ABSTRACT
Background
The QUAZAR AML-001 trial (NCT01757535) showed survival benefits with the maintenance treatment of oral azacitidine(CC-486) for acute myeloid leukemia(AML) in first complete remission. We conducted a cost-effectiveness analysis to explore the costs and benefits of oral azacitidine in AML.
Methods
We constructed a Markov model to evaluate the economic value of oral azacitidine. The time horizon was 10-years. The health utility scores and until prices of medical costs were acquired from previous studies and GoodRX. The transition probabilities were derived from the survival curves of the QUAZAR AML-001 study. Outcomes were measured in quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER).
Results
Compared with placebo, oral azacitidine improved 0.39 QALY, with an increasing cost of $458,928.66. The ICER of oral azacitidine is $1,176,740.15(P < 0.05). Deterministic sensitivity analysis showed that the price of oral azacitidine has a significant impact on ICERs (P < 0.05). Probability sensitivity analysis showed that the probability of cost-effectiveness for oral azacitidine is 0.
Conclusion
In the United States, oral azacitidine is unlikely to be cost-effective for AML patients at current prices.
Clinical trial registration
The trial is registered at ClinicalTrials.gov (CT.gov identifier: NCT01757535)
1. Introduction
Acute myeloid leukemia (AML) is a progressive hematological malignancy. It is the most common type of acute leukemia in adults. The median age at diagnosis was approximately 68 years [Citation1]. According to the American National Cancer Institute estimates, there were approximately 19,940 newly diagnosed AML patients and 11,180 related deaths in the United States in 2020 [Citation2]. The incidence rate of AML may also increase with an increase in the average life expectancy. With the combination of targeted therapy and chemotherapy, the rate of complete remission (CR) has greatly improved [Citation3–5]. High relapse rates remain the most important cause of treatment failure. Studies have found that the relapse rate is over 60% in high-risk AML patients, leading to a 5-year survival rate of 20%–25% [Citation5–9].
Allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT) is a potential cure for AML. But many AML patients are not suitable for allo-HSCT due to age, comorbidities, or lack of donors with matching human leukocyte antigen (HLA) [Citation8,Citation10]. Consolidation chemotherapy with cytarabine can improve overall survival (OS), but a high dose of cytarabine also causes severe treatment-associated toxicity [Citation11,Citation12].
Oral azacitidine (CC-486, onureg) is an oral hypomethylating agent, that can continuously bind DNA and RNA and carry out persistent epigenetic regulation. The primary mechanism of the drug is the hypomethylation of DNA and direct cytotoxicity to abnormal hematopoietic cells in the bone marrow [Citation13–15]. Recently, a phase 3 study (QUAZAR AML-001) was reported. This study randomly assigned patients unfit for allo-HSCT to oral azacitidine or placebo groups in their first complete remission after intensive chemotherapy, and found that maintenance therapy with oral azacitidine significantly improved relapse-free survival (RFS) and overall survival (OS) in AML patients. Compared to placebo, OS in the oral azacitidine group improved from 14.8 months to 24.7. RFS was also prolonged to 10.2 months. For most subgroups, including patients with poor cytogenetic risk at diagnosis, oral azacitidine tends to have favorable outcomes [Citation16].
Based on these promising results, the food and drug administration (FDA) of United States has approved the use of oral azacitidine for the maintenance treatment of adult patients with AML in their first complete remission [Citation17]. However, AML is already associated with a substantial economic burden, estimated in the US at $386,077 in treated patients and $79,382 in newly diagnosed patients in 2018 [Citation18]. The price of oral azacitidine is about $22,098.57 for 14 tablets. The acquisition costs of oral azacitidine may be difficult for patients and payers to bear, especially under the influence of the pandemic. Thus, we performed this cost-effective analysis to explore the economic value of oral azacitidine.
2. Methods
2.1. Patients and intervention
A cost-effectiveness model was used to simulate the treatment course of patients with AML in their first remission in the QUAZAR AML-001 trial [Citation16]. The baseline characteristics of the patients were the same as those enrolled in the QUAZAR AML-001 trial [Citation16]. The total number of participants in the clinical trial was 472, coming from 23 countries and regions, with a median age of 68 years. Most patients had de novo AML (91%) with an ECOG performance of 0–1 (92%). Patients classified as intermediate cytogenetic risk at diagnosis were 406 (86%). The main reasons for patients not receiving allo-HSCT were as follows: age (65%), comorbidities (22%) and lack of donors (15%). All patients were randomly assigned to the oral azacitidine maintenance therapy group and placebo group at a ratio of 1:1. Treatment in the two groups was as follows: (1) oral azacitidine group: oral azacitidine 300 mg administered once daily on day 1 through 14 of every repeated 28-day cycle. (2) placebo group: the same dose of placebo every two weeks. Baseline characteristics were similar between the two groups. The treatment plan above was given to patients until unacceptable toxicity and disease progression(>15% blasts in the bone marrow) [Citation16].
2.2. Model construction
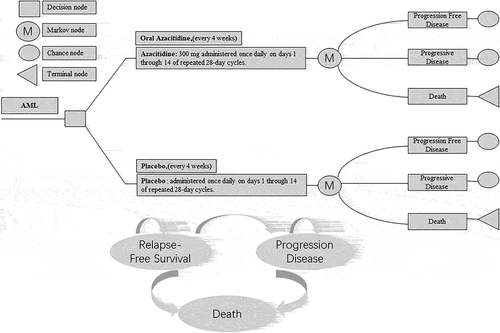
We established our decision analysis Markov model using TreeAge Pro 2021 software (TreeAge, Williamstown, MA). This model was used to calculate the ten-year costs and survival benefits of oral azacitidine maintenance therapy. The model consists of three mutually incompatible health states: progression free disease, progressive disease and death, as shown in .
Figure 1. Abbreviated decision tree and Markov model used to compare two strategies for treating AML patients at their first remission.
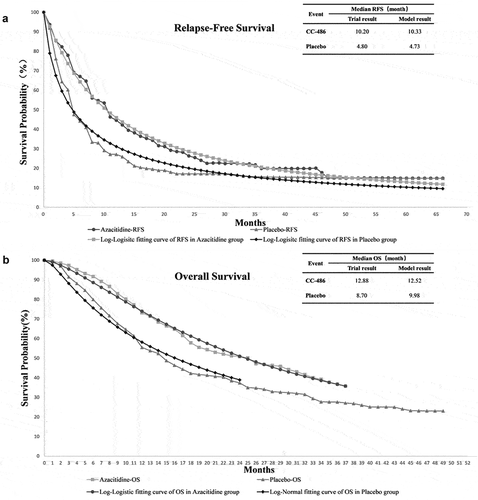
We used WebPlot-Digitizer software (version 4.2; https://apps.automeris. io/wpd/index.zh_CN.html) to extract the survival data from the published OS and PFS curves of the QUAZAR AML-001 trial [Citation16]. These survival data were then used to fit parametric survival models using the algorithm derived by Hoyle et al [Citation19]. The fitting degree of the Log-logistic survival model and the QUAZAR AML-001 trial is shown in . Based on the log-logistic model, the transition probabilities from RFS to PD and PD to death in each cycle were calculated using the formula used in the published cost-effective analysis in the Markov model [Citation20]. The US life tables were applied to obtain the mortality rate due to other causes for each age group [Citation21].
2.3. Cost and utility
All cost data are evaluated from the perspective of US payers. We only analyzed the direct medical cost, which mainly included the cost of drug acquisition, management of severe treatment-associated adverse events (AEs), drug administration, cost of subsequent treatment and cost of regular tests during the treatment course. We collected these costs from GoodRx and published literature [Citation22–25]. The detailed cost data are listed in . The post-relapse treatment costs were estimated as the weighted average cost of different treatment modalities of the subsequent-line therapy described in the QUAZAR AML-001 trial [Citation16].
Table 1. Key clinical and health preference data
According to the US consumer price index, costs associated with healthcare services were inflated to 2021 values [Citation31]. The discount rate was set to 3% per year [Citation23]. We searched previously published literature concerning the quality of life of AML patients in each state of health [Citation32,Citation33]. Utility values for DFS and recurrence were 0.80 and 0.67, respectively ().
2.4. Sensitive analysis
To avoid the uncertainty of the parameters applied in our study, we set ± 30% as a varied range of variables. We also used the gamma distribution for costs and beta distributions for health utility values. A tornado diagram was used to evaluate the uncertainty of all parameters in our study including treatment efficacy, utilities, and cost. Sensitivity analyses were conducted by 1,000 Monte Carlo simulations to calculate the probabilistic of which treatment method patients may choose under the willingness to pay(WTP). The WTP was set as $150,000, as in previous studies. The results of the probabilistic sensitivity analyses were expressed as cost-effectiveness acceptability curves.
3. Results
3.1. Base-case analysis
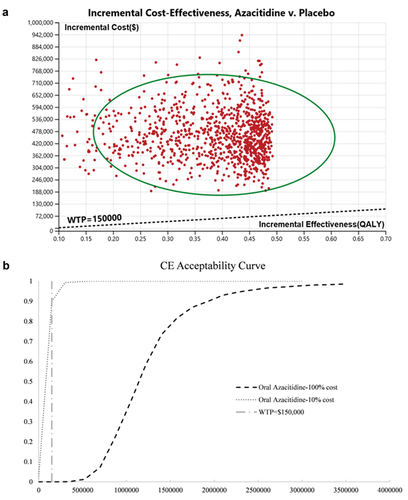
Our model showed that patients treated with oral azacitidine maintenance therapy were associated with an improvement of 0.39 quality adjusted life years (QALYs) compared with placebo (2.09 vs 1.7 QALYs, respectively). The overall life years (LYs) in azacitidine group also improved from 2.27 to 2.77. However, the lifetime overall health care costs in the oral azacitidine group were also higher than those in the placebo group ($566,129.37 vs $107,200.71, respectively). The incremental cost was $458,928.66. Therefore, the ICER for oral azacitidine maintenance therapy was $1,176,740.15 per QALY, which is above the willingness-to-pay (WTP) threshold of $150,000 per QALY ().
Table 2. Summary of cost and outcome results in the base-case analysis
3.2. Sensitivity analysis
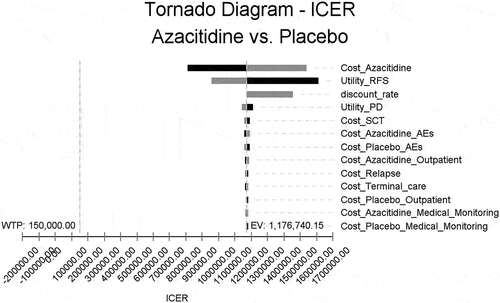
The results of the one-way sensitivity analyses are shown in . The cost of oral azacitidine has a significant influence on the incremental cost effectiveness ratio (ICER). The ICER range of oral azacitidine changed from $1,145,239.3 to 1,873,222.88. The other parameters include the discount rate, cost of the managing adverse event of oral azacitidine and placebo. All the ICERs remain above the WTP of $150,000 per QALY.
Figure 3. Tornado diagram of the incremental cost effectiveness ratio (ICER) of oral azacitidine for different model input parameters in the United States. RFS, relapse-free survival; PD, progression disease; SCT, Stem cell transplantation.
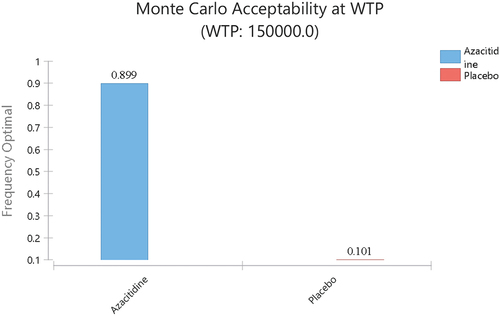
In our probability sensitive analysis, incremental cost effectiveness scatterplot and cost-effectiveness acceptability curves showed that, when oral azacitidine is sold at the current price, people could not afford the expense of the drug under the current WTP (). The Monte Carlos analysis shows that, when the price is cut to 10%, from $1578.47 per 300 mg to $157.85, most AML patients (89.9%) may consider oral azacitidine as maintenance therapy ().
Figure 4. Probabilistic sensitivity analysis for cost effectiveness of treatment strategies for AML patients at their first remission. Result of 1000 bootstraps was generated in the probabilistic sensitivity analysis. Each dot represents the lifetime discounted incremental cost and QALYs of one bootstrap sample. The dotted line indicates willingness-to-pay threshold of US$150000(A). Cost-effectiveness acceptable curves are showing the cost-effective probability of oral azacitidine at different prices. The dotted vertical lines represent the willingness to pay thresholds(B).
4. Discussion
In this study, we explored the cost effectiveness of oral azacitidine maintenance treatment in AML patients during their first remission using a Markov model. Our model showed that the incremental cost-effectiveness ratio of oral azacitidine is $1,176,740.15, which is not cost-effective under current pricing. The average sales price of oral azacitidine should reduce to $236.77 per tablet to lower the ICER to <$150k/QLAY.
The estimated incidence rate of AML is 4.3 in the United States per 100,000 population at risk [Citation2]. Approximately 50% of patients can achieve CR after induction treatment [Citation10].We assume that half of these patients could not be treated with high-dose consolidation chemotherapy and received oral azacitidine as maintenance therapy. Therefore, if the drug is widely applied at current prices, it would add about $1006 million per year to total health care costs, far beyond the affordability of medical expenses.
Azacitidine exerts its anti-tumor effects by inhibiting DNA methyltransferase [Citation34,Citation35]. However, azacitidine alone has only amodest effect in newly diagnosed AML patients, with an overall response rate about 20% [Citation3]. During induction chemotherapy, azacitidine is now used as a combination therapy with other target drugs to reduce tumor burden [Citation3,Citation12,Citation34,Citation36]. Combining azacitidine with venetoclax has been proven effective as an induction treatment in AML patients who are not fit for intensive chemotherapy [Citation12]. When AML patients who cannot be treated with high-dose chemotherapy achieved CR after induction therapy, azacitidine or decitabine is recommended as maintenance therapy by the National Comprehensive Cancer Network (NCCN) before the approval of oral azacitidine. In September 2020, the US FDA approved oral azacitidine in the maintenance therapy for AML at their first remission based on the QUAZAR AML-001 trial [Citation16,Citation17].
Previous cost-effective analyses concerning azacitidine in AML were mainly intravenous or subcutaneous azacitidine [Citation32,Citation37]. Levy and Coyle’ s cost-effectiveness analysis evaluated the economic benefit of azacitidine in AML patients whose blast cells in the bone marrow are below 30% or over 30% in Canada, respectively. Their studies were based on a third-party payer perspective. They found that azacitidine is only cost-effective when patients’ blast cells are below 30% [Citation32,Citation37]. A previous cost-effective analysis found that the combination of venetoclax and azacitidine is not likely to be cost-effective as an induction therapy for newly diagnosed AML patients under current prices [Citation38].
Our research found that, like other newly approved anti-cancer drugs, such as venetoclax and daratumumab, oral azacitidine is unlikely to be cost-effective at the current price [Citation38,Citation39]. This can be attributed to the limited survival benefit and high prices of these drugs for advanced or progressive cancer patients. Although the administration issued the American Patients First policy to cut drug prices in May 2018 [Citation40], due to lack of transparency, the drug price in America is still high [Citation41].Under the influence of COVID-19, the world economy has shrunk and financial pressure on health has increased significantly. Thus, a reasonable price setting or charity drug donation scheme is needed to access innovative treatments.
Our research has the following advantages. First, the data in our cost-effectiveness analysis were based on a large-size, phase III, double-blind trial that directly compared azacitidine maintenance treatment in AML patients at their first remission [Citation16]. The estimated RFS and OS curves in our model closely fit the original results reported in that trial. Second, we searched the latest medical cost from published medical care costs analyzed using the latest data from Centers for Medicare & Medicaid Services(CMS), the largest public payer in the US.
There are also some limitations in our study. First, there are inherent limitations in cost-effectiveness models, because the availability of data used to populate the model. Although our efficacy inputs were based on a phase 3 randomized trial, there is uncertainty for costs in real world. Second, we only calculated directly medical costs. Indirect costs, such as losses due to hospital visit or transportation expenses were not included. Third, although the proportion of patients receiving different treatment modalities after recurrence was mentioned in the research, the detailed treatment regimens were not included. Therefore, we used the cost of post-progressed treatment from the published literature to calculate the subsequent treatment costs.
5. Conclusion
Oral azacitidine is unlikely to be cost-effective for AML patients at their first remission after intensive chemotherapy from the perspective of the United States’ payer at its current price. New pricing, identification of appropriate patients, charity drug donation schemes, and new insurance strategies are needed to support cost-effective treatment measures.
Author contributions
T Niu put forward the idea of this study. J Zhu analyzed and interpreted the data generated in the clinic trail. J Wang helped to search the cost data. J Zhu and Q Wu performed the analysis and contributed equally in writing the manuscript. All authors read and approved the final manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclosure statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Shallis RM, Wang R, Davidoff A, et al. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019 Jul;36:70–87. doi:https://doi.org/10.1016/j.blre.2019.04.005.
- Institute NC. Cancer stat facts: acute myeloid leukemia (AML) 2020 [Cited 2021 Jun 3.]. Available from: https://seer.cancer.gov/statfacts/html/amyl.html
- DiNardo CD, Schuh AC, Stein EM, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukemia (AG221-AML-005): a single-arm, phase 1b, randomized phase 2 trial. Lancet Oncol. 2021 Nov;22(11):1597–1608.
- Cortes JE, Lin TL, Uy GL, et al. Quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) analysis of CPX-351 versus 7 + 3 in older adults with newly diagnosed high-risk/secondary AML. J Hematol Oncol. 2021 Jul 13 14(1):110.
- Huls G, Chitu DA, Havelange V, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019 Mar 28 133(13):1457–1464.
- Medeiros BC, Chan SM, Daver NG, et al. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am J Hematol. 2019 Jul;94(7):803–811.
- Schlenk RF, Frech P, Weber D, et al. Impact of pretreatment characteristics and salvage strategy on outcome in patients with relapsed acute myeloid leukemia. Leukemia. 2017 May;31(5):1217–1220.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017 Jan 26 129(4):424–447.
- DiNardo CD, Lachowiez CA, Takahashi K, et al. venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed and relapsed or refractory acute myeloid leukemia. J Clin Oncol. 2021 Sep 1 39(25):2768–2778.
- Molica M, Breccia M, Foa R, et al. Maintenance therapy in AML: the past, the present and the future. Am J Hematol. 2019 Nov;94(11):1254–1265.
- Blum W, Sanford BL, Klisovic R, et al. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 cancer and leukemia group B study (CALGB 10503). Leukemia. 2017 Jan;31(1):34–39.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020 Aug 13 383(7):617–629.
- Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011 Jun 20 29(18):2521–2527.
- Laille E, Shi T, Garcia-Manero G, et al. Pharmacokinetics and pharmacodynamics with extended dosing of cc-486 in patients with hematologic malignancies. PLoS One. 2015;10(8):e0135520.
- Savona MR, Kolibaba K, Conkling P, et al. Extended dosing with CC-486 (oral azacitidine) in patients with myeloid malignancies. Am J Hematol. 2018 Oct;93(10):1199–1206.
- Wei AH, Döhner H, Pocock C, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020 Dec 24 383(26):2526–2537.
- FDA. FDA approves Onureg (azacitidine tablets) for acute myeloid leukemia Sep 2020 [Cited 2021 Jun 2]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-onureg-azacitidine-tablets-acute-myeloid-leukemia
- Stein EM, Bonifacio G, Latremouille-Viau D, et al. Treatment patterns, healthcare resource utilization, and costs in patients with acute myeloid leukemia in commercially insured and medicare populations. J Med Econ. 2018 Jun;21(6):556–563.
- Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011 Oct 10; 11:139
- Diaby V, Adunlin G, Montero AJ. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014 Feb;32(2):101–108.
- Arias E, Bastian B, Xu J, et al. U.S. State Life Tables, 2018. Natl Vital Stat Rep. 2021 Mar;70(1):1–18.
- Tallman M, Lo-Coco F, Barnes G, et al. Cost-effectiveness analysis of treating acute promyelocytic leukemia patients with arsenic trioxide and retinoic acid in the United States. Clin Lymphoma Myeloma Leuk. 2015 Dec;15(12):771–777.
- Stein E, Xie J, Duchesneau E, et al. cost effectiveness of midostaurin in the treatment of newly diagnosed flt3-mutated acute myeloid leukemia in the United States. PharmacoEconomics. 2019 Feb;37(2):239–253.
- Stein EM, Yang M, Guerin A, et al. Assessing utility values for treatment-related health states of acute myeloid leukemia in the United States. Health Qual Life Outcomes. 2018 Sep 21 16(1):193.
- Bell JA, Galaznik A, Farrelly E, et al. Economic burden of elderly patients with acute myeloid leukemia treated in routine clinical care in the United States. Leuk Res. 2018 Aug;71:27–33.
- Drugs C [Accessed June 2,2021].Available from: https://www.drugs.com/price-guide/onureg.
- Wong W, Yim Y, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PloS one. 2018;13(4):e0196007.
- GoodRx. [Cited 2021 Jun]. Available from: www.goodrx.com.
- Hill G, Barron R, Fust K, et al. Primary vs secondary prophylaxis with pegfilgrastim for the reduction of febrile neutropenia risk in patients receiving chemotherapy for non-Hodgkin’s lymphoma: cost-effectiveness analyses. Journal of Medical Economics. 2014;17(1):32–42.
- Kornblith AB, Herndon JE 2nd, Silverman LR, et al. Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized phase III trial: a cancer and leukemia group B study. J Clin Oncol. 2002 May 15 20(10):2441–2452.
- Labor. UDo. Calculators. 2018 [ Accessed June 2,2021]. Available from: http://www.bls.gov/data/#calculators
- Levy AR, Zou D, Risebrough N, et al. Cost-effectiveness in Canada of azacitidine for the treatment of higher-risk myelodysplastic syndromes. Curr Oncol. 2014 Feb;21(1):e29–40.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. Jama. 2016 Sep 13 316(10):1093–1103.
- Sato T, Issa JJ, Kropf P. DNA hypomethylating drugs in cancer therapy. Cold Spring Harb Perspect Med. 2017 May 1; 7(5):a026948.
- Zhang W, Xu J. DNA methyltransferases and their roles in tumorigenesis. Biomark Res. 2017;5:1.
- Cojocari D, Smith BN, Purkal JJ, et al. Pevonedistat and azacitidine upregulate NOXA (PMAIP1) to increase sensitivity to venetoclax in preclinical models of acute myeloid leukemia. Haematologica. 2021 Apr 15;https://doi.org/10.3324/haematol.2020.272609
- Coyle D, Villeneuve PJA. Economic Evaluation Of Azacitidine In Elderly Patients With Acute Myeloid Leukemia With High Blast Counts. Pharmacoecon Open. 2020 Jun;4(2):297–305.
- Patel KK, Zeidan AM, Shallis RM, et al. Cost-effectiveness of azacitidine and venetoclax in unfit patients with previously untreated acute myeloid leukemia. Blood Adv. 2021 Feb 23 5(4):994–1002.
- Cao Y, Zhao L, Zhang T, et al. Cost-effectiveness analysis of adding daratumumab to bortezomib, melphalan, and prednisone for untreated multiple myeloma. Front Pharmacol. 2021;12:608685.
- Burki TK A new strategy to reduce US drug prices. Lancet Oncol. 2018 May 17;18(S1470–2045):30374–7.
- Prasad V, Jesús K D, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017 Jun;14(6):381–390.