1. Introduction
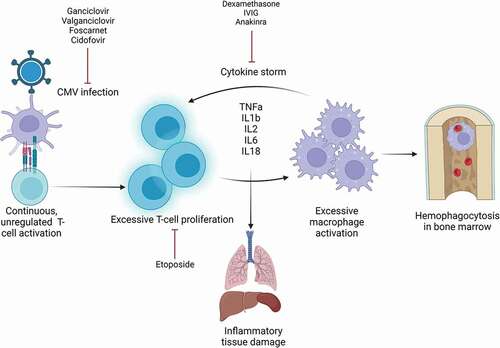
Hemophagocytic lymphohistiocytosis (HLH) is an acute hematological condition caused by uncontrolled overactivation of the patient’s immune system. HLH is an umbrella term for conditions with similar pathophysiology, rather than a single disorder. Underlying or triggering cause, clinical presentation, and course of disease exhibit great heterogeneity. The core clinical findings of HLH are persistent fever and splenomegaly, combined with laboratory findings of cytopenias, extreme hyperferritinemia, elevated triglycerides, and low fibrinogen. Elevated serum interleukin-2 receptor (IL-2 r) is a parameter with high sensitivity for HLH [Citation1,Citation2]. Liver infection and elevated lactate dehydrogenase (LDH) are also commonly seen [Citation3]. Histiocytosis and hemophagocytosis can be found in the bone marrow or in other organs. The pathogenesis of HLH is an unregulated, continuous immune activation, causing a cytokine storm and excessive macrophage activation. Interferon-γ (INF-γ), tumor necrosis factor-α (TNF-α), IL-1, IL-2, IL-6, and IL-18 are central proinflammatory cytokines in HLH pathophysiology [Citation4,Citation5]. Untreated, the hyperactivation causes inflammatory tissue damage (). This can result in multiorgan failure, and untreated HLH has a high mortality rate [Citation6]. The condition is divided into primary and secondary HLH. Primary HLH is caused by genetic mutations and often debuts within the first years of life. In secondary HLH, the immune system is triggered by exogenous factors. Infectious agents are among the most common triggers in secondary HLH, and members of the herpesvirus family are the most frequent viral agents [Citation7]. This included Epstein-Barr virus (EBV), herpes simplex virus (HSV), and cytomegalovirus (CMV). CMV transmission often takes place early in life. Positive serology in the adult population is relatively common, as CMV infection is often asymptomatic or subclinical [Citation8]. However, new treatment approaches in modern medicine, including immunosuppressive treatment and transplantation, have in recent decades raised concern and awareness regarding development of more severe CMV disease. One of the most feared complications is development of hyperinflammation conditions, such as HLH. Hence, HLH is a concomitant condition to CMV infection physician working with immunosuppressive patients should be aware of.
Figure 1. Pathophysiology and treatment targets for CMV-induced HLH.
2. Current knowledge
Immunosuppression is a common denominator for patients at risk for CMV-induced HLH. The main conditions associated with HLH induced by CMV are listed in . The use of immunosuppressive therapy is the standard approach for many of the conditions listed. Inflammatory bowel disease (IBD) appears to be particularly linked to increased HLH risk, especially when treated with immunosuppressants like azathioprine, mesalazine, or TNF-α inhibitors [Citation9,Citation10]. A similar, but less frequent, occurrence of CMV-induced HLH is seen in patients with autoimmune diseases, such as systemic lupus erythematosus (SLE) and myasthenia gravis [Citation11]. Immunosuppressive therapy is frequently used in this patient group as well. The main cause of HLH predisposition in these cases can be the disease itself, the use of immunosuppressants, or a combination of the two factors. This is also relevant for immunosuppression due to solid organ or stem cell transplantation. Other comorbidities at risk of HLH development are malignant disease and concomitant infections such as HIV or endocarditis [Citation12–15]. Primary immunodeficiency should be considered in the absence of immunosuppressive risk factors [Citation16]. Although rare, HLH can occur in immunocompetent individuals [Citation17]. Complications due to CMV infection occur both in primary infection and by reactivation of previously undergone infection. Hence, serology including both CMV IgM and IgG, in addition to CMV transcription levels detected by polymerase chain reaction (PCR) for viral load, can be used to differentiate primary infection from reactivation [Citation18].
Table 1. The conditions most commonly associated with CMV-induced HLH.
Initial HLH symptoms can be unspecific and often resemble bacterial infections or sepsis. Rapid recognition, prompt diagnostic work, and initiation of treatment are important prognostic factors. The Histiocyte Society has developed a set of diagnostic criteria, based on the HLH-2004 study [Citation13,Citation19]. The criteria cover some of the core findings of HLH, and a minimum of five of eight criteria must be met to establish a diagnosis. The treatment protocol for secondary HLH in adults is based on the HLH-94 study and recommends eight-week immunomodulating treatment with corticosteroids, with or without additional treatment with etoposide or methotrexate [Citation14,Citation20,Citation21] (). Treatment should always include intervention of underlying cause when possible. Hence, in viral-associated HLH, additional antiviral treatment is regarded beneficial (). First-line treatment of CMV infection is ganciclovir or its oral prodrug valganciclovir, while foscarnet and cidofovir are alternatives if resistance or unmanageable side effects are present [Citation22]. The importance of antiviral treatment is further highlighted when considering potential damage caused by long-term use of the immunomodulating treatment agents of HLH therapy. An important risk of prolonged immunomodulating treatment is possible reactivation of the triggering infection [Citation7]. The therapeutic challenge of suppressing hyperinflammation while maintaining control of the underlying infection must be considered when treating HLH patients.
3. Future aspects and research topics
Increased use of immunomodulating treatment in IBD and other autoimmune diseases, in malignant diseases, and in the setting of organ and allogeneic hematopoietic stem cell transplantation (allo-HSCT) creates patient groups more vulnerable to complications related to CMV infection and thereby a small, but significant, secondary increased risk of HLH development. As CMV-associated HLH is a rare complication, adequate immunosuppressive treatment of the primary condition should be prioritized. Monitoring for CMV activation by measuring CMV-DNA transcript by PCR techniques is considered mandatory in some settings, such as allo-HSCT [Citation22]. However, an important measure to prevent CMV-associated HLH is awareness of the correlation, to ensure early recognition of CMV disease and HLH development in immunocompromised patients. This is perhaps the best way to reduce development of fulminant disease with high mortality rates in risk patients.
As stated, the link between IBD and viral HLH seems to be especially strong, and the use of immunomodulating treatment of IBD is a likely cause. In addition, CMV-induced HLH in autoimmune patients occurs in a lesser degree, despite the use of immunomodulating treatment. The reason for the particularly increased risk in IBD patients is a subject for future research, and increased knowledge may improve prevention of HLH in these patients.
Furthermore, the diagnostic value of the HLH-2004 criteria is limited. Although the cutoff is set to the presence of five or more criteria, there seems to be a considerable inconsistency in the number of criteria present in described cases of HLH in the literature [Citation11]. The HLH-2004 criteria list important signs of HLH, although they lack some high-frequent findings such as liver infection. The only histopathological element of the HLH diagnostic criteria is hemophagocytosis in bone marrow or other tissue. Although a common finding in HLH patients, hemophagocytosis is not always present at the time of diagnosis [Citation7,Citation23]. There have been cases describing HLH where hemophagocytosis was not found upon investigation [Citation17,Citation24], and one case-controlled study on the subject concluded that hemophagocytosis in bone marrow had relatively low specificity for HLH [Citation25]. Establishing a HLH diagnosis is mainly based on overall clinical presentation and an individual evaluation of each patient. Serum IL-2r is recognized in the literature as a parameter with high sensitivity for HLH. Increased serum IL-2r is frequently seen in HLH and has both diagnostic and prognostic value as it can also be used in treatment monitoring [Citation1,Citation2]. The importance of IL-2r should be emphasized in future improvement of HLH diagnostic. As stated, many clinical features of HLH mimic other conditions, such as sepsis and hematological malignancy. HScore is another diagnostic tool that aims to evaluate the risk of an individual having HLH and contributes to distinguishing HLH from conditions with similar clinical presentation [Citation2,Citation26]. Both the HLH-2004 clinical criteria and Hscore can be helpful for rapid identification of patients with HLH. However, an even more precise diagnostic tool, adapted to the heterogeneity of the patient group, could further increase the diagnostic specificity with the aim of making the diagnostic process less divergent. The current HLH diagnostic criteria should be considered guidelines rather than absolute criteria. A revision of the criteria should probably include infection of liver enzymes and elevated LDH as diagnostic findings [Citation3].
Molecular diagnostic can be performed by detecting genetic mutations associated with HLH development, although genetic mutations are more relevant in primary HLH [Citation14]. There is no equivalent molecular diagnostic tool for secondary HLH described in the literature, although genetic vulnerability of HLH development may exist. Within the field of next-generation sequencing and high throughput data, new clinical studies should emphasize these aspects of secondary HLH as well.
Future HLH treatment is an ongoing topic, as the current treatment protocol is essentially based on studies performed decades ago. A review of literature shows a tendency of increased use of biological treatment and less use of etoposide. Divithotawela et al. describe the use of the IL-1 receptor antagonist, anakinra, in a case of CMV-associated HLH [Citation27], and Bami et al. report the use of anakinra for secondary HLH in six children [Citation28]. The use of anakinra in the literature is sparse and limited to recent cases, although with favorable outcomes reported. More research on treatment involving anakinra for secondary HLH could further investigate the effect. The use of intravenous gamma globulin (IVIG) in cases of viral HLH is a debated, but potentially efficient agent in HLH treatment. IVIG is not included in the current treatment protocol, but several case reports in the literature show positive outcomes with the use of IVIG [Citation11]. However, IVIG as part of CMV-induced HLH treatment is mostly described in single or small case reports in the current literature. More research is needed to document the use of IVIG in treatment of viral HLH. Rituximab is known to be efficient in EBV-induced HLH, but there is no equivalent specific immunomodulating treatment for CMV-induced HLH. Etoposide in addition to immunomodulation is listed as an option in the HLH treatment protocol, but a review of literature revealed few cases involving treatment with etoposide [Citation11]. Wohlfarth et al. reported a series of patients treated with different etoposide-free regimens, including treatment with anakinra and IVIG, with a survival rate of 50% [Citation29]. Etoposide is known to be efficient in HLH treatment, but other agents may be equally effective with less side effects. More research on etoposide-free treatment regimens is required. Furthermore, recent case reports describe positive outcomes with treatment with the janus kinase (JAK) inhibitor ruxolitinib, either as monotherapy or by adding low doses of ruxolitinib to standard HLH treatment. Ruxolitinib has an inhibitory effect on proinflammatory cytokines, which seems to be effective in targeting HLH pathophysiology [Citation30,Citation31]. Proven efficiency of new treatment options such as anakinra, ruxolitinib, and IVIG may call for revision of the current protocol to optimize HLH treatment.
4. Summary
As immunocompromised patients have an overall greater risk of HLH development than the general population, reported cases of CMV-induced HLH in the literature have a predominance of patients with immunocompromising predispositions. Specifically, IBD is described as a high-risk condition, as well as other autoimmune diseases, malignancy, and organ or stem cell transplantation. The HLH diagnostic criteria are suitable for disease recognition, although with a limited diagnostic value. The literature suggests that the cutoff at five fulfilled criteria is not transferable to the heterogenous patient group. There is currently no precise diagnostic tool for secondary HLH equivalent to molecular diagnostic in primary HLH. The current treatment protocol for CMV-induced HLH consists of combining antiviral and immunomodulating treatment. Alternative treatment options in accordance with current understanding of HLH pathophysiology is an ongoing research topic. Successful use of biological therapy in recent literature encourages future research on the subject to further improve HLH treatment.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A peer reviewer of this manuscript has received consulting fees from Sobi inc. Peer reviewers of this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529–2534.
- Lin M, Park S, Hayden A, et al. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Ann Hematol. 2017;96(8):1241–1251.
- La Rosee P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477.
- Osugi Y, Hara J, Tagawa S, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89(11):4100–4103.
- Zinter MS, Hermiston ML. Calming the storm in HLH. Blood. 2019;134(2):103–104.
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34(4):101515.
- Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program. 2013;2013:605–611.
- Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235(2):288–297.
- Brambilla B, Barbosa AM, Scholze CDS, et al. Hemophagocytic lymphohistiocytosis and inflammatory bowel disease: case report and systematic review. Inflamm Intest Dis. 2020;5(2):49–58.
- Li Y, Xia X, Zhang J, et al. Haemophagocytic lymphohistiocytosis in inflammatory bowel disease with virus infection. Prz Gastroenterol. 2015;10(2):78–82.
- Rolsdorph LÅ, Mosevoll KA, Helgeland L, et al. Concomitant hemophagocytic lymphohistiocytosis and cytomegalovirus disease: a case based systemic review. Front Med (Lausanne). 2022;9. DOI:10.3389/fmed.2022.819465.
- Reiner AP, Spivak JL. Hematophagic histiocytosis. A report of 23 new patients and a review of the literature. Medicine (Baltimore). 1988;67(6):369–388.
- Allen CE, McClain KL. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematology Am Soc Hematol Educ Program. 2015;2015:177–182.
- Jordan MB, Allen CE, Weitzman S, et al. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–4052.
- Fardet L, Lambotte O, Meynard JL, et al. Reactive haemophagocytic syndrome in 58 HIV-1-infected patients: clinical features, underlying diseases and prognosis. AIDS. 2010;24(9):1299–1306.
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66(11):e27929.
- Atim-Oluk M. Cytomegalovirus associated haemophagocytic lymphohistiocytosis in the immunocompetent adult managed according to HLH-2004 diagnostic using clinical and serological means only. Eur J Microbiol Immunol (Bp). 2013;3(1):81–89.
- Dioverti MV, Razonable RR, Hayden RT. Cytomegalovirus. Microbiol Spectr. 2016;4(4). DOI:10.1128/microbiolspec.DMIH2-0022-2015
- Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL study group of the histiocyte society. Semin Oncol. 1991;18(1):29–33.
- Henter JI, Aricò M, Egeler RM, et al. HLH-94: a treatment protocol for hemophagocytic lymphohistiocytosis. HLH study group of the histiocyte society. Med Pediatr Oncol. 1997;28(5):342–347.
- Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125(19):2908–2914.
- Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(8):e260–e272.
- Morimoto A, Nakazawa Y, Ishii E. Hemophagocytic lymphohistiocytosis: pathogenesis, diagnosis, and management. Pediatr Int. 2016;58(9):817–825.
- Ozbalak M, Mastanzade MG, Gurel E, et al. Cytomegalovirus reactivation during adult acute lymphoblastic leukemia maintenance: do we underestimate (un)expected guest of pediatric approach? Am J Blood Res. 2021;11(1):118–122.
- Goel S, Polski JM, Imran H. Sensitivity and specificity of bone marrow hemophagocytosis in hemophagocytic lymphohistiocytosis. Ann Clin Lab Sci. 2012;42(1):21–25.
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620.
- Divithotawela C, Garrett P, Westall G, et al. Successful treatment of cytomegalovirus associated hemophagocytic lymphohistiocytosis with the interleukin 1 inhibitor - anakinra. Respirol Case Rep. 2016;4(1):4–6.
- Bami S, Vagrecha A, Soberman D, et al. The use of anakinra in the treatment of secondary hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2020;67(11):e28581.
- Wohlfarth P, Agis H, Gualdoni GA, et al. Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically Ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med. 2019;34(9):723–731.
- Wang H, Gu J, Liang X, et al. Low dose ruxolitinib plus HLH-94 protocol: a potential choice for secondary HLH. Semin Hematol. 2020;57(1):26–30.
- Hansen S, Alduaij W, Biggs CM, et al. Ruxolitinib as adjunctive therapy for secondary hemophagocytic lymphohistiocytosis: a case series. Eur J Haematol. 2021;106(5):654–661.
