ABSTRACT
Introduction
A systematic literature review was conducted to understand disease burden in patients with relapsed/refractory classical Hodgkin lymphoma (R/R cHL).
Areas covered
Embase®, PubMed®, and Cochrane were searched for records from 2001 to 2020 in accordance with PRISMA guidelines. A total of 13,257 abstracts and 1731 papers were screened; 144 studies were identified. cHL accounted for 0.5% of all cancers, with 4‒66.7% of cases progressing to R/R disease (studies with >500 patients); this range varied across countries. Quality of life (QoL) was assessed via EORTC-QLQ-C30 (n = 7), EQ-5D (n = 5), SF-36 (n = 3), FACIT-F (n = 1), and MFI (n = 1) questionnaires. In general, pembrolizumab and other programmed cell death protein-1 inhibitors improved QoL scores. Brentuximab vedotin showed mixed outcomes, and high-dose therapy (HDT) and autologous stem-cell rescue (ASCR) showed worsening functionality/symptoms. Economic burden studies (n = 21) reported increased costs and health care resource in R/R cHL. Across clinical guidelines (n = 13) and treatment pattern studies (n = 46), HDT followed by ASCR was recommended as initial R/R cHL treatment. Pembrolizumab and nivolumab were frequently recommended for patients relapsing following HDT/ASCR.
Expert Opinion
Despite recent treatment advances, patients with R/R cHL continue to report reduced quality of life. Unmet medical needs remain, particularly with respect to slowing disease progression and identifying the best treatment approaches for improving longer-term survival and quality of life. This systematic literature review provides an extensive overview of the current landscape in patients with R/R cHL, focusing on four key areas: epidemiology, QoL, economic burden, and disease management. These findings will be useful to those with an interest in managing patients with R/R cHL or in designing future studies.
1. Introduction
Hodgkin lymphoma (HL) is a rare lymphoid malignancy involving large multinucleated cells derived from B lymphocytes, known as Hodgkin/Reed-Sternberg cells [Citation1]. HL is divided into two major categories based on morphology and immunophenotype differences: classical Hodgkin lymphoma (cHL) and Nodular Lymphocyte Predominant Hodgkin lymphoma (NLPHL). Approximately 95% of diagnosed patients have cHL [Citation2]. HL accounts for 0.5% of all new cancer cases globally, and the average annual age-standardized rate is 0.98 per 100,000 population [Citation3,Citation4].
First-line (1L) treatment of cHL with multi-agent chemotherapy plus radiotherapy is associated with very good overall survival rates [Citation2]; however, up to 30% of patients progress or relapse within 10 years and require salvage therapy [Citation5]. High-dose therapy (HDT) followed by autologous stem cell rescue (ASCR) is recommended as initial treatment for patients with relapsed/refractory (R/R) cHL [Citation5,Citation6]. This approach is successful in approximately 50% of treated patients [Citation5,Citation6]. Brentuximab vedotin may be effective as a consolidation therapy in patients that relapse following HDT/ASCR [Citation7,Citation8]. Pembrolizumab [Citation9] and other programmed cell death protein-1 (PD-1) inhibitors [Citation10] may also be effective in patients who continue to relapse, regardless of prior treatment or evidence of chemoresistant cHL.
Despite these treatment advances in R/R cHL, patients continue to have reduced quality of life (QoL), highlighting a need to focus on QoL of initial treatment decisions for long-term survival [Citation11]. Unmet medical needs remain, particularly with respect to identifying markers of disease progression and in the best treatment approaches for improving longer-term survival. Considering these issues, the objective of this systematic literature review (SLR) was to understand and quantify the burden of disease in patients with R/R cHL. To our knowledge, there are no comprehensive SLRs in this area to date; it is hoped that this SLR will address gaps in our understanding of R/R cHL, particularly with respect to epidemiology, economic burden, QoL, and treatment patterns.
2. Methods
The SLR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation12].
2.1. Data sources
Embase®, PubMed®, the Cochrane Central Register of Controlled Trials (CENTRAL), the UK National Health Service Economic Evaluation Database (NHS EED), and the Database of Abstracts of Reviews of Effects (DARE) were searched for R/R cHL records from 1 January 2001 to 21 February 2020, using targeted keyword searches, to identify studies exploring disease burden and evolving treatment patterns. Disease burden was defined as the impact of the disease of the patient population, measured using epidemiological, economic, and QoL indicators. Economic evaluations were performed from 2015 to 2020 to capture recent cost data associated with newer cHL therapies. Bibliographies of published SLRs were also screened to supplement the database searches.
2.2. Study and patient selection
Eligibility criteria included adult and pediatric patients of any ethnicity, race, or gender with any type of HL. Patients could be receiving any pharmacological intervention. Studies could take place in any country, but only those published in English were included. The type of HL was also recorded.
Searches were carried out to identify evidence in four areas of interest. These included epidemiology (cohort, case-control, cross-sectional, or database studies), QoL (cohort, case-control, cross-sectional, database, or randomized controlled trials), economic burden (cost studies/surveys/analyses, cost/economic burden of illness, database studies collecting cost data (e.g. claims databases and hospital records), resource use data, database/registry-based studies, questionnaires/surveys, cost-effectiveness, cost utility and cost-minimization analyses, budget impact models, and cost-consequence and cost-benefit analyses), and disease management (treatment guidelines, management algorithms, long-term observational, and real-world studies).
Studies estimating the impact of treatments on QoL and studies estimating the impact of cHL on patients (not related to treatments; disease in general) were considered for inclusion. However, the major focus was on assessing the treatment impact. Treatments approved by the Food and Drug Administration for treatment of R/R cHL within the search period of the SLR included brentuximab vedotin (approved August 2011; considered after failure of ASCR or ≥2 prior multi-agent chemotherapy regimens in patients who are not ASCR candidates); nivolumab (approved May 2016; prescribed to patients who have relapsed or progressed after ASCR or treatment with brentuximab vedotin); pembrolizumab (approved March 2017; considered in patients who have failed ≥3 prior therapies).
2.3. Search strategy, procedures, and information extracted
All records were downloaded into a systematic review database. The first screening (titles and abstracts) and the second screening (full-text papers) were undertaken by a single reviewer followed by a quality check from a second independent reviewer. One reviewer then conducted data extraction, which was validated by an independent reviewer. Where more than one publication was identified as describing a single study, the data were compiled into a single entry to avoid double counting of patients and studies. The methodology is summarized in Supplemental Figure 1. The search strategies used for Embase® and PubMed® are shown in Supplemental Table 1. Data were extracted into tables summarizing available evidence on R/R cHL in relation to epidemiology, QoL, economic burden, and disease management. Where a large number of studies were identified, tabulated data were presented according to study size (i.e. patient cohort). This was based on an arbitrary threshold, which is shown in the results tables. The use of studies with larger sample sizes also reduces the impact of inferential statistics. This enables generalization from a study sample that more closely resembles the population.
3. Results
A total of 13,257 abstracts and 1731 papers were screened. Overall, 144 relevant studies (including 60 abstracts) were identified. The flow chart is summarized in Supplemental Figure 2.
3.1. Epidemiology review
The epidemiology search identified 60 relevant studies (Supplemental Figure 2A). Fifty-six studies included relapse or recurrence and/or mortality data (relapsed after first-line [1L]: 15 [26.8%]; relapsed after second-line plus [2L+]: 39 [69.6%]; relapsed after 1L, refractory to 1L, relapsed after 2L+, refractory to 2L+: 1 [1.8%]; relapsed/refractory after 1L: 1 [1.8%]), and four studies reported prevalence of HL (not shown). The proportion of patients with HL/cHL progressing to R/R disease ranged from 14.9% to 66.7% in Europe, from 12.5% to 51.5% in the United States, and from 4% to 9% in Canada/United States in studies with >500 patients ( and Supplemental Table 2) [Citation13–19]. It ranged from 10.0% to 66.0% in South America (n = 7; all <500 patients), from 5.7% to 40.0% in Asia (n = 4; all <500 patients), and from 20.0% to 37.8% in the Middle East (n = 4; all <500 patients), respectively.
Figure 1. Proportion of patients* with CHL progressing after treatment in studies with >500 patients.1L, first-line of treatment; 2L/2L+, second-line and/or later line of treatment; 3L, third-line of treatment; classical Hodgkin lymphoma; HL, Hodgkin lymphoma. *Where ranges were reported, the highest value is shown. cHL patients (Farruggia 2019; Myers, 2018); HL patients (Mehta 2012; Josting 2005; Martinez 2017; Sureda 2013).
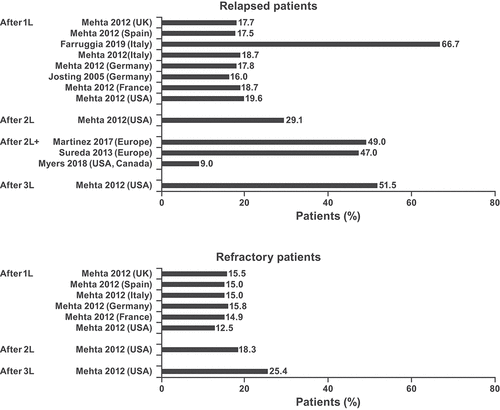
The mortality rate ranged from 5.0% to 19.8% in Europe and was 30.6% in North America/Canada in studies with >500 patients ( and Supplemental Table 2) [Citation13–19]. It ranged from 5.5% to 64.0% (South America), from 1.6% to 4.5% (Asia), and from 2.2% to 60.0% (Middle East) (all <500 patients).
Curative HDT/ASCR remains the standard of care for patients with R/R cHL/HL who are ASCR-eligible; however, the largest epidemiology study to date (n = 2261) estimated that 47% of patients with HL who receive ASCR will relapse within 15 years [Citation17]. A total of nine studies from all those identified (n = 144) provided information on the line of therapy based on ASCR use prior to relapse. The studies revealed that a significant number of patients (median age: 32‒57 years) received ASCR but still relapsed () [Citation16,Citation19–26].
Table 1. Summary of treatment use in HL/cHL studies with >250 patients.
3.2. QoL review
The humanistic search identified 16 studies with QoL data (Supplemental Figure 2B). These studies were from 18 countries and had diverse methodologies (observational, n = 8 studies; survey-based, n = 4; single-arm, n = 3; randomized, n = 1) with sample size ranging from 7 to 329 patients.
QoL was found to be assessed via five different tools; the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30; n = 7 studies), EuroQoL five dimensions questionnaire (EQ-5D; n = 5), 36-Item Short Form Survey (SF-36; n = 3), Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F; n = 1), and Multidimensional Fatigue Inventory (MFI; n = 1), with a focus on treatment impact.
Studies with QoL data and >75 patients are shown in [Citation24,Citation27–35]. In general, treatment with HDT/ASCR was associated with reduced functioning and worsening of some symptoms with an elevated risk of second malignancies [Citation28–30]. Pembrolizumab [Citation32], nivolumab [Citation31,Citation33], and sintilimab [Citation35] were associated with improvements in QoL across EORTC-QLQ-C30 and EQ-5D scales. Brentuximab vedotin showed mixed results, with one study (<75 patients with R/R HL) reporting an improvement in QoL [Citation36], and another study (>75 patients with R/R cHL) reporting a deterioration [Citation24].
Table 2. Summary of QoL data in HL/cHL studies with >75 patients.
3.3. Economic review
The economic burden searches identified a total of 32 studies (Supplemental Figure 2C). Cost and health care resource utilization data were reported in 21 studies, and economic evaluations were reported in 11 studies; 10 estimated the cost-effectiveness of treatment and one assessed budget impact. However, there is limited evidence (two studies) on the impact of R/R cHL on indirect costs.
3.3.1. Economic burden
Ten of the 21 studies identified reported economic burden specific to R/R cHL. These studies showed a substantial cost burden associated with R/R cHL. Front-line treatment failure health care costs were projected to be at least $535,846 (€450,752*) per patient with R/R cHL over 5 years [Citation37]. Mean costs per line of therapy were higher in relapsing patients with cHL: US$77,219/€64,958* (2L, relapsed) and US$59,442/€50,004* (third-line, relapsed) versus US$21,956/€18,470* (1L, non-progressive), and costs increased seven- to eight-fold (vs. 1L therapy) after allogenic or autologous transplantation [Citation38]. A real-world study was conducted to determine direct treatment costs of brentuximab vedotin (alone or after ASCR) or chemotherapy (after ASCR failure) in patients with R/R cHL. The mean costs per patient per month were higher for chemotherapy (US$51,245/€43,112*) and brentuximab vedotin plus ASCR (US$33,782/€28,421*) than brentuximab vedotin alone (US$28,313/€23,821). Inpatient, outpatient, and pharmacy costs represented approximately 69%, 16%, and 15% of total costs for chemotherapy; 34%, 16%, and 51% for brentuximab vedotin plus ASCR; and 21%, 14%, and 65% for brentuximab vedotin alone [Citation22]. These data highlight the impact that newer treatments have on reducing other management costs associated with R/R cHL, but a similar study has not been performed with PD-1 inhibitors to our knowledge or in ASCR-ineligible patients. Several other studies have shown that the length of hospital stays was variable and ranged from 3.1 to 48 days for cHL/HL [Citation39,Citation40]. There was, however, agreement in US and European studies that surveillance scanning for cHL was associated with high cost and limited utility [Citation41–44].
*1 US$ = 0.841 €
3.3.2. Cost-effectiveness
Data from cost-effectiveness studies suggest that brentuximab vedotin, nivolumab, and pembrolizumab are cost-effective treatments in relapsed patients, but the findings were affected by health care settings in different countries; four of these studies were specific to R/R cHL () [Citation45–54]. Brentuximab vedotin was likely to be cost-effective compared with standard of care in the United States [Citation51], although brentuximab vedotin consolidation therapy was not cost-effective versus active surveillance plus salvage therapy [Citation46]. Brentuximab vedotin exceeded the threshold for cost-effectiveness in the UK, but its cost was modest relative to other orphan drugs [Citation47]. Pembrolizumab was a cost-effective alternative to brentuximab vedotin [Citation45] in the United States, and nivolumab was cost-effective versus the standard of care in the UK [Citation50].
Table 3. Summary of results from cost-effectiveness studies in patients with HL/cHL.
3.4. Disease management review
The disease management search identified 13 treatment guidelines (United States = 2, Canada = 2, UK = 3, Europe = 5, and Australia = 1), and 46 treatment pattern studies from 17 countries (Supplemental Figure 2D). Across the clinical practice guidelines identified for patients with R/R cHL, including those from the United States [Citation55,Citation56] and Europe [Citation2,Citation20,Citation57,Citation58], HDT followed by ASCR was recommended as initial treatment unless contraindicated or in ineligible patients. Treatment options in patients with R/R cHL who have failed or are ineligible for ASCR are limited to salvage chemotherapies and off-label use of brentuximab vedotin as monotherapy or in combination with bendamustine or nivolumab [Citation2,Citation55]. Pembrolizumab and nivolumab were commonly recommended as subsequent therapy options for patients with cHL who relapsed or progressed following HDT/ASCR and post-transplant brentuximab vedotin, or those who relapsed/progressed after three or more lines of systemic therapy [Citation2,Citation55].
Real-world treatment patterns for relapsed patients are shown in [Citation20,Citation21,Citation23,Citation25,Citation26,Citation39,Citation59–63]. The most commonly used 2L treatment in the United States was HDT/ASCR (56% in one cohort) [Citation26]. A variety of treatments were used as 2L+ including brentuximab vedotin-based regimens (4–62%) and PD-1 inhibitors (11–73%). Similar findings were observed in later lines of therapy.
Table 4. Real-world treatment patterns reported in HL/cHL studies with >200 patients.
4. Discussion
This paper provides an extensive overview of the current landscape in patients with R/R cHL focusing on four key areas: epidemiology, QoL, economic burden, and disease management, which include clinical guidelines and real-world treatment patterns.
The epidemiology review showed that many patients progress to R/R disease at some point; it ranged from 4% to 66.7% in studies with more than 500 patients. The largest study showed that 47% of patients who received ASCR relapsed within 15 years [Citation17]. In patients who progress on ASCR, the median overall survival was found to be 25 months, and 5-year survival was <20% [Citation64]. In addition, a further 15–30% of patients are ineligible for ASCR due to inadequate response or progressive disease, unacceptable toxicity, or failure of stem cell collection [Citation65]. Unfortunately, there was limited information on ASCR-eligible versus ineligible cohorts in the studies identified. The epidemiology search was also impacted by limited reporting of prevalence and incidence data, and little data specific to patients with R/R cHL. The studies did not adequately summarize the parameters affecting the incidence or mortality estimates, meaning that we could not identify predictors for these outcomes.
The QoL review showed that pembrolizumab and other PD-1 inhibitors were associated with QoL improvements across different instruments in patients with R/R cHL [Citation31–33]. Sintilimab was shown to have numerical improvements on EQ-5D-5L visual analog score and EORTC-QLQ-C30 (overall health and QoL scores) and better outcomes in responders than nonresponders (defined according to complete or partial remission on computed tomography [CT] and magnetic resonance imaging [MRI] according to the International Working Group 2007 criteria) in post-hoc analyses [Citation35]. Brentuximab vedotin showed mixed positive-negative results, and treatment with HDT and ASCR was associated with reduced functionality and worsening of some symptoms [Citation24,Citation28,Citation36]. However, many studies examining QoL tended to be small and were not randomized or controlled, suggesting limited power to draw conclusions.
Although rare, HL still has a huge economic impact. It has been calculated to have the second highest societal cost per death (of 19 common cancers measured) due to the relatively large proportion of working-age individuals who are diagnosed compared with other cancers [Citation66]. When costs were measured by the present value of lifetime earnings lost per death, HL was the second most costly cancer per death at US$544,118 (€457,850*) [Citation66].
The economic review supported this; it found that progression to R/R disease significantly increased health care costs. PD-1 inhibitors and brentuximab vedotin appeared to be cost-effective across many health care scenarios in different countries. These findings were clinically relevant as most of these studies were conducted in the R/R cHL cohort, which increased the reliability of the estimates. However, these studies were generally limited to retrospective considerations of direct treatment costs, with little information available on indirect and intangible costs.
The disease management review showed that there was some consensus on the initial treatment approach to R/R cHL across countries, notably HDT/ASCR, but there was less agreement in best practice approach to subsequent therapies or combinations. A similar pattern emerged in the real-world studies. This is an important area where further discussions are warranted. Given the high number of patients that progress after HDT/ASCR and the poor survival in these patients, analyzing the data to show the impact of treatment choices on outcomes would be extremely useful, but data were too limited to enable this. In patients who progress after 1L therapy and are ASCR-ineligible, 2L treatment is limited to salvage therapies, which produce high response rates, but they are associated with burdensome tolerability profiles. Unfortunately, few studies have also examined treatment patterns in later lines of therapy.
Although these reviews provide an extensive overview of R/R cHL, there are some general limitations. It was difficult to distinguish between HL and cHL in some studies, and patients with HL were included in some ranges due to limited data. The reported ranges were also very wide between studies; this reflects many factors including differences in study design, disease severity and other clinical characteristics, and country-specific health care systems. However, it was not possible to adjust for these differences. Due to the size of the review, only studies with larger patient numbers were shown in the tables; these thresholds were arbitrary.
5. Conclusion
This paper provides an extensive overview of the current landscape in patients with R/R cHL. It highlights areas where further studies and improvements are warranted, particularly to distinguish issues specific to R/R cHL from the overall HL cohort. Many patients develop R/R cHL; it is associated with a substantial cost burden, but this needs to be quantified beyond direct medical costs with additional investigations on resource use globally. Despite treatments being available for R/R cHL, there also remains an unmet need to slow disease progression and improve QoL as some of the existing therapies reported impaired QoL. Real-world treatment pattern studies highlighted the need for further assessment of strategies and outcomes after R/R cHL based on the range of treatments used. It is hoped that some of these findings will be useful to those with an interest in managing patients with R/R cHL or in designing future studies.
6. Expert opinion
Despite recent treatment advances, up to 30% of patients with classical Hodgkin lymphoma (cHL) are refractory to first-line chemotherapy treatments and relapse within 10 years. Relapsed/refractory (R/R) cHL is associated with increased morbidity and mortality, reduced quality of life (QoL), and poor outcomes. Unmet medical needs remain, particularly regarding slowing disease progression and identifying the best treatment approaches for improving longer-term survival and QoL. This systematic literature review has provided an extensive overview of the current landscape in patients with R/R cHL and focused on four key areas: epidemiology, QoL, economic burden, and disease management.
Results from epidemiology studies with more than 500 patients showed that 4–66.7% of patients with cHL progress to R/R disease at some point during the disease trajectory, and this was associated with a mortality rate of 5–30.6%. The proportion of patients progressing to R/R cHL was affected by numerous factors, including prior treatment strategies; 66.7% of patients developed R/R cHL after 1L therapy, whereas only 4% of patients progressed after 2L treatment. While curative high-dose therapy/autologous stem-cell rescue (HDT/ASCR) remains the standard of care for patients with R/R cHL who are ASCR eligible, the largest study to date showed that 47% of patients who received ASCR relapsed within 15 years. In those patients who continued to progress on ASCR, the median overall survival was found to be 25 months; 5-year survival was <20%. These data suggest that the optimal treatment strategy for patients who do not respond to ASCR is yet to be elucidated.
Overall, the QoL review found that pembrolizumab and other PD-1 inhibitors were associated with QoL improvements across different tools in patients with R/R cHL. Conversely, treatment with HDT and ASCR was associated with reduced functionality and worsening of some symptoms, with an elevated risk of second malignancies. Brentuximab vedotin showed mixed results across studies; some patients reported improvements in QoL, whereas others noted a deterioration. While there remains an unmet need to slow disease progression and improve response rates, patient QoL must be considered an important factor when selecting treatment strategies for R/R cHL.
Although rare, cHL still has a huge economic impact. It has been calculated to have the second highest societal cost per death (of 19 common cancers measured) due to the relatively large proportion of working-age individuals who are diagnosed, when compared with other cancers. The current economic review supported this finding that progression to R/R disease significantly increases health care costs. Despite this, PD-1 inhibitors and brentuximab vedotin appeared to be cost-effective across many health care scenarios in different countries.
Generally, the disease management review found that there was some consensus on the initial treatment approach to R/R cHL across countries, notably HDT/ASCR, but there was less agreement on the best-practice approach to subsequent therapies or combinations. A similar pattern has emerged in real-world studies. Given the high number of patients that progress after HDT/ASCR and the poor survival in these patients, studies assessing the impact of treatment choices on outcomes may offer valuable data to support improved management of patients with R/R cHL.
Although many patients will develop R/R cHL, the disease remains associated with poor outcomes and substantial cost burden. The identification of reliable markers of disease progression and real-world studies assessing the best treatment approaches for improving longer-term survival are fundamentally required to improve patient management and reduce direct medical costs.
Article highlights
This systematic literature review (SLR) looked at overall disease burden in patients with relapsed/refractory classical Hodgkin lymphoma (R/R cHL).
Many patients develop R/R cHL; it is associated with a substantial cost burden, but this needs to be quantified beyond direct medical costs with additional investigations on resource use globally.
Despite treatments being available for R/R cHL, there also remains an unmet need to slow disease progression and improve quality of life (QoL) as some of the existing therapies reported impaired QoL.
Real-world treatment pattern studies highlighted the need for further assessment of strategies and outcomes after R/R cHL based on the range of treatments used.
It is hoped that this SLR will be useful in identifying areas where further research is warranted.
Declaration of Interest
M Raut and I Hiscock are employees of Merck & Co., Inc. G Singh, S Sharma, and N Pilkhwal are employees of Parexel International. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the study design, data interpretation, and reviewed and approved all manuscript drafts, including the final draft.
Supplemental Material
Download MS Word (793.8 KB)Acknowledgments
Editorial and writing support was provided by Parexel International and was funded by Merck & Co., Inc.
Data availability statement
Data can be obtained via a written request to the corresponding author.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17474086.2022.2080050
Additional information
Funding
References
- Connors JM, Cozen W, Steidl C, et al. Hodgkin lymphoma. Nat Rev Dis Primers. 2020;6(1):61.
- Eichenauer DA, Aleman BMP, André M, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv19–iv29.
- GLOBOCAN. GLOBOCAN 2018: estimated cancer incidence, mortality, and prevalence worldwide in 2018. Available from: https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=population&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1. Cited 2021 Apr 21.
- National cancer institute: Surveillance epidemiology, and end results program (SEER). 2021. Cancer stat facts: Hodgkin lymphoma. Available from: https://seer.cancer.gov/statfacts/html/hodg.html. Cited 2021 Apr 21.
- Grimm SE, Fayter D, Ramaekers BLT, et al. Pembrolizumab for treating relapsed or refractory classical Hodgkin lymphoma: An evidence review group perspective of a nice single technology appraisal. Pharmacoeconomics. 2019;37(10):1195–1207.
- National Institute for Health and Care Excellence. March 2017. Health technology appraisal. Pembrolizumab for treating relapsed or refractory classical Hodgkin lymphoma. Available from: https://www.nice.org.uk/guidance/ta540/documents/final-scope. Cited 2021 Apr 21.
- Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862.
- Moskowitz CH, Walewski J, Nademanee A, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639–2642.
- Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144–1153.
- Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428–1439.
- Linendoll N, Saunders T, Burns R, et al. Health-related quality of life in Hodgkin lymphoma: a systematic review. Health Qual Life Outcomes. 2016;14(1):114.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Mehta J, Abbe A, Trask PC, et al. Epidemiology projection trends for Hodgkin lymphoma patients in the U.S. and Europe. Blood. 2012;120(21):4782.
- Farruggia P, Puccio G, Locatelli F, et al. Classical pediatric Hodgkin lymphoma in very young patients: the Italian experience. Leuk Lymphoma. 2019;60(3):696–702.
- Josting A, Nogova L, Franklin J, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin’s lymphoma: a retrospective analysis from the German Hodgkin lymphoma study group. J Clin Oncol. 2005;23(7):1522–1529.
- Martinez C, Gayoso J, Canals C, et al. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: a registry study of the lymphoma working party of the European society for blood and marrow transplantation. J Clin Oncol. 2017;35(30):3425–3432.
- Sureda A, Boumendil A, Sieniawski M, et al. Secondary malignancies after autologous stem cell transplantation in patients with relapsed/refractory Hodgkin’s lymphoma. A retrospective analysis on behalf of the lymphoma working party of the European Society for Blood and Marrow Transplantation (EBMT). Blood. 2013;122 (21) :4636.
- Myers RM, Hill BT, Shaw BE, et al. Long-term outcomes among 2-year survivors of autologous hematopoietic cell transplantation for Hodgkin and diffuse large b-cell lymphoma. Cancer. 2018;124(4):816–825.
- Dulery R, Reman O, Boumemdil A, et al. Outcome analysis of high-dose chemotherapy followed by autologous stem cell transplantation in patients with Hodgkin lymphoma: A francophone society of bone marrow transplantation and cellular therapy study. Blood. 2016;128(22):3458. DOI:https://doi.org/10.1182/blood.V128.22.3458.3458.
- Bröckelmann PJ, Eichenauer DA, Jakob T, et al. Hodgkin lymphoma in adults. Dtsch Arztebl Int. 2018;115(31–32):535–540.
- Byrne K, Juarez-Garcia A, Hallworth P, et al. Later line drug treatment patterns of classical Hodgkin’s lymphoma (cHL) patients in Canada, France, Germany and the United Kingdom. Haematologica. 2017;102 e2 :83.
- Chen C, Johnston K, Szabo S, et al. Economic burden in US patients with relapsed or refractory classical Hodgkin lymphoma treated with brentuximab vedotin or chemotherapy after failure of autologous hematopoietic cell transplantation. Blood. 2017;130 (Supplement_1) :4705.
- Collins GP, Rueda A, Salles G, et al. Management of Hodgkin lymphoma in the era of brentuximab vedotin: real-world data from five European countries. Leuk Lymphoma. 2018;59(9):2113–2120.
- Ramsey SD, Nademanee A, Masszi T, et al. Quality of life results from a phase 3 study of brentuximab vedotin consolidation following autologous haematopoietic stem cell transplant for persons with Hodgkin lymphoma. Br J Haematol. 2016;175(5):860–867.
- Szabo SM, Juarez-Garcia A, Johnston KM, et al. Real world treatment patterns among an incident cohort of patients with Hodgkin lymphoma in the United States. Value Health. 2017;20 (5) :A124–A124.
- Szabo SM, Hirji I, Johnston KM, et al. Treatment patterns and costs of care for patients with relapsed and refractory Hodgkin lymphoma treated with brentuximab vedotin in the United States: a retrospective cohort study. PLoS One. 2017;12(10):e0180261.
- Ruffer JU, Flechtner H, Tralls P, et al. Fatigue in long-term survivors of Hodgkin’s lymphoma; a report from the German Hodgkin lymphoma study group (GHSG). Eur J Cancer. 2003;39(15):2179–2186.
- Goodman KA, Riedel E, Serrano V, et al. Long-term effects of high-dose chemotherapy and radiation for relapsed and refractory Hodgkin’s lymphoma. J Clin Oncol. 2008;26(32):5240–5247.
- Magagnoli M, Anastasia A, Mencaglia M, et al. Psychosocial functioning in Hodgkin’s lymphoma survivors after ifosfamide, gemcitabine, vinorelbine (IGEV) and high-dose chemotherapy with autologous haematopoietic stem cell infusion. Bone Marrow Transplant. 2010;45:S108–S108.
- Minn AY, Riedel E, Halpern J, et al. Long-term outcomes after high dose therapy and autologous haematopoietic cell rescue for refractory/relapsed Hodgkin lymphoma. Br J Haematol. 2012;159(3):329–339.
- Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–1294.
- von Tresckow B, Fanale M, Ardeshna KM, et al. Patient-reported outcomes in KEYNOTE-087, a phase 2 study of pembrolizumab in patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2019;60(11):2705–2711.
- Lepik K, Mikhaylova N, Kondakova E, et al. Response to nivolumab as ≥3rd line therapy in pts with relapsed/refractory classical Hodgkin’s lymphoma (cHL) and its impact on quality of life in responders and nonresponders. Hematol Oncol. 2019;37 (Supplement_2) :495.
- Kreissl S, Goergen H, Muller H, et al. Survivors’ perspectives on risks and benefits of Hodgkin lymphoma treatment: results of a survey by the German Hodgkin study group. Leuk Lymphoma. 2019;60(6):1389–1398.
- Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6(1):e12–e19.
- Ionova T, Afanasiev B, Andrievskih M, et al. Response to brentuximab vedotin and quality of life in patients with relapsed/refractory Hodgkin lymphoma (RR HL) in the real world setting. Blood. 2019;134(Supplement_1):5296.
- Bonafede M, Feliciano J, Cai Q, et al. Real-world analysis of cost, health care resource utilization, and supportive care in Hodgkin lymphoma patients with frontline failure. Clinicoecon Outcomes Res. 2018;10:629–641.
- Yasenchak CA, Tseng WY, Yap M, et al. Economic impact of disease progression following front-line therapy in classical Hodgkin lymphoma. Leuk Lymphoma. 2015;56(11):3143–3149.
- Laliberté F, Raut M, Duh M, et al. Real-world healthcare resource utilization (HRU) of classical Hodgkin lymphoma (cHL) patients (PTS) treated with anti-PD1 checkpoint inhibitors in the United States (US). Hematol Oncol. 2019;37:495–496.
- Radford J, McKay P, Malladi R, et al. Treatment pathways and resource use associated with recurrent Hodgkin lymphoma after autologous stem cell transplantation. Bone Marrow Transplant. 2017;52(3):452–454.
- Lee AI, Zuckerman DS, Van den Abbeele AD, et al. Surveillance imaging of Hodgkin lymphoma patients in first remission: a clinical and economic analysis. Cancer. 2010;116(16):3835–3842.
- Patel V, Buckstein M, Perini R, et al. Computed tomography and positron emission tomography/computed tomography surveillance after combined modality treatment of supradiaphragmatic Hodgkin lymphoma: a clinical and economic perspective. Leuk Lymphoma. 2013;54(10):2168–2176.
- Pingali SR, Jewell SW, Havlat L, et al. Limited utility of routine surveillance imaging for classical Hodgkin lymphoma patients in first complete remission. Cancer. 2014;120(14):2122–2129.
- El-Galaly TC, Mylam KJ, Brown P, et al. Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica. 2012;97(6):931–936.
- Large S, Hettle R, Balakumaran A, et al. Cost-effectiveness of pembrolizumab versus brentuximab vedotin for patients with relapsed or refractory classical Hodgkin’s lymphoma: a United States payer perspective. Med Econ. 2018:1–10. doi:https://doi.org/10.1080/13696998.2018.1534738.
- Hui L, von Keudell G, Wang R, et al. Cost-effectiveness analysis of consolidation with brentuximab vedotin for high-risk Hodgkin lymphoma after autologous stem cell transplantation. Cancer. 2017;123(19):3763–3771.
- Parker C, Woods B, Eaton J, et al. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma post-autologous stem cell transplant: a cost-effectiveness analysis in Scotland. J Med Econ. 2017;20(1):8–18.
- Babashov V, Begen MA, Mangel J. Economic evaluation of brentuximab vedotin for persistent Hodgkin lymphoma. Current Oncol. 2017;24(1):e6.
- Tan M, Lozano-Ortega G, Szabo S, et al. Cost effectiveness of nivolumab for the treatment of relapsed/refractory classical Hodgkin lymphoma (cHL) after failure of autologous stem cell transplantation (ASCT) and treatment with brentuximab vedotin (BV) treatment in Australia. Blood. 2017;130 (Supplement_1) :2166.
- Jones B, Ward T, Harrison J, et al. The cost-effectiveness of nivolumab for the treatment of people with relapsed or refractory classical Hodgkin lymphoma following autologous stem cell transplant and brentuximab vedotin. Value Health. 2017;20(9):A433–A433.
- Patidar M, Campbell JD. To evaluate the cost-effectiveness of brentuximab vedotin in patients with relapsed/refractory Hodgkin lymphoma who have received chemotherapy/chemotherapy with stem cell transplantation as first line therapy from a US healthcare perspective. Value Health. 2017;20 (5) :A359–A359.
- Romero Prada ME, Roa Cardenas NC, Vasquez Melo EC, et al. Cost-effectiveness of using brentuximab vedotin in patients with recurrent Hodgkin lymphoma or refractory to transplant. Value Health. 2016;19(7):A733.
- Khachatryan GR, Fedyaev DV, Avxentyeva MV. Cost-effectiveness analysis of brentuximab vedotin in adults with relapsed or refractory Hodgkin’s lymphoma. Value Health. 2016;19 (7) :A734.
- Ringkvist J Engstrom, A. The cost-effectiveness of brentuximab vedotin as consolidation treatment after autologous stem-cell transplantation in patients with Hodgkin lymphoma and increased risk of relapse. Value Health. 2017;20(9):A437.
- NCCN National Comprehensive Cancer Network®. Clinical practice guidelines in oncology (NCCN guidelines). Hodgkin Lymphoma. 2020; Version 1.2020.
- Perales MA, Ceberio I, Armand P, et al. Role of cytotoxic therapy with hematopoietic cell transplantation in the treatment of Hodgkin lymphoma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21(6):971–983.
- Rueda Domínguez A, Alfaro Lizaso J, de la Cruz Merino L, et al. SEOM clinical guidelines for the treatment of Hodgkin’s lymphoma. Clin Transl Oncol. 2015;17(12):1005–1013.
- Eccersley L, Iyengar S, Townsend W, et al. Pan London haemato-oncology clinical guidelines. Lymphoid malignancies. part 1: Hodgkin lymphoma. January 2020. RM Partners West London Cancer Alliance. SELCA. South East London Cancer Alliance. NHS North Central and East London Cancer Alliance; https://rmpartners.nhs.uk/wp-content/uploads/2020/01/Pan-London-Hodgkin-Guidelines-Jan-2020.pdf Accessed 21 April 2021.
- Badar T, Epperla N, Szabo A, et al. Trends in postrelapse survival in classic Hodgkin lymphoma patients after experiencing therapy failure following auto-HCT. Blood Adv. 2020;4(1):47–54.
- Shao C, Liu J, Zhou W, et al. Treatment patterns, health care resource utilization, and costs in patients with relapsed/refractory Hodgkin lymphoma treated with brentuximab vedotin. Leuk Lymphoma. 2019;60(4):947–954.
- Gajra A, Klink AJ, Nabhan C. Utilization of immune checkpoint inhibitors for relapsed/refractory classical Hodgkin lymphoma (R/R cHL) in oncology community practices. Blood. 2019;134(Supplement_1):4057.
- Feliciano J, Way N, Engley G, et al. Real world evidence in relapsed/refractory classical Hodgkin lymphoma patients who are ineligible for stem cell transplant in the United States (US). Blood. 2018;132(Supplement 1):2268.
- Gerrie AS, Power MM, Shepherd JD, et al. Chemoresistance can be overcome with high-dose chemotherapy and autologous stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Ann Oncol. 2014;25(11) :2218–2223.
- Moskowitz AJ, Perales MA, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146(2):158–163.
- Vassilakopoulos TP, Angelopoulou MK. Advanced and relapsed/refractory Hodgkin lymphoma: what has been achieved during the last 50 years. Semin Hematol. 2013;50(1):4–14.
- Bradley CJ, Yabroff KR, Dahman B, et al. Productivity costs of cancer mortality in the United States: 2000-2020. J Natl Cancer Inst. 2008;100(24):1763–1770.
