ABSTRACT
Background
FVIII replacement is standard treatment for hemophilia A without inhibitors, but non-factor therapies, such as emicizumab, are changing the treatment landscape. We explore the ramifications of switching treatment.
Methods
Pharmacy database data (July 2017–May 2020) from patients with hemophilia A without inhibitors who switched to rurioctocog alfa pegol or emicizumab prophylaxis after ≥6 months’ prophylaxis with another FVIII product were analyzed for total mean weekly consumption, dosing frequency, product wastage, and ABR.
Results
Post-switch mean weekly consumption of prophylactic rurioctocog alfa pegol and emicizumab were 6224 IU/kg and 109 mg, respectively. Dosing schedules for emicizumab were primarily weekly (48.2%) and every 2 weeks (40.0%). Most patients in the rurioctocog alfa pegol cohort received treatment twice-weekly (83.3%). Mean product wastage of emicizumab (8.4%) was significantly higher versus rurioctocog alfa pegol (−0.3%; P < 0.001). Mean annualized emicizumab and rurioctocog alfa pegol wastage during prophylaxis was 330.82 mg and −974.80 IU, respectively. ABR change was not significantly different (P = 0.527) for patients switching to emicizumab (−1.05) or rurioctocog alfa pegol (−1.53).
Conclusions
Bleed rates were similar for patients receiving prophylaxis with emicizumab or rurioctocog alfa pegol after switching from prophylaxis with another FVIII. However, wastage associated with dispensing inaccuracies was greater with emicizumab.
1. Introduction
Hemophilia A (HA) is an X-linked congenital bleeding disorder caused by deficiency of coagulation factor VIII (FVIII) [Citation1]. Regular replacement therapy (prophylaxis) with infusion of plasma-derived or recombinant FVIII concentrates has been the mainstay of hemophilia treatment and is recommended over on-demand (episodic) therapy to maintain hemostasis and prevent bleeding [Citation1]. Extended–half-life (EHL) FVIII concentrates have reduced the treatment burden of prophylaxis and allowed for maintenance of higher factor trough levels for improved bleed prevention [Citation1].
Rurioctocog alfa pegol [ADYNOVATE (Antihemophilic Factor {Recombinant}, Pegylated)] is a full-length, recombinant EHL FVIII manufactured by attaching a branched polyethylene glycol molecule to octocog alfa [ADVATE (Antihemophilic Factor {Recombinant})], a standard half-life (SHL) FVIII [Citation2]. It is indicated for on-demand treatment and routine prophylaxis [40–55 IU/kg body weight, twice weekly (BIW)], adjusted per clinical response [Citation3]. Efficacy of rurioctocog alfa pegol for prophylaxis was demonstrated in open-label, phase II/III clinical trials, in which 38–40% of patients with severe HA had no bleeding episodes over 6 months with BIW prophylaxis [Citation4,Citation5]. Among previously treated patients aged 12–65 years, the median annualized bleed rate (ABR) was 1.9 versus 41.5 for patients treated on demand [Citation4]. Emerging real-world evidence also supports its use in prophylaxis [Citation6,Citation7].
The treatment landscape has expanded with the availability of non-factor therapies, such as emicizumab, a bispecific monoclonal antibody that mimics activated FVIII by bridging activated factors IX and X. Emicizumab is the first non-factor therapy approved for routine prophylaxis in patients with HA, with or without inhibitors [Citation8]. It is administered by subcutaneous injection and has an elimination half-life of almost 1 month [Citation8]. In the HAVEN 3 trial, 152 patients with severe HA without inhibitors were randomized to maintenance prophylaxis with emicizumab 1.5 mg/kg weekly (QW) or 3 mg/kg every 2 weeks (Q2W), or no prophylaxis. The prophylaxis arm was associated with a 96–97% reduction in ABR [Citation9].
Real-world data suggest that initiating or switching to emicizumab prophylaxis provides clinical benefits similar to those observed in clinical trials [Citation10]. Additionally, a 2020 policy report by the Institute for Clinical and Economic Review recommends emicizumab as the preferred agent for prophylaxis for many patients [Citation11]. However, the clinical impact of switching from other FVIII products to emicizumab or EHL FVIII in US clinical practice remains underexplored; there are no studies comparing product utilization, including wastage with emicizumab or FVIII concentrates for prophylaxis.
Our objective was to evaluate and compare the utilization, wastage, and clinical effectiveness (ABR) of prophylactic treatment with emicizumab and FVIII (rurioctocog alfa pegol and octocog alfa) in patients with moderate-to-severe hemophilia A without inhibitors in US clinical practice. Breakthrough bleeding and the utilization of additional FVIII for management of breakthrough bleeding were also compared before and after switching to rurioctocog alfa pegol or emicizumab from other FVIII prophylaxis products.
2. Methods
2.1. Patients and study design
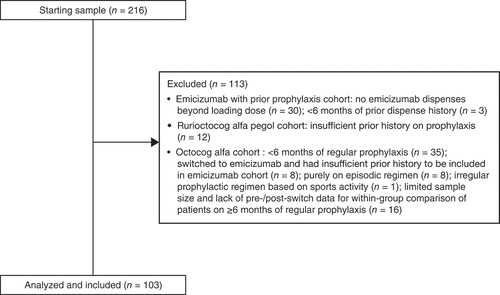
This study included male patients with moderate-to-severe hemophilia A (FVIII level ≤5%) who had received prophylaxis with emicizumab after ≥6 months of prophylactic FVIII (emicizumab cohort); prophylaxis with rurioctocog alfa pegol after ≥6 months of prophylactic FVIII (rurioctocog alfa pegol cohort); or ≥6 months of prophylaxis with octocog alfa without switching to other FVIII prophylaxis during the study period (octocog alfa cohort; ). Patients with evidence of inhibitors to FVIII during the entire pre-index period (≥6 months) were excluded. Data for the octocog alfa cohort are excluded from this report because of the limited sample size and lack of pre-/post-switch data for a within-group comparison.
Figure 1. Study design.
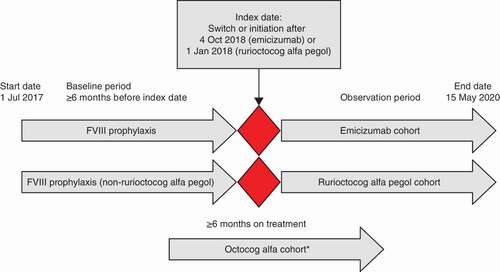
This retrospective, observational cohort study used data from the Trio Health Specialty Pharmacy Partners (SPP) database from July 2017 to May 2020.
Patients who switched to emicizumab or rurioctocog alfa pegol had to have initiated emicizumab after 4 October 2018 and rurioctocog alfa pegol after 1 January 2018 (because a limited number of patients initiated rurioctocog alfa pegol after 4 October 2018, date of treatment initiation was extended past 1 January 2018). The index date was defined as the date of rurioctocog alfa pegol or emicizumab prophylaxis initiation, the baseline period as ≥6 months before the index date and the observation period as the index date through the end of the study period. Patient characteristics, prophylaxis frequency, prescriptions, doses dispensed, patient bleeds, and bleed medication were analyzed.
This study met the criteria for institutional review board exemption. Data were stripped of personally identifiable information.
2.2. Study outcomes and analyses
Primary outcomes included total mean weekly consumption of FVIII and emicizumab, dosing frequency and product wastage.
Total mean weekly consumption included FVIII and emicizumab used for regular prophylaxis and additional FVIII used to manage breakthrough bleeding. FVIII utilization to manage breakthrough bleeding for medical procedures was defined as any additional infusions attributed to ‘Medical test/Procedure,’ ‘Immunization,’ ‘Dental Procedure,’ or ‘Surgical Procedure.’ FVIII utilization to manage breakthrough bleeding for bleed treatment/preventive measures included any additional infusion not attributed to the above medical procedure category, including ‘Fall,’ ‘Injury,’ ‘Spontaneous,’ ‘Physical activity/Sports,’ and ‘Preventive Treatment.’ Utilization for treatment of breakthrough bleeding was based on the number of infusions required for bleeding resolution and the dose listed in the bleed log or closest on-demand prescription before the bleed.
Prophylactic frequency was determined using dispensing and prescription data. If the prescription included prophylactic and on-demand FVIII, dispensed prophylactic FVIII was calculated as the number of vials dispensed to maintain the scheduled prophylaxis regimen. Dosing frequency was based on dosing schedule data.
Wastage of emicizumab and rurioctocog alfa pegol prophylaxis was defined as extraneous drug in each vial based on the prescribed dose versus dispensed drug in the vial. Product wastage was quantified as total wastage divided by total prescribed prophylaxis. Annualized wastage in milligrams or IU of product was defined as observed wastage during follow-up divided by follow-up in years. If a prescription included prophylactic and additional FVIII to manage breakthrough bleeding, dispensed prophylactic FVIII was calculated as the number of vials dispensed to maintain the scheduled prophylaxis regimen.
Secondary outcomes included all breakthrough bleeds, including but not limited to treated bleeds evaluated as ABRs. All bleeds, joint bleeds, spontaneous, trauma/injury bleeds, and spontaneous joint bleeds were included. Target joints were defined as single joints with three or more spontaneous bleeds within a consecutive 6-month period. ABR data, including the date, location, and cause of the bleed and the medication used to treat it, were collected from patient bleeding logs and calculated from all infusions attributed to a bleed.
2.3. Statistical methods
Patient characteristics and treatment dose/utilization parameters were summarized using descriptive statistics, including mean, 95% confidence interval (CI), range, median (min; max), number, and proportion. Patient age was de-identified into 5-year buckets; continuous estimates were not available.
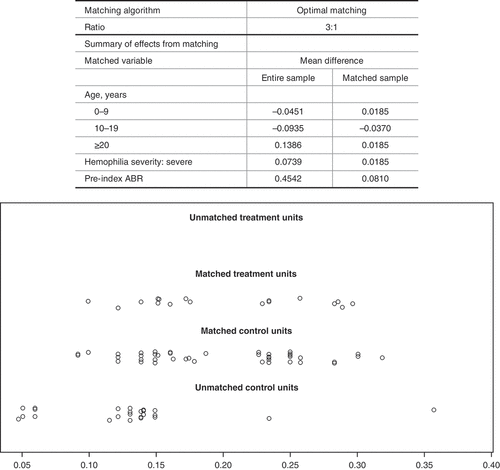
To reduce the effect of baseline differences in the emicizumab and rurioctocog alfa pegol cohorts for between-group comparisons of post-index utilization and changes in ABR, propensity-score matching was performed based on age, hemophilia severity, and pre-index ABR (). Target joints were excluded from matching, as data capture was limited.
Total mean weekly consumption was assessed using t-tests and dosing frequency using Fisher’s exact test. Within-group comparisons were assessed using paired t-tests.
ABR estimates and CIs were generated using Poisson regression with fixed effects for pre- versus post-index date, group, and interaction term between these effects. CIs for change in ABR after index date were generated using bootstrapping with 5000 replicates. Statistical tests on ABR estimates were limited to overall ABR. Subset analyses of patients in the rurioctocog alfa pegol cohort who switched after 4 October 2018 were conducted to verify a lack of temporal effect on product switch after emicizumab approval. Point estimates and CIs for this subset were compared with results for the overall rurioctocog alfa pegol cohort, with no statistical testing or matching. Pre-index outcomes were evaluated for the baseline period ≥6 months before switching treatment and post-index outcomes for the outcomes period ≥6 months after switching.
3. Results
3.1. Patient population and prophylaxis dosing frequency
Data were obtained for 216 patients treated by 314 prescribers at 87 practices across 40 states in the United States (one national specialty pharmacy). Ninety-seven patients were excluded because of insufficient pre- or post-switch treatment dispensing history (). An additional 16 patients who received ≥6 months of prophylaxis with octocog alfa were also excluded because of the lack of pre-/post-switch data for within-group comparison. The final analysis included 85 patients in the emicizumab with 6 months prior FVIII prophylaxis group, including 54 patients in the rurioctocog alfa pegol-matched emicizumab subset of that cohort and 18 in the rurioctocog alfa pegol with 6 months prior FVIII prophylaxis group.
Patients in the emicizumab cohort were primarily on QW (48.2%, all patients; 46.3%, matched cohort) and Q2W (40.0%, all patients; 42.6%, matched cohort) dosing schedules; the most common regimen for the remaining patients was every 4 weeks (Q4W; 10.6%). In the rurioctocog alfa pegol cohort, most patients received treatment BIW (83.3%), with remaining patients receiving treatment every 2, 3, or 5 days (5.6% each).
3.2. Patient characteristics and treatment utilization
Compared with the overall emicizumab prophylaxis cohort, patients in the rurioctocog alfa pegol cohort tended to be older (aged ≥20, 44.4% vs 30.6%; P = 0.526) and a higher proportion had severe Haemophilia (94.4% vs 87.1%, P = 0.687; FVIII <1%) (). Among patients with target joint data, the rurioctocog alfa pegol cohort had a significantly higher proportion of patients with ≥2 target joints (36% vs 9%; P = 0.025).
Table 1. Patient demographic and characteristics at baseline.
Total mean weekly consumption of prophylactic FVIII and emicizumab and additional FVIII to manage breakthrough bleeding are shown in . Pre-index emicizumab patients had slightly lower mean [standard deviation (SD)] weekly total FVIII consumption than rurioctocog alfa pegol patients [7635 (4655) IU vs 7740 (4129) IU]; however, in the matched emicizumab cohort, total FVIII consumption (SD) was higher than in the rurioctocog alfa pegol cohort [8644 (4724) IU vs 7740 (4129) IU]. Post-index, overall consumption of additional FVIII to manage breakthrough bleeding decreased in all three cohorts versus pre-index consumption.
Table 2. Total mean weekly consumption of on-demand and prophylactic FVIII and emicizumab.
Total mean weekly consumption (SD) of FVIII for breakthrough bleed treatment or prevention post-index was higher in the rurioctocog alfa pegol cohort [171 (396) IU] than in the overall [94 (198) IU] and matched [127 (232) IU] emicizumab cohorts, but this was largely driven by a single rurioctocog alfa pegol patient who required 92,340 IU to resolve a trauma-related bleed. After excluding this outlier, on-demand weekly use of additional FVIII in the rurioctocog alfa pegol cohort was 81 (117) IU.
Total mean weekly consumption (SD) of prophylactic rurioctocog alfa pegol post-index was 6224 (2939) IU/kg; mean emicizumab consumption was 109 (67) mg/kg in the overall emicizumab cohort and 124 (72) mg/kg in the matched emicizumab cohort.
3.3. Wastage
includes proportional wastage of FVIII and emicizumab prophylaxis. Some patients had negative wastage, as dispensed FVIII prophylaxis was less than the prescribed dose. Unmatched and matched emicizumab cohorts had slightly higher mean wastage of FVIII prophylaxis before the index date versus the rurioctocog alfa pegol cohort (1% unmatched, 0.38% matched vs −0.3% rurioctocog alfa pegol).
Table 3. Proportional wastage of FVIII and emicizumab prophylaxis.
After the index date, mean product wastage of emicizumab (emicizumab cohort, 8.4%; matched emicizumab cohort, 6.0%) was significantly higher versus rurioctocog alfa pegol (−0.3%; P < 0.001 for each comparison).
The increase in product wastage in the emicizumab cohort post-index versus pre-index was statistically significant in both the unmatched (+7.41%; P < 0.001) and matched (+5.61%; P < 0.001) cohorts, whereas no difference in wastage was observed in the rurioctocog alfa pegol cohort (+0.00%; P = 0.997).
The mean annualized wastage of emicizumab during prophylaxis was 330.82 mg. Emicizumab wastage was correlated with the frequency of administration, with more frequent administrations having higher wastage (429.69 mg QW; 206.70 mg Q2W; and 119.18 mg Q4W). The mean annualized wastage of rurioctocog alfa pegol during prophylaxis was −974.80 IU.
3.4. Clinical efficacy
Patients in the rurioctocog alfa pegol cohort had higher pre-index ABR than the emicizumab cohort (2.19 vs 1.68). After matching based on age, hemophilia severity and pre-index ABR, this difference decreased (2.19 vs 2.06; ). Patients in the rurioctocog alfa pegol cohort had higher pre-switch mean joint ABR (1.36 vs 0.89) and trauma/injury ABR (1.42 vs 0.98) than the matched emicizumab group but lower spontaneous bleeds (0.58 vs 0.86) and spontaneous joint bleeds (0.39 vs 0.50).
Table 4. Observed ABR during pre- and post-treatment switch periods and change in ABR after switching.
Post-index, no differences were observed between cohorts for all bleeds and for each bleed type, suggesting similar treatment effectiveness. ABR decreased post-index for patients switching to emicizumab (−1.05 unmatched; −1.36 matched) and to rurioctocog alfa pegol (−1.53) and was not significantly different between either the unmatched (P = 0.527) or matched (P = 0.738) cohorts. In the rurioctocog alfa pegol cohort, ABR reduction was mostly driven by a decrease in joint bleeds (−0.98) and bleeds caused by trauma/injury (−1.14). The largest decreases in the emicizumab cohorts were for spontaneous (−0.44 unmatched; −0.55 matched) and trauma/injury (−0.43 unmatched; −0.59 matched) bleeds. Across all patients, 22 of the 836 total bleed events were related to procedures (11 surgical, 3 medical tests, and 8 dental).
4. Discussion
We evaluated the effects of switching patients with moderate-to-severe hemophilia A to prophylaxis with rurioctocog alfa pegol or emicizumab in US clinical practice, with a focus on clinical effectiveness, treatment utilization, and product wastage.
ABRs were similar for rurioctocog alfa pegol and emicizumab during the post-switch follow-up period, suggesting similar clinical effectiveness. After using propensity-score matching to account for baseline differences between treatment groups, overall ABR, joint bleeds, and bleeds associated with trauma/injury were comparable for rurioctocog alfa pegol and emicizumab. ABRs decreased from the pre-switch period for both treatments, with no significant differences between cohorts. Patients in the rurioctocog alfa pegol cohort did not have a significant decrease in spontaneous bleeds post switch, perhaps because of the inflated standard error from a limited sample size (n = 18).
These data showing similar bleed rates during emicizumab and rurioctocog alfa pegol prophylaxis contrast with analyses in which the ABR was compared before and after switching to emicizumab from FVIII prophylaxis. A subgroup of patients in the HAVEN 3 study showed a 68% reduction in ABR after switching to emicizumab from previous FVIII prophylaxis (with mainly SHL products) in a non-interventional study [Citation9,Citation12]. However, only half of the patients had relatively high (≥80%) FVIII regimen adherence and the findings might have been affected by selection bias, as there was no comparator group of patients who had switched to another FVIII product [Citation12]. In a real-world analysis of 93 patients, the ABR reduced from 4.4 in patients with inhibitors and from 1.6 in patients without inhibitors to 0.4 for both groups after switching to emicizumab from previous FVIII treatment (mostly an SHL) [Citation10]. However, only 84% of patients were on a prophylactic FVIII regimen before switching.
A network meta-analysis showed a lower total treated bleed rate with emicizumab versus FVIII prophylaxis [rate ratio (RR) 0.36; 95% credible interval (Crl), 0.13–0.95] [Citation13]. However, this analysis only included 4 to 10 studies with high heterogeneity and focused on treated bleeds, which were not systematically assessed in previous studies.
Our findings show a reduction in bleeding rates with rurioctocog alfa pegol after switching from other FVIII prophylaxis, which aligns with other real-world analyses [Citation6,Citation7]. In a retrospective US-based analysis of 56 people with HA, the overall mean ABR reduced by 71% (range, 5.8–1.7; P < 0.001) after switching to rurioctocog alfa pegol from another prophylactic recombinant FVIII products and 20 patients had no bleeding events over a median of 1 year [Citation6].
Product wastage with emicizumab prophylaxis was significantly higher versus rurioctocog alfa pegol prophylaxis, with wastage positively associated with emicizumab dosing frequency. Emicizumab wastage should be considered within the context of the benefits associated with emicizumab; in addition to bleed control, these include quality-of-life improvements and subcutaneous administration [Citation14].
The finding of reduced product wastage with rurioctocog alfa pegol versus emicizumab prophylaxis is consistent with previous research showing that a broader selection of dosage strengths is associated with improved dispensing accuracy of hemophilia A treatment. Product wastage increases the cost of prophylactic treatment for HA. Zhou et al. developed a Markov state transition model to predict the short- and long-term clinical and economic outcomes of emicizumab versus SHL recombinant FVIII prophylaxis and estimated there were fewer treated bleeds and treated joint bleeds in patients with severe HA receiving emicizumab across all time horizons; additionally, emicizumab was associated with lower direct costs [Citation15]. Efficacy inputs to the model included a much lower ABR for treated bleeds with emicizumab versus FVIII prophylaxis (1.5 vs 4.8 for patients with severe HA without inhibitors) based on non-interventional study data for FVIII prophylaxis. Base-case unit prices were also done per wholesale cost rather than average sales price, favoring emicizumab, which has a lower discount. Additionally, the model assigned all patients to either FVIII or emicizumab at 1 year of age, which eventually causes mixing of populations (patients with and without inhibitors), as patients start developing inhibitors.
Our analysis has methodological strengths that support the comparative efficacy of emicizumab and rurioctocog alfa pegol for prophylaxis. In particular, the bidirectional switch to and concurrent analysis of emicizumab and rurioctocog alfa pegol cohorts minimizes the effect of patient selection bias to either treatment alone; propensity-score matching was used to reduce the effect of baseline differences between the cohorts. HA severity and number of target joints were lower in the unmatched emicizumab versus rurioctocog alfa pegol cohort, which may reflect differences in appropriate patient types for each treatment. The greater number of target joints in the rurioctocog alfa pegol cohort could also reflect the higher average age of patients. The focus on all bleeds rather than treated bleeds enhances the clinical relevance of these findings for physicians.
The limitations of this study are typical of retrospective observational studies. Patients were non-randomized, observers were non-blinded and the rurioctocog alfa pegol group size was relatively small. Some bleed data in the Trio Health SPP database were obtained directly from patients and caregivers and are subject to recall limitations.
5. Conclusions
To our knowledge, this is the first study to assess effectiveness of emicizumab versus rurioctocog alfa pegol prophylaxis in clinical practice while also assessing product wastage and utilization of additional FVIII to manage breakthrough bleeding. Bleed rates were similar for patients receiving prophylaxis with emicizumab or rurioctocog alfa pegol after switching from prophylaxis with another FVIII in real-world clinical practice, suggesting similar efficacy of the treatments. However, wastage associated with dispensing inaccuracies was greater with emicizumab.
Declaration of interests
S Sun is an employee and shareholder of Takeda. A Frick is an employee of Trio Health Inc. and has received study funding from Takeda. V Balasa serves on speaker bureaus for Takeda, CSL, and Sanofi Genzyme. JC Roberts has received research support from Takeda and Genentech; has participated in consulting/advisory boards for Genentech, Sanofi Genzyme, Takeda, Octapharma, Novo Nordisk and Pfizer; and has participated in speaker bureaus for Sanofi Genzyme, Novo Nordisk, Octapharma, and Takeda.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
S Sun and A Frick developed the study concept and initial data analysis. V Balasa and JC Roberts participated in the writing, review, and revision of the manuscript. All authors reviewed data and contributed to developing and editing the final manuscript. All authors gave final approval of this version to be published and agree to be accountable for all aspects of this work.
Acknowledgments
Writing assistance was provided by Zela Keuylian of Parexel, with funding from Takeda (Takeda Development Center Americas Inc., a Takeda company, Lexington, MA, USA).
Additional information
Funding
References
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158.
- Aledort L, Mannucci PM, Schramm W, et al. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17(6):479–486.
- ADYNOVATE (Antihemophilic Factor (Recombinant)) [ package insert]. Shire Website; [ updated 2021 Jun; cited 2021 Sept 1. Available from: https://www.shirecontent.com/PI/PDFs/ADYNOVATE_USA_ENG.pdf
- Konkle BA, Stasyshyn O, Chowdary P, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078–1085.
- Mullins ES, Stasyshyn O, Alvarez-Roman MT, et al. Extended half-life pegylated, full-length recombinant factor VIII for prophylaxis in children with severe haemophilia A. Haemophilia. 2017;23(2):238–246.
- Aledort L, Milligan S, Watt M, et al. A retrospective observational study of rurioctocog alfa pegol in clinical practice in the United States. J Manag Care Spec Pharm. 2020;26(4):492–503.
- Journeycake J, Caicedo J, Cheng D, et al. Clinical outcomes in patients with haemophilia A after switching to rurioctocog alfa pegol prophylaxis in the ATHN 2 study. Haemophilia. 2021;27(suppl 2):ABS070.
- HEMLIBRA (emicizumab-kxwh) injection. Prescribing information. South San Francisco, CA: Genentech, Inc; 2021.
- Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811–822.
- McCary I, Guelcher C, Kuhn J, et al. Real-world use of emicizumab in patients with haemophilia A: bleeding outcomes and surgical procedures. Haemophilia. 2020;26(4):631–636.
- Institute for Clinical and Economic Review. Valoctocogene roxaparvovec and emicizumab for hemophilia A without inhibitors: final policy recommendations; 2020 [cited 2021 Sept 20]. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_Hemophilia-A_Key-Policy-Recommendations_112020.pdf
- Kruse-Jarres R, Oldenburg J, Santagostino E, et al. Bleeding and safety outcomes in persons with haemophilia A without inhibitors: results from a prospective non-interventional study in a real-world setting. Haemophilia. 2019;25(2):213–220.
- Reyes A, Revil C, Niggli M, et al. Efficacy of emicizumab prophylaxis versus factor VIII prophylaxis for treatment of hemophilia A without inhibitors: network meta-analysis and sub-group analyses of the intra-patient comparison of the HAVEN 3 trial. Curr Med Res Opin. 2019;35(12):2079–2087.
- Le Quellec S. Clinical evidence and safety profile of emicizumab for the management of children with hemophilia A. Drug Des Devel Ther. 2020;14:469–481.
- Zhou ZY, Raimundo K, Patel AM, et al. Model of short- and long-term outcomes of emicizumab prophylaxis treatment for persons with hemophilia A. J Manag Care Spec Pharm. 2020;26(9):1109–1120.