1. Introduction
Our understanding of the pathobiology of multiple myeloma (MM) and its treatment have progressed substantially over past decades. While risk stratification including characterization of cytogenetic abnormalities is standard practice, the therapeutic armamentarium of proteasome inhibitors (PIs), immunomodulatory agents (IMiDs), and monoclonal antibodies (mAbs) continues to be deployed in response to prognostic considerations rather than any specific biological vulnerability identified in MM. The oral BH3 mimetic venetoclax is currently undergoing evaluation in MM with early results indicating particular activity in a subset of patients with BCL-2-dependent disease. Venetoclax offers hope as the first precision therapy in MM, though a careful application of biomarker-driven patient selection and rational combination strategies are likely to be necessary to fulfill this promise.
2. Role of BCL-2 in MM and pre-clinical development of venetoclax
The BCL-2 family of proteins are key regulators of apoptosis: the pro-apoptotic BH3 proteins (BIM, BID) activate BAX/BAK in the outer mitochondrial membrane leading to cytochrome c release and cell death, and these are inhibited by the anti-apoptotic proteins MCL-1, BCL-2, and BCL-XL [Citation1] ()). Venetoclax is an oral BH3 mimetic with a high binding affinity to BCL-2, avoiding off-target effects from BCL-XL binding that restricted the development of similar agents [Citation2] ()). It has significant clinical activity across a range of hematology malignancies including chronic lymphocytic leukemia and acute myeloid leukemia.
Figure 1. Targeting BCL-2 with venetoclax-based regimens in MM (a) BCL-2 family proteins regulate the intrinsic apoptotic pathway which can be divided into anti-apoptotic and pro-apoptotic members. Anti-apoptotic proteins bind and sequester pro-apoptotic proteins via the BH3 domain, thus promoting survival of MM cells. (b) BCL-2 can be overexpressed in MM, where it preferentially sequesters high levels of the pro-apoptotic proteins BIM and BAX. Such cells are poised to initiate apoptosis upon the release of sufficient quantities of the pro-apoptotic proteins, a state referred to as ‘primed for death’ by BCL-2 inhibition. Venetoclax can competitively displace BIM and BAX to trigger MM cell death, however other anti-apoptotic proteins such as BCL-XL and MCL-1 can act as resistance factors by capturing the pro-apoptotic proteins released by venetoclax. (c) Venetoclax can induce cell death as a single agent in MM cells that have an innate dependency upon BCL-2 for cell survival, such as those harboring the t(11;14) translocation. Venetoclax activity in MM can also be potentiated through combination with agents that increase BCL-2 dependency, target known resistance factors, and/or have complementary anti-myeloma mechanisms of action (MOA), including the glucocorticoid dexamethasone, proteasome inhibitors bortezomib and carfilzomib, and CD38 monoclonal antibody daratumumab, respectively. CDC, complement-dependent cytotoxicity; ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis
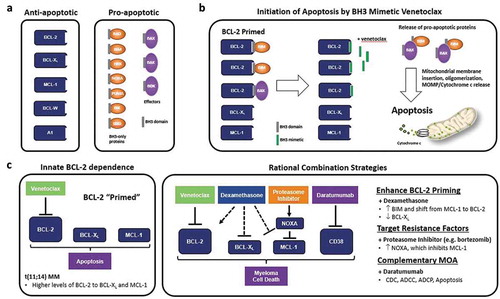
BCL-2 family proteins play a key role in B-cell development and differentiation into plasma cells. The role of anti-apoptotic proteins in MM is dynamic and heterogeneous, with Touzeau et al. [Citation3] demonstrating the different combinations of dependencies on one or more of MCL-1, BCL-2, and BCL-XL in cell lines and patient samples. Increased dependency upon BCL-2 for MM cell survival has been found in a subset of cases and correlated with the specific cytogenetic abnormality involving rearrangement of chromosomes 11 and 14, namely t(11;14) [Citation4]. Although the underlying mechanism of this co-occurrence is not well understood, t(11;14) MM possesses a unique ‘B-cell-like’ biology that may result in its increased dependency upon BCL-2 for survival [Citation5]. t(11;14) is found in approximately 15–20% of MM patients at diagnosis and has an intermediate prognosis in the modern era, though interestingly up to 50% of plasma cell leukemias (PCL) harbor t(11;14) and which generally have a poor prognosis [Citation5]. The t(11;14) translocation also occurs in approximately 50% of patients with light chain (AL) amyloidosis and is an independent adverse prognostic marker [Citation6,Citation7]. The differentiated biology of t(11;14)-positive plasma cell dyscrasias with respect to BCL-2 dependency offers a unique opportunity for a targeted therapeutic approach with venetoclax-based regimens.
Pre-clinical results confirmed that increased sensitivity to venetoclax in human MM cell lines and primary MM samples positive for t(11;14) correlated with higher expression of BCL-2 than BCL-XL and MCL-1, and functional dependence on BCL-2 by BH3 profiling [Citation3,Citation4,Citation8]. In vitro testing also revealed that the activity of venetoclax could be further potentiated by combinations with the glucocorticoid dexamethasone and the proteasome inhibitor bortezomib [Citation9,Citation10] ()).
3. Early clinical trial experience with venetoclax in MM
The promising pre-clinical data for venetoclax has led to a series of clinical trials in both t(11;14) selected and unselected patients with relapsed/refractory MM (RRMM) ().
Table 1. Evaluation of venetoclax in relapsed/refractory multiple myeloma: results in key biomarker subgroups from selected phase 1–3 clinical trials
The M13-367 study of venetoclax monotherapy enrolled 30 patients in the dose escalation cohort and a further 36 patients in safety expansion [Citation11]. All patients with RRMM had received PIs and IMiDs with a median of five prior lines of therapy. The overall response rate (ORR) for all patients was 21%. The proportion of patients with t(11;14) was somewhat over-represented compared to the general MM population though not specifically selected for, comprising 30 of 66 patients (45%). Patients with t(11;14) also comprised most of the responders (12 of 14), resulting in notably significant responses in this group with ORR 40% and 27% achieving a very good partial response (VGPR) or better. The median time to progression in the t(11;14) group was 6.6 months (95% CI 3.9–10.2 months), compared to 1.9 months (95% CI 1.2–2.3 months) in the non-t(11;14) group.
The M12-901 study combined venetoclax with bortezomib and dexamethasone, with 54 patients in the dose escalation arm and 12 in the safety expansion [Citation12]. Patients had received a median of 3 prior therapies and 39% were refractory to bortezomib with 80% previously exposed. The ORR was 67%, with 42% achieving a VGPR or better, while in 30 patients with 1–3 lines of therapy and not refractory to bortezomib the ORR was 97% (74% VGPR or better). Notably, patients with t(11;14) (n = 9) had a similar response (ORR 78%) compared to the non-t(11;14) group (n = 57) (ORR 65%). The most common toxicities in both phase 1 studies were gastrointestinal and hematological, with no cases of tumor lysis syndrome.
4. Biomarker-guided development of venetoclax in MM
The targeting of specific cytogenetic and molecular subgroups with specific drugs has long been a therapeutic goal, though with inconsistent results reflecting the challenge of tumoral heterogeneity and clonal evolution (). Umbrella trials are underway that may identify genomic subgroups that most benefit from new therapies, but highlights the need to select the right patient for the right drug based on validated predictive markers. The development of venetoclax as an exemplar of targeted therapy in MM offers both opportunities and pitfalls in selecting the optimal patient population and combination regimen based upon clinical and biological parameters.
Table 2. Table of other targeted cytogenetic and molecular subgroups in myeloma
In the phase 1 trial of single agent venetoclax, BCL-2 (BCL2), BCL-XL (BCL2L1), and MCL-1 (MCL1) gene expression was analyzed in 44 patients who had available baseline bone marrow samples [Citation11]. The ratios of BCL2:MCL1 and BCL2:BCL2L1 expression were significantly higher in responders, with high BCL2:BCL2L1 more prevalent in patients with t(11;14) but also identifying responders within this subgroup: the ORR in the high expression group (n = 9) was 88% compared with 20% in the low expression group (n = 15), thus implicating BCL-XL as a key resistance factor to venetoclax clinical activity within the t(11;14) subgroup. As noted above, dexamethasone is hypothesized to enhance ‘BCL-2 priming’, a state where BCL-2 maintains cell survival by sequestering high levels of BIM, providing rationale for use as a combination agent with venetoclax in t(11;14) MM [Citation9] ()). Indeed, an increased ORR of 60% (phase 1; n = 20) and 48% (phase 2; n = 31) was observed in patients with t(11;14) RRMM who received venetoclax in combination with dexamethasone [Citation14]. These data corroborate a potential synergism between BCL-2 inhibition and corticosteroids as reported in vitro, and support the ongoing investigation of VenDex in the phase 3 CANOVA trial (NCT03539744) in patients with t(11;14) RRMM [Citation15] ().
Based upon results from the M12-901 study, the phase 3 BELLINI trial randomized patients 2:1 to venetoclax or placebo in combination with bortezomib and dexamethasone [Citation16]. Of 291 patients, 194 were randomized to the venetoclax arm and 97 to the placebo. Venetoclax significantly improved progression free survival (22.4 months) compared with placebo (11.5 months), with p = 0.010 and HR 0.63 (95% CI, 0.443–0.897). Unexpectedly the venetoclax arm also had a lower overall survival, with the median not reached in either arm but favoring the placebo (HR 2.03 (95% CI 1.04–3.95)). At a median follow-up of 28.6 months, there were 64 (33%) deaths in the venetoclax arm vs 24 (25%) in placebo mainly due to an increase in infectious deaths at the time of disease progression. In the subgroup of patients with t(11;14) (n = 35), there was significant progression free survival benefit (not reached vs 9.5 months, p = 0.002; HR = 0.110 (95% CI, 0.022–0.560)) without increased mortality on venetoclax. In a post-hoc analysis, high BCL2 expression also identified a patient population that derived a PFS benefit without increased mortality with venetoclax. The median progression-free survival in patients with high BCL2 gene expression (n = 98) was 22.4 months (95% CI 22.4–not estimable) with venetoclax and 9.9 months (9.0–14.0) with placebo (HR 0.24 [95% CI 0.12–0.48]; p < 0 · 0001) [Citation16]. This was noticeably contrasted by poorer outcomes in the low BCL2 and non-t(11;14) group which had lower overall survival rates compared with placebo (n = 130, HR = 3.13, 95% CI = 1.2–8.13, p = 0.019) [Citation16]. The distinct benefit/risk profiles observed in the BELLINI trial and real-world experience with venetoclax [Citation17] provide compelling rationale for a biomarker-driven approach to patient selection that reflects disease biology and critical evaluation of the optimal combination regimen.
Venetoclax has also been combined with carfilzomib in the M15-538 phase 2 study, with an ORR of 78% (n = 49) and no new safety signals present compared to prior phase 1 trials [Citation18]. Of the 13 patients with t(11;14), the ORR was 92% with 75% achieving VGPR or better. Given the strong preliminary signal in t(11;14) patients and the results from the BELLINI trial, the study plans to enroll approximately 65 patients in an expansion cohort (randomized, open label) to gain further safety and efficacy data in this key subgroup (). Additionally, the M15-654 study evaluated the preliminary safety and tolerability of venetoclax in combination with daratumumab and dexamethasone (VenDd) in patients with t(11;14) RRMM and VenDd with bortezomib (VenDVd) in cytogenetically unselected patients with RRMM [Citation19]. The ORR was 96% (96% achieving VGPR or better) with VenDd (n = 24) and 92% (79% achieving VGPR or better) with VenDVd (n = 24). The preliminary results from this study suggest that the combination of venetoclax with agents of complementary mechanisms of action (i.e. daratumumab) may also be a promising strategy in MM ()). Given the promising results with VenDd in patients with t(11;14) RRMM, this study continues enrolling patients with t(11;14) RRMM to a randomized, open-label expansion cohort that will evaluate VenDd with a DVd control arm to contextualize safety results and confirm efficacy ().
5. Conclusion
Venetoclax is amongst the first therapies in MM that has shown significant and lasting responses in a defined biological subset of patients in a phase 3 clinical trial. The benefit observed to date in patients with t(11;14) RRMM has enabled ongoing prospective trials to confirm safety and efficacy in this subgroup of patients. If ongoing studies are successful, venetoclax has the potential to be the first biomarker-driven therapy for RRMM. Elevated levels of BCL2 gene expression may also identify a patient population beyond t(11;14) who may derive benefit from a venetoclax-based therapy. Prospective clinical trials will be needed to confirm the BELLINI findings in patients high BCL2 RRMM. A deeper understanding of intra-patient tumor heterogeneity and biologically informed combination strategies should guide these studies. Nevertheless, the use of a biomarker-driven strategy in prospective clinical trials is the most promising path to demonstrate safe and effective use of venetoclax in MM and ultimately paving the way to precision medicine in MM.
Declaration of interest
S Harrison receives consultancy fees from, is on the advisory board for and an investigator for Abbvie; Celgene and Janssen; receives honoraria and research funding from Celgene and Janssen. J Ross has employment with AbbVie and may hold stock or other options. P Moreau receives consultancy and honoraria fees from Sanofi, Janssen, Amgen, Celgene/Bristol-Myers Squibb and Abbvie; honoraria from Takeda and Novartis. SK Kumar receives Research funding for clinical trials to the institution from Abbvie, Amgen, BMS, Carsgen, Janssen, KITE, Merck, Medimmune, Novartis, Roche-Genentech, Takeda, and Tenebio; is on the consulting/advisory board for (with no personal payments) Abbvie, Amgen, BMS, Janssen, Roche-Genentech, Takeda; and (with personal payment) Oncopeptides, Beigene. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Kale J, Osterlund EJ, Andrews DWBCL-2. family proteins: changing partners in the dance towards death. Cell Death Differ. 2018 Jan;25(1):65–80.
- Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013 Feb;19(2):202–208.
- Touzeau C, Ryan J, Guerriero J, et al. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia. 2016 Mar;30(3):761–764.
- Touzeau C, Dousset C, Le Gouill S, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014 Jan;28(1):210–212.
- Jelinek T, Mihalyova J, Kascak M, et al. Single-agent venetoclax induces MRD-negative response in relapsed primary plasma cell leukemia with t(11;14). Am J Hematol. 2019 Jan;94(1):E35–E37.
- Bryce AH, Ketterling RP, Gertz MA, et al. Translocation t(11;14) and survival of patients with light chain (AL) amyloidosis. Haematologica. 2009 Mar;94(3):380–386.
- Warsame R, Kumar SK, Gertz MA, et al. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer J. 2015 May;1(5):e310.
- Punnoose EA, Leverson JD, Peale F, et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016 May;15(5):1132–1144.
- Matulis SM, Gupta VA, Nooka AK, et al. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia. 2016 May;30(5):1086–1093.
- Qin JZ, Ziffra J, Stennett L, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005 Jul 15;65(14):6282–6293.
- Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017 Nov 30;130(22):2401–2409.
- Moreau P, Chanan-Khan A, Roberts AW, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017 Nov 30;130(22):2392–2400.
- Scheid C, Reece D, Beksac M, et al. Phase 2 study of dovitinib in patients with relapsed or refractory multiple myeloma with or without t(4;14) translocation. Eur J Haematol. 2015 Oct;95(4):316–324.
- Kaufman JL, Gasparetto C, Schjesvold FH, et al. Phase I/II study evaluating the safety and efficacy of venetoclax in combination with dexamethasone as targeted therapy for patients with t(11;14) relapsed/refractory multiple myeloma. Blood. 2019;134(Supplement_1):926.
- Mateos M-V, Moreau P, Dimopoulos MA, et al. A phase III, randomized, multicenter, open-label study of venetoclax or pomalidomide in combination with dexamethasone in patients with t(11;14)-positive relapsed/refractory multiple myeloma. J clin oncol. 2020;38(15_suppl):TPS8554–TPS8554.
- Kumar SK, Harrison SJ, Cavo M, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020 Dec;21; (12):1630-1642.
- Jelinek T, Popkova T, Duras J, et al. Venetoclax plus bortezomib and dexamethasone in heavily pretreated end-stage myeloma patients without t(11;14): a real-world cohort. Hematol Oncol. 2020 Aug;38(3):412–414.
- Costa LC, Stadtmauer EA, Morgan G, et al. Phase 2 study of venetoclax plus carfilzomib and dexamethasone in patients with relapsed/refractory multiple myeloma. EHA Lib. 2019 06 15;266992:PS1375.
- Kaufman JL, Baz RC, Harrison SJ, et al. Updated analysis of a phase I/II study of venetoclax in combination with daratumumab and dexamethasone, ± bortezomib, in patients with relapsed/refractory multiple myeloma. J clin oncol. 2020;38(15_suppl):8511.
