1. Introduction
Until recently, treatment options for higher risk (HR)-myelodysplastic syndrome (MDS) patients ineligible for allogeneic hematopoietic stem cell transplantation (allo-HSCT) were limited, with hypomethylating agents (HMA) azacitidine and decitabine representing the standard of care [Citation1,Citation2]. Response rates to these agents are around 50%, responses can be very slow and all patients invariably relapse or progress. Prognosis after failure of HMAs is exceptionally poor, with a median overall survival (OS) of 4–6 months [Citation3]. Since azacitidine is the only drug shown to prolong survival in MDS, there is a high clinical need for new therapeutic options for this patient group. The oral selective BCL-2 inhibitor venetoclax is currently undergoing evaluation in both first-line therapy as well as salvage therapy after HMA failure in HR-MDS with early results offering hope as a potentially new and effective treatment strategy within this patient cohort.
2. Role of BCL-2 in myeloid malignancies
The BCL-2 family proteins are key regulators of the mitochondrial apoptosis pathway ()). Activation of pro-apoptotic effectors BAX/BAK via pro-apoptotic BH3 proteins (BIM, BID) leads to mitochondrial outer membrane permeabilization, cytochrome C release and cell death. Sequestering BIM and other BH3 or effector proteins by anti-apoptotic proteins such as BCL-2, MCL-1, and BCL-XL effectively prevents cells from initiating apoptosis () [Citation4]. Venetoclax as an oral BH3 mimetic binds with high affinity to the BH3-binding groove of BCL-2, displacing BIM and other BH3 proteins that are normally sequestered by BCL-2 (). This specific BCL-2-inhibition causes rapid tumor cell apoptosis [Citation5].
Figure 1. Targeting BCL-2 in MDS. (a) Anti- and pro-apoptotic members of the BCL-2 family proteins as regulators of the mitochondrial pathway of apoptosis. (b) Cancer cell survival: BCL-2 overexpression sequestering high levels of pro-apoptotic proteins prevents cells from initiating apoptosis. (c) Induction of apoptosis: displacing BIM and BAK by venetoclax allows pro-apoptotic proteins to initiate apoptosis. (d) Suggested synergy between hypomethylating agents and venetoclax. (e) Decreasing basal apoptosis rate with increasing BCL-2 dependency and suggested higher venetoclax sensitivity upon disease progression in MDS
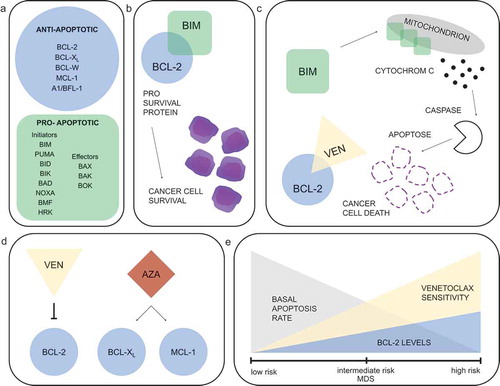
Many cancer cells express high levels of BCL-2 and are dependent on BCL-2 for survival [Citation6,Citation7]. In acute myeloid leukemia (AML) over-expression of BCL-2 is associated with increased survival of leukemic cells, resistance to chemotherapy and shorter overall survival (OS), making BCL-2 a compelling target for antileukemic therapy [Citation6,Citation8]. First results from a phase 1b trial showed high remission rates of 67% for venetoclax in elderly AML patients when combined either with decitabine or azacitidine [Citation9]. This was followed by two randomized placebo-controlled phase III trials showing improved remission rates and prolonged OS for the combination of venetoclax with either azacitidine [Citation10] or low-dose cytarabine [Citation11] in comparison to the placebo arm, leading to approval of venetoclax in these combinations for treatment of AML patients unfit for intensive chemotherapy. Given these encouraging results, interest has grown in evaluating venetoclax for HR-MDS as well.
3. Pre-clinical data of venetoclax in MDS
Myelodysplastic syndromes are clonal hematopoietic stem/progenitor cells (HSPC) disorders, characterized by bone marrow hypercellularity, ineffective hematopoiesis with dysplastic features, and increased risk of leukemic transformation. The paradox of most patients presenting with peripheral blood cytopenias despite a hypercellular bone marrow has been attributed to excessive intramedullary apoptosis of HSPC in lower risk (LR)-MDS. Several studies have demonstrated reduced BCL-2 expression and increased levels of apoptosis in HSPC from patients with early MDS subtypes compared to more advanced disease stages or healthy controls, supporting a potential role of the BCL-2-related proteins in the pathogenesis of deregulated apoptosis [Citation12–14]. In particular, apoptosis exceeds proliferation with an apoptosis: proliferation ratio of 2.08 [1.15–3.63] in LR-MDS patients, whereas in disease progression this ratio is equivocal due to increasing proliferation [Citation14]. Interestingly, transformation from MDS to AML is associated with inhibition of apoptosis rather than increased proliferation. In addition, increased pro-apoptotic (BAX/BAK) versus anti-apoptotic (BCL-2, BCL-X) BCL-2-related protein ratios were noted in LR-MDS compared to healthy controls. Disease progression was associated with significantly reduced ratios, primarily resulting from increased BCL-2 expression, exemplifying that HSPC in MDS acquire apoptotic resistance on disease progression ().
HSPC from HR-MDS or AML patient samples are uniquely susceptible to BCL-2 inhibition in vitro, with pronounced apoptosis inducible either by ABT-737 (which binds to BCL-XL and BCL-W and BCL-2) or by venetoclax, while HSPC from LR-MDS samples or healthy controls are much less sensitive [Citation15]. Notably, venetoclax still efficiently targeted HSPC in advanced MDS patient samples in vitro, even when harboring adverse mutation profiles, such as ASXL1, RUNX1, TP53, and EZH2, indicating apoptotic vulnerabilities in HR-MDS are likely independent from underlying somatic mutations [Citation16]. Of note, RNA-interference drug modifier screens identified BCL-2 family proteins as potential azacitidine-sensitizing targets, providing a rationale for combining venetoclax with HMAs and preclinical studies have shown that treatment with BCL-2 inhibitors renders leukemic cells more susceptible to HMAs [Citation17]. Azacitidine treatment indirectly increases sensitivity to BCL‑2 inhibition in HR-MDS cells by modifying the relative levels of BCL-2 family members, indicating synergistic effects of BCL-2 inhibition and HMA treatment (). A recent in vitro study has additionally demonstrated that venetoclax and azacitidine in combination effectively target malignant cells in MDS and AML patients, even at lower doses of azacitidine and after HMA failure, while sparing healthy hematopoiesis [Citation18].
4. Clinical trials with venetoclax in MDS
Preclinical studies have provided a rationale to evaluate venetoclax as a therapeutic agent specifically in HR-MDS. However, advanced MDS is defined by usually profound and lasting cytopenias and patients are generally older and more vulnerable to drug toxicities. As HMAs are known to increase cytopenias during the first few treatment cycles, the combination with venetoclax has significant potential for cumulative toxicity and myelosuppression. Multiple clinical trials with venetoclax in HR-MDS are currently underway (). The first phase 1b/2 trials in treatment naive as well as HMA pretreated HR-MDS have finished accrual and preliminary reports show impressive remission rates. Responses are swift with a median time to response of 1–2 months, representing a major advantage over treatment with HMA alone. In a dose-escalation phase 1b study [NCT02942290] in treatment naive HR-MDS, 78 patients were treated with azacitidine (75 mg/m2, d1-7) and venetoclax. Venetoclax was initially given at a dose of 400 and 800 mg/d for 28 days, but was later amended to an escalating dose regimen (100, 200, and 400 mg) for only 14/28 days per cycle due to excessive toxicity [Citation19]. The overall response rate (ORR) was 77%, including complete remission (CR) and marrow CR (mCR) achieved by 42% and 35% of patients, respectively. The median OS was 27.5 months among all patients. For those patients who received 400 mg venetoclax, OS was not reached. Importantly, 65% of patients dependent on red blood cell or platelet transfusions at study entry achieved transfusion independence. However, adverse events (AEs) were common with neutropenia (51%) and thrombocytopenia (30%) being the most common ≥ grade 3 AEs. Febrile neutropenia was the most common serious AE (42%) and the 30-day mortality rate was 2% [Citation19]. Two-thirds of patients required a cycle delay for a median of 15 days and 55% experienced two or more venetoclax dose interruptions. Dose reduction of azacitidine was also necessary in 30% of patients. This illustrates that despite high responses, HR-MDS patients constitute a vulnerable patient population in terms of toxicities and the optimal dosing regimen may not have been determined yet. Results of this phase Ib study led to initiation of a phase III, placebo-controlled trial [NCT04401748], which is currently enrolling patients with HR-MDS. The outcome of this trial will be crucial to definitely determine whether the combination of venetoclax and azacitidine is superior to azacitidine alone, especially in light of the fact that recent encouraging results from early MDS trials with promising new agents (e.g., APR-246) have not been borne out in subsequent randomized phase III trials. Additionally, it is worth mentioning that high mCR rates as seen in the phase I trials do not necessarily translate into a survival benefit, especially if hematologic improvement (which is an extremely relevant clinical outcome parameter for HR-MDS patients) is offset by prolonged myelosuppression. This dilemma was recently highlighted in a larger retrospective analysis of HR-MDS patients treated with different modalities and evaluated according to standard IWG 2006 criteria [Citation20].
Table 1. Selection of current clinical trials with venetoclax in MDS
Table 2. Selection of clinical trials with venetoclax in myeloid malignancies including MDS
For relapsed/refractory (r/r) myeloid malignancies, trials evaluating venetoclax in combination with HMAs [NCT03404193 and NCT 02966782, ] are also under investigation. Preliminary phase 1b results of r/r MDS patients after HMA failure treated with venetoclax monotherapy (400 or 800 mg d1-28) or in combination with azacitidine (75 mg/m2, d1-7; escalating doses of venetoclax (100, 200, and 400 mg) for 14 days of 28-day cycles) showed promising results in the combination arm with an ORR of 40% observed in 15 (CR:3, mCR: 12) of 37 evaluable patients [Citation21]. Median time to response was 1.2 months (range 0.7–6.3) and 12-months OS estimate was 65%. Hematologic ≥ grade 3 AEs were common with febrile neutropenia and pneumonia being the most common serious AEs. A retrospective study recently confirmed high rates of mCR (59%) and hematologic improvement (41%) upon combination treatment with venetoclax and HMA in heavily pretreated HR-MDS patients [Citation22]. Notably, 62% of all responding patients in this smaller cohort were able to proceed to allo-HSCT, which was associated with prolonged survival. However, higher rates of treatment discontinuation (>20%) due to adverse events were also observed compared to data in AML patients [Citation9].
Finally, there are currently several other promising treatment options for HR-MDS patients being explored in combination with HMAs besides venetoclax worth mentioning here. The NEDD8-activating enzyme inhibitor pevonedistat in combination with azacitidine has shown high overall response rates in a randomized phase II trial [Citation23] and seems to induce less myelosuppression than venetoclax, which might prove to be key for this patient population. Monoclonal antibodies targeting either TIM3 (sabalitomab) or CD47 (magrolimab) in combination with azacitidine are now in phase III trials with results eagerly anticipated. Early combination trials of venetoclax with HMAs and antibodies or pevonedistat are also beginning accrual ( trial 5, trial 10). And oral HMA derivatives such as ASTX-727 (oral decitabine and cedazuridine) and ASTX-030 (oral azacitidine and cedazuridine) are now entering clinical trials and offer the possibility of a completely oral therapy, which may potentially also be combined with venetoclax (, trial 4), as can the IRAK4 kinase inhibitor CA-4948 (, trial 3).
5. Future considerations and conclusions
Given the high response rates as well as swiftness of responses in both treatment naive and HMA pretreated HR-MDS, the combination of venetoclax and azacitidine certainly has potential to become an additional effective treatment option for this patient group, perhaps even as a backbone to which other agents, such as IDH inhibitors or monoclonal antibodies may be added. The short time to first response seen in r/r MDS patients also allows this combination to serve as a bridge to allo-HSCT, offering a potential for cure for more patients. However, until the results of the ongoing randomized phase III trial conclusively determine superior overall survival compared to azacitidine alone, a definite conclusion on the efficacy of venetoclax + azacitidine for HR-MDS cannot be drawn.
Furthermore, in light of the necessarydose delays and reductions observed in the phase 1 trials as well as the high rate of clinically relevant and prolonged neutropenia, it will be crucial to determine the optimal dosing schedule of this combination allowing for maximum benefit without undue toxicity in this vulnerable patient population.
Patients need to be closely monitored and receive adequate supportive care, including antifungals, during prolonged neutropenia, which requires dose adaptation of venetoclax. It may well be that only a carefully selected HR-MDS patient population will prove eligible for this combination therapy in the future, for example, those that are younger, with less severe cytopenias or those with a chance to proceed to allogeneic transplant. To more adequately resolve this question, incorporation of exploratory biomarkers of response into future clinical trials may be useful to identify HR-MDS patients most likely to benefit from venetoclax.
Declaration of interest
KS Götze received research funding from BMS/Celgene, and has participated in advisory boards for BMS/Celgene and Abbvie. All other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Additional information
Funding
References
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232.
- Kantarjian H, Issa J-PJ, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803.
- Prébet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–3327.
- Cheng EH, Wei MC, Weiler S, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711.
- Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–375.
- Delia D, Aiello A, Soligo D, et al. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood. 1992;79:1291–1298.
- Valentin R, Grabow S, Davids MS. The rise of apoptosis: targeting apoptosis in hematologic malignancies. Blood. 2018;132:1248–1264.
- Campos L, Rouault JP, Sabido O, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–3096.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–629.
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135:2137–2145.
- Rajapaksa R, Ginzton N, Rott LS, et al. Altered oncoprotein expression and apoptosis in myelodysplastic syndrome marrow cells. Blood. 1996;88:4275–4287.
- Bincoletto C, Saad ST, Soares da Silva E, et al. Autonomous proliferation and bcl-2 expression involving haematopoietic cells in patients with myelodysplastic syndrome. Br J Cancer. 1998;78:621–624.
- Parker JE, Mufti GJ, Rasool F, et al. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood. 2000;96:3932–3938.
- Jilg S, Reidel V, Müller-Thomas C, et al. Blockade of BCL-2 proteins efficiently induces apoptosis in progenitor cells of high-risk myelodysplastic syndromes patients. Leukemia. 2016;30:112–123.
- Reidel V, Kauschinger J, Hauch RT, et al. Selective inhibition of BCL-2 is a promising target in patients with high-risk myelodysplastic syndromes and adverse mutational profile. Oncotarget. 2018;9:17270–17281.
- Bogenberger JM, Kornblau SM, Pierceall WE, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28:1657–1665.
- Jilg S, Hauch RT, Kauschinger J, et al. Venetoclax with azacitidine targets refractory MDS but spares healthy hematopoiesis at tailored dose. Exp Hematol Oncol. 2019;8:9.
- Garcia JS, Wei AH, Borate U, et al. Safety, efficacy, and patient-reported outcomes of venetoclax in combination with azacitidine for the treatment of patients with higher-risk myelodysplastic syndrome: a Phase 1b study. Blood. 2020;136:55–57.
- Komrokji RS, Al Ali NH, Sallman D, et al. Validation of International Working Group response criteria in higher-risk myelodysplastic syndromes: a report on behalf of the MDS clinical research consortium. Cancer Med. 2021;10:447–453.
- Zeidan AM, Pollyea DA, Garcia JS, et al. A phase 1b study evaluating the safety and efficacy of venetoclax as monotherapy or in combination with azacitidine for the treatment of relapsed/refractory myelodysplastic syndrome. Blood. 2019;134:565.
- Ball BJ, Famulare CA, Stein EM, et al. Venetoclax and hypomethylating agents (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020;4:2866–2870.
- Sekeres MA, Watts J, Radinoff A, et al. Randomized phase 2 trial of pevonedistat plus azacitidine versus azacitidine for higher-risk MDS/CMML or low-blast AML. Leukemia. 2021;35:2119–2124.
