ABSTRACT
Introduction
Monochorionic twins may develop fetal anemia when blood is unequally distributed via the placental vascular anastomoses. This review focuses on the causes of fetal anemia in complicated monochorionic twins and highlights the differences in management and outcome.
Areas covered
Fetal anemia can occur in the context of twin anemia polycythemia sequence (TAPS), chronic twin–twin transfusion syndrome (TTTS) and acute peripartum TTTS, and in cotwins after single fetal demise. Diagnosis of fetal anemia is based on abnormal Doppler ultrasound measurements. Management options include fetoscopic laser surgery, intrauterine blood transfusion, or expectant management, depending on the type of complication and the severity of the disease. In all complications, fetal anemia may lead to perinatal mortality, neonatal morbidity, severe cerebral injury, and long-term neurodevelopmental impairment. In TAPS specifically, anemic donors may also show bilateral deafness.
Expert opinion
Knowledge on the diagnosis and optimal treatment in TTTS is nowadays widespread, but caregivers often fail to distinguish TAPS from acute peripartum TTTS at birth. A full blood count including reticulocyte count is required, and placental dye injection is extremely helpful to reach the correct diagnosis and establish the optimal management.
1. Introduction
Fetal anemia is a rare, but serious condition in the unborn child, which can result in severe cerebral damage and perinatal death. Fetal anemia can be caused by immune-related causes, such as hemolytic disease of the fetus and newborn, and nonimmune related causes including Parvo B12 infection, hemoglobinopathies, and feto-maternal transfusion. Monochorionic (MC) twin pregnancies are exposed to these risks as well, but have an even higher chance to develop fetal anemia, because they share one fetal blood circulation, which can lead to various complications. In this review, we will describe the pathophysiology, diagnosis, management options, and outcome for the most important conditions responsible for fetal anemia in MC twins.
2. MC twins
There are two types of twins: dizygotic (fraternal) and monozygotic (identical). Dizygotic twins always have their own placenta (dichorionic twins). In monozygotic twins, the number of placentas depends on the timing of embryonic division. When division occurs within the first 3 days after fertilization, the identical twins will be dichorionic as well and have their own placenta. In two-third of the identical twins, division occurs after 3 days from fertilization, resulting in a MC twin pregnancy, meaning that the fetuses share a placenta and have their blood circulation connected to each other by vascular connections at the placental surface. In contrast, dichorionic twins almost never have vascular connections. These vascular connections, also called anastomoses, allow blood to flow bidirectionally between the two fetuses. There are generally two types of anastomoses: unidirectional (arterio-venous (AV), veno-arterial (VA)), and bidirectional (arterio-arterial (AA)/veno-venous (VV)). AV anastomoses are also called deep anastomoses as they run deep through capillary networks in a placental cotyledon. AA and VV anastomoses are called superficial anastomoses as they remain on the placental surface of the placenta without running through a cotyledon. In contrast to deep anastomoses, superficial anastomoses have a very low vascular resistance allowing blood to flow more freely from one side of the placenta to the other. In approximately 15% of MC twins, the anastomoses can lead to unequal blood distribution between the two fetuses [Citation1]. Depending on the size and type of the anastomoses, this can lead to different types of life-threatening conditions, which can all present with fetal anemia. If the anastomoses are small (and particularly of the AV type), this can lead to twin anemia polycythemia sequence (TAPS) [Citation2]. If these AV anastomoses are relatively large, unequal blood flow can result in chronic twin–twin transfusion syndrome (TTTS). Acute peripartum TTTS can occur only in the presence of large superficial AA or VV anastomoses allowing large amount of blood to flow quickly from one twin to the other. Fetal anemia can also occur after single fetal demise due to acute exsanguination in the cotwin. An overview of the prevalence of fetal anemia and type of fetal anemia for the different complications can be viewed in .
Table 1. Prevalence and type of fetal anemia for different types of MC twin complications.
3. TAPS
TAPS is the most prevalent cause of fetal anemia in MC twins. The condition is relatively new (and therefore sometimes mistaken for chronic TTTS). The pathophysiology was first described by our research group in 2006 [Citation2]. TAPS arises from slow unbalanced blood transfusion through minuscule vascular AV anastomoses at the placental surface, causing the donor twin to become chronically anemic and the recipient twin to become chronically polycythemic. TAPS may develop spontaneously in 3–5% of MC twins (spontaneous TAPS), or can occur iatrogenically in up to 16% of TTTS twins treated with laser surgery, when minuscule anastomoses are missed (post-laser TAPS) [Citation3–6]. As TAPS develops without maternal complaints, active ultrasound screening for fetal anemia and polycythemia is crucial. Antenatal identification is achieved by performing ultrasound Doppler examinations measuring the middle cerebral artery peak systolic velocity (MCA-PSV), a method that was introduced by Mari et al. to detect fetal anemia based on erythrocyte allommunization in singletons [Citation7]. Klaritsch et al. proposed MCA-PSV reference ranges for MC twins [Citation8]. In TAPS, the MCA-PSV value in the donor will be high, as a sign of a hyperdynamic blood circulation caused by chronic anemia. In the recipient twin, the MCA-PSV will be lower due to chronic polycythemia. An MCA-PSV difference >0.5 multiples of the median (MoM) is indicative of TAPS. TAPS can be classified according to the classification system by Tollenaar et al. [Citation9] (). As TAPS is a rare disease, studies investigating the best MCA-PSV criteria in TAPS are generally based on small numbers, hampering the generalizability of the results. Therefore, the most optimal MCA-PSV criterion of the diagnosis of TAPS is still under investigation. Aside from abnormal MCA-PSV values, TAPS twins frequently show additional ultrasound markers that can support the diagnosis () [Citation10]. First, the placenta in TAPS can have a dichotomous appearance with a hyperechogenic, hydropic placental territory for the TAPS donor and a hypoechogenic flattened placental territory for the TAPS recipient. Second, TAPS donors can demonstrate additional signs of anemia such as echogenic bowels and cardiomegaly, as the anemic and hypoxic environment demands higher cardiac output to be able to supply the body with enough oxygen. Third, in TAPS recipients a starry-sky aspect of the liver can be observed, with clearly identified hyperechogenic portal venules that project star-like against a hypoechogenic liver parenchyma.
Figure 1. (a) Sonographic image of placental dichotomy, with hyperechogenic hydropic placental share for the TAPS donor and flattened hypoechogenic placental share for the TAPS recipient. (b) Cardiomegaly in the TAPS donor. (c) Starry-sky liver in the TAPS recipient.
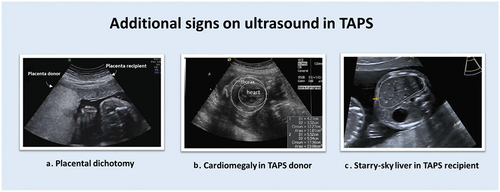
Table 2. Antenatal classification system for TAPS.
Antenatal management options for TAPS include expectant management, prenatal delivery, intrauterine transfusion (IUT) with or without a partial exchange transfusion (PET), fetoscopic laser surgery, or selective reduction. With expectant management, no intrauterine treatment is performed, but the twins are closely monitored with MCA-PSV measurements. Expectant management is usually opted in mild or stable cases of TAPS. Preterm delivery can be a choice when intrauterine treatment is not feasible and when prolonging the TAPS pregnancy is expected to be more detrimental to the health of the twins than the consequences of prematurity. With an IUT, the TAPS donor will be provided with red blood cells to temporarily correct fetal anemia. In case of severe polycythemia in the recipient twin, an IUT in the donor can be combined with a PET in the recipient to reduce the hyperviscosity/polycythemia. During a PET, 5–10 ml of the recipient’s blood will be removed slowly and will be preplaced with saline, repeatedly. IUT (with PET) is not a definitive treatment, and only a temporary solution and therefore reintervention might be required. Fetoscopic laser surgery is the only treatment option that tackles the cause of TAPS. Laser surgery is an endoscopic intrauterine procedure during which the tiny anastomoses are identified and then coagulated. Selective reduction can be considered in severe cases of TAPS, when there are structural anomalies, or when other treatment options are infeasible, and is aimed at sacrificing one twin in order to increase the chances for healthy survival in the cotwin. The best treatment option for TAPS is still under investigation. We are currently performing an international multicenter randomized controlled trial (TAPS trial), comparing laser surgery to standard treatment (IUT/(±PET), expectant management, preterm delivery) (anonymized) (Clincialtrials.org number NCT04432168)
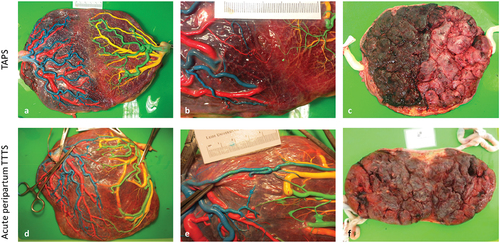
At birth, TAPS has a classical clinical presentation with a pale anemic TAPS donor and a plethoric polycythemic TAPS recipient (). Some cases of TAPS are still missed antenatally. This can be due to lacking MCA-PSV measurements in the routine follow-up of MC twins, due to engagement of the fetal head into the pelvic canal which hampers adequate MCA-PSV assessment, or due to false-negative MCA-PSV measurements. Therefore, postnatal criteria to diagnose TAPS have also been established. The first criterion is an inter-twin hemoglobin (Hb) difference >8 g/dL [Citation11]. To distinguish TAPS from the similarly presenting acute peripartum TTTS, two additional criteria have been added. The first is a reticulocyte count ratio >1.7. The reticulocyte count ratio can be calculated by dividing the reticulocyte count (in promille (‰) or percentage (%), not in absolute values) of the donor twin by the reticulocyte count of the recipient twin. In TAPS, there will be reticulocytosis in the donor twin due to chronic anemia, resulting in an increased ratio. The other additional criterion is the presence of only minuscule (diameter <1 mm) placental anastomoses, mainly AV anastomoses, detected through color dye injection of the placenta ()[Citation2]. AA are found in 19% of TAPS placentas [Citation12]. VV anastomoses are even more scarce and are detected in 7%. The maternal side of the placenta typically shows a color difference, with a pale (sometimes hydropic) placental share for the donor twin and a plethoric placental share for the recipient twin [Citation13].
Figure 2. A MC twin pair with spontaneous twin anemia polycythemia sequence illustrating the characteristic large skin color difference.
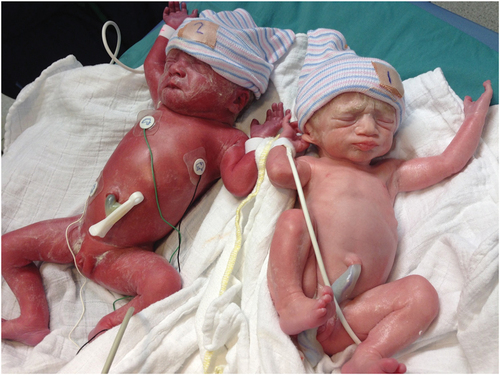
Figure 3. An overview of placental differences between TAPS (upper row) and acute peripartum TTTS (lower row). Placentas have been injected with colored dye. Blue (arteries) and red (veins) were used for the first-born twin and green (arteries) and yellow (veins) were used for the second-born twin. (a) Fetal side of the a placenta from spontaneous TAPS. The left placental share belongs to the recipient twin, the right placental share belongs to the donor twin. (b) Color dye injection revealed only one minuscule veno-arterial anastomosis (red-green). (c) The maternal side of the TAPS placenta shows a striking color difference with a plethoric share for the recipient and a pale share for the donor. (d) Fetal side of a placenta from a acute peripartum TTTS twin. The left placental share belongs to the recipient twin, the left placental share to the donor twin. (e) Color dye injection revealed numerous large anastomoses including an arterio-arterial anastomosis (blue-green). (f) The maternal side of the acute peripartum TTTS placenta shows no color difference.
Perinatal mortality in TAPS occurs in 9% of spontaneous TAPS twins and in 18% of post-laser TAPS twins, with TAPS donors having a fourfold increased risk [Citation12,Citation14]. Neonatal complications in TAPS range from isolated Hb differences to severe cerebral injury and even neonatal death. TAPS donor twins may be born severely anemic, and might need multiple red blood cell transfusions to treat anemia. Importantly, as donor twins have been chronically suffering from anemia, the transfusion needs to be administered slowly in order to prevent cardiac decompensation. In TAPS cases with mild anemia and sufficient erythropoiesis, blood transfusion might not be needed. Aside from anemia, TAPS donor twins can also suffer from hypoalbuminemia and low total protein, possibly as a result of chronic loss into the recipients’ circulation [Citation15]. Moreover, TAPS donors more frequently born with leukopenia than TAPS recipients, with an associated increased risk for early onset neonatal sepsis [Citation16]. TAPS donors can also suffer from metabolic complications at birth. A recent study from our center showed that TAPS donors have lower pH values and higher lactate in the first hours after birth, likely as a reflection of chronic intrauterine hypoxia due to anemia [Citation17]. Additionally, TAPS donors are more often diagnosed with hypoglycemia in the first 24 h after birth. Lastly, short-term renal disfunction has also been described in approximately a quarter of TAPS donors [Citation18].
TAPS recipients on the other hand might suffer from polycythemia hyperviscosity syndrome, needing hyperhydration therapy, or in more severe cases, requiring a partial exchange transfusion. Polycythemia hyperviscosity syndrome might in rare situations also result in skin necrosis and limb ischemia [Citation19]. In addition, TAPS recipients often present with thrombocytopenia at birth, possibly due to impaired bone marrow production secondary to tissue hypoxia and impaired spleen perfusion [Citation11]. Both anemia and polycythemia might lead to severe brain damage. This is reported in 4% of spontaneous TAPS twins and in 11% of post-laser TAPS twins, with similar risks for donor and recipients [Citation12,Citation14]. As most TAPS twins are delivered prematurely (at a median gestational age of 32 weeks), they might suffer from associated severe neonatal sequalae in up to 40% [Citation12,Citation14]. Long-term follow-up in spontaneous TAPS twins shows that especially donors are at risk for severe neurodevelopmental impairment (18% in donors compared to 3% of recipients) [Citation20]. Aside from cognitive delay (mild or severe), which is seen in 41% of donors, 15% of donors also suffered from bilateral deafness. Deafness always occurred in the form of auditory neuropathy spectrum disorder (ANSD), in which the outer ear is intact, but the inner hair cells, connecting synapses, or auditory nerve is damaged. This form of deafness is only detected with automatic auditory brainstem response (AABR) at birth. Importantly, ANSD will be missed with the standard neonatal hearing screening using otoacoustic emissions. To facilitate adequate treatment and prevent a delay in speech and language development, neonatal AABR screening for all twins diagnosed with TAPS is strongly recommended.
4. TTTS
There are two types of TTTS: chronic TTTS and acute peripartum TTTS. Chronic TTTS, generally referred to as simply ‘TTTS,’ is a condition that develops in the second trimester of pregnancy [Citation1]. Acute peripartum TTTS is a rarer form of TTTS, which may develop during delivery in previously uncomplicated MC twin pregnancies [Citation21]. In both conditions, fetal anemia can be detected.
4.1. Chronic TTTS
Chronic TTTS develops due to unbalanced transfusion through relatively large placental anastomoses at the surface of the placenta [Citation22]. This causes the TTTS donor to become hypovolemic, leading to oliguria and oligohydramnios or even anhydramnios. The excess of blood in the TTTS recipient twins results in hypervolemia, causing polyuria and polyhydramnios. Moreover, hypervolemia may also lead to cardiac decompensation in the recipient. Chronic TTTS can develop in up to 10% of MC pregnancies [Citation1]. The time of onset ranges between 14 and 30 weeks of gestation, with a median gestation of 20 weeks. The difference in amniotic fluid, also called twin oligohydraminos polyhydramnios sequence (TOPS), is pathognomic for TTTS. The severity of the condition is antenatally classified according to the Eurofetus or Quintero criteria [Citation23,Citation24]. As chronic TTTS concerns a large, but equal loss of erythrocytes and plasma, TTTS donors are hypovolemic, but often not anemic [Citation25]. However, a subgroup of TTTS cases may present with anemia in the TTTS donor twin, and polycythemia in the TTTS recipient twin [Citation26–28]. Similar to TAPS, TTTS twins may show a large MCA-PSV difference between the fetuses, combined with cardiomegaly in the donor twin, starry sky liver in the recipient twin, and placental dichotomy [Citation28]. Multiple research groups have investigated the prevalence of anemia–polycythemia (AP) in TTTS cases prior and found that it ranged from 2.4% to 15%, depending on MCA-PSV criteria [Citation26–28]. Interestingly, TTTS+AP cases had a later diagnosis of TTTS, and demonstrated a lower number and smaller size of anastomoses, comparable with the pathophysiology and placenta angioarchitecture of TAPS [Citation26–28]. The best treatment for TTTS – regardless of the presence of anemia in the TTTS donor twin – is fetoscopic laser coagulation of the placental anastomoses. Laser is the best option in cases with a stage 2 or higher [Citation29], and in TTTS stage 1 with maternal complaints or cervical shortening [Citation30]. The preferred coagulation technique is the Solomon technique, in which the anastomoses are coagulated one by one, whereafter a laser line is drawn from one placental margin to the other, connecting all coagulation spots. This approach reduces the chance of recurrent TTTS and post-laser TAPS [Citation6]. After laser surgery, some twins might show an MCA-PSV >1.5 MoM in one of the twins, suggestive of fetal anemia due to a missed anastomosis [Citation31]. However, most of the time this is not due to an incomplete laser, but due to hemodynamic re-equilibration after separation of the shared circulation. If the laser is complete, the high MCA-PSV may return to normal within weeks. The introduction of fetoscopic laser surgery has drastically improved the prognosis of TTTS. Without treatment, the mortality ranged from 75% to 100% [Citation32]. Now, overall survival is around 75%; survival of at least one survivor is reported in 85–87% of cases, and in 60–64% both children may survive [Citation6,Citation29]. In late cases of TTTS (gestation >28 weeks), laser surgery could be considered but is often not preferred, due to the large distance to the placenta, the blurry amniotic fluid, and the size of the anastomoses. In those cases, amniodrainage can be performed to prolong pregnancy. When fetoscopic laser surgery is technically infeasible, or in case of brain injury or congenital abnormalities, selective reduction can be an option to increase the chances for healthy survival in the cotwin. After laser treatment, the twins are usually delivered at a median gestational age of 32 weeks.
Color-dye injection of the placenta in TTTS is vital to understand the pathogenesis of the disease. In TTTS, there are relatively large AV anastomoses [Citation22]. AA anastomoses are scarce in TTTS [Citation33]. It is hypothesized that AA anastomoses can compensate for differences in blood volumes due to their bidirectional character. The absence of an AA anastomosis can therefore potentially contribute to the onset and progression of TTTS. In contrast, VV anastomoses are more frequently found in TTTS placentas than in placentas from uncomplicated MC twins, but their exact role in the onset of the condition is not well understood [Citation34]. In cases treated with laser surgery, examination of the laser line is important to check for completeness of the procedure. In case of post-laser TAPS, one should look for minuscule anastomoses that are usually located at the margin of the placenta, where they are more easily missed [Citation35].
Although outcome in TTTS has improved, neonatal morbidity still remains twice as high compared to the outcome of uncomplicated MC twins (26% vs. 13%, respectively) [Citation36]. Neonatal morbidity in TTTS treated with laser is mostly related to the degree of prematurity and includes respiratory distress syndrome, patent ductus arteriosus, necrotizing enterocolitis, and retinopathy of prematurity. Severe cerebral injury is detected in 5–10% of the treated TTTS survivors and can be a consequence of the TTTS itself or can be related to prematurity [Citation36,Citation37]. We therefore recommend routine cranial ultrasound examinations in all TTTS survivors at birth to rule out a severe cerebral injury. Neonatal mortality still occurs in approximately 5–8% of liveborn infants treated with laser, mostly as the result of extreme prematurity [Citation6,Citation36]. Severe long-term neurodevelopmental impairment is seen in 12% of TTTS survivors treated with laser surgery, with no difference between donor and recipients [Citation38,Citation39].
4.2. Acute peripartum TTTS
Acute peripartum TTTS is a rare complication that can develop in up to 2% of MC twins during delivery [Citation21]. In acute peripartum TTTS, there is an abrupt shift of blood through large bidirectional superficial anastomoses from one fetus to the other, causing the donor twin to become anemic and hypovolemic, and the recipient twin to become polycythemic and hypervolemic. The underlying mechanism for this acute transfer of blood is not fully understood. A possible explanation might be that the uterine contractions combined with relative positioning of the fetuses might result in sudden differences in blood pressure, thereby generating a pressure gradient that affects the blood flow between the twins. Strict antenatal diagnostic criteria have not been established for acute peripartum TTTS. Since the onset of the condition occurs very quickly, there is no TOPS. Cardiotocography (CTG) registration during labor may show a sinusoidal pattern in the donor twin, indicating fetal anemia. However, not all cases of acute peripartum TTTS show abnormalities on CTG registration, so absence of this should not rule out the diagnosis.
At birth, the donor twin in acute peripartum TTTS is pale and might suffer from hypovolemic shock. Neonatal management in acute peripartum TTTS donors differs from management in TAPS donors. Whereas TAPS donors should be transfused slowly to prevent cardiac decompensation, acute peripartum TTTS donors require prompt fluid resuscitation (including blood transfusion(s)) in order to restore the blood circulation. The recipient twin in acute peripartum TTTS is born with a plethoric skin color and might need a partial exchange transfusion to treat polycythemia. As neonatal management vastly differs between TAPS and acute peripartum TTTS, distinction at birth is crucial. The quickest way to get a hint toward the right diagnosis is by inspecting the maternal side of the placenta, shortly after birth of the twins. In contrast to TAPS, the maternal side of the placenta in acute peripartum TTTS will be equally colored and will not show redness discordance ()[Citation40]. After this examination, the placenta can be injected with color dye. Whereas TAPS is characterized by only minuscule anastomoses, in acute peripartum TTTS there needs to be at least one large (diameter >1 mm) bidirectional anastomosis (AA or VV) at the placental surface that allows a large and rapid transfusion [Citation21]. Furthermore, in acute peripartum TTTS, there is no reticulocytosis in the anemic donor, as the abrupt onset of anemia did not allow erythropoiesis to take place. Therefore, reticulocyte count ratio is <1.7. Due to the rarity of the disease, studies investigating the outcome in acute peripartum TTTS are scarce. Neonatal morbidity in acute peripartum TTTS includes perinatal asphyxia in up to a third of the donor twins due to acute hemorrhaging [Citation21]. Neonatal mortality occurred in 8%. To date, there are no studies available on the long-term outcome of twins with acute peripartum TTTS.
5. Peri-mortem transfusion
Fetal anemia may also develop after single fetal demise (sFD) in an MC twin. When one of the twins dies, its heart will stop beating, causing an abrupt drop in blood pressure. This will create an unbalanced pressure gradient, leading to acute exsanguination from the living cotwin through placental anastomoses into the low-pressure circulation of the demised twin. In the surviving cotwin, this may result in hypovolemia, hypotonia, acute anemia, and hypoxic-ischemic shock. Consequently, perimortem transfusion is associated with high rates mortality and morbidity. Perinatal mortality occurs in 16% of cotwins after sFD [Citation41]. In 26–32% of surviving cotwins, severe cerebral injury is detected, which may present as hypoxic-ischemic lesions, hemorrhagic lesions, or other anomalies secondary to a vascular disturbance, such as optic nerve hypoplasia [Citation41–43]. In addition, acute perimortem transfusion may also lead to severe renal damage, with subsequent acute and chronic renal failure, potentially leading to neonatal death [Citation44,Citation45]. After single fetal demise, 23% of survivors may suffer from long-term neurodevelopmental impairment, mainly due to cerebral palsy [Citation41].
Interestingly, not all cotwins suffer from excessive hemorrhaging following sFD. Intrauterine blood sampling after single fetal demise showed that 50% of MC twin survivors show fetal anemia [Citation46]. Possibly, the type, size, and number of placental anastomoses play a role. Similar to TAPS and chronic TTTS, MCA-PSV ultrasound Doppler is used for diagnosis of fetal anemia after sFD. Antenatal management depends on the gestational age and the presence of fetal anemia [Citation46]. When the pregnancy is preterm and there are no signs of fetal anemia, a conservative strategy including close ultrasound monitoring is recommended. In preterm pregnancies with fetal anemia, a rescue IUT in the cotwin can be envisaged, but the benefit is questionable as IUT because usually too late to avoid the hypoxic brain injury, as acute exsanguination as already occured [Citation47]. Additionally, a fetal MRI at least 3 weeks after fetal death can be done to check for severe brain damage in the cotwin in all preterm cases. Immediate delivery after sFD is not advised in preterm gestations, as perinatal transition and (extreme) prematurity may potentially damage the already vulnerable brain of the cotwin. In sFD cases that are term or near term (close to 36 weeks), an IUT is usually technically challenging, and therefore a delivery – either induced or via cesarean section – should be pursued.
As sFD is associated with high rates of adverse outcome, preventive intrauterine treatment can be performed to reduce the risk of perinatal mortality and morbidity. In MC twins with imminent fetal death, a selective feticide (using radiofrequency ablation, bipolar cord occlusion, fetoscopic laser surgery, or interstitial laser coagulation) can be a management option to establish a relatively safe and controlled demise, thereby preventing exsanguination in the healthy cotwin. After selective reduction, perinatal mortality in the cotwin ranges up to 33%, and severe neonatal morbidity is 12% [Citation48].
6. Conclusion
In conclusion, MC twins are at increased risk for fetal anemia when blood is unequally distributed via the anastomoses at the surface of the shared placenta. Fetal anemia can be seen in the context of TAPS, chronic TTTS and acute peripartum TTTS, and in the cotwin after single fetal demise. Diagnosis of fetal anemia in MC twins is based on MCA-PSV Doppler measurements and may be accompanied by the presence of cardiomegaly, a hydropic hypoechogenic placenta, echogenic bowels, and fetal hydrops. In anemic donors in acute peripartum TTTS, CTG registration might show a sinusoidal pattern. Antenatal management of anemia in MC twins includes fetoscopic laser surgery, IUT, expectant management or delivery, depending on the type of complication and the severity of the disease. At birth, a full blood count including Hb and reticulocyte count (‰ or %) and placental examination is crucial to differentiate between TAPS and acute peripartum TTTS. In all complications, fetal anemia may lead to perinatal mortality, neonatal morbidity, severe cerebral damage, and long-term neurodevelopmental impairment. In TAPS specifically, anemic donors also show an increased risk of bilateral deafness. Routine postnatal follow-up including neonatal brain imaging, AABR hearing testing (only in TAPS), and long-term neurodevelopmental assessment is crucial to detect developmental abnormalities and to facilitate timely treatment.
7. Expert opinion
MC twins may develop fetal anemia when blood is unequally distributed via the vascular anastomoses at the surface of the shared placenta. Fetal anemia can be detected in the context of TAPS, chronic TTTS and acute peripartum TTTS, and in cotwins after single fetal demise. An increased MCA-PSV Doppler measurement is the cornerstone in the diagnosis of fetal anemia, supported by additional ultrasound signs including cardiomegaly, echogenic bowel, and fetal hydrops. Additionally, in TAPS, the placenta can show dichotomy. Treatment options for fetal anemia in MC twins may vary, depending on the complication. In TAPS, the best antenatal treatment is not clear. Management options include fetoscopic laser surgery, intrauterine blood transfusion, expectant management, or delivery. For chronic TTTS, the best treatment is laser surgery. In acute peripartum TTTS, prenatal treatment is not an option, as this complication occurs during labor. Fetal anemia in the co-twin after single fetal demise can be treated with a rescue IUT, although its benefit is doubtful as hypoxic brain injury has already occurred. To distinguish between TAPS and acute peripartum TTTS at birth, it is of utmost importance to perform a full blood count including hemoglobin and reticulocyte count (‰ or %). Furthermore, the maternal side of the placenta should be checked for redness discordance, and the placental vessels should be injected with color dye to investigate the type and size of anastomoses. In the past, most cases presenting at birth with an anemic and a polycythemic twin have often wrongly been labeled as due to acute peripartum TTTS. Given the higher prevalence of TAPS, the majority of these cases were probably due to TAPS. Nowadays, diagnosis of TAPS should be reached more frequently. However, TAPS is a relatively ‘new disease’ and the correct diagnosis is still often missed as many perinatologists throughout the world are yet unaware of this disease. In daily practice, reticulocyte counts are often not measured and only few centers perform placenta dye injection. Therefore a clear distinction between acute peripartum TTTS and TAPS cannot be made and a definitive diagnosis cannot be reached. More awareness should be created throughout the perinatal community worldwide in order to reach the correct diagnosis and to manage these cases promptly and adequately.
Finally, in all complications, fetal anemia may lead to perinatal mortality, neonatal morbidity, severe cerebral injury, and long-term neurodevelopmental impairment. In TAPS specifically, anemic donors may also show bilateral deafness. Routine postnatal follow-up including neonatal brain imaging, automated auditory brainstem response hearing testing (only in TAPS), and long-term neurodevelopmental assessment is crucial to detect developmental abnormalities and to facilitate timely treatment.
Article highlights
Monochorionic twins are often at risk of severe complications, including fetal anemia.
Fetal anemia can be due to anemia polycythemia sequence (TAPS), chronic twin–twin transfusion syndrome (TTTS) and acute peripartum TTTS, and in cotwins after single fetal demise.
Antenatal diagnosis can be reached through Doppler ultrasound measurements.
Postnatal diagnosis should include reticulocyte count measurements to distinguish between acute and chronic forms of anemia.
Placental dye injection is important to understand the causes and pathophysiology of the various complications.
Caregivers should be aware of the high risk (15%) of bilateral deafness in TAPS donors and perform the right test at birth (BERA) to avoid permanent speech development delay.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol. 2008;199(5):514 e1–8.
- Lopriore E, Middeldorp JM, Oepkes D, et al. Twin anemia-polycythemia sequence in two monochorionic twin pairs without oligo-polyhydramnios sequence. Placenta. 2007;28(1):47–51.
- Gucciardo L, Lewi L, Vaast P, et al. Twin anemia polycythemia sequence from a prenatal perspective. Prenat Diagn. 2010;30(5):438–442.
- Knijnenburg PJC, Slaghekke F, Tollenaar LSA, et al. Incidence of and risk factors for residual anastomoses in twin-twin transfusion syndrome treated with laser surgery: a 15-year single-center experience. Fetal Diagn Ther. 2019;45(1):13–20.
- Robyr R, Lewi L, Salomon LJ, et al. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2006;194(3):796–803.
- Slaghekke F, Lopriore E, Lewi L, et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: an open-label randomised controlled trial. Lancet. 2014;383(9935):2144–2151.
- Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative group for Doppler assessment of the blood velocity in anemic fetuses. N Engl J Med. 2000;342(1):9–14.
- Klaritsch P, Deprest J, Van Mieghem T, et al. Reference ranges for middle cerebral artery peak systolic velocity in monochorionic diamniotic twins: a longitudinal study. Ultrasound Obstet Gynecol. 2009;34(2):149–154.
- Tollenaar LSA, Lopriore E, Middeldorp JM, et al. Improved prediction of twin anemia-polycythemia sequence by delta middle cerebral artery peak systolic velocity: new antenatal classification system. Ultrasound Obstet Gynecol. 2019;53(6):788–793.
- Tollenaar LSA, Lopriore E, Middeldorp JM, et al. Prevalence of placental dichotomy, fetal cardiomegaly and starry-sky liver in twin anemia polycythemia sequence. Ultrasound Obstet Gynecol. 2019;53(6):788–793. 10.1002/uog.20096.
- Lopriore E, Slaghekke F, Oepkes D, et al. Hematological characteristics in neonates with twin anemia-polycythemia sequence (TAPS). Prenat Diagn. 2010;30(3):251–255.
- Tollenaar LSA, Slaghekke F, Lewi L, et al. Spontaneous twin anemia polycythemia sequence: diagnosis, management, and outcome in an international cohort of 249 cases. Am J Obstet Gynecol. 2021;224(2):213.e1–213.e11.
- Tollenaar LS, Zhao DP, Middeldorp JM, et al. Color difference in placentas with twin anemia-polycythemia sequence: an additional diagnostic criterion? Fetal Diagn Ther. 2016;40(2):123–127.
- Tollenaar LSA, Lopriore E, Stefano F, et al. Post-laser twin anemia polycythemia sequence: diagnosis, management, and outcome in an international cohort of 164 cases. J Clin Med. 2020;9(6):1759.
- Verbeek L, Slaghekke F, Hulzebos CV, et al. Hypoalbuminemia in donors with twin anemia-polycythemia sequence: a matched case-control study. Fetal Diagn Ther. 2013;33(4):241–245.
- Visser GL, Tollenaar LA, Bekker V, et al. Leukocyte Counts and other hematological values in twin-twin transfusion syndrome and twin anemia-polycythemia sequence. Fetal Diagn Ther. 2020;47(2):123–128.
- van de Sande MJA, Lopriore E, Verweij EJT, et al. Lactate acidosis and hypoglycaemia in twin anaemia polycythemia sequence donors. Arch Dis Child Fetal Neonatal Ed. 2022;fetalneonatal-2022–323964. 10.1136/archdischild-2022-323964.
- Verbeek L, Slaghekke F, Favre R, et al. Short-TERM postnatal renal function in twin anemia-polycythemia sequence. Fetal Diagn Ther. 2016;39(3):192–197.
- Lopriore E, Lewi L, Oepkes D, et al. In utero acquired limb ischemia in monochorionic twins with and without twin-to-twin transfusion syndrome. Prenat Diagn. 2008;28(9):800–804.
- Tollenaar LSA, Lopriore E, Slaghekke F, et al. High risk of long-term neurodevelopmental impairment in donor twins with spontaneous twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol. 2020;55(1):39–46.
- Lopriore E, Holtkamp N, Sueters M, et al. Acute peripartum twin-twin transfusion syndrome: incidence, risk factors, placental characteristics and neonatal outcome. J Obstet Gynaecol Res. 2014;40(1):18–24.
- Zhao DP, de Villiers SF, Slaghekke F, et al. Prevalence, size, number and localization of vascular anastomoses in monochorionic placentas. Placenta. 2013;34(7):589–593.
- Society for Maternal-Fetal M, Simpson LL. Twin-twin transfusion syndrome. Am J Obstet Gynecol. 2013;208(1):3–18.
- Quintero RA, Dickinson JE, Morales WJ, et al. Stage-based treatment of twin-twin transfusion syndrome. Am J Obstet Gynecol. 2003;188(5):1333–1340.
- Denbow M, Fogliani R, Kyle P, et al. Haematological indices at fetal blood sampling in monochorionic pregnancies complicated by feto-fetal transfusion syndrome. Prenat Diagn. 1998;18(9):941–946.
- Donepudi R, Papanna R, Snowise S, et al. Does anemia-polycythemia complicating twin-twin transfusion syndrome affect outcome after fetoscopic laser surgery? Ultrasound Obstet Gynecol. 2016;47(3):340–344.
- Van Winden KR, Quintero R, Kontopoulos EV, et al. Pre-operative twin anemia/polycythemia in the setting of twin-twin transfusion syndrome (TTTS). Fetal Diagn Ther. 2015;37(4):274–280.
- Tollenaar LSA, Slaghekke F, Van Klink JMM, et al. Twin-twin transfusion syndrome with anemia-polycythemia: prevalence, characteristics, and outcome. J Clin Med. 2019;8(8):1129.
- Senat MV, Deprest J, Boulvain M, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004;351(2):136–144.
- Stirnemann J, Slaghekke F, Khalek N, et al. Intrauterine fetoscopic laser surgery versus expectant management in stage 1 twin-to-twin transfusion syndrome: an international randomized trial. Am J Obstet Gynecol. 2021;224(5):528 e1–528 e12.
- Gijtenbeek M, Haak MC, Huberts TJP, et al. Perioperative fetal hemodynamic changes in twin-twin transfusion syndrome and neurodevelopmental outcome at two years of age. Prenat Diagn. 2020;40(7):825–830.
- Berghella V, Kaufmann M. Natural history of twin-twin transfusion syndrome. J Reprod Med. 2001;46(5):480–484.
- de Villiers SF, Slaghekke F, Middeldorp JM, et al. Arterio-arterial vascular anastomoses in monochorionic placentas with and without twin-twin transfusion syndrome. Placenta. 2012;33(8):652–654.
- Zhao DP, Cambiaso O, Otaño L, et al. Veno-venous anastomoses in twin-twin transfusion syndrome: a multicenter study. Placenta. 2015;36(8):911–914.
- de Villiers SF, Slaghekke F, Middeldorp JM, et al. Placental characteristics in monochorionic twins with spontaneous versus post-laser twin anemia-polycythemia sequence. Placenta. 2013;34(5):456–459.
- Lopriore E, Middeldorp JM, Sueters M, et al. Neonatal outcome in twin-to-twin transfusion syndrome treated with fetoscopic laser occlusion of vascular anastomoses. J Pediatr. 2005;147(5):597–602.
- Spruijt M, Steggerda S, Rath M, et al. Cerebral injury in twin-twin transfusion syndrome treated with fetoscopic laser surgery. Obstet Gynecol. 2012;120(1):15–20.
- Knijnenburg PJC, Spruijt MS, Jansen L, et al. Neurodevelopmental trajectories of preterm born survivors of twin-twin transfusion syndrome: from birth to 5 years of age. J Pediatr. 2022 e1;240:51–57.
- Spruijt MS, Lopriore E, Tan RNGB, et al. Long-Term neurodevelopmental outcome in twin-to-twin transfusion syndrome: is there still room for improvement? J Clin Med. 2019;8(8):1226.
- Tollenaar LSA, Zhao DP, Middeldorp JM, et al. Can color difference on the maternal side of the placenta distinguish between acute peripartum twin-twin transfusion syndrome and twin anemia-polycythemia sequence? Placenta. 2017;57:189–193.
- Hillman SC, Morris RK, Kilby MD. Co-twin prognosis after single fetal death: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(4):928–940.
- Hu LS, Caire J, Twickler DM. MR findings of complicated multifetal gestations. Pediatr Radiol. 2006;36(1):76–81.
- van Klink JM, van Steenis A, Steggerda SJ, et al. Single fetal demise in monochorionic pregnancies: incidence and patterns of cerebral injury. Ultrasound Obstet Gynecol. 2015;45(3):294–300.
- Genova L, Sueters M, van Steenis A, et al. Renal failure after single fetal demise in monochorionic twins: incidence and description of a case. Fetal Diagn Ther. 2014;35(4):302–305.
- Lin IJ, Chen CH, Wang TM, et al. Infants of twin pregnancies with one twin demise in the uterus: a retrospective study. Acta Paediatr Taiwan. 1999;40(2):92–96.
- Senat MV, Bernard J-P, Loizeau S, et al. Management of single fetal death in twin-to-twin transfusion syndrome: a role for fetal blood sampling. Ultrasound Obstet Gynecol. 2002;20(4):360–363.
- Tedjawirja VN, van Klink JM, Haak MC, et al. Questionable benefit of intrauterine transfusion following single fetal death in monochorionic twin pregnancy. Ultrasound Obstet Gynecol. 2022;59(6):824–825.
- van den Bos EM, van Klink JMM, Middeldorp JM, et al. Perinatal outcome after selective feticide in monochorionic twin pregnancies. Ultrasound Obstet Gynecol. 2013;41(6):653–658.
