ABSTRACT
Introduction
Survival outcomes of children with relapsed/refractory (r/r) acute leukemia remain poor. Novel expensive treatments have been developed to improve their outcomes, yet, limited evidence exists about cost-effectiveness of alternative treatment strategies.
Areas covered
A systematic review was conducted to summarize health-economic evidence about costs/cost-effectiveness of treating r/r acute leukemia in children/young adults. We searched Medline, Embase, and Cochrane databases until August 13th, 2021. Eligible articles included peer-reviewed original studies addressing r/r pediatric/young-adult acute lymphoblastic leukemia (ALL), and acute myeloid leukemia (AML). Quality assessment was conducted using Consolidated Health Economics Evaluation Reporting Standards (CHEERS) checklist.
Expert Opinion
The majority of papers focused on CAR-T cell therapy, which is still a novel treatment for r/r ALL, and was found to be cost-effective, yet, there remain concerns over its long-term effectiveness, affordability, and equity in access. The next best treatment option is Blinatumomab, followed by Clofarabine therapy, whereas FLA-IDA salvage chemotherapy provides least value for money. The quality of evidence is moderate to high, with limited generalizability of findings due to high variability in outcomes obtained from modeling studies. Limited studies evaluated r/r AML. We provide recommendations to deliver cost-effective treatments in real-world contexts, with implications for healthcare policy and practice.
1. Introduction
Leukemia is the most common cancer in children accounting for approximately 35% of all childhood cancers [Citation1]. Around 75% of leukemia among children are acute lymphoblastic leukemia (ALL), and the remaining cases are mostly acute myeloid leukemia (AML) [Citation1]. The 5-year overall survival in children with newly diagnosed ALL has improved dramatically reaching almost 90% [Citation2], yet, 15–20% of patients relapse, and around 2% of patients do not respond to treatment (refractory to induction chemotherapy) [Citation2–4]. Relapsed ALL is considered the fourth most common childhood malignancy with poor survival post-relapse (<40%), which requires intensive treatment including salvage chemotherapy, stem cell transplant (SCT), and/or other newer drugs [Citation4–6]. Over the past decade, a number of novel but expensive drugs (such as immunotherapeutic agents) have been developed, changing the treatment landscape for children with relapsed ALL [Citation7,Citation8]. Therefore, current treatment strategies incur higher costs, with greater hospital admissions and longer in-patient stays [Citation9].
It is estimated that 30% of children with AML will relapse [Citation10], and their outcomes remain poor despite intensive therapy, with overall survival at 3 to 5 years of about 40% [Citation11]. Similar to ALL, costs of treating relapsed AML are very high due to expensive intensive therapy, where inpatient hospitalization is the largest cost driver [Citation12].
Treatment of relapsed acute leukemia in children is highly desirable, yet the outcomes are not always rewarding and can impose great economic and financial burden on health systems. Therefore, there is a need to determine which treatment strategies are the most cost-effective and represent the greatest value for money, especially in resource-limited setting [Citation13,Citation14]. Although a previous systematic review by Russell et al. (2013) summarized the evidence about economic evaluation of pediatric cancer treatment, it did not address cost-effectiveness of treating relapsed leukemia [Citation14]. Besides, there were no former systematic literature review studies specifically focused on costs/cost-effectiveness of refractory/relapsed ALL/AML in children/young adults, creating a gap in this area.
The objective of this systematic review is to establish the health-economic evidence base for costs and cost-effectiveness of treatment for relapsed/refractory (r/r) acute leukemia in children and young adults from the health system/payer perspective, through summarizing the published evidence, and comparing costs and effects of the alternative treatment interventions.
2. Methods
We followed the guidelines by the Center for Reviews and Dissemination (CRD) for conducting systematic reviews of economic evaluation [Citation15] and the Campbell and Cochrane Economic Methods Group (CCEMG) guidance for incorporating economics evidence [Citation13]. This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [Citation16] shown in Supplementary methods (S1).
2.1. Search strategy
A comprehensive search was conducted from inception to 12 August 2021, in the following databases: Medline (OvidSP) [1946-present] Embase (OvidSP) [1974-present], Cochrane Database Reviews & Cochrane Central Register of Controlled Trials (Cochrane Library, Wiley) [Issue 8 of 12, August 2021], Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS-EED) & Health Technology Assessment Database (HTA) (Center for Reviews & Dissemination https://www.crd.york.ac.uk/CRDWeb/) [inception to 31/03/2015], Science Citation Index & Social Science Citation Index (Web of Science Core Collection) [1900-present] and Cost-Effectiveness Analysis Registry (http://healtheconomics.tuftsmedicalcenter.org/cear2n/search/search.aspx). Detailed search strategy is presented in Supplementary methods (S2). The search strategy was developed by a medical librarian (NR), and consisted of title/abstract keywords and subject headings to describe the key concepts of ‘children,’ ‘leukaemia,’ and ‘relapse.’ The search was restricted to English language; no publication limits were applied. We also screened reference list of included articles (snowball search), and duplicate articles were removed in Endnote 20. Full electronic searches are shown in Supplementary methods (S3).
2.2. Eligibility criteria and study selection
We included studies with the following inclusion criteria: (1) relapsed acute leukemia in children and young adults (up to 25 years old) (2) analysis of the cost, cost-effectiveness analysis (CEA), cost-utility (CUA), or cost-benefit of any interventions to treat relapsed/refractory (r/r) acute leukemia, including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML); (3) measurement of economic costs, cost per life year gained (LYG) or quality-adjusted life year (QALY) gained, cost per life saved, cost per Disability-Adjusted Life Year (DALY) averted, incremental cost-effectiveness ratio (ICER), or any other measures of economic evaluations without restrictions.
We excluded studies that only reported clinical effectiveness in terms of survival outcomes, quality of life (QOL), patient-reported outcomes, and utilities without cost analysis. The following study types were also excluded: qualitative studies, conference abstracts, reviews, editorials, commentaries, or methodological articles. Bibliographies of review articles were searched for including relevant studies.
One author (RS) screened the initial search results for potential inclusion based on title and abstract. Then, two authors (RS and NSB) conducted the full-text review of the selected articles based on the above-mentioned inclusion/exclusion criteria. Authors with extensive clinical background in pediatric oncology (AE, IS, SM) decided on the eligibility criteria to make sure the included studies were clinically relevant. Disagreements between the authors were resolved by discussion and consensus in the presence of senior authors (CH, AE, JO).
2.3. Data extraction
Data extraction was performed on a pilot-tested standardized form on Microsoft Excel by two authors (RS and NSB) and reviewed by two other authors (IS and SM). The form was structured based on the format and guidelines used to summarize findings of economic evaluations studies, including the NHS Economic Evaluation Database (NHS EED) [Citation17], the CCEMG [Citation13], the ‘Consolidated Health Economics Evaluation Reporting Standards (CHEERS)’ statement [Citation18], and data items included in published studies [Citation14,Citation19]. Data extraction form is included in Supplementary methods (S4). We focused our analysis on the healthcare/payer perspective solely for purposes of comparability and consistency, to compare costs/cost-effectiveness of the available treatment options across different health care/payer systems in different countries and contexts. Whereas costs from the societal perspective were not included as they provide greater source of variation based on community preferences and varying definitions of cost of lost productivity across different countries and contexts.
2.4. Quality assessment
Two authors reviewed and appraised the methodological quality of the included studies (RS and NSB), using the CHEERS checklist [Citation18]. The CHEERS checklist (Supplementary methods (S5)) evaluates 24 key items recommended by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) [Citation20] to be reported in economic evaluations. To validate quality assessment, the process was independently checked for completeness and accuracy by two other reviewers (IS and SM). Discordance in quality assessment was resolved by discussion with senior author (CH).
2.5. Data synthesis and analysis
We summarized the main characteristics and results of the included studies by tabulating the characteristics and key findings of the included studies. This was supported by a narrative summary that compared the main results among the included studies. We reported the original costs and reference year of treatment interventions as presented in the included articles. Then, we converted all cost outcomes to USD (2019) using the World Bank exchange rates (for currencies in different countries) [Citation21], then we inflated using US inflation rates to the reference year (2019) based on the World Bank GDP deflator [Citation22] We reported median (IQR), mean (±SD) values, and ranges of costs, LY gained, and QALY gained. We calculated the incremental costs and ICERs between the different treatment strategies in a standardized currency and reference year (USD 2019), to allow for comparability of results among the included studies. We also calculated the median values and range of the CHEERS checklist scores of the included studies.
3. Results
3.1. Search results and characteristics of included studies
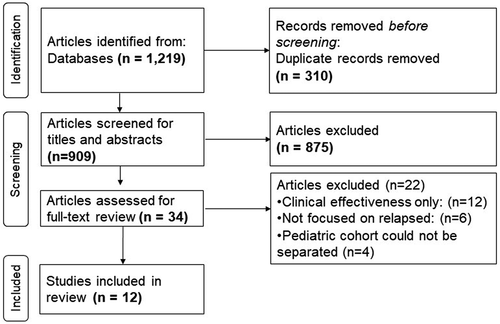
Our search identified 1,219 articles, which yielded 909 articles after removing duplicates. Thirty-four articles were eligible for full-text review, of which 12 met full inclusion criteria [Citation23–34]. shows a flow diagram of included/excluded studies. The characteristics of the 12 included articles are summarized in . All articles were conducted in high-income countries (HICs), and none were reported from resource-limited settings in low- and middle-income countries (LMICs). Ten studies [Citation25–34] (83.3%) addressed r/r pediatric ALL, and only two studies [Citation23,Citation24] evaluated costs/cost-effectiveness of r/r ALL/AML. All studies reported CEA or CUA except for the study by Yang et al. (2020) [Citation31] which only analyzed cost outcomes, and Schulthess et al. (2021) [Citation32] which reported cost and clinical outcomes separately (). Only two studies addressed societal costs (data not extracted) [Citation27,Citation29], in addition to the healthcare/payer costs.
Table 1. Characteristics of included studies
The majority of studies (10 out of 12) [Citation24–31,Citation33,Citation34] used decision-analytic models, while two [Citation23,Citation32] analyzed real-world data using cohort/observational study designs. For r/r ALL/AML, Lin et al (2010) [Citation23] compared allogeneic peripheral blood stem cell transplant (PBSCT) versus BMT, and Maser et al. (2020) [Citation24] compared levofloxacin prophylaxis versus no prophylaxis. Ten studies [Citation25–34] evaluated CAR-T cell therapy (tisagenlecleucel, tisa-cel) for r/r ALL as the main intervention, compared to any of the other comparators: Blinatumomab (Blina); Clofarabine therapy [Clofarabine monotherapy (Clo–M)] or Clofarabine combination therapy (Clo–C; Clofarabine/cyclophosphamide/etoposide); FLA-IDA salvage chemotherapy (Fludarabine/cytarabine/idarubicin) to achieve complete remission (CR), then moving forward to bone marrow transplant (BMT) or hematopoietic stem-cell transplantation (HSCT) as a general approach (). Six studies evaluated cost-effectiveness of CAR-T cell therapy (tisagenlecleucel) in pediatrics and young adults (up to 25 years), as it was approved by the FDA and indicated for this age-group [Citation26,Citation28,Citation29,Citation32–34].
3.2. Quality of included studies
of study characteristics shows CHEERS checklist score for each of the included studies, with median CHEERS checklist score of 19.5 (Range: 15.5–21.5) out of 24. Overall quality of included studies is moderate to high (detailed scoring in Supplementary Table (S1)). Three studies were of moderate quality with CHEERS score below the median as follows: 15.5 for Yang et al. (2020) [Citation31], 17.5 for Schulthess et al. (2021) [Citation32], and 19 for Thielen et al. (2020) [Citation29], while the remaining studies were of high quality. The most common methodological errors included: not describing population subgroups or characterizing heterogenicity to explain variations in costs and outcomes; not reporting method for adjusting unit costs; and not explaining why they chose a specific decision-analytic model. Besides, in some studies, the authors did not report potential conflict of interest (despite the study being funded) nor the role of the funder. Detailed CHEERS checklist scoring for each study is shown in Supplementary Table (S1).
3.3. Approach to cost-effectiveness analyses
For studies that developed decision-analytic models, economic analyses were based on a lifetime horizon, while observational studies reported follow-up periods (). Discounting of costs and benefits was done at 3% discount rate in five studies [Citation25–28,Citation32] at the base-case scenario, while five other studies [Citation24,Citation29,Citation30,Citation33,Citation34] used different discount rates, and no discounting was reported in two studies [Citation23,Citation31] (). We summarized the key model input parameters in Supplementary Table (S2), including sources of clinical evidence (cited in Supplementary references), utilities (quality of life), and listed drug(s) price. Evidence for clinical effects and utility data were sourced from a combination of clinical trials and published literature as outlined in Supplementary Table (S2). List drugs’ prices of the main and comparator treatment interventions varied among studies conducted in different countries. Cost categories for each treatment intervention were also summarized for each study, which contributed to different cost outputs. The majority of studies (n = 11, 91.6%) conducted sensitivity (uncertainty) analysis as either deterministic and/or probabilistic analyses, or scenario analysis (Supplementary Table (S3)).
Table 2. Costs, clinical outcomes, and cost-effectiveness estimates to treat relapsed/refractory acute leukemia
3.4. Costs, benefits, and cost-effectiveness estimates
summarizes key information about costs (in actual currency as reported in each study), clinical effects, and cost-effectiveness of alternative treatment strategies; main intervention versus comparator(s). Willingness-to-pay (WTP) thresholds varied among studies. Lin et al (2010) [Citation23] reported that in r/r acute leukemia (ALL/AML), the probability of treatment success was higher (23.5%) in the BMT group compared to 18.8% in the PBSCT group, which was cost-effective in standard-risk patients. Levofloxacin prophylaxis showed cost savings of $5,059 per QALY gained in children receiving intensive chemotherapy, as reported by Maser et al. (2020) [Citation24], compared to not administering levofloxacin. CAR-T cell therapy was found to be more cost-effective compared to the other alternative treatment strategies in nine studies () [Citation25–31,Citation33,Citation34]. Whereas, Schulthess et al. (2021) [Citation32] reported lower 3-year relapse-free survival (RFS) for CAR-T cell therapy (46%, 95% CI: 8–79%) at a higher cost, compared to 68% (95% CI: 46–83%) with hematopoietic cell transplant (HCT), due to higher tumor burden in CAR-T cell cohort.
summarizes outcomes from nine studies, reporting standardized costs (adjusted to USD 2019), QALYs gained, incremental costs and QALYs gained, and ICERs for CAR–T cell therapy (tisa-cel) compared to alternative treatment interventions. QALYs gained ranged between 5.5 and 16.76 for CAR-T cell therapy, 2.07–3.57 for Blina, 1.7–8.58 for Clo-C, 0.49–3.12 for Clo-M, 0.39–0.46 for FLA-IDA, and was 3.46 for chemotherapy/HSCT (). Blinatumomab achieved higher QALYs gained (better clinical benefit) compared to Clo-M and Clo-C therapies [Citation25,Citation29,Citation33,Citation34]. Whereas, FLA-IDA showed the least favorable clinical outcome (lowest QALYs gained), compared to the other interventions as reported by Santasusana et al. (2020) [Citation28] and Moradi-Lakeh et al. (2021) [Citation34]. shows the QALYs gained by the alternative treatment options as reported in each study. ICER for CAR-T cell therapy relative to other comparators ranged from $18,753 – $157,026/QALY gained, with varying outcomes on sensitivity analysis shown in Supplementary Table (S3).
Figure 2. QALYs gained for CAR-T cell therapy vs. alternative interventions to treat relapsed/refractory pediatric ALL.
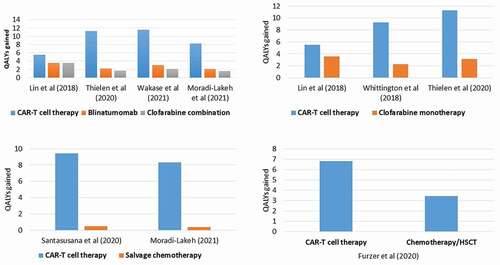
Table 3. Costs (USD 2019) and QALYs gained of CAR-T cell therapy and comparator(s) to treat relapsed/refractory ALL
The estimated costs of CAR-T cell therapy included all cost components of treatment and not just the listing price of tisagenlecleucel, calculated over a life-time horizon. Median cost (USD 2019) for CAR-T cell therapy in the reported studies was $561,075 (IQR: $464,335–$612,779), and mean cost was $577,631 (SD: ± $180,000), where highest cost estimates were reported by Sarkar et al. (2019) [Citation27] (, Supplementary Figure 1). The main cost driver was the drug list price, and other top three cost drivers of ‘additional cost’ included adverse events (AE) management (51.6%), inpatient and ICU admissions not attributed to AE (42.1%), and laboratory tests and procedures (3.8%) (Supplementary Table (S2)). We noted that studies conducted from the US health system/payer perspective had higher CAR-T cell therapy costs (median $612,779; IQR: $596,748–$694,644) (mean: $700,647 SD ±$177,357) compared to studies conducted in other countries (median: $458,121; IQR: $428,841–$482,975) (mean $454,616; SD ±60,103) (Supplementary Figure 2). Clinical benefit (LYG and QALYs gained) from CAR-T cell therapy varied among studies as shown in Supplementary Figure 3.
4. Discussion
Our findings summarize the existing evidence on the costs and cost-effectiveness of treatment interventions for relapsed/refractory acute leukemia (ALL and AML) in children/young adults. To our knowledge, this systematic review provides the most comprehensive and updated results about the costs, clinical benefits, and cost-effectiveness of the alternative treatment options for r/r acute leukemia in children, with objective quality appraisal of the generated evidence. The majority of the included studies (10 out 12) evaluated costs/cost-effectiveness of CAR-T cell therapy compared to other treatment strategies for r/r ALL, whereas only two studies addressed r/r acute leukemia (ALL/AML) considering different approaches.
The majority of the papers focused on CAR-T cell therapy, which is still a novel treatment for r/r ALL. The high upfront cost of CAR-T cell therapy (drug cost only is 475,000 USD) raises important consideration about the cost-effectiveness and value for money on the longer term for this expensive treatment option. Therefore, there was a greater need to conduct cost-effectiveness analysis for CAR-T cell therapy to justify its high-cost relative to the health benefits gained during the drug approval process. However, it is notable that although earlier treatments were used to treat r/r ALL, very few publications analyzed their costs and cost-effectiveness creating a significant evidence gap. There is sufficient evidence to suggest that CAR-T cell therapy (tisagenlecleucel) may provide survival gains and is a cost-effective strategy to treat r/r ALL in children compared with other currently available therapies. Nevertheless, there remain serious concerns over the uncertainty of its long-term effectiveness [Citation23]. At the most optimistic scenario (40% 5-year RFS), tisagenlecleucel would result in significant survival gains (12.1 life-years) with good economic value (<$100,000/QALY), however, with lower long-term effects (20–0% 5-year RFS), its economic value is reduced and may not meet the accepted cost-effectiveness thresholds [Citation23]. Other concerns include affordability of the drug price, equity in access for children residing in LMICs [Citation35–37], and the serious side effects which occur in majority of patients [Citation38]. Outcomes-based pricing could be a good option, where reimbursement depends on achieving and maintaining favorable clinical outcomes (initial remission) [Citation23].
The best parameter for health economic analysis is costs (expressed in dollars–USD in a standardized reference year) per each QALY gained, as this parameter takes into consideration the costs of treatment in a standardized currency (USD 2019), and QALY gained as a composite outcome measure taking into account survival rate and utility scores (quality of life) for each treatment option. CAR-T cell therapy showed the greatest cost/QALYs gained compared to the other alternatives. The next best treatment option was Blinatumomab which showed the greatest QALYs gained relative to cost, followed by Clo–C, and Clo–M regimens (at lower/comparable costs to Blinatumomab) [Citation25,Citation29,Citation34], whereas FLA-IDA salvage chemotherapy showed the least favorable clinical outcomes at comparable costs [Citation34]. There is poor evidence about the cost-effectiveness of treating r/r pediatric AML, due to limited published articles. Lin et al. (2010) [Citation23] conducted comparative economic evaluation of allogeneic PBSCT versus BMT in r/r ALL/AML in children and found that BMT was more favorable for standard risk patients. Maser et al. (2020) [Citation24] evaluated cost-effectiveness of prophylaxis with Levofloxacin antibiotic for relapse ALL and AML, and the reported findings showed that it was cost-saving in children receiving intensive chemotherapy. Quality appraisal using the CHEERS checklist showed moderate-to-high quality of the generated evidence, as the included modeling studies followed good economic evaluation practices (good internal validity). Whereas, the external validity is poor, due to limited confidence in generalizability and applicability of the findings as the majority of the included studies (10 out of 12) were modeled studies, which utilized clinical trial data from limited sample numbers. This combined with major variations in other input parameters across the studies, led to high levels of uncertainty in the outcomes.
4.1. Sources of variability and addressing uncertainty
Despite consistency in the main findings that CAR-T cell therapy was more cost-effective than the other comparators to treat r/r ALL in children, there was a large variation among the included studies in the estimated costs, clinical outcomes, and ICERs of tisagenlecleucel and the alternative treatment options. Variation in costs is likely common as they vary across countries due to differences in list prices of drugs, hospital costs, and varying contexts and practices [Citation39]. This could explain the higher costs of CAR-T cell therapy in studies conducted from the US health system perspective, compared to studies conducted in other countries (Canada, Japan, Netherlands, Spain, Switzerland), where the list price of tisagenlecleucel (Novartis’s Kymriah) in the US ($475,000) is higher than in the other countries. Other potential reasons for variation in costs among studies include: discount rate of costs, costing perspective and context, time horizon, and the included cost categories.
Heterogeneity of findings among studies can also be contributed to difference in key model inputs and parameters, different types of decision-analytic models used with varying assumptions, and inconsistency in the sources of clinical evidence (survival outcomes and utilities). We summarized these key model inputs in Supplementary Table (S2) to show how the variability in model inputs lead to variation in model outputs. Besides, findings from modeled studies differed from those reported by pragmatic studies using real-world data [Citation23,Citation32].
Uncertainty of results was addressed by most of the modeled studies by conducting deterministic and/or probabilistic sensitivity analyses or scenario analysis. Model results remained robust to alternative model assumptions and inputs, where CAR-T cell therapy remained more cost-effective than the comparators, in studies reported by Whittington et al. (2018) [Citation26], Santasusana et al. (2020) [Citation28], Furzer et al. (2020) [Citation30], Wakase et al. (2021) [Citation33], and Moradi-Lakeh et al. (2021) [Citation34]. Whereas, CAR-T cell therapy was no longer cost-effective in sensitivity analysis for the study conducted by Sarkar et al. (2019) when the 1-year survival was assumed to be 57.8% (instead of 76% in the base-case model), if the complete remission rate of CAR-T recipients decreased from 81% to 56.2%, or if the health utility of disease-free survivors decreased from 0.94 to 0.66 [Citation27]. Also, Thielen et al. (2020) [Citation29] reported that tisa-cel was less cost-effective with a shorter time-horizon (20 years versus lifetime horizon). The most influential input parameters that most affected the results included: discount rate for costs and benefits (0–5%; 3% base-case), cost of tis-cel, starting age (1–25 years; 12 years base-case), time horizon (20 years; vs lifetime in the base-case), and different overall survival parametric functions [Citation27–29].
4.2. Comparison with published literature
To our knowledge, this is the first systematic review that summarizes the health economic evidence about costs and cost-effectiveness of treatment with a focus on r/r acute leukemia in children/young adults, contrary to other reviews in the literature, which had different scopes and perspectives. Other published systematic reviews addressed economic evaluation of CAR-T cell therapy but were not focused solely on relapsed pediatric acute leukemia. A systematic review by Gye et al. (2022) [Citation39] conducted health technology assessments (HTA) of CAR T-cell therapies in the young compared with older patients, summarizing the evidence generated by HTA agencies (not peer-reviewed original studies) [Citation39]. It included 14 HTA evaluations; five for pediatric ALL evaluating tisagenlecleucel, and nine for adult diffuse large B-cell lymphoma (DLBCL) evaluating axicabtagene ciloleucel [Citation39]. The authors also identified sources of variability in outcomes similar to those reported by our systematic review findings. Another systematic review performed by Petrou et al. (2019) [Citation40] assessed the economic evaluations of tisagenlecleucel and axicabtagene in ALL and large B cell lymphoma [Citation40]. This review only evaluated three peer-reviewed full-text articles and three abstracts about r/r ALL, and included expert opinion which suggested that these products demonstrate a potentially favorable cost-effectiveness ratio, yet, their budget impact is very high and more affirmative clinical data are imperative in order to mitigate uncertainty [Citation40].
Another review article discussed the health economic aspects CAR T-cell therapies for hematological cancers was conducted by Heine et al. (2020) [Citation41], which conducted a review of list prices, HTA reports, budget impact analysis dossiers, and published cost-effectiveness analyses. It also forecasted the 10-year health expenditures on CAR T-cells for several hematological cancers in selected European Union (EU) countries [Citation41]. Heine et al. (2020) only evaluated cost-effectiveness of tisagenlecleucel in six published articles [Citation41]. None of the above-mentioned systematic reviews focused on the other alternative treatment options for relapsed r/r pediatric ALL, to allow for comparability of results. We also found a conference abstract by Pechlivanoglou et al. (2021) that compared two alternative treatments for r/r ALL in children, apart from CAR-T cell therapy [Citation42]. Pechlivanoglou et al. (2021) reported that Blinatumomab was cost-effective compared to standard intensive chemotherapy (followed by SCT) for high-risk relapse ALL (relapse within 18 months from diagnosis) [Citation42]. There were no identified systematic reviews in the literature addressing cost-effectiveness of r/r pediatric AML treatments, identifying a substantial gap in knowledge in this area. A systematic review of clinical effectiveness only exists by Hoffman et al. (2021) [Citation43], which evaluated clinical outcomes of relapsed AML patients, without reflecting on costs/cost-effectiveness of treatment [Citation43]. Although a novel targeted therapy (Gemtuzumab ozogamicin) was approved in 2017 in the US to treat patients ≥ 2 years of age with r/r CD33+ AML, its budget impact analysis was mainly evaluated in adults and limited data exist for r/r pediatric CD33+ AML patients [Citation44].
4.3. Limitations
Despite the valuable evidence generated from this systematic review, some limitations exist. First, the limited number of published original studies that compared the costs and cost-effectiveness of the available treatment strategies for r/r pediatric ALL, and the substantial lack of published literature for r/r pediatric AML. Second, the majority of studies were modeled with high levels of uncertainty in cost estimates, and with study outcomes greatly affected by model input parameters and assumptions. As previously outlined by Gye et al. (2022) [Citation39], although there was consistency in the clinical evidence from clinical trials and the literature, variability in ICERs (mainly QALYs) was attributed to the different methods used to extrapolate overall survival data beyond the trial period [Citation39]. Thus, variability in costs and outcomes result in weak external validity due to limited confidence in the generalizability of the results. Third, there were no studies conducted in LMICs, therefore, the cost-effectiveness and affordability of CAR-T cell therapy and alternative treatments remain unknown, limiting the applicability and equity in access to resource-limited contexts. Although data from developing countries could not be included because these advanced treatments for r/r pediatric acute leukemia are hardly available, yet, salvage chemotherapy, and HSCT are reasonably available across these countries and sibling donor/matched unrelated donor/haploidentical donors are extensively used. Estimated mean cost of allogenic HSCT for ALL is 11,053 (SD: ±2,817) in a developing country [Citation45], nevertheless, limited data exist about cost-effectiveness of HSCT in LMIC settings. Fourth, there was no subgroup analysis for r/r ALL patients by age-group, risk, site, time, number of relapses, or tumor burden in any of the included studies.
4.4. Implications for practice and policy
From a policy perspective, findings from this systematic review could encourage policy-makers to find innovate ways for value-based payment systems, through making deals with pharmaceutical industry (manufacturer) to lower drug prices of CAR-T cell therapy and other advanced treatment options. Policy-makers in LMICs should advocate promoting equity and access to novel treatments for r/r pediatric ALL at an affordable price, and include the cost-effective treatment strategies within national health insurance plans. From a research perspective, this systematic review has identified gaps in existing knowledge that would pave the way for future research in key areas including: limited cost-effectiveness studies for r/r pediatric AML; limited pragmatic studies for r/r pediatric ALL using real-world data (RWD); and lack of subgroup analysis. From a clinical perspective, clinicians can use findings from this review to treat r/r ALL in children in a more cost-effective manner, based on the available and affordable treatment options in their contexts.
Key recommendations to deliver cost-effective treatment for r/r pediatric acute leukemia include:
Determine cost-effectiveness of different treatment strategies for r/r AML in children.
Study long-term cost-effectiveness of treating r/r ALL from longitudinal cohorts to provide more robust results and reduce the uncertainty in outcomes observed in modeling studies.
Identify the most cost-effective treatment option(s) for each subgroup of r/r ALL patients, stratified by risk, site, time of relapse, and disease burden.
Guide clinicians to make better informed decisions regarding r/r ALL and adopt more cost-effective treatment strategies, based on real-world evidence.
Engage policy- and decision-makers to set cost-effectiveness (WTP) thresholds for introducing novel treatment interventions for r/r ALL in resource-limited settings in LMICs.
Evaluate the applicability of adopting novel treatment interventions for r/r ALL in LMICs, and negotiating prices with pharmaceutical industry to allow affordability.
5. Conclusions
CAR-T cell therapy is the primary candidate in treating r/r ALL and based on the generated evidence it seems like a cost-effective treatment strategy for r/r ALL in children and young adults compared to other treatment strategies. The next best treatment option was Blinatumomab, followed by Clofarabine therapy (Clo–C and Clo–M), whereas FLA-IDA salvage chemotherapy (with/without HSCT) provides the least value for money. The overall quality of the generated evidence is moderate to high, with limited confidence in generalizability and applicability of findings due to the high variability in outcomes among the included studies. Further research is needed to determine the long-term cost-effectiveness of r/r ALL in real-world contexts, stratified by risk, site, time of relapse and disease burden, and to evaluate cost-effectiveness of r/r AML treatments to address the gap in current knowledge. Policymakers should find innovative approaches to allow affordability and applicability in clinical practice and promote equity of access to novel treatments for patients residing in resource-limited settings in LMICs.
6. Expert opinion
The generated evidence from this systematic review highlights important insights to deliver cost-effective treatment for children/young adults with relapsed/refractory acute leukemia in real-world contexts. The quality of evidence is moderate to high (good internal validity), yet, external validity is poor, due to limited confidence in generalizability and applicability of the findings in clinical practice as the majority of were modeling studies with high variability in outcomes. We provided evidence-based recommendations with implications for healthcare policy and clinical/research practice, to help translate knowledge into practice and address the identified gaps in knowledge.
Key areas to improve the methodological limitations of the selected studies in our systematic review include: generating real-world evidence (RWE) from observational/cohort studies, rather than modeling studies, which provide high levels of uncertainty and variability in outcomes; study the long-term cost-effectiveness of the alternative treatment strategies for r/r pediatric acute leukemia to provide more robust results on the longer term; study the cost-effectiveness and affordability of novel treatments in resource-limited contexts in low- and middle-income countries (LMICs), where the majority of children of cancer reside, to promote equity in access to the unprivileged populations; finally, overcome the limitation in all included studies, which did not report any subgroup analysis for r/r ALL patients by make population sub-group stratification by risk, site, time of relapse, and tumor burden, to allow tailoring of the most cost-effective therapies to targeted patient subgroups.
In fact, there is good potential to improve the above-mentioned areas through conducting further research in health economic evaluation, and health policy. Policy-makers are encouraged to find innovative approaches to promote affordability of novel expensive treatments for r/r pediatric acute leukemia, such as through making negotiations and deals with pharmaceutical industry (manufacturer) to lower drug prices of CAR-T cell therapy and other advanced treatment options. Also, policy- and decision-makers should advocate promoting equity of access of novel treatments to children residing in resource-limited settings in LMICs, through setting cost-effectiveness (willingness to pay), and introducing the most cost-effective treatment interventions in national health policy plans to treat relapsed/refractory pediatric acute leukemia.
Other promising areas to promote the field of pediatric acute leukemia include finding effective and cost-effective treatment strategies for the high-risk ALL and AML patients which are at high risk of relapse or not responding to treatment (refractory to induction chemotherapy), in order to find ways to avoid disease relapse before it happens, whenever possible.
It is expected that the field of improving survival outcomes for relapsed/refractory pediatric acute leukemia will evolve in the future. Further study of the impact of advanced novel treatments in real-world contexts would promote clinical practice within the next five or ten years. This could be achieved through conducting pragmatic studies based on real-world data, and reducing manufacturing costs of CAR-T cell therapy and other novel treatments for r/r pediatric acute leukemia, which would eventually lead to lowered drug prices, allowing for better affordability in the different contexts and countries, including resource-limited settings in LMICs. The generated evidence from this systematic review will greatly contribute to evolving and promoting the field of relapsed/refractory acute leukemia in children/young adults within the next five years.
Article highlights
The generated evidence suggests that CAR-T cell therapy is a cost-effective treatment for r/r pediatric ALL compared to other treatment strategies, yet, serious concerns remain over uncertainty of its long-term effectiveness in real-world contexts, affordability of drug price, and equity in access for patients residing in LMICs.
The next best treatment option is Blinatumomab, followed by Clofarabine therapy, whereas FLA-IDA salvage chemotherapy provided the least value for money.
There is a gap in knowledge about cost-effectiveness of treatment for r/r Acute Myeloid Leukemia (AML), due to limited published studies.
Levofloxacin prophylaxis is cost-saving during intensive chemotherapy for r/r acute leukemia.
The quality of generated evidence is moderate to high, with limited generalizability and applicability of findings due to high variability in outcomes among studies.
Declaration of interest
R Soliman was supported by Egypt Cancer Network (ECN). C Heneghan is funded by the National Institute for Health Research (NIHR) School for Primary Care Research [Project Number 390] and NIHR Oxford BRC. C Heneghan also received expenses for his media work and the WHO. The views expressed are those of the author and not necessarily those of the NIHR, the NHS, or the Department of Health.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (108 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17474086.2022.2069096
Additional information
Funding
References
- American Cancer Society (ACS). Key statistics for childhood leukemia. ACS. cited 2021 Jul 28 at https://www.cancer.org/cancer/leukemia-in-children/about/key-statistics.html
- Sun W, Malvar J, Sposto R, et al. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: therapeutic advances in childhood leukemia & lymphoma study. Leukemia. 2018;32:2316–2325.
- Martin A, Morgan E, Hijiya N. Relapsed or refractory pediatric acute lymphoblastic leukemia: current and emerging treatments. Paediatr Drugs. 2012;14(6):377–387.
- Locatelli F, Schrappe M, Bernardo M, et al. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;4:2.
- Scalo F, Rascati KL. Trends and issues in oncology costs. Expert Rev. Pharmacoecon. Outcomes Res. 2014;14:35–44.
- Chhatwal J, Mathisen M, Kantarjian H. Are high drug prices for hematologic malignancies justified? A critical analysis. Cancer. 2015;121:3372–3379.
- Pui H, Evans E. A 50‐year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50:185–196.
- Stephen PH, Elizabeth AR. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. 2020;136:15.
- Kaul S, Korgenski EK, Ying J, et al. A retrospective analysis of treatment-related hospitalization costs of pediatric, adolescent, and young adult acute lymphoblastic leukemia. Cancer Med. 2016;5(2):221–229.
- Rasche M, Steidel E, Zimmermann M, et al. Second relapse of pediatric patients with acute myeloid leukemia: a report on current treatment strategies and outcome of the AML-BFM study group. Cancers (Basel). 2021;13(4):789.
- Hoffman AE, Schoonmade LJ, Kaspers GJL. Pediatric relapsed acute myeloid leukemia: a systematic review. Expert Rev Anticancer Ther. 2021;21(1):45–52.
- Forsythe A, Sandman KE. What does the economic burden of acute myeloid leukemia treatment look like for the next decade? An analysis of key findings, challenges and recommendations. J Blood Med. 2021;12:245–255.
- Shemilt I, Mugford M, Byford S, et al. Chapter 15: incorporating economics evidence. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. UK: The Cochrane Collaboration; 2011. p. 449–80. Version 5.1.0 (cited March 2011). The Cochrane Collaboration. Accessed on 28 Jul 2021 at www.handbook.cochrane.org
- Russell HV, Panchal J, Vonville H, et al. Economic evaluation of pediatric cancer treatment: a systematic literature review. Pediatrics. 2013;131(1):e273–87.
- Centre for Reviews and Dissemination (CRD). Systematic reviews: CRD’s guidance for undertaking reviews in healthcare. York, UK: University of York; 2009.
- PRISMA Group: Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;51:264–269.
- Craig D, Rice S. NHS economic evaluation database handbook: centre for reviews and dissemination. York, UK: University of York; 2007.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Eff Resour Alloc. 2013;11(1):6.
- Fung A, Horton S, Zabih V, et al. Cost and cost-effectiveness of childhood cancer treatment in low-income and middle-income countries: a systematic review. BMJ Glob Health. 2019;4:e001825.
- The Professional Society for Health Economics and Outcomes Research (ISPOR). cited 2021 Nov 10 th. https://www.ispor.org/home
- The World Bank Indicators. Official exchange rate (LCU per US$, period average). World Bank Group. cited 2020 Aug 28th. Available at: https://data.worldbank.org/indicator/PA.NUS.FCRF?view=chart
- The World Bank Indicators. GDP deflator (base year varies by country). world bank group. cited Aug 28, 2020. Available at 2020 Aug 28: https://data.worldbank.org/indicator/NY.GDP.DEFL.ZS
- Lin YF, Lairson DR, Chan W, et al. The costs and cost-effectiveness of allogeneic peripheral blood stem cell transplantation versus bone marrow transplantation in pediatric patients with acute leukemia. Biol Blood Marrow Transplant. 2010;16(9):1272–1281.
- Maser B, Pelland-Marcotte M-C, Alexander S, et al. Levofloxacin prophylaxis in hospitalized children with leukemia: a cost-utility analysis. Pediatr Blood Cancer. 2020;67:e28643.
- Lin JK, Lerman BJ, Barnes JI, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36(32):3192–3202. 10.
- Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and value of chimeric antigen receptor T-cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018;172(12):1161–1168. 1.
- Sarkar RR, Gloude NJ, Schiff D, et al. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111(7):719–726. 1.
- Santasusana R, Saldaña A, García-Muñoz N, et al. Cost-effectiveness analysis of tisagenlecleucel in the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia in children and young adults in Spain. Clinicoecon Outcomes Res. 2020;12:253–264.
- Thielen FW, van Dongen-leunis A, Arons AMM, et al. Cost-effectiveness of anti-CD19 chimeric antigen receptor T-Cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. A societal view. Eur J Haematol. 2020;105(2):203–215.
- Furzer J, Sumit G, Paul C, et al. Cost-effectiveness of tisagenlecleucel vs standard care in high-risk relapsed pediatric acute lymphoblastic leukemia in Canada. JAMA Oncol. 2020;6(3):393–401.
- Yang H, Hao Y, Qi CZ, et al. Estimation of total costs in pediatric and young adult patients with relapsed or refractory acute lymphoblastic leukemia receiving tisagenlecleucel from a U.S. hospital’s perspective. J Manag Care Spec Pharm. 2020;26(8):971–980.
- Schulthess D, Gassull D, Makady A, et al. Are CAR-T therapies living up to their hype? A study using real-world data in two cohorts to determine how well they are actually working in practice compared with bone marrow transplants. BMJ Evid Based Med. 2021;26(3):98–102.
- Wakase S, Teshima T, Zhang J, et al. Cost-effectiveness analysis of tisagenlecleucel for the treatment of pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia in Japan. Transplant Cell Ther. 2021;27(3):241.e1–241.e11.
- Moradi-Lakeh M, Yaghoubi M, Seitz P, et al. Cost-effectiveness of tisagenlecleucel in paediatric acute lymphoblastic leukaemia (pALL) and adult diffuse large B-cell lymphoma (DLBCL) in Switzerland. Adv Ther. 2021;38(6):3427–3443.
- Fiorenza S, Ritchie DS, Ramsey SD, et al. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplant. 2020;55:1706–1715.
- Pepper MS, Alessandrini M, Pope A, et al. Cell and gene therapies at the forefront of innovative medical care: implications for South Africa. S Afr Med J. 2018;109(1):20–22. 13.
- Cornetta K, Patel K, Wanjiku CM, et al. Equitable access to gene therapy: a call to action for the American society of gene and cell therapy. Mol Ther. 2018;26(12):2715–2716. 5.
- European Medicines Agency (EMA). Kymriah (tisagenlecleucel). What are the risks associated with Kymriah? cited 2022 Jan 20th. https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah
- Gye A, Goodall S, De Abreu L. A systematic review of health technology assessments of chimeric antigen receptor T-cell therapies in young compared with older patients. Value Health. 2022;25(1):47–58.
- Petrou P. Is it a Chimera? A systematic review of the economic evaluations of CAR-T cell therapy. Expert Rev Pharmacoecon Outcomes Res. 2019;19(5):529–536.
- Heine R, Thielen FW, Koopmanschap M, et al. Health economic aspects of chimeric antigen receptor T-cell therapies for hematological cancers: present and future. Hemasphere. 2021;5(2):e524.
- Pechlivanoglou P, Luu L, Li Q, et al. Blinatumomab is cost-effective compared to standard chemotherapy for children with high-risk relapses of acute lymphoblastic leukemia: a cost-effectiveness analysis using population-based healthcare data. Blood. 2021;38(1):565.
- Hoffman A, Schoonmade L, Kaspers G. Pediatric relapsed acute myeloid leukemia: a systematic review. Expert Rev Anticancer Ther. 2021;21(1):45–52.
- Mamolo C, Welch V, Walter RB, et al. Budget impact analysis of gemtuzumab ozogamicin for the treatment of CD33-positive acute myeloid leukemia. Pharmacoeconomics. 2021;39(1):121–131.
- Jaime-Pérez JC, Heredia-Salazar AC, Cantú-Rodríguez OG, et al. Cost structure and clinical outcome of a stem cell transplantation program in a developing country: the experience in northeast Mexico. Oncologist. 2015;20(4):386–392.