPP treatment regimens.
3.4.7. Adverse events commonly associated with RT
Radiotherapy is a common component of frontline treatment of patients with HL (either as adjuvant or consolidation therapy) and was used in majority of the studies included in the review (details provided in Supplementary Table 14 and 15). While studies have reported commonly observed AEs, no direct association with RT was reported in any study. The AEs from the included studies that have been commonly observed in the literature as being linked to RT are therefore presented in Supplementary Table 20. Long-term AEs such as cardiac and lung toxicity were frequently reported in the included studies. Cardiovascular toxicity was reported in 16 studies and ranged from 0% to 10% with ABVD [Citation18,Citation98] and 0–15% with BEACOPP [Citation21]. Grade 3/4 cardiovascular toxicity ranged from 1% to 15% [Citation21,Citation47]. Similarly, pulmonary toxicities were reported in 21 studies and ranged from 0% to 36% [Citation39,Citation101], with 0–24.5% of patients reporting Grade 3/4 toxicity [Citation21,Citation33,Citation62,Citation64,Citation78]. The most commonly reported short-term adverse effects were nausea/vomiting and infections. In one study, Grade 3 and 4 nausea/vomiting was reported in 18% of patients [Citation21]. Infection was reported in 16 studies in 0–42% of patients [Citation59,Citation68].
3.5. Feasibility assessment for indirect treatment comparisons
The SLR identified 10 RCTs (eight non-PET and two PET-guided studies) and three retrospective studies that assessed a BEACOPPESC-containing regimen . Based on data for A+AVD from ECHELON-1, no BEACOPPESC studies were suitable for inclusion in a conventional ITC of BEACOPPESC with A+AVD as front-line therapy in patients with Stage III–IV HL. The reasons for unsuitability for inclusion for each study in a conventional ITC are listed in . Heterogeneity was also observed across trials in terms of the key baseline patient characteristics (e.g. age, gender, Eastern Cooperative Oncology Group [ECOG] Performance Status score), as well as definitions of reported outcomes – any study not reporting Stage III–IV patient outcome data were too dissimilar to the ECHELON-1 trial to ensure appropriate patient outcome data would be compared (similarity assumption). Only LYSA H34 and EORTC 20,012 reported this subgroup data . ECHELON-1 compared A+AVD with 6xABVD. However, the only ABVD treatment arms in LYSA H34 and EORTC 20,012 were 8xABVD. Even if an assumption was made that these treatment arms were similar enough to be pooled into a single treatment node, the BEACOPP arm in each study was, not the comparator of interest, BEACOPPESC. BEACOPPESC has been shown to have greater efficacy in advanced-staged HL compared with BEACOPPBASE [Citation7]. Consequently, two assumptions about the equivalence of treatment regimens were required to conduct an ITC:
Considering 8xABVD (LYSA H34, EORTC 20,012) clinically equivalent to 6xABVD (ECHELON-1)
Considering the 4xBEACOPPESC + 4xBEACOPPBASE treatment arm clinically equivalent to BEACOPPESC
Table 5. Feasibility assessment of identified trials for indirect treatment comparison of BEACOPPESC with A+AVD.
These assumptions were considered not clinically justifiable, due to potential differences in the efficacy and safety profiles of the regimens, which could lead to considerable bias in the analysis results; the longer 8xABVD regimen may result in a greater number of AEs and discontinuations and thus could bias the safety results in favor of BEACOPPESC. Therefore, a conventional ITC was not considered feasible. In the absence of a suitable common comparator in the trials, an alternative approach would be to consider an unanchored matching-adjusted indirect comparison (MAIC) or simulated treatment comparison (STC) analysis. However, the strong assumption on which this method is based is that all treatment effect modifiers and prognostic factors are accounted for in the model. This is considered very difficult, the only factors that are available for matching are those reported in the baseline characteristics table in the comparator trial. This assumption also cannot hold if there are unobserved differences between treatment arms that impact patient outcome; an unknown amount of residual bias may be introduced to the results, making it difficult to justify that this approach will produce a less biased estimate of the relative treatment effect than a conventional ITC [Citation126].
In the context of the current data set, the only study identified as potentially suitable to conduct an unanchored indirect comparison of A+AVD with BEACOPPESC was HD18 which reported relevant outcome data in the Stage III–IV patient subgroup for BEACOPPESC treatment. However, in the absence of baseline characteristic data for the Stage III–IV subgroup from the HD18 trial, it would not be possible to match the Stage III–IV group exactly. Matching patient characteristics are one of the key steps of an unanchored MAIC or STC, and therefore these analyses were also not considered feasible.
4. Discussion
The aim of this SLR was to assess the published evidence for the efficacy and safety of treatments for newly-diagnosed advanced-stage HL. Specifically, the front-line treatments for HL, ABVD and BEACOPP, with and without RT, in addition to the newly approved therapy A+AVD. This SLR was conducted according to PRISMA guidelines, however, there were some limitations. The same efficacy outcomes were not reported for all included studies and treatment regimens were not always consistent between trials, with some involving different cycles of treatment. This review focused primarily on 5-year OS and PFS rates as these were the most commonly reported. However, not all studies assessed these specific outcomes, and some reported values at different time-points, limiting comparisons between studies. A particular focus was Stage IV disease or extra-nodal involvement, however, subgroup analyses for these populations were not reported for most studies. Inclusion criteria were largely consistent across studies included in the SLR. Most studies included patients aged ≥18–60 years, with the exception of a small number of studies that also included patients aged >60 years. The focus of this SLR was to assess the efficacy and safety of current standard of care frontline therapies used in the treatment of patients with advanced HL and therefore other therapies such as Stanford V and COPP are discussed briefly. Additionally, results of a Phase II study investigating BEACOPP backbone plus brentuximab vedotin have been presented; however, this therapy has not been described in detail as results from the Phase III trial (HD21) are pending.
Results from included studies showed high efficacy for ABVD and BEACOPP. Several of the studies observed lower complete response, OS, FFS, and FFP rates in patients with Stage IV treated with MOPP/ABVD compared with other disease stages [Citation25,Citation37,Citation83]. Progression-free survival rates were also lower in patients with Stage IV HL treated with ABVD/BEACOPP [Citation68] and A+AVD/AVBD [Citation64,Citation69,Citation71], compared with less-advanced disease. Following treatment with MOPP and/or ABVD, patients with extra-nodal involvement had lower complete response, FFP, FFS, and OS rates than those with no or less extra-nodal involvement [Citation25,Citation48,Citation82]. However, in a study of patients treated with BEACOPPESC or R-BEACOPPESC, 3-year estimates for PFS and OS remained largely unchanged in patients with Stage IV vs Stage II–IV disease.
The current review has similarities with a recent Cochrane review by Skoetz et al., 2017 comparing the efficacy and safety of BEACOPP vs ABVD for treatment of HL [Citation6]; however, there are several key differences which are of relevance when considering the results of each review (Supplementary Table 21). Five RCTs [Citation26,Citation32,Citation35,Citation59,Citation127] were identified by the Skoetz review, all of which met the criteria for inclusion in the current review, with the exception of HD14, which enrolled patients with early disease (Stage IA, IB, IIA, or IIB), and was therefore outside the scope of our review [Citation127]. Results from the Skoetz meta-analysis indicate that BEACOPPESC was associated with improvements in both OS and PFS vs ABVD [Citation6]. A network meta-analysis was not performed in the current review. Results from individual trials that compared directly ABVD and BEACOPP did not find significant differences in 4-, 5-, or 7-year survival [Citation21,Citation26,Citation46,Citation59,Citation122]. However, a significant difference in 10-year OS across treatment arms (ABVD, BEACOPPBASE, and BEACOPPESC) was observed (p < 0.001) [Citation32], and there were significantly better 5-year PFS rates after treatment with BEACOPP regimens (BEACOPP4+4 and BEACOPP4+2), compared with ABVD (p < 0.05) [Citation26,Citation46,Citation122]. The current review found one additional head-to-head RCT that was not included in the Skoetz review [Citation21], which was part of the HD9 trial, but included only patients aged 66–75 [Citation21] and was excluded as it did not include BEACOPPESC, a regimen that is not well tolerated in older patients [Citation6,Citation21,Citation32]. In contrast to the original study, older patients treated with BEACOPPBASE did not have significantly higher OS and complete response rates compared with the alternating ABVD/COPP regimen [Citation21,Citation32].
Although ABVD and BEACOPP are efficacious treatment options for advanced-stage HL, both are associated with several tolerability issues and short- and long-term side effects. The Skoetz review found that use of BEACOPP resulted in a statistically significant increase in incidence of AML and MDS (p = 0.02), and BEACOPPESC in an increase in Grade 3/4 hematological events compared with ABVD [Citation6]. This was also true in the additional studies found in our review. Elderly patients treated with BEACOPPBASE had higher rates of Grade 4 acute toxicity vs ABVD/COPP [Citation21]. Mounier et al., 2014 showed higher rates of anemia and leukopenia in the BEACOPP4+4 treatment group vs ABVD [Citation46]. Chemotherapy-induced neutropenia is another common side-effect of HL treatment, and can result in dose-modifications or treatment delays [Citation35,Citation128]. One study reported neutropenia in 90% of patients treated with BEACOPP4+4 [Citation84]. This compares with results from the Skoetz meta-analysis, in which patients treated with BEACOPPESC vs ABVD had a statistically significant increase in neutropenia (p < 0.0001) [Citation6]. Risk of neutropenia can be managed using G-CSF [Citation125], which in our review ranged from use as primary prophylaxis [Citation19] and use with certain treatments such as BEACOPPESC [Citation84], to secondary prophylaxis for patients with severe neutropenia [Citation39]. ABVD and BEACOPP may also be associated with side effects not identified in this review. For example, alkylating agents in the BEACOPP regimen can affect fertility [Citation129].
Radiotherapy is also associated with a range of early (skin changes, fatigue, dry mouth, nausea, and diarrhea) and late (hypothyroidism) adverse effects [Citation8]. Secondary malignancies are higher in patients treated with ABVD that receive RT, compared with those that do not; as patients receiving BEACOPPESC compared with ABVD require less additional RT, they may be less likely to develop solid tumors [Citation130]. The studies identified did not report direct associations of toxicity with RT; however, many treatment-related AEs were reported that have commonly been linked to RT, including nausea, vomiting, infections, skin-related complications, cardiovascular, and pulmonary toxicity. Chemotherapy agents may exacerbate these RT-associated conditions; use of anthracyclines, such as doxorubicin, are associated with an increased risk of heart failure [Citation129,Citation131].
The most serious long-term effect of RT is increased risk of secondary malignancy, which was reported in 8.3% and 1.7–4% of patients treated with ABVD [Citation83] and BEACOPP [Citation62,Citation68,Citation84], respectively. Consistent with published data, AML and NHL were the most commonly observed secondary malignancies [Citation8]. The incidence of secondary malignancies is higher in patients who had combined RT and chemotherapy, vs RT alone [Citation132]; however, this was not specifically reported in the included studies.
Previous studies have associated bleomycin with pulmonary toxicity [Citation9,Citation10]. Eleven studies in our review identified a causal relationship between pulmonary toxicity and bleomycin [Citation26,Citation30,Citation33,Citation38,Citation41,Citation42,Citation48,Citation68,Citation91,Citation93,Citation101,Citation124]. G-CSF may increase the incidence of bleomycin-associated lung toxicity [Citation9,Citation133]. Bleomycin discontinuation, or dose-reduction, resulted in fewer adverse events such as pulmonary toxicity, without a significant effect on efficacy (OS and PFS) [Citation48,Citation68,Citation91,Citation93].
Recently, PET response-adapted therapies have been assessed to improve efficacy and avoid toxicity. In addition to four studies reporting patients’ response to PET response-adapted therapy [Citation60,Citation61,Citation68,Citation73,Citation78], two recent SLRs have investigated PET and HL; one on interim PET results and prognosis concluded that negative interim PET results have a large advantage in terms of OS (moderate certainty evidence) [Citation134]. A review of PET-adapted therapy for HL recommended a PET treatment approach as standard of care for advanced HL [Citation135].
Superior efficacy results were reported for A+AVD vs ABVD in the ECHELON-1 trial based on 2-year modified PFS. Modified PFS, in addition to disease progression or death, included modified progression (defined as evidence of noncomplete response after the completion of frontline chemotherapy based on independently assessed PET results, followed by subsequent anticancer therapy) as an event, which more accurately assesses the curative potential of frontline chemotherapy for HL than the standard endpoint PFS. The conventional end point of PFS does not accurately assess a cure by frontline chemotherapy; PFS would not recognize the persistence of disease when it does not cause progression or death, a state which requires further intervention [Citation64].
Patients aged ≥60 years with HL have limited treatment options and inferior survival due to treatment-related toxicities and comorbidities. Brentuximab vedotin plus dacarbazine may be a frontline option for patients aged ≥60 years based on tolerability and response duration observed in a Phase II trial [Citation117]. Brentuximab vedotin plus bendamustine is not considered a tolerable treatment regimen for older patients, as treatment was stopped prematurely due to an unacceptably high rate of SAEs and deaths, highlighting the importance of dedicated trials evaluating novel regimens in elderly patients. However, A+AVD is a treatment option for older patients with advanced HL, as it provides a tolerable treatment option with equivalent efficacy to ABVD [Citation64]. In the ECHELON-1 study, lower rates of pulmonary toxicity were reported for A+AVD vs ABVD. Although higher rates of myelotoxicity and neuropathy were observed in the A+AVD vs ABVD arm, it was noted that myelotoxicity can be ameliorated with prophylactic G-CSF and neurotoxicity was largely reversible.
Although good efficacy outcomes can be achieved in most patients with advanced-stage HL with ABVD and BEACOPP, patients with Stage IV disease, extra-nodal involvement, or elderly patients are likely to benefit from treatments with enhanced efficacy. There is significant scope for improvements in safety and tolerability for all patients, particularly with regards to nausea/vomiting, neutropenia, and RT-associated AEs. Brentuximab vedotin has been recently approved for the treatment of Stage III (US)/Stage IV (US and Europe) HL, in combination with AVD (A+AVD), which does not contain bleomycin and provides an additional option for clinicians. No head-to-head trial data comparing A+AVD with BEACOPPESC are available, and based on available published evidence, it is not possible to compare these two therapies by ITC. However, the safety and efficacy of A+AVD and ABVD were directly compared in the Phase III RCT ECHELON-1 and demonstrated that PFS (based on 2-year modified PFS) and OS were higher and pulmonary toxicity was decreased in the A+AVD vs ABVD group [Citation64]. Furthermore, the treatment benefit was higher for patients with Stage IV vs III HL and for patients with extra-nodal involvement [Citation64,Citation69,Citation71].
5. Conclusion
ABVD and BEACOPP are efficacious treatment options for advanced-stage HL, with reported 5-year OS rates ranging from 60–97% and 84–99%, and 5-year PFS rates ranging from 58–81% and 83–96%, respectively. However, both treatments are associated with several tolerability issues and short- and long-term side effects, particularly with regards to nausea/vomiting, neutropenia, and RT-associated AEs. New therapies, such as A+AVD, may provide a more tolerable treatment option for patients with advanced stage HL.
Author contributions
Mehul Dalal: Conceptualization, Project administration, methodology, Writing – Review & Editing. Jatin Gupta: Methodology, investigation, validation, Writing – Review & Editing. Kim price: Supervision, Writing – Review & Editing. Athanasios Zomas: Writing – Review & Editing. Harry Miao: Writing – Review & Editing. Ajibade Ashaye: Project administration, Supervision, validation, Writing – Review & Editing.
Declaration of interest
M Dalal, A Ashaye, A Zomas and H Miao are currently employed by Takeda and owns stock in the aforementioned. J Gupta is currently employed by Decision Resources Group, who were commissioned to conduct the systematic review by Takeda. K Price is a former employee of Decision Resources Group, who were commissioned to conduct the systematic review by Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (581.8 KB)Acknowledgments
The authors would like to thank Laura Eaton (Decision Resources Group) for writing assistance.
Data availability statement
All data generated or analyzed during this study are included in this published article as supplementary information.
Supplementary data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2008;9(1):nrc2542.
- Eichenauer DA, Aleman BMP, Andre M, et al. Hodgkin lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct 1;29(Suppl 4):iv19–iv29.
- Weber AL, Rahemtullah A, Ferry JA. Hodgkin and non-Hodgkin lymphoma of the head and neck: clinical, pathologic, and imaging evaluation. Neuroimaging Clin N Am. 2003 Aug;13(3):371–392.
- Rathore B, Kadin ME. Hodgkin’s lymphoma therapy: past, present, and future. Expert Opin Pharmacother. 2010;11(17):2891–2906.
- DeVita VT Jr., Carbone PP. Treatment of Hodgkin’s disease. Med Ann District Columbia. 1967 Apr;36(4):232–234. passim.
- Skoetz N, Will A, Monsef I, et al. Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2017 May 25;5:CD007941.
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003 Jun 12;348(24):2386–2395.
- Evens AM, Hutchings M, Diehl V. Treatment of Hodgkin lymphoma: the past, present, and future. Nature Clin Pract Oncol. 2008;5(9):543–556.
- Martin WG, Ristow KM, Habermann TM, et al. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005 Oct 20;23(30):7614–7620.
- Canellos GP, Duggan D, Johnson J, et al. How important is bleomycin in the adriamycin + bleomycin + vinblastine + dacarbazine regimen? J Clin Oncol. 2004 Apr 15;22(8):1532–1533.
- Federico M, Luminari S, Pellegrini C, et al. Brentuximab vedotin followed by ABVD ± radiotherapy in patients with previously untreated Hodgkin lymphoma: final results of a pilot phase II study. Haematologica. 2016 Apr;101(4):e139–41.
- Forero-Torres A, Holkova B, Goldschmidt J, et al. Phase 2 study of frontline brentuximab vedotin monotherapy in Hodgkin lymphoma patients aged 60 years and older. Blood. 2015 Dec 24;126(26):2798–2804.
- US Food and Drug Administration. FDA news release. FDA expands approval of Adcetris for first-line treatment of Stage III or IV classical Hodgkin lymphoma in combination with chemotherapy. Available at: https://wwwfdagov/news-events/press-announcements/fda-expands-approval-adcetris-first-line-treatment-stage-iii-or-iv-classical-hodgkin-lymphoma . 2018.
- Takeda. European Commission Approves ADCETRIS® (brentuximab vedotin) with AVD, the first new treatment in decades for adults with previously untreated CD30+ stage IV Hodgkin lymphoma. News release Feb 2019. Available at https://wwwtakedacom/newsroom/newsreleases/2019/european-commission-approves-adcetris-with-avd-for-adults-with-previously-untreated-cd30-stage-iv-hodgkin-lymphoma/. 2019.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Ann Intern Med. 2009;151(4):264–269.
- National Institute of Clinical Excellence N. Single technology appraisal: user guide for company evidence submission template. 2017 [cited 2020]. Available from: https://www.nice.org.uk/process/pmg24/chapter/instructions-for-companies.
- (CRD). CfRaD. Systematic reviews CRD’s guidance for undertaking reviews in health care 2009 [cited 2020]. Available from: http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf
- Anselmo AP, Cavalieri E, Enrici RM, et al. Combined modality therapy in advanced Hodgkin’s disease: a report on 218 patients with a median follow-up of eight years. Haematologica. 1998 Jul;83(7):645–650.
- Arakelyan N, Berthou C, Desablens B, et al. Early versus late intensification for patients with high-risk Hodgkin lymphoma-3 cycles of intensive chemotherapy plus low-dose lymph node radiation therapy versus 4 cycles of combined doxorubicin, bleomycin, vinblastine, and dacarbazine plus myeloablative chemotherapy with autologous stem cell transplantation: five-year results of a randomized trial on behalf of the GOELAMS Group. Cancer. 2008 Dec 15;113(12):3323–3330.
- Aviles A, Neri N, Nambo MJ, et al. Late cardiac toxicity secondary to treatment in Hodgkin’s disease. A study comparing doxorubicin, epirubicin and mitoxantrone in combined therapy. Leukemia Lymphoma. 2005;46(7):1023–1028.
- Ballova V, Ruffer JU, Haverkamp H, et al. A prospectively randomized trial carried out by the German Hodgkin study group (GHSG) for elderly patients with advanced Hodgkin’s disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly). Ann Oncol. 2005 Jan;16(1):124–131.
- Bjorkholm M, Axdorph U, Grimfors G, et al. Fixed versus response-adapted MOPP/ABVD chemotherapy in Hodgkin’s disease. A prospective randomized trial. Ann Oncol. 1995 Nov;6(9):895–899.
- Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage Hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin study group. J Clin Oncol. 2011 Nov 10;29(32):4234–4242.
- Bonadonna G, Valagussa P, Santoro A. Alternating non-cross-resistant combination chemotherapy or MOPP in stage IV Hodgkin’s disease. A report of 8-year results. Ann Intern Med. 1986 Jun;104(6):739–746.
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992 Nov 19;327(21):1478–1484.
- Carde P, Karrasch M, Fortpied C, et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, international prognostic score >/= 3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 intergroup trial. J Clin Oncol. 2016 Jun 10;34(17):2028–2036.
- Casasnovas O, Brice P, Bouabdallah R, et al. Randomized phase III study comparing an early pet driven treatment de-escalation to a not pet-monitored strategy in patients with advanced stages Hodgkin lymphoma: interim analysis of the AHL2011 lysa study. Blood. 2015 Dec 03;126(23):577.
- Chisesi T, Federico M, Levis A, et al. ABVD versus Stanford V versus MEC in unfavourable Hodgkin’s lymphoma: results of a randomised trial. Ann Oncol. 2002;13(SUPPL. 1):102–106.
- Chisesi T, Bellei M, Luminari S, et al. Long-term follow-up analysis of HD9601 trial comparing ABVD versus Stanford V versus MOPP/EBV/CAD in patients with newly diagnosed advanced-stage Hodgkin’s lymphoma: a study from the Intergruppo Italiano Linfomi. J Clin Oncol. 2011 Nov 10;29(32):4227–4233.
- Connors JM, Klimo P, Adams G, et al. Treatment of advanced Hodgkin’s disease with chemotherapy–comparison of MOPP/ABV hybrid regimen with alternating courses of MOPP and ABVD: a report from the national cancer institute of Canada clinical trials group. J Clin Oncol. 1997 Apr;15(4):1638–1645.
- Diehl V, Franklin J, Hasenclever D, et al. BEACOPP, a new dose-escalated and accelerated regimen, is at least as effective as COPP/ABVD in patients with advanced-stage Hodgkin’s lymphoma: interim report from a trial of the German Hodgkin’s lymphoma study group. J Clin Oncol. 1998 Dec;16(12):3810–3821.
- Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009 Sep 20;27(27):4548–4554.
- Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol. 2003 Feb 15;21(4):607–614.
- Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial.[erratum appears in lancet. Lancet. 2012 May 12;379(9828):1791–1799.
- Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: results from the HD2000 Gruppo Italiano per lo studio dei linfomi trial. J Clin Oncol. 2009 Feb 10;27(5):805–811.
- Merli F, Luminari S, Gobbi PG, et al. Long-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: A study by fondazione Italiana Linfomi. J Clin Oncol. 2016;34(11):1175–1181.
- Ferme C, Lepage E, Brice P, et al. Combined chemotherapy-radiotherapy in advanced Hodgkin’s disease: results of a prospective clinical trial with 70 stage IIIB-IV patients. Int J Radiat Oncol Biol Phys. 1993 Jun 15;26(3):397–405.
- Glick JH, Young ML, Harrington D, et al. MOPP/ABV hybrid chemotherapy for advanced Hodgkin’s disease significantly improves failure-free and overall survival: the 8-year results of the intergroup trial. J Clin Oncol. 1998 Jan;16(1):19–26.
- Gobbi PG, Levis A, Chisesi T, et al. ABVD versus modified stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin’s lymphoma: final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol. 2005 Dec 20;23(36):9198–9207.
- Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the eastern cooperative oncology group (E2496). J Clin Oncol. 2013 Feb 20;31(6):684–691.
- Evens AM, Hong F, Gordon LI, et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013 Apr;161(1):76–86.
- Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s lymphoma: united kingdom national cancer research institute lymphoma group study ISRCTN 64141244. J Clin Oncol. 2009 Nov 10;27(32):5390–5396.
- Johnson PW, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: results of the United Kingdom lymphoma group LY09 Trial (ISRCTN97144519). J Clin Oncol. 2005 Dec 20;23(36):9208–9218.
- Kriachok IA, Titorenko IB, Novosad OI, et al. Preliminary results of prospective randomized multicentre study ‘beacopp-14 versus beacoppescalated in patients with hodgkins lymphoma from poor-prognosis group’. Hematol Oncol. 2013;31:125–126.
- Machiels JP, Ferrant A, Martiat P, et al. A prospective randomized study of two alternating, non cross-resistant chemotherapies for advanced Hodgkin’s disease. Acta Clin Belg. 1992;47(4):244–250.
- Mounier N, Brice P, Bologna S, et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles > 4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann Oncol. 2014 Aug 01;25(8):1622–1628.
- Sieber M, Tesch H, Pfistner B, et al. Treatment of advanced Hodgkin’s disease with COPP/ABV/IMEP versus COPP/ABVD and consolidating radiotherapy: final results of the German Hodgkin’s lymphoma study group HD6 trial. Ann Oncol. 2004 Feb;15(2):276–282.
- Viviani S, Bonadonna G, Santoro A, et al. Alternating versus hybrid MOPP-ABVD in Hodgkin’s disease: the milan experience. Ann Oncol. 1991;2(2):55–62.
- Aviles A, Guzman R, Talavera A, et al. Randomized study for the treatment of adult advanced Hodgkin’s disease: epirubicin, vinblastine, bleomycin, and dacarbazine (EVBD) versus mitoxantrone, vinblastine, bleomycin, and dacarbazine (MVBD). Med Pediatr Oncol. 1994;22(3):168–172.
- Aviles A, Cleto S, Neri N, et al. Treatment of advanced Hodgkin’s disease: EBVD versus intensive brief chemotherapy. Leuk Lymphoma. 2003 Aug;44(8):1361–1365.
- Djeridane M, Oudard S, Escoffre-Barbe M, et al. Treatment of patients with advanced or bulky Hodgkin disease with a 12-week doxorubicin, bleomycin, vinblastine, and dacarbazine-like chemotherapy regimen followed by extended-field, full-dose radiotherapy: long-term results of the groupe ouest et est des leucemies et autres maladies de sang H90-A/B multicenter randomized trial. Cancer. 2002 Nov 15;95(10):2169–2179.
- Gobbi PG, Broglia C, Berte R, et al. Lomustine and melphalan cannot be replaced by cyclophosphamide and etoposide without reducing efficacy in MOPPEBVCAD chemotherapy for advanced Hodgkin’s disease. Haematologica. 2000 Jul;85(7):722–728.
- Grozea PN, De Persio EJ, Coltman CA, et al. A southwest oncology group: chemotherapy versus chemotherapy plus radiotherapy in treatment of stage III Hodgkin’s disease. Recent results cancer res Fortschr der Krebsforschung Progrès dans les recherches sur le cancer. 1982;80:83–91.
- Hancock BW, Gregory WM, Cullen MH, et al. ChIVPP alternating with PABIOE is superior to PABIOE alone in the initial treatment of advanced Hodgkin’s disease: results of a british national lymphoma investigation/central lymphoma group randomized controlled trial. Br J Cancer. 2001 May 18;84(10):1293–1300.
- Holte H, Mella O, Wist E, et al. ChlVPP is as effective as alternating ChlVPP/ABOD in advanced stage Hodgkin’s disease. Acta Oncologica. 1996;35(8):73–80.
- Longo DL, Duffey PL, DeVita VT, et al. Treatment of advanced-stage Hodgkin’s disease: alternating noncrossresistant MOPP/CABS is not superior to MOPP. J Clin Oncol. 1991 Aug;9(8):1409–1420.
- Pavlovsky S, Santarelli MT, Muriel FS, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage III-IV A & B Hodgkin’s disease. Ann Oncol. 1992 Jul;3(7):533–537.
- Radford JA, Rohatiner AZ, Ryder WD, et al. ChlVPP/EVA hybrid versus the weekly VAPEC-B regimen for previously untreated Hodgkin’s disease. J Clin Oncol. 2002 Jul 1;20(13):2988–2994.
- Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011 Jul 21;365(3):203–212.
- Borchmann P, Goergen H, Kobe C, et al. Treatment reduction in patients with advanced-stage hodgkin lymphoma and negative interim pet: final results of the international, randomized phase 3 trial HD18 by the German hodgkin study group. In: Presidential Symposium:European Hematology Association; 2017June23. Abstract S150
- Borchmann P, Goergen H, Kobe C, et al. ebeacopp with or without rituximab in interim-pet-positive advanced-stage Hodgkin lymphoma: updated results of the international, randomized phase 3 GHSG HD18 trial. Hematol Oncol. 2017;35(S2):65.
- Borchmann P, Goergen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin study group. Lancet. 2017 Oct 20;390(10114):2790–2802.
- Borchmann P, Haverkamp H, Lohri A, et al. Progression-free survival of early interim PET-positive patients with advanced stage Hodgkin’s lymphoma treated with BEACOPPescalated alone or in combination with rituximab (HD18): an open-label, international, randomised phase 3 study by the German Hodgkin study group. Lancet Oncol. 2017 Apr;18(4):454–463.
- Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018 Jan 25;378(4):331–344.
- Chen R, Ansell S, Gallamini A, et al. Brentuximab vedotin with chemotherapy for stage iii or iv Hodgkin lymphoma: impact of cycle 2 pet result on modified progression-free survival. Abstract PS1172. European Hematology Association 2018 Conference. Sweden. 2018.
- Chen RW, Ansell SM, Gallamini A, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin lymphoma (HL): impact of cycle 2 PET result on modified progression-free survival (mPFS). Abstract 7539. 2018 ASCO Annual Meeting. United States. 2018.
- Trotman J, Fosså A, Federico M, et al. response-adjusted therapy for advanced Hodgkin lymphoma (Rathl) trial: longer follow up confirms efficacy of de-escalation after a negative interim pet scan (CRUK/07/033). Hematol Oncol. 2017;35(S2):65–67.
- Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016 Jun 23;374(25):2419–2429.
- Ramchandren R, Advani RH, Ansell SM, et al. Brentuximab vedotin (BV) plus chemotherapy in patients with newly diagnosed advanced stage Hodgkin lymphoma (HL): north American results. J Clin Oncol. 2018;36(15_suppl):7541.
- Straus DJ, Collins GP, Walewski JA, et al. Improving outcomes with brentuximab vedotin (BV) plus chemotherapy in patients with newly diagnosed advanced stage Hodgkin lymphoma. J Clin Oncol. 2018;36(15_suppl):7534.
- Hutchings M, Radford J, Gallamini A, et al. Brentuximab vedotin plus chemotherapy in high risk advanced-stage classical Hodgkin lymphoma (CHL) patients: results of pre-specified sub-group analyses from the ECHELON-1 STUDY. European Hematology Association. Abstract S112. Sweden. 2018.
- Casasnovas O, Brice P, Bouabdallah R, et al. Final analysis of the AHL2011 randomized phase iii LYSA study comparing an early pet driven treatment de-escalation to a not pet-monitored strategy in patients with advanced stages Hodgkin lymphoma. Abstract S110. European Hematology Association. Sweden. 2018.
- Casasnovas O, Brice P, Bouabdallah R, et al. Randomized phase III study comparing an early PET driven treatment de-escalation to a not PET-monitored strategy in patients with advanced stages Hodgkin lymphoma: final analysis of the AHL2011 LYSA study. J Clin Oncol. 2018;36(15_suppl):7503.
- Kobe C, Goergen H, Fuchs M, et al. Treatment reduction in patients with advanced-stage Hodgkin lymphoma and negative interim FDG-PET: final results of the international, randomized, phase 3 HD18 trial by the german Hodgkin study group. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 2):S119–S956.
- Kreissl SGH, Kobe C, Fuchs M, et al. Treatment reduction in patients with advanced-stage-Hodgkin-lymphoma and negative interim PET: final results of the international randomized phase 3 trial HD18 by the German Hodgkin study group. Abstracts Oncol Res Treat. 2017;40(suppl 3): 1–245. Abstract V684.
- Eichenauer DA, Plutschow A, Kreissl S, et al. Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin’s lymphoma: final analysis of a phase 2 randomised trial by the German Hodgkin study group. Lancet Oncol. 2017 Dec;18(12):1680–1687.
- Gallamini A, Radford J, Jurczak W, et al. Frontline brentuximab vedotin plus chemotherapy exhibits superior modified progression-free survival vs chemotherapy alone in patients with stage iii or iv Hodgkin lymphoma: phase 3 ECHELON-1 study. European Hematology Association. Abstact PS1182. Sweden. 2018.
- Gallamini A, Tarella C, Viviani S, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 trial. J Clin Oncol. 2018 Feb 10;36(5):454–462.
- von Tresckow B, Kreissl S, Haverkamp H, et al. Beacoppescalated followed by radiotherapy of initial bulk or residual disease in advanced stage Hodgkin lymphoma: long-term follow up of the HD9 and HD12 trials of the German Hodgkin study group. T001. Haematologica: 10th International Symposium on Hodgkin Lymphoma. Germany. 2016.
- Aleknavicius E, Valuckas KP, Aleknaviciene B, et al. [Treatment results of Hodgkin’s lymphoma]. [Lithuanian]. [Hodzkino limfomos gydymo rezultatai.]. Medicina (Kaunas). 2009;45(8):615–623.
- Andjelic BM, Mihaljevic BS, Jakovic LR. ABVD as the treatment option in advanced Hodgkin’s lymphoma patients older than 45 years. Pathol Oncol Res. 2012 Jul;18(3):675–680.
- Andjelic B, Antic D, Jakovic L, et al. A single institution experience on 314 newly diagnosed advanced Hodgkin lymphoma patients: the role of ABVD in daily practice. Eur J Haematol. 2014 Nov;93(5):392–399.
- Andrieu JM, Jais JP, Colonna P, et al. Ten-year results of a strategy combining three cycles of ABVD and high-dose extended irradiation for treating Hodgkin’s disease at advanced stages. Ann Oncol. 1998 Feb;9(2):195–203.
- Belada D, Stepankova P, Sykorova A, et al. Ten years’ experience with four cycles of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, procarbazine (BEACOPP)-escalated followed by four cycles of baseline-dose BEACOPP in patients with advanced stage Hodgkin lymphoma: A single-center, retrospective study. Leukemia Lymphoma. 2015 Jul 01;56(7):2013–2018.
- Cabrera ME, Garcia H, Lois V, et al. Hodgkin lymphoma in Chile: experience of the national adult cancer program. [Spanish]. [Linfoma de Hodkin en Chile: experiencia de 15 anos del programa nacional de cancer del adulto.]. Revista Medica De Chile. 2007 Mar;135(3):341–350.
- Chichoune S, Touati S, Menia H, et al. Outcome of high risk Hodgkin’s lymphoma in the south-EST of Algeria. Haematologica. 2013 Jun 01;98:573.
- Fossa A, Fiskvik IH, Kolstad A, et al. Two escalated followed by six standard BEACOPP in advanced-stage high-risk classical Hodgkin lymphoma: high cure rates but increased risk of aseptic osteonecrosis. Ann Oncol. 2012 May;23(5):1254–1259.
- Gallamini A, Patti C, Viviani S, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol. 2011 Mar;152(5):551–560.
- Ganesan P, Rajendranath R, Kannan K, et al. Phase II study of interim PET-CT-guided response-adapted therapy in advanced Hodgkin’s lymphoma. Ann Oncol. 2015 Jun;26(6):1170–1174.
- Gupta S, Lapuz C, Holliday E, et al. Excellent prognosis in stage iii and iv Hodgkin lymphoma treated with ABVD with complete response on pet. Hematol Oncol. 2015;33:258.
- Haverkamp H, Boll B, Eichenauer DA, et al. Impact of bleomycin and vincristine dose reductions in patients with advanced Hodgkin lymphoma treated with BEACOPP: an analysis of the German Hodgkin study group HD12 and HD15 trials. J Clin Oncol. 2015 Aug 1;33(22):2430–2436.
- Jain H, Sengar M, Nair R, et al. Treatment results in advanced stage Hodgkins lymphoma: a retrospective study. J Postgrad Med. 2015 Apr 01;61(2):88–91.
- Jalali A, Ha FJ, Chong G, et al. Hodgkin lymphoma: an Australian experience of ABVD chemotherapy in the modern era. Ann Hematol. 2016 Apr 01;95(5):809–816.
- Kedmi M, Apel A, Davidson T, et al. High risk advanced stage Hodgkin lymphoma is well controlled with 2 cycles of escalated beacopp followed by 4 cycles of ABVD in patients who rapidly achieve metabolic CR on interim PET/CT scan. Blood Conference: 56th Annual Meeting of the American Society of Hematology, United States 2018. Abstract. ASH. 2014;124(21):4442.
- Lester EP, Petroni GR, Barcos M, et al. Cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide (CHOPE) for advanced-stage Hodgkin’s disease: CALGB 8856. Cancer Invest. 2001;19(5):447–458.
- Markova J, Kahraman D, Kobe C, et al. Role of [18F]-fluoro-2-deoxy-D-glucose positron emission tomography in early and late therapy assessment of patients with advanced Hodgkin lymphoma treated with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone. Leuk Lymphoma. 2012 Jan;53(1):64–70.
- Miltenyi Z, Barna S, Garai I, et al. Prognostic value of interim and restaging PET/CT in Hodgkin lymphoma. Results of the CHEAP (Chemotherapy effectiveness assessment by PET/CT) study - Long term observation. Neoplasma. 2015;62(4):627–634.
- Russo F, Corazzelli G, Frigeri F, et al. A phase II study of dose-dense and dose-intense ABVD (ABVDDD-DI) without consolidation radiotherapy in patients with advanced Hodgkin lymphoma. Br J Haematol. 2014 Jul;166(1):118–129.
- Tao YX, Sun SY, Kang SY, et al. Comparison of dose-dense ABVD and standard ABVD in the treatment of early unfavorable and advanced Hodgkin’s lymphoma: A retrospective analysis. J Huazhong Univ Sci Technol Med Sci. 2014 April;34(2):260–264.
- Wongso D, Fuchs M, Plutschow A, et al. Treatment-related mortality in patients with advanced-stage Hodgkin lymphoma: an analysis of the german Hodgkin study group. J Clin Oncol. 2013 Aug 1;31(22):2819–2824.
- Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013 Dec;14(13):1348–1356.
- Edwards-Bennett SM, Jacks LM, Moskowitz CH, et al. Stanford V program for locally extensive and advanced Hodgkin lymphoma: the memorial sloan-kettering cancer center experience. Ann Oncol. 2010 Mar;21(3):574–581.
- Gobbi PG, Pieresca C, Ghirardelli ML, et al. Long-term results from MOPPEBVCAD chemotherapy with optional limited radiotherapy in advanced Hodgkin’s disease. Blood. 1998 Apr 15;91(8):2704–2712.
- Gobbi PG, Broglia C, Levis A, et al. MOPPEBVCAD chemotherapy with limited and conditioned radiotherapy in advanced Hodgkin’s lymphoma: 10-year results, late toxicity, and second tumors. Clin Cancer Res off J Am Assoc Cancer Res. 2006 Jan 15;12(2):529–535.
- Horning SJ, Williams J, Bartlett NL, et al. Assessment of the stanford V regimen and consolidative radiotherapy for bulky and advanced Hodgkin’s disease: eastern cooperative oncology group pilot study E1492. J Clin Oncol. 2000 Mar;18(5):972–980.
- Levis A, Anselmo AP, Ambrosetti A, et al. VEPEMB in elderly Hodgkin’s lymphoma patients. Results from an Intergruppo Italiano Linfomi (IIL) study. Ann Oncol. 2004 Jan;15(1):123–128.
- Martinelli G, Cocorocchio E, Peccatori F, et al. ChlVPP/ABVVP, a first line ‘hybrid’ combination chemotherapy for advanced Hodgkin’s lymphoma: a retrospective analysis. Br J Haematol. 2004 Jun;125(5):584–589.
- Saletti P, Zucca E, Gueneau M, et al. ChlVPP/ABV-VP16 hybrid regimen for advanced Hodgkin’s disease: a study in 36 patients. Leukemia Lymphoma. 1999;33(3–4):313–319.
- Wiernik PH, Hong F, Glick JH, et al. Radiation therapy compared with chemotherapy for consolidation of chemotherapy-induced remission of advanced Hodgkin lymphoma: a study by the eastern co-operative oncology group (E1476) with >20 years follow-up. Leuk Lymphoma. 2009 Oct;50(10):1632–1641.
- Younes A, Oki Y, McLaughlin P, et al. Phase 2 study of rituximab plusABVD in patients with newly diagnosed classical Hodgkin lymphoma. Blood. 2012;119(18):4123–4128.
- Russo F, Aloj L, Svanera G, et al. Dose-dense/dose-intense ABVD in advanced-stage Hodgkin’s lymphoma: a long-term follow up study. P009. Haematologica: 10th International Symposium on Hodgkin Lymphoma, Cologne, Germany, Oct 22- 25.2016.
- Andjelic B, Antic D, Jakovic L, et al. Advanced Hodgkin lymphoma patients without large tumor mass a new prognostic score identifies patients with favourable outcome. PB 1892. 22nd Congress of the European Hematology Association. Spain. 2017.
- Carras S, Dubois B, Senecal D, et al. Interim PET response-adapted strategy in untreated advanced stage Hodgkin lymphoma: results of GOELAMS LH 2007 phase 2 multicentric trial. Clin Lymphoma Myeloma Leuk. 2018 Mar;18(3):191–198.
- Damlaj M, Al-Zahrani M, Syed G, et al. Escalation from ABVD following positive interim functional imaging improves progression free survival but not overall survival in advanced classical Hodgkin lymphoma - a real world analysis. Blood. 2017;130(Suppl 1):2799.
- Dann EJ, Bairey O, Bar-Shalom R, et al. Modification of initial therapy in early and advanced Hodgkin lymphoma, based on interim PET/CT is beneficial: a prospective multicentre trial of 355 patients. Br J Haematol. 2017 Sep;178(5):709–718.
- Evens AM, Hamlin PA, Nabhan C, et al. Sequential brentuximab vedotin and adriamycin, vinblastine, and dacarbazine for older patients with untreated Hodgkin lymphoma: findings from a phase ii window study. P001. Haematologica: 10th International Symposium on Hodgkin Lymphoma. Germany. 2016.
- Friedberg JW, Forero-Torres A, Bordoni RE, et al. Frontline brentuximab vedotin in combination with dacarbazine or bendamustine in patients aged >/=60 years with HL. Blood. 2017 Dec 28;130(26):2829–2837.
- C Ea L, O’Brien PC, Capp AL, et al. Outcomes and relapse patterns following chemotherapy in advanced Hodgkin lymphoma in the positron emission tomography era. Blood Lymphat Cancer. 2018;8:13–20.
- Marr KC, Connors JM, Savage KJ, et al. ABVD chemotherapy with reduced radiation therapy rates in children, adolescents and young adults with all stages of Hodgkin lymphoma. Ann Oncol. 2017 Apr 1;28(4):849–854.
- Russell J, Collins A, Fowler A, et al. Advanced Hodgkin lymphoma in the east of England cancer network: A 10-year comparative analysis of outcomes for ABVD and escalated-BEACOPP treated patients aged 16 to 59. Hematol Oncol. 2017;35(S2):318–319.
- Zaucha JM, Malkowski B, Chauvie S, et al. The predictive role of interim PET after the first chemotherapy cycle and sequential evaluation of response to ABVD in Hodgkin’s lymphoma patients-the polish lymphoma research group (PLRG) observational study. Ann Oncol. 2017 Dec 1;28(12):3051–3057.
- Federico M, Bellei M, Cheson BD. BEACOPP or no BEACOPP? Lancet Oncol. 2013 Nov;14(12):e487–8.
- Evens AM, Advani RH, Helenowski IB, et al. Multicenter phase II study of sequential brentuximab vedotin and doxorubicin, vinblastine, and dacarbazine chemotherapy for older patients with untreated classical Hodgkin lymphoma. J Clin Oncol. 2018 Oct 20;36(30):3015–3022.
- Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012 Jun 20;30(18):2183–2189.
- Rueda A, Sevilla I, Guma J, et al. Secondary prophylactic G-CSF (filgrastim) administration in chemotherapy of stage I and II Hodgkin’s lymphoma with ABVD. Leuk Lymphoma. 2001 Apr;41(3–4):353–358.
- Phillippo DM, Ades AE, Dias S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submission to NICE. 2016 [cited 2020]. Available from http://nicedsu.org.uk/
- von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin study group HD14 trial. J Clin Oncol. 2012 Mar 20;30(9):907–913.
- Vakkalanka B, Link BK. Neutropenia and neutropenic complications in ABVD chemotherapy for Hodgkin lymphoma. Adv Hematol. 2011;2011:656013.
- Gotti M, Fiaccadori V, Bono E, et al. Therapy-related late adverse events in Hodgkin’s lymphoma. Lymphoma. 2013;2013:7.
- Eichenauer DA, Becker I, Monsef I, et al. Secondary malignant neoplasms, progression-free survival and overall survival in patients treated for Hodgkin lymphoma: a systematic review and meta-analysis of randomized clinical trials. Haematologica. 2017 Oct;102(10):1748–1757.
- van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017 Apr 20;129(16):2257–2265.
- Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002 Sep 15;100(6):1989–1996.
- Martin WG, Habermann TM, Colgan JP, et al. G-CSF administration increases pulmonary toxicity for Hodgkin’s disease patients treated with bleomycin-containing chemotherapy. Blood. 2004;104(11):1317.
- Aldin A, Umlauff L, Estcourt LJ, et al. Interim PET-results for prognosis in adults with Hodgkin lymphoma: a systematic review and meta-analysis of prognostic factor studies. Cochrane Database Syst Rev. 2019 Sep;16(9):Cd012643.
- Amitai I, Gurion R, Vidal L, et al. PET-adapted therapy for advanced Hodgkin lymphoma - systematic review. Acta Oncol. 2018 Jun;57(6):765–772.