ABSTRACT
Background
The National Hemophilia Foundation (NHF) conducted extensive, inclusive community consultations to guide prioritization of research in coming decades in alignment with its mission to find cures and address and prevent complications enabling people and families with blood disorders to thrive.
Research Design and Methods
With the American Thrombosis and Hemostasis Network, NHF recruited multidisciplinary expert working groups (WG) to distill the community-identified priorities into concrete research questions and score their feasibility, impact, and risk. WG6 was charged with identifying the infrastructure, workforce development, and funding and resources to facilitate the prioritized research. Community input on conclusions was gathered at the NHF State of the Science Research Summit.
Results
WG6 detailed a minimal research capacity infrastructure threshold, and opportunities to enable its attainment, for bleeding disorders centers to participate in prospective, multicenter national registries. They identified challenges and opportunities to recruit, retain, and train the diverse multidisciplinary care and research workforce required into the future. Innovative collaborative approaches to trial design, resource networking, and funding to surmount obstacles facing research in rare disorders were elucidated.
Conclusions
The innovations in infrastructure, workforce development, and resources and funding proposed herein may contribute to facilitating a National Research Blueprint for Inherited Bleeding Disorders.
Plain Language Summary
Research is critical to advancing the diagnosis and care of people with inherited bleeding disorders (PWIBD). This research requires significant infrastructure, including people and resources. Hemophilia treatment centers (HTC) need many different skilled care professionals including doctors, nurses, and other providers; also statisticians, data managers, and other experts to process patients’ clinical information into research. Attracting diverse qualified professionals to the clinical and research work requires long-term planning, recruiting individuals in training programs and retaining them as they become experts. Research infrastructure includes physical servers running database software, networks that link them, and the environment in which these components function. US Centers for Disease Control and Prevention (CDC) and American Thrombosis and Hemostasis Network (ATHN) coordinate and fund data collection at HTCs on the health and well-being of thousands of PWIBD into a registry used in research studies.
National Hemophilia Foundation (NHF) and ATHN asked our group of health care professionals, technology experts, and lived experience experts (LEE) to identify the infrastructure, workforce, and resources needed to do the research most important to PWIBD. We identified the types of CDC/ATHN studies all HTCs should be able to perform, and the physical and human infrastructure this requires. We prioritized finding the best clinical trial designs to study inherited bleeding disorders, identifying ways to share personnel and tools between HTCs, and innovating how research is governed and funded. Involving LEEs in designing, managing, and carrying out research will be key in conducting research to improve the lives of PWIBD.
1. Introduction
1.1. Inherited bleeding disorders care and research networks
Hemophilia treatment centers (HTC) in the US have evolved to provide specialized multidisciplinary care not only to boys and men with hemophilia, but also a broad spectrum of individuals with inherited bleeding disorders (BD) [Citation1–3]. HTCs partner with people with inherited BDs [Citation4] through shared decision-making [Citation2,Citation5] with a core HTC team, composed of a medical director or hematologist, registered nurse, physical therapist, and social worker, and an extended team including an internist, pediatrician, orthopedic surgeon, oral surgeon/dentist, obstetrician/gynecologist, psychologist, genetic counselor, educational/vocational rehabilitation counselor, pharmacist, nutritionist, and visiting nurse [Citation2]. Standardized specialized coagulation laboratories and blood banks provide essential support to the comprehensive approach [Citation6,Citation7], which also features education, training, advocacy [Citation2], counseling, surveillance, and outpatient pharmacy services [Citation2,Citation5]. As at least 30% of people with inherited BDs receive care outside HTCs, by the term ‘HTC’ we include ‘non-HTC’ clinics, staff, and patients, and underscore the need to identify, engage, and include them in the inherited BDs research network.
The network of >140 centers of the US HTC Network (USHTCN) receives grant funding from the Health Resources and Services Administration (HRSA) [Citation8], and is organized into eight regions, each with a core center that disburses funds and ensures compliance with core grant objectives and requirements [Citation6–8]. Importantly, the majority of HTCs utilize revenue from the HRSA 340B Drug Pricing Program, providing pharmacy discounted medications, to finance 90% of support staff and services [Citation2,Citation9,Citation10]. The American Thrombosis and Hemostasis Network (ATHN) funds a secure national database, the ATHNdataest, and coordinates network data collection [Citation11] in collaboration with Centers for Disease Control and Prevention (CDC) [Citation12] to facilitate collaborative research at multiple HTCs. Their standardized integrated systems, including the ATHN Transcends natural history study with hemophilia, von Willebrand disease, and congenital platelet disorders cohorts, harmonizes data collection, simplifies contracts, utilizes a single institutional review board (IRB), and maintains a centralized specimen biorepository [Citation13].
1.2. Community-identified research priorities
The mission of the National Hemophilia Foundation (NHF), the largest patient advocacy organization serving people with inherited BDs, is to find cures and address and prevent complications through research, education, and advocacy enabling people and families to thrive [Citation14]. Recognizing research as a cornerstone in achieving this mission, in 2020 NHF initiated a collaboration with ATHN to develop a National Research Blueprint for Inherited Bleeding Disorders [Citation15], to prioritize and facilitate research most beneficial to its constituency [Citation16].
NHF and ATHN began with extensive, inclusive community consultations, soliciting input from people who live with inherited BDs and their families, true lived experience experts (LEE) [Citation17], physicians, nurses, other health care professionals (HCP), researchers, federal agency partners, advocacy organization leaders, foundations, and industry representatives, all invested in minimizing the burden of inherited BDs [Citation15,Citation18]. Analysis of resulting data by the NHF/ATHN State of the Science (SOS) Research Summit Steering Committee (SC) identified priorities pertaining to specific BDs and others spanning all inherited BDs [Citation15]. The need for a national research plan was clear; the SC recruited six multidisciplinary working groups (WG) to distill the top research questions from the community concerns in specific focus areas [Citation19–24].
Pursuing a national research blueprint requires a national research infrastructure, capacity building, and acculturation to facilitate and optimize this work, particularly [Citation15,Citation16,Citation18]:
Sustaining and expanding the comprehensive care model to improve access to care and strive toward health equity
Ensuring HTC financial stability to deliver comprehensive care and engage in research
National patient-centered data collection
Multidisciplinary team science drawing on expertise from within and outside the inherited BDs scientific community
Hypothesis-driven, feasible basic research, observational studies, clinical trials, and implementation trials
Expansion of an inclusive, diverse, and well-trained expert workforce, which practices cultural humility and linguistically appropriate care
Regional, national, and international collaborations
Strategic partnerships that leverage existing infrastructure and common goals
To explore these opportunities () the SC convened and charged WG6 with examining the key questions:
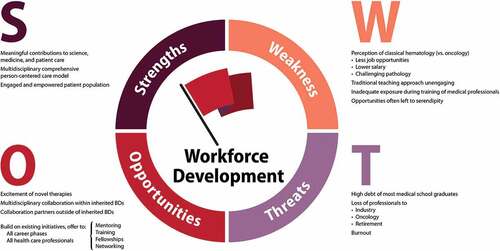
Figure 1. Working Group 6 Facilitating Priority Research in the Inherited Bleeding Disorders Community schematic of community-identified opportunities.BD: bleeding disorder, HTC: hemophilia treatment center, IRB: institutional review board, NHF: National Hemophilia Foundation
How can we build and fund a research network that is centered on care delivery and designed to reduce the burden of participation?
How can we encourage more trainees to join our professional community so that people with inherited BDs are assured care providers well into the future?
How can we retain and engage our current workforce?
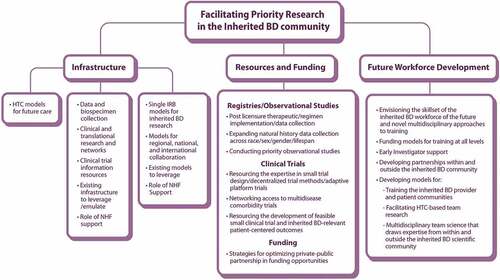
WG6 approached their task as three subgroups: Infrastructure, Workforce Development, and Resources and Funding, recognizing the significant overlap between these domains.
1.3. Infrastructure
WGs 1–5 [Citation19–23] underscored an urgent need for basic and translational research examining foundational biology, elucidating pathologies, developing diagnostics and therapeutics, and conducting large-scale natural history studies characterizing phenotype, genotype, and response to existing therapeutics across the lifespan. Facilitating this research requires a basic infrastructure framework [Citation25] as well as a research network, not limited to HTC comprehensive care teams, to develop and evaluate novel drugs in patient-centric clinical translational trials.
For this initiative to be successful, LEEs must be at the center of the research effort and the research team [Citation17]. The team includes investigators, research nurses, data managers, IRB coordinators, laboratory staff, and clinical research coordinators, with each HTC staffing these positions as their comprehensive team permits. They may avail themselves of a multiplicity of websites and digital tools including electronic medical records (EMR); electronic case report forms (eCRF); trial management software; interactive web response systems (IWRS); and local and/or central IRBs. Infrastructure elements can be shared across trials and sites to increase efficiency; each site’s infrastructure capacity, whether site-specific or through access to common infrastructure, will determine research participation.
1.4. Workforce development
To ensure and expand access to high quality care for all people with inherited BDs at HTCs [Citation2,Citation26–29], concerted efforts to recruit, train, and retain a diverse, highly skilled multidisciplinary workforce will be critical. The corps of adult and pediatric hematologists must be fortified to meet growing demand [Citation30–32]. The inherited BDs workforce must reflect community diversity to avoid perpetuating obstacles to diagnosis, treatment, and meaningful research [Citation19,Citation20]. Enhanced physician and provider knowledge and comfort discussing all aspects of BDs (e.g. menstruation) requires education and training for all levels of the workforce, including throughout HTCs, and the engagement of obstetricians/gynecologists in provision of care [Citation33–35]. Access to care must be promoted and its social determinants; such as living rurally, socioeconomic status, and race; examined to advance health equity for all [Citation36,Citation37]. Optimal care delivery will require enhanced sensitivity and understanding of the realities of marginalized and minoritized populations and their experiences with health care (e.g. transgender people, underserved minorities) [Citation20,Citation38,Citation39]. Researchers must demonstrate respect and sensitivity to socioeconomic demographics and determinants of health to build trust with underrepresented populations, encouraging participation in research studies that have meaning for them [Citation40,Citation41]. The inherited BDs workforce, and the professionals with whom they collaborate (e.g. pharmacists, nurses) must be recruited, educated, and trained to deliver health care and engage in research that is truly inclusive and equitable, inspires trust, and is meaningful to its constituents [Citation20].
The priorities identified by WGs 1–5 [Citation19–23] may best be addressed through research embedded in, or at least in collaboration with, HTCs, requiring further expansion of the HTC workforce. Professionals primarily engaged in care, (e.g. pharmacists, nurses) must be trained in research fundamentals and the specific tasks they can each contribute. Additional expertise such as laboratory scientists, data managers, statisticians, informaticians, etc. must be recruited to complement the care team. A culture that values research and the contributions of all team members, including LEEs, to research must be established. The research workforce must extend to collaborative networks sharing expertise, training, and resources (human, material, and financial) between HTCs and inherited BDs research initiatives, and further to related areas of investigation.
1.5. Resources and funding
Identifying, procuring, and managing the diverse, sustainable funding and other resources and recruiting, retaining, and training the workforce necessary to facilitate the prioritized inherited BDs research requires specific expertise, building upon successful models and innovating novel initiatives. Scientifically well-designed research studies, quality and uniform data collection, aligned and integrated sources and databases, use of technological advances to mine maximum information from existing records, and identification of the most meaningful metrics and predictive models all must be explored [Citation42,Citation43]. Innovations such as adaptive platform trials, embedding trials in medical records, and de-centralizing trial participation also offer opportunities to optimize the platforms and study designs that underpin research.
Well-managed partnerships with national data capture organizations and industry will help facilitate the conduct of post-approval surveillance studies. Important contributions from the inherited BDs community and its LEEs are essential to ensuring the research effort is feasible and meaningful. Stewarding these relationships; working to develop, engage, and amplify the community voice; and fostering an understanding of research are important investments [Citation17]. Though not new to health care, working with LEEs to design, perform, encourage and participate in research is new to BDs research teams [Citation44–46]. LEEs, clinicians, scientists, and HCPs all need support to form a functional and successful research network [Citation17].
WG6 presented their preliminary conclusions to the community at the NHF SOS Research Summit in September 2021. Summit feedback and discussions informed the results and discussion offered herein to support a National Research Blueprint for Inherited Bleeding Disorders [Citation15,Citation16,Citation18].
2. Methods
2.1. Working Group 6 composition
Three cochairs were recruited to lead the WG6 subgroups and worked with the SC to populate them with the expertise required (). This included three LEEs, experts in living with inherited BDs [Citation17], NHF board and staff members, and a leader of another patient organization (World Federation of Hemophilia [WFH]). Multidisciplinary comprehensive care team professionals included hematologists (adult and pediatric), an advanced practice provider, a social worker, and a clinical research coordinator. Many WG members are active in inherited BDs research, while several were recruited from other specializations including artificial intelligence, machine learning, compliance, and research administration. Two health policy and public health experts and representatives of industry, ATHN, a federal agency, and the NHF SOS Research Summit Steering and Advisory Committees completed the group. This diversity and depth of expertise fueled comprehensive and constructive deliberations.
Table 1. Members of Working Group 6, by subgroup.
2.2. Ways of working
WG6 functioned as three largely independent subgroups (), meeting virtually, weekly, via the Microsoft Teams (also used for document sharing) and Zoom platforms from March to July 2021. In the Infrastructure and Workforce Development subgroups, full subgroup discussions were supplemented by written summaries of individual members’ specific expertise on subtopics solicited by the cochairs. Together these yielded priority lists which were then scored and ranked. The Resources and Funding subgroup seeded discussions with invited presentations from guests/colleagues with specific expertise critical to setting up and conducting research and securing resources for a clinical research network. Presentations were followed by active discussion and question and answer sessions, summarized in weekly minutes.
Each subgroup included a LEE as a full member and equal participant in all discussions and initiatives [Citation17]. An NHF staff member accompanied the LEEs throughout the process, meeting with them regularly, individually and as a group, to empower them to fully participate, recognizing that the context had the potential to be intimidating or overwhelming. The cochairs actively encouraged their input and all WG members contributed to constructive, respectful, and inclusive discussion that valued all perspectives. The WG6 cochairs met regularly and attended occasional meetings with all other WG chairs to share ideas. WG6 members from the NHF SOS Research Summit Advisory and Steering Committees also facilitated alignment with other WGs.
2.3. Feasibility-impact-risk scoring
The NHF charge to all WGs included scoring the feasibility, impact, and risk of the priorities they identified against a predefined criteria matrix [Citation15,Citation18,Citation24]. The Infrastructure and Resources and Funding subgroups were provided one matrix and asked to sum the scores across the three dimensions, while Workforce Development were to evaluate impact only (Supplementary Table S1). Members of the Infrastructure subgroup each contributed priorities from their area of expertise, then each scored all priorities, and the average, maximum, minimum, and standard deviations of the individual scores were calculated yielding the final ranking. Consensus discussions of the Workforce Development subgroup over the three months yielded a list of priorities, the nature of which precluded meaningful scoring with the predefined matrix. Instead, through further consensus discussions, the subgroup evaluated the impact, cost, and timescale of each priority and categorized them into top, moderate, and low priority tiers. The Resources and Funding subgroup generated a priority list of work to be done, refined it as a group, and then ranked the list with each member invited to review and comment. The list was scored on the basis of the 21 questions detailed in the scoring matrix (Suppl Table S1A).
2.4. NHF State of the Science Research Summit
All WGs sought feedback from the inherited BDs community on their deliberations and conclusions at the NHF SOS Research Summit, held (virtually) September 12–15, 2021 [Citation15]. Over 880 delegates attended the free event, including LEEs, physicians, researchers, multidisciplinary care team professionals, and federal and industry partners. Ziva Mann, MA provided a LEE perspective on facilitating priority research. A plenary address on learning health systems by Charles Bailey, MD, PhD, and presentations of WG6ʹs findings by its cochairs, lead to a lively panel discussion with the preceding speakers moderated by Shannon L. Carpenter, MD, MS fielding questions and comments from Summit delegates [Citation15,Citation18]. This panel discussion and the final panel discussion concluding the Summit informed the Discussion and Conclusions reported herein.
3. Results
3.1. Infrastructure
Examination of the infrastructure required to advance the inherited BDs research prioritized by the SOS Research Summit and National Research Blueprint centered upon HTCs as the home of care delivery and research. The subgroup considered the different types of HTCs and their existing research capacities, identified a target minimal research capability for all HTCs, and discussed the site-specific and common infrastructure elements needed to bring all HTCs to this level, and to promote their further development along the research continuum.
3.1.1. Foundational infrastructure requirements for inherited bleeding disorders research
The subgroup recognized that individual HTCs across the US are currently engaged in diverse types of research, with varying levels of supporting infrastructure. They identified four types of HTCs and ATHN-affiliated BDs comprehensive care centers, each with distinct research infrastructure challenges and opportunities:
Federally supported academic centers located within adult or pediatric university-based hospitals
Federally supported centers located within adult and pediatric hospitals, but without university affiliation
Federally supported centers located in private clinics, not affiliated with a hospital
Centers that are not federally supported
The subgroup derived a pyramid model of types of research currently conducted at HTCs, with increasing infrastructure requirements (). At the base of the pyramid are local retrospective studies; most HTCs can design such a study and collect the necessary data, though perhaps on a limited scale. They may be conducted without dedicated research staff and facilities and be exempt from IRB approval. Registries and longitudinal natural history studies require more personnel and infrastructure and while many HTCs do participate in these, not all have the capacity. Clinical trials sponsored by industry partners (e.g. pharmaceutical companies) are more complex and require significant research capabilities, but participation usually entails considerable funds and resources which facilitate their undertaking. Investigator-initiated clinical studies are very resource intensive as is basic translational research.
Figure 2. Pyramidal depiction of types of research, with increasing infrastructure requirements. The star indicates the proposed target minimal research infrastructure threshold for all HTCs in the USHTCN. ATHN: American thrombosis and hemostasis network, HTC: hemophilia treatment center, USHTCN: United States Hemophilia Treatment Center Network.
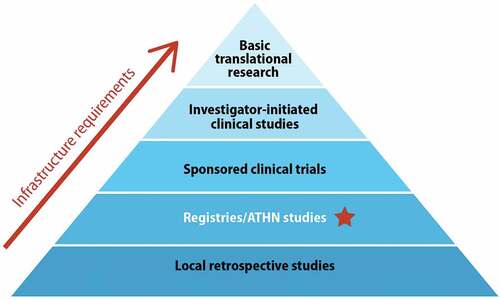
Registries and longitudinal natural history studies, with standardized data and biospecimen collection involving many centers to maximize cohort size, have been highlighted in the research priorities of each the four BD-specific WGs [Citation19,Citation21–23]. The subgroup, therefore, proposes the second tier of the research pyramid () as the target minimal research infrastructure threshold for all HTCs, such that they may all participate in prospective, multicenter registries like CDC Community Counts [Citation12], ATHNdataset [Citation47], and at least one cohort of ATHN Transcends [Citation13]. This threshold aligns with the community-supported lifespan registry underpinning the research network proposed by the Resources and Funding WG6 subgroup (see below).
Services, processes, and tools supporting participation in clinical studies vary widely between HTCs. Some centers lack dedicated research staff and generous comprehensive care team professionals conduct minimal data collection in addition to their full clinical workload, essentially as volunteers. Centers at the other extreme enjoy the support of entire research units at their affiliated institutions, providing personnel and facilities to manage IRB and regulatory requirements, conduct sample collection and analyses, perform data entry and analyses, and coordinate contracts and consents, and can participate in most levels of the research pyramid. Centers that participate in industry-sponsored trials or receive grants for investigator-initiated studies may be able to contribute some of the resulting infrastructure to support additional studies.
Fundamental to the success of research at any HTC is buy-in from the whole team. HTC leadership (e.g. Directors) should foster a culture emphasizing the importance of research and ensure that all team members (even those not directly involved in research) support the research mission. Educational initiatives to demonstrate the value of contributing to research, and training to equip all comprehensive care and research team professionals with the skills to successfully carry out their tasks, are essential infrastructure elements. Educational support for HTC Directors on how to foster such a culture should be an ongoing initiative. Clearly defining the roles and responsibilities of individual research team members is critical, especially if they also have clinical professional obligations. Equally important is securing protected, remunerated time for them to fulfill their research responsibilities.
The services, processes, and tools required for an HTC to participate in tier 2 of the research pyramid, that is registries and natural history studies, are summarized in . Some of these elements are site-specific and must be secured at each individual HTC. Many may, potentially, be provided centrally as common resources to multiple centers. A hub of common resources can reduce the burden on local research personnel and participants, and standardize processes and data collection increasing comparability, and streamlining efficiencies.
Table 2. Infrastructure requirements to participate in tier 2 research: registries and natural history studies.
Bringing all HTCs to tier 2 of the research capacity pyramid will require equitable, not necessarily equal, investment. Centers requiring more resources should receive more than those whose research infrastructure is already better established. Improved and expanded centralized common infrastructure will benefit all HTCs and may be expected to facilitate progression of most HTCs’ research capabilities. Reaching tier 2 of the pyramid may not be immediately realistic for some HTCs requiring an initial investment to bolster a research culture and training to establish infrastructure and expertise. HTCs may benefit from clinical research [Citation48]. Contributing to tier 2 scientifically rigorous registries and longitudinal studies should be available and strongly encouraged to all HTCs.
Delineation of an infrastructure framework for each tier in the pyramid, and opportunities to facilitate attainment of needed infrastructure, should enable HTCs to evaluate their current capabilities and identify priority investments or acquisitions in order to progress to the next level of research participation. This could include a centralized compilation of funding opportunities and costs associated with various infrastructure elements; standardized templates for contracts, and IRB, regulatory and consent requirements; a hub for best practice communities and training opportunities; a network to facilitate connection of research professionals with personnel needs; guidance on research prioritization, study design, and inclusive participant recruitment; a roster of ongoing and proposed research studies; and a forum to debate potential research proposals.
The Infrastructure subgroup recognizes that developing the research capacity of HTCs requires resources, funding, and workforce development, emphasizing the importance of advancing the priorities of all three WG6 subgroups.
3.1.2. Feasibility-impact-risk scoring of priority infrastructure questions
The Infrastructure subgroup summarized their deliberations with a set of eight priority questions, ranked by their average total scores (, ) in the preassigned feasibility-impact-risk matrix [Citation24]. Further details of the evaluation of each question against the criteria for all three dimensions (Suppl Table S1A), including score averages, maxima, minima, and standard deviations are reported in Suppl Table S2. Priorities 7 and 8 reflect the overlap in the needs identified by this subgroup with the primary focus areas of the two other WG6 subgroups.
Figure 3. Plot of feasibility, impact, and risk scores of WG6 Infrastructure and Resources and Funding top priorities.
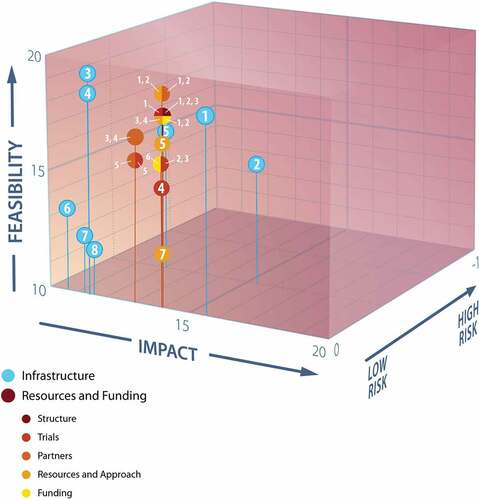
Table 3. Priority questions scored for feasibility, impact, and risk by the WG6 Infrastructure subgroup.
3.2. Workforce development
3.2.1. Challenges and opportunities in developing a sustainable expert workforce
This subgroup examined key issues impacting the development of the sustainable, diverse, expert workforce required for multidisciplinary comprehensive care and research in the inherited BDs community to thrive (Suppl Table S3). They considered recruitment, retention, mentorship, and networking. They explored the skillset and membership of the workforce required to carry out team science, including expertise from outside the inherited BDs community. They looked at how to facilitate HTC-based team research including funding models and novel and multidisciplinary approaches to the training of diverse, qualified HTC professionals. In so doing, they identified a number of strengths, weaknesses, opportunities, and threats to the development of this workforce ().
3.2.2. Prioritizing workforce development initiatives
The subgroup deliberations resulted in 14 initiatives key to the development of a sustainable diverse, expert workforce, falling into (often more than one of) 5 themes ():
Study the problem
Include non-physicians
Attract a diverse group of new people
Retention and workforce development
Funding
Table 4. Three tiers of priority initiatives, falling into five themes, identified by the WG6 Workforce Development subgroup.
The priority initiatives did not lend themselves well to scoring against the predefined impact criteria (Suppl Table S1B) [Citation24], therefore, through consensus discussions the subgroup distilled them into near term, mid-term, and long-term priority tiers; classing the impact and cost of each as either high or low ().
Common presumptions of caregiver and staff shortages, in HTCs and across hematology, must be investigated to detail the true needs and the factors contributing to them. There is evidence of an impending shortage of adult hematologists [Citation30–32], however it is not clear whether this extends to pediatric hematologists, whether there are correlations with urban versus rural geography, to what extent recruitment versus retention issues are responsible, what role funding and/or academic opportunities play, etc. A lack of workforce diversity and inclusivity is readily apparent, and most centers encounter insufficient staffing as an obstacle to their desired level of clinical and laboratory-based research participation. Resolving these issues also requires careful characterization. Collecting quantitative data to clearly define the problem is the subgroup’s top priority. Too often problems are studied, but the attention ends there; therefore, this must be accompanied by analysis informing a clear and actionable plan to address current barriers and drivers, complete with ongoing monitoring at local, regional, and national levels.
The majority of the remaining top tier priority initiatives concern funding, are expected to have a high impact at low cost, and to be relatively easy to implement in the short term. These include knowledge sharing to empower all centers to optimize their capacity to invest revenue from the 340B Drug Pricing Program [Citation9] back into clinical care and research to enhance patient care. The subgroup identified several federal and government agency, foundation, philanthropic, society, and industry resources, not limited to the hematology sphere, that should be expanded to include all multidisciplinary team professionals (not just clinicians) at all career stages (Suppl Table S3). A single website consolidating information on all opportunities would be enormously helpful in facilitating maximum capitalization on these opportunities. Hematology professional organizations should embrace inclusivity with membership reflective of all the professions that contribute to comprehensive care. The subgroup also identified the importance of raising the profile of hematology, both publicly and among HCPs potentially making career direction choices, for example through inclusion as a category in the U.S. News & World Report evaluation of hospitals [Citation50], which they propose may be achieved through lobbying.
The second priority tier consists primarily of high cost, high impact initiatives that can be implemented in the mid-term. A number of partnerships between leading inherited BDs community organizations have proven fruitful (e.g. the Hemostasis & Thrombosis Research Society [HTRS] with the Foundation for Women & Girls with Blood Disorders [FWGBD] [Citation51]; ATHN with its community partners [Citation52]; and NHF with the American Society of Hematology [ASH], International Society on Thrombosis and Hemostasis [ISTH], and WFH for the development of clinical practice guidelines [Citation53,Citation54]). The subgroup encourages these and additional collaborations to create training programs targeted to identified needs. These could match centers with specific expertise to those seeking to acquire it, bringing trainees to the expert centers [Citation55] or sending the experts out to train professionals locally. Here too, opportunities must not be restricted to clinicians but reflect the multidisciplinary nature of the comprehensive care and research team, and include all career phases. Funding opportunities must be created that specifically address the workforce development needs of the clinical and basic, non-traditional and cross-disciplinary, and collaborative beyond the traditional specializations of inherited BDs, research prioritized by WGs 1–5 [Citation19–23]. Mentorship has proven to be a determining factor in hematology career selection [Citation31]. The prioritization of existing networking programs for students, trainees, and junior faculty, the creation of additional opportunities (including capitalizing on social media and virtual innovations), and the recognition, funding [Citation49], and training of mentors should yield a good return on investment.
The third priority tier features new, primarily long-term initiatives that will likely involve high costs but are also high impact. The Workforce Development subgroup proposes the development of hub and spoke style regional HTC networks that align education, translation, and implementation research excellence providing a workforce with new comprehensive skillsets to the networked centers. Areas of specialization might include bioinformatics and clinical informatics to exploit next generation sequencing or EMRs, clinical research expertise such as the design and conduct of adaptive platform trials, or specific laboratory techniques such as advanced molecular biology including genome editing and gene therapy. Cross-disciplinary relationships with individuals and societies that share interests with inherited BDs research, within and outside of hematology (e.g. immunology, rheumatology, emergency medicine, trauma surgery), should be fostered and the cross-pollination of diverse expertise encouraged. Recruitment and retention educational and promotional strategies must be innovative and inclusive. The inherited BDs workforce must reflect the community for which it cares; it must be trained to meet the needs of underserved communities and to deliver health care in an inclusive and equitable manner.
3.3. Resources and funding
In seeking to identify priorities for the facilitation of inherited BDs research, the Resources and Funding subgroup discussed issues concerning registries and observational studies such as post-licensure therapies and regimen implementation, data collection, and the most relevant patient-centered outcomes. They also considered how to integrate meaningful patient-centered outcomes into clinical trials, how to resource expertise in small trial design, decentralized trial methods, and adaptive platform trials; how to network access to multi-disease comorbidity trials; and how to develop the resources needed to make small clinical trials feasible. Strategies for optimizing private-public partnerships and funding opportunities were also discussed. Fourteen invited presentations providing in-depth expertise on specific aspects of data collection, clinical trials, community partnerships, and funding enriched the discussions (Suppl Table S4).
3.3.1. An innovative inherited bleeding disorders research network
The subgroup envisioned a future in which inherited BDs clinical research is conducted within the USHTCN [Citation8] and rooted in a community-supported lifetime registry (). The network would capitalize upon machine learning and EMRs to enable observational and interventional investigations of all people with inherited BDs, facilitating the research prioritized by the five other WGs [Citation19–23].
Figure 5. HTC network organization of inherited BDs research rooted in a community-supported lifespan registry, envisioned by WG6 Resources and Funding subgroup. BD: bleeding disorder, EMR: electronic medical record, HTC: hemophilia treatment center, ML: machine learning, WG: working group
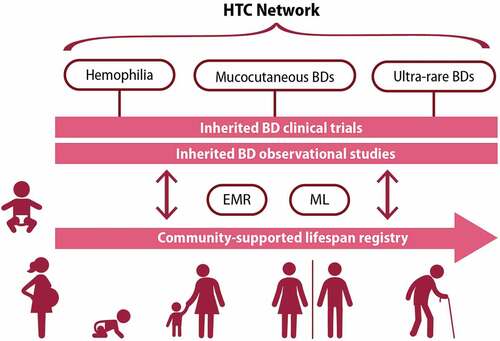
A person with an inherited BD could enter the community registry at birth (or immediately upon identification of a BD) and their data collected using EMRs and machine learning, all within the HTC network. Data collection would include common data elements (CDE) and parent- or patient-reported outcomes (PROs). At any point, any individual could join an observational study or trial, based on symptoms, classification, or complication. This could be a prevention trial, interventional investigation, or post-licensure phase 4 trial. Upon completion of the study they could return to the registry. The registry database must be built to ensure the robustness and reliability of historic data, capture the information required to answer clinical questions, provide time-sensitive linked datasets, and allow open-label comparisons. Perhaps most importantly, it must maintain high standards of data privacy and confidentiality, patient protections, and informed consent. Critically, all of this would occur under the umbrella of the HTC with research embedded alongside the delivery of care.
The subgroup identified important partners and resources required to enable and sustain the proposed lifespan registry and research network:
Community: LEEs must be involved in research planning and design, in decision-making, and in the engagement of the research network with the community
HTC professionals: all of the multidisciplinary HTC professionals (e.g. principal investigators, nurses, data managers) must be part of a research culture that values, enables, and validates all of their contributions
Clinical research organization: the involvement of these specialized professionals may facilitate the incorporation of decentralization and remote virtual approaches, lowering some barriers to research participation
Common trial resources: shared centralized IRBs, master protocols, and platform trials will decrease the participation burden on individual centers
Clinical trial research forum: a joint exploration of trial concepts and innovative approaches such as decentralized or virtual trial protocols, and using historic controls, registries, and expert opinion, will help optimize trial design
Trialists: specialized expertise regarding small study population trial design, statistics, and epidemiology constitute valuable additions to the clinical expertise of HTC multidisciplinary professionals
Network database: CDEs, including PROs, must be collected systematically following standardized protocols into a shared data repository
Biorepository: standardized protocols for sample collection, handling, and analysis, and a shared biobank will help maximize learnings from small populations
Information technology (IT) partners: the complexities of machine learning and sophisticated data collection, including from EMRs, require specialized expertise and support
Foundations: sustainable funding for research may rely on foundations
- In addition to contributing its invaluable relationship with the community to engagement with the research network, the subgroup proposes a particular role for NHF in overseeing the funding of the network.
- The specialized expertise of the FWGBD and HTRS in models for funding research and the accompanying workforce training will be an important asset.
3.3.2. Innovations in clinical trial design
The subgroup explored clinical trial innovations, as traditional stand alone, parallel-group, randomized, controlled studies tend to be inefficient, costly, and take too long to generate results especially in rare and ultra-rare disorders [Citation21,Citation56,Citation57]. The opportunities offered by decentralized clinical trial designs [Citation58] have been highlighted in the context of the COVID pandemic. Varying from fully virtual to operating via intermediaries, features such as remote and telemedicine visits, at-home drug delivery and monitoring, and less restrictive selection criteria increase their inclusivity and convenience, and decrease the burden on research participants while collecting real-world data to support translatable results. These qualities make them particularly attractive for the study of rare disorders in heterogenous widely geographically distributed small populations.
Adaptive platform trials [Citation59] constitute another appealing innovation for tackling these challenges. Designed to study multiple interventions in a disorder in a perpetual manner, intervention arms are added or dropped from the trial on the basis of a predefined decision algorithm that assesses their success or futility through frequent interim analyses of continuously updated outcomes data. This flexibility facilitates a focus on treatment of the disorder, avoids stopping and starting of individual trials, and incentivizes participants who may feel less concerned that they will remain in a futile study arm. Industry partners may benefit from early data availability and post-marketing surveillance studies, and investigators and mentors from more opportunities to publish results. Importantly, adaptive platform trials lend themselves well to the integration of community input during the design phase and throughout.
3.3.3. Three potential research network models
The subgroup proposes three possible models to provide the requisite resources for the inherited BDs research HTC network they envision (). All three models are based upon a network of eight to ten regional research HTCs, working in collaboration with the individual HTCs in their region [Citation8]. The first model () features a single data coordinating center (DCC) that provides all of the required advanced technical expertise to the regional centers, such as innovative clinical trial design (e.g. decentralized, adaptive platform trial), biological sample banking, and the database and IT support. The DCC would also provide one central point of contact for the inherited BDs community, foundations, and community-based organizations.
Figure 6. Potential models for an inherited BDs research network in which eight to ten regional research HTCs, collaborating with their respective network of local HTCs, are supported by a) an external data coordinating center, b) the diverse capabilities contained within the regional research HTCs made available to the entire network, or c) a hybrid of self-contained and externally provided expertise.BD: bleeding disorder, Biorep: biorepository, CCC: clinical coordinating center, CDE: common data elements, DCC: data coordinating center, EMR: electronic medical record, HTC: hemophilia treatment center, IRB: institutional review board, IT: informatic technology, ML: machine learning, R-HTC: regional research HTC, stats: statistics
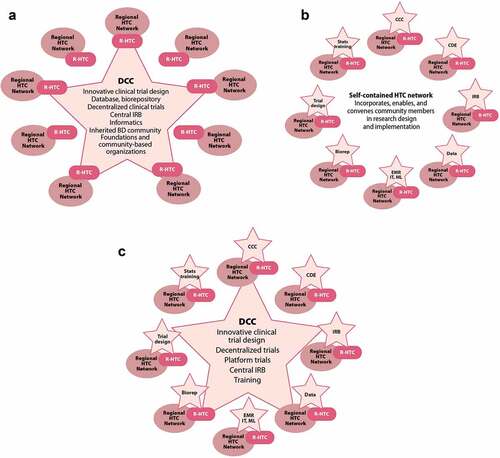
The second model () features clinical coordinating centers (CCC) at every regional research HTC. The CCCs draw upon the expertise (e.g. clinical trial design, informatics, machine learning) at each of the regional HTCs such that the network as a whole is self-contained and self-sufficient. This model incorporates, enables, and convenes members of the inherited BDs community into each phase of research design and implementation. The third model () is a hybrid of the first two, with CCCs at each regional HTC to capitalize on the expertise available (e.g. local IRB, statistics, a biorepository), with a central DCC furnishing only the most specialized expertise otherwise lacking (e.g. innovative clinical trial design, centralized IRB, specific training).
Appropriate governance of the research network could be assured by a steering committee and a protocol review committee; with federal agency partners, the inherited BDs community, NHF, HTRS, ATHN, and FWGBD all providing external advisory oversight; and NHF also providing fiscal oversight. The inherited BDs community must also be integrally involved in the strategic and execution decisions that ensure the sustainability of the research network, which will rely upon a variety of valuable partners ().
Table 5. Opportunities for diverse partners in ensuring the sustainability of an inherited BDs research network proposed by the WG6 Resources and Funding subgroup.
3.3.4. Feasibility-impact-risk scoring of priority resources and funding initiatives
The Resources and Funding subgroup derived 22 concrete specific initiatives, from the above deliberations, falling into structure, trials, partners, resources and approach, and funding categories (). They evaluated each against the preassigned feasibility-impact-risk matrix (Suppl Table S1A) [Citation24]. Full scoring details of each initiative across all criteria for all three dimensions are reported in Suppl Table S5; sub-total scores of each dimension permitted comparative three-dimensional plotting (); while summing these yielded the scores to rank priorities within categories ().
Table 6. Feasibility-impact-risk scored priority initiatives identified by the WG6 Resources and Funding subgroup.
4. Discussion
Five WGs tasked with distilling actionable research questions in specific inherited BDs domains from the community priorities revealed by extensive consultations reported detailed and ambitious agendas to the NHF SOS Research Summit and in this supplement [Citation16,Citation19–23]. Their priorities in basic and translational foundational research, large-scale longitudinal natural history studies, innovative approaches to developing and capitalizing on novel therapies, harnessing technology, regional and national (and international) networking, collaborating with other specialties and seeking out external expertise, flexibility and streamlining in regulatory affairs, characterizing and dismantling barriers to equitable access, centering mental health and quality-of-life, and systems optimization have the potential to advance transformational community-based health care and health equity for all people with inherited BDs [Citation16].
4.1. Limitations
WG6 was charged with identifying priorities in infrastructure, workforce development, resources and funding to facilitate the emerging research agenda [Citation15,Citation24]. Applying the predefined scoring matrixes provided to evaluate the feasibility, impact, and risk of each priority (Suppl Table S1) [Citation24] proved challenging as some initiatives were not well characterized by these criteria. The Infrastructure and Resources and Funding subgroups applied the matrixes to the best of their ability (Suppl Tables S2 and S5), thus ranking their priorities (). The Workforce Development initiatives proved incompatible with such scoring; the subgroup instead evaluated associated cost, impact, and timescale to generate three priority tiers (). Despite these procedural challenges, each subgroup identified and ranked top initiatives to benefit the inherited BDs research endeavor.
As predominantly rare and ultra-rare disorders, research into inherited BDs faces particular challenges [Citation21,Citation60]. The characterization of their pathology, natural history, and meaningful end points is often limited; the population of potential research participants is small, heterogeneous, and usually widely dispersed geographically; and there is a lack of widespread expertise to identify them [Citation56,Citation61]. Without collaborative coordination, investigators may even find themselves competing with other studies to recruit the few available participants. The limited (sometimes negligible) return on investment for industry of developing novel therapeutics for a tiny market can be prohibitive [Citation62].
Facilitating the prioritized inherited BDs research [Citation16,Citation19–23], in the face of these challenges, requires strategic, integrated, and innovative approaches to workforce development, infrastructure, and resources and funding.
4.2. Capitalize and grow
Many excellent initiatives and resources exist to facilitate inherited BDs research. The identification of all available training, mentoring, infrastructure, and funding opportunities in centralized listings and sharing of how best to capitalize on them would encourage maximum uptake. Successful initiatives should be expanded and innovations from other disciplines adopted. Coordination and communication will be key.
Most HTCs use 340B revenue for essential care salaries [Citation10] which, with appropriate approvals, has the potential to support research initiatives [Citation63]. The inherited BDs community must take full advantage of opportunities such as the National Institutes of Health (NIH) development grants targeting research professionals at various stages of their careers [Citation64] (e.g. K12: Institutional Clinical Scientist Awards for fellows wishing to conduct research with a faculty mentor and K24: Midcareer Investigator Award in Patient-Oriented Research to provide support for protected time to devote to patient-oriented research and to act as research mentors) [Citation49,Citation65] and advocate for their expansion to include a greater range of the professionals essential to the multidisciplinary care and research team [Citation66], through all career stages. Similarly, programs to increase workforce diversity [Citation67–69] must be grown and example taken from successful initiatives in other disciplines [Citation70]. The WFH International Hemophilia Training Center Fellowship program brings multidisciplinary care team professionals from developing countries to train for several weeks with corresponding professionals in highly resourced expert centers [Citation55]. In a recent review of 50 years of this program, 86% of its over 750 fellows reported that their center changed the way they manage BDs as a result of their training, >95% had shared their new skills with others in their home country, and 5 years later 91% of fellows continued to work in BDs care [Citation55]. A similar approach offering mini-sabbaticals, or touring expert consultants, for HTC professionals to acquire advanced research skills might also encourage them to pursue a long career in the field and boost the research capacity of many HTCs.
Incorporating existing infrastructure such as registries and research networks into future research initiatives must be favored over investing scarce resources (human and financial) in their duplication. The majority of people with inherited BDs in the US receive care at an HTC linked to over 140 others in a national network, the USHTCN [Citation3]. Many of these centers participate in national research projects with ATHN and CDC [Citation12,Citation13]. NHF’s Community Voices in Research (CVR) initiative is a community-powered registry facilitating direct access for researchers to people with inherited BDs and the data that they report [Citation71]. Partners in Bleeding Disorders Education [Citation72] and NHF [Citation73] offer extensive educational resources (in-person and online) to the multidisciplinary professionals of the comprehensive care team associated with HTCs and those delivering BDs care outside of the HTC setting. Harmonizing future research with these valuable existing resources will require an initial investment of effort and good will, which will pay off many times over in the efficiencies gained avoiding redundancy or competition.
4.3. Cooperate, collaborate, and integrate
Advancing individual interests or initiatives in isolation is inefficient. To achieve the bold research ambitions of WGs1–5 [Citation19–23], the inherited BDs community must come together and cooperate among themselves, collaborate with diverse and new partners, and integrate efforts to reach common goals.
Research should be embedded in HTCs, and non-HTCs, alongside care, rooted in the same values of the patient, or LEE, as partner and a multidisciplinary approach [Citation4,Citation5]. The well-being and outcomes of importance to people with inherited BDs should drive research priorities and conduct, as they do care. The USHTCN provides an excellent foundation for the coordination of priorities, resources, and initiatives. Whether through a more or less centralized model (), with its combined expertise, volume of potential research participants, and infrastructure the network can embark upon more powerful studies than most centers could on their own. Regional and national cooperation to share common and decentralized infrastructure elements will increase the number of HTCs with the foundational research capacity required to participate in network initiatives (), and aid others in continuing their progression along the capacity continuum (). Communities of practice, in which peers share their successful approaches to common challenges and learn together from leaders in the field, will help all teams to elevate their research competencies.
Intertwined training and research initiatives will advance one another. The need for additional personnel to grow research capacity constitutes an opportunity to train diverse professionals at different stages of their career. The excitement of working in a collaborative team with a highly engaged community in the discovery and implementation of life-changing diagnostics and therapies may attract and retain valuable talent. Partnerships with expertise not (extensively) found within the inherited BDs community, such as artificial intelligence or machine learning, advanced clinical trial design, diversity and equity, and other medical specialties such as gynecology and obstetrics or cardiovascular morbidities, will be necessary to design and execute the prioritized research. These partnerships may also attract new talent to join the inherited BDs research and care workforce.
A collaborative approach is needed to fund the research and its network; the NHF may be well positioned to develop a coordinated and integrated business model, consulting the expertise of valued partners such as HTRS and FWGBD. Government agencies and grants, institutions, professional associations, patient organizations, philanthropic individuals, foundations, industry, and insurers all currently contribute to inherited BDs research, in a piece meal fashion. Coordination of these sources, and sharing of the elements they fund where possible, would be expected to create considerable efficiencies, enabling more ambitious studies. These studies, judiciously designed to generate meaningful data with the minimum delay () and at lower cost than conventional trials, would in turn attract more support from well-resourced partners and likely the enrollment of more research participants. Industry partners may also recognize the value of collaborating in funding the training of a diverse research workforce in this context [Citation74,Citation75].
The inherited BDs community and its LEEs must be integrated throughout the research network and processes. Their lived experiences drive the urgency and direction and help to align priorities for better health outcomes through collaborative research. They have important roles to play in the governance of the network and in policy development and advocacy [Citation17,Citation76]. Training programs should capitalize upon the unique expertise of LEEs involving them in content development and delivery [Citation76], and LEEs interested in pursuing careers in inherited BDs care and/or research should be actively encouraged and supported. LEEs have important expertise to contribute to trial design, emphasizing patient-important outcomes and the feasibility and acceptability of practical aspects. They are also essential to liaison with the entire inherited BDs community, which is much larger than just those individuals closely engaged with an HTC or active in patient organizations. LEEs can inform effective communication of the excitement and value of research and its results to the very people it is intended to serve. Many people with inherited BDs receiving care at an HTC have family members or social contacts, impacted by the same disorder, who do not. Especially within marginalized and minoritized populations, they are invaluable allies who can open doors to trust building and relationships that engage a larger and more diverse community.
4.4. Innovation, evaluation, and agility
The joint vision for inherited BDs research of the WGs and the delegates attending the SOS Research Summit is far reaching and perhaps even daunting [Citation16]. WG6 proposes that attaining this vision will be facilitated by an approach that starts small, learns continuously, and evolves rapidly, much like an adaptive clinical trial design. Some research priorities identified by WGs 1–5 [Citation19–23] may be tackled almost immediately. Several may be selected as pilot projects driving the establishment of networks, collaborations, infrastructure, and processes. Close observation, as they proceed, will provide important learnings feeding constant refinements to improve efficiency and effectiveness. Completing these initial projects, will pave the way for larger more ambitious projects, to be conducted in the same fashion, with each experience contributing to optimization of shared infrastructure, training and recruitment/retention strategies, and study design and execution.
The evolving research network approach should embrace innovation and integrate expertise from outside the inherited BDs domain. Important advances in decentralization of trial design and the role of telemedicine and digital solutions in care delivery and trial conduct were made during the COVID pandemic [Citation77]. These study designs, which appear to be more resilient to changes in enrollment and attrition, may help to reduce barriers to trial participation, and improve participant burden, convenience, inclusion, and data quality [Citation57,Citation78]. Digital solutions are more amenable to language localization and accessibility which may prove particularly useful in rare disorder trials enrolling participants internationally [Citation57]. There may also be opportunities to capitalize on decentralized and digital solutions to advance training objectives [Citation79] and access to care [Citation78] especially in rural and remote locations. Implementation of these tools must, however, avoid perpetuating or exacerbating disparities and health inequity, with an awareness that less than two in three US adults who identify as Hispanic or African American have broadband internet at home, with similar patterns for those with lower income or education levels [Citation80,Citation81].
Agile new adaptive platform trials are being designed to better meet the needs of rare disorders research [Citation82,Citation83] and personalized medicine to maximize treatment effects within trials [Citation84]. Artificial intelligence and machine learning offer advanced techniques to maximize information gleaned from available data [Citation85,Citation86], the value of which, and challenges to, are amplified in rare disorders with scarce, heterogenous, and geographically disbursed research participants [Citation87–90]. The integration of artificial intelligence into any health care process must be done with explicit awareness of, and efforts to eliminate, algorithmic bias which may be introduced in the algorithm design, previous data collection, coding, and/or selection [Citation91,Citation92]. A responsive strategy of ongoing evaluation, learning, and implementation of learnings will allow the inherited BDs research network to capitalize on rapidly evolving technological and other advances. LEEs have an essential role to play here too, ensuring that the research topics and approaches prioritized align with what matters most to the community, and are appropriate to their lived realities.
LEE Perspective
WG6 was tasked with working out how a future research network might look. We thought through who and what the network would need, how it would engage people, and how we would all work together. Most of all, we discussed what it means for a research network to be community-led and possible roles for people and families like us, living with inherited BDs. Could we design research? Be part of a team doing research? Or take leadership roles, overseeing the network, guiding it to focus on the research that people and families need most? We knew that research often has a hierarchy, but the research we need to see in our lifetimes will come from all of us working and thinking together: researchers, health care providers, parents, people living with BDs, local community leaders, foundations, and more. As a team we agreed we would prioritize partnership, building it into the network’s DNA. We dug deep to see what was needed to make this network accessible to everyone. We learned about different ways to design clinical trials, and about research that is community-based/community-driven. We discussed ways to develop training, recruitment, and retention to build stronger, more complete research teams. We heard from experts about measuring authentic partnership between researchers and people with lived experience. It was extraordinarily meaningful to see us all come together around a network that will engage, support, and be centered on partnership – to have all of our voices heard and valued – as we plan for our future.
5. Conclusions
WGs 1–5 of the SOS Research Summit initiative distilled a vast array of actionable research endeavors from community priorities [Citation19–23]. Bringing to fruition the research with the greatest potential to transform the lives of people with inherited BDs requires the intentional establishment of a research culture that embraces innovation and collaboration [Citation16].
This ambitious agenda can only be achieved through the alignment of all stakeholders, and the contribution of the unique expertise of each. The multidisciplinary care team and the research team must work in synchronicity, sharing personnel and expertise, and enabling one another to excel in their pursuit of the best possible care and outcomes for people with inherited BDs. LEEs must lie at the heart of the research endeavor, their expertise valued and capitalized on throughout the research process [Citation17]. Complementing the rich experience of inherited BDs scientists and treaters with cutting edge expertise in data collection, management, and mining, and in trial design will vastly expand research horizons. Collaboration with industry and regulatory partners will also be essential to realizing the therapeutic potential of exciting scientific advances, and to optimizing their impact in the real world. Existing opportunities to train professionals in research skills must be expanded across the disciplines and career phases, and beyond inherited BDs to adjacent specialties, with an emphasis on improving diversity, equity, and inclusion in the workforce.
Collaboration between research centers will be as important as collaboration within. The challenges typically faced by research into rare disorders can only be addressed by pooling and sharing resources, expertise, and opportunities. The entire research network will benefit from a maximum number of individual centers participating in joint studies. The establishment of the minimal infrastructure threshold for participation in national registries and natural history observational studies will facilitate joint efforts to ensure access to the necessary staff, processes, and tools. A common awareness of the infrastructure requirements for different types of research and how to build that capacity will facilitate the progression of all centers along the continuum. Fostering a culture that values research and emphasizes its importance in improving the lives and outcomes of people with inherited BDs will engender efforts to advance research capacity across the board. Industry, business, and community partners must come together and apply the same rigor, innovation, efficiencies, and collaboration to securing a sustainable financial foundation for the research that will advance health equity for all people with inherited BDs.
List of Abbreviations
| ASH | = | American Society of Hematology |
| ATHN | = | American Thrombosis and Hemostasis Network |
| BD | = | Bleeding disorder |
| CCC | = | Clinical coordinating center |
| CDC | = | US Centers for Disease Control and Prevention |
| CDE | = | Common data element |
| CVR | = | Community Voices in Research |
| DCC | = | Data coordinating center |
| eCRF | = | Electronic case report form |
| EMR | = | Electronic medical record |
| F-I-R | = | Feasibility-impact-risk |
| FWGBD | = | Foundation for Women & Girls with Blood Disorders |
| HCP | = | Health care professional |
| HRSA | = | Health Resources and Services Administration |
| HTC | = | Hemophilia treatment center |
| HTRS | = | Hemostasis & Thrombosis Research Society |
| HUGS | = | Hematology Utilization Group Study |
| IRB | = | Institutional review board |
| ISTH | = | International Society on Thrombosis and Haemostasis |
| IT | = | Information technology |
| IWRS | = | Interactive web response system |
| LEE | = | Lived experience expert |
| NHF | = | National Hemophilia Foundation |
| NIH | = | National Institutes of Health |
| PRO | = | Patient-reported outcome |
| RFA | = | Request for applications |
| SC | = | Steering Committee |
| SOS | = | State of the Science |
| US | = | United States |
| USHTCN | = | United State Hemophilia Treatment Center Network |
| WFH | = | World Federation of Hemophilia |
| WG | = | Working group |
Declaration of interest
The authors are integrated members of the inherited bleeding disorders community: people with inherited bleeding disorders, their family members, healthcare providers and researchers (including physicians, nurses, physical therapists, pharmacists, social workers/psychologists, geneticists/genetic counselors, etc.), industry partners, government officials/regulators, local community organization representatives, and others.
Margaret V. Ragni discloses: Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: NHF, MASAC, FWGBD. Jordan A. Shavit discloses: All support for the present manuscript: Henry and Mala Dorfman Family Professorship in Pediatric Hematology/Oncology; Consulting fees: Sanofi, Takeda, Genentech, HEMA Biologics; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Hemostasis and Thrombosis Research Society Director and VP. Adam Cuker discloses: All support for the present manuscript: NHF; Royalties or licenses: UpToDate; Consulting fees: Synergy. Amber Federizo discloses: Grants or contracts from any entity: Octapharma, HTCNV of NV; Consulting fees: Octapharma, Sanofi; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Octapharma, Sanofi; Support for attending meetings and/or travel: Octapharma, Sanofi; Participation on a Data Safety Monitoring Board or Advisory Board: Octapharma, Sanofi; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Chair Nevada State Rare Disease Advisory Council; Receipt of equipment, materials, drugs, medical writing, gifts or other services: Octapharma; Other financial or non-financial interests: Sanofi. Bruce Ewenstein discloses: Stock or stock options: Takeda. Ellis Neufeld discloses: Consulting fees: Genentech, Pfizer; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Octapharma, Takeda; Participation on a Data Safety Monitoring Board or Advisory Board: Bayer, Acceleron/Merck, SOBI/DOVA, Genentech, Novo Nordisk, Acceleron Pharma; Stock or stock options: Saliogen. Emily Bisson discloses: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Medscape/WebMD; Support for attending meetings and/or travel: NHF for BDC attendance. Emma Neely discloses: All support for the present manuscript: National Hemophilia Foundation. Glaivy Batsuli discloses: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Kedrion, Bio Products Laboratories; Participation on a Data Safety Monitoring Board or Advisory Board: Genentech. Glenn F. Pierce discloses: Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: World Federation of Hemophilia VP Medical, NHF MASAC. Leslie Raffini discloses: Participation on a Data Safety Monitoring Board or Advisory Board: Genentech. Lindsey A. George discloses: Grants or contracts from any entity: AskBio; Royalties or licenses: AskBio; Consulting fees: Pfizer, Bayer, Spark, Biomarin; Participation on a Data Safety Monitoring Board or Advisory Board: Avrobio (DSMB); Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: STRM.Bio (Scientific Advisory Board). Lynn Malec discloses: Consulting fees: Sanofi, Sobi, Spark, CSL, Novo Nordisk, Takeda; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: National Hemophilia Foundation. Michael Denne discloses: Stock or stock options: Takeda; Other financial or non-financial interests: Employee of Takeda. Neil Frick discloses: Employee of NHF. Randall Curtis discloses: All support for the present manuscript: University of Southern California, Institute for Policy Advancement, Ltd.; Consulting fees: HUGS, PROBE; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Global Blood Disorders Foundation; Support for attending meetings and/or travel: National Hemophilia Foundation (NHF), Hematology Utilization Group Study (HUGS), Patient Reported Outcomes, Burdens and Experiences (PROBE); Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Hemophilia Foundation of Northern California, Center for Inherited Blood Disorders. Roger J. Lewis discloses: Other financial or non-financial interests: Berry Consultants, LLC. Shannon L. Carpenter discloses: Participation on a Data Safety Monitoring Board or Advisory Board: Kedrion, Novo Nordisk, Genentech. Shawn M. Jobe discloses: Consulting fees: Sanofi, Takeda, Roche, Octapharma, CSL Behring; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Sanofi; Support for attending meetings and/or travel: Sanofi, Takeda, Roche, Octapharma, CSL Behring. Steven W. Pipe discloses: Consulting fees: Apcintex, ASC Therapeutics, Bayer, Biomarin, CSL Behring, GenVentiv, HEMA Biologics, Freeline, LFB, Novo Nordisk, Pfizer, Regeneron/Intellia, Roche/Genentech, Sanofi, Takeda, Spark Therapeutics, uniQure. Christopher James Langmead discloses: Grants or contracts from any entity: AMD hardware allocation, Pittsburgh Health Data Alliance; Consulting fees: Lifeware Labs, Amgen; Patents planned, issued or pending: Carnegie Mellon (US Patent App. 16/296,088).
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MVR, JS, and GY led the working group recruitment and organization, and subgroup discussions and analysis. All authors contributed to discussions and analysis. MVR, JS, and GY led manuscript preparation, all authors offered input and contributed to revisions of the manuscript, approved the final version to be published, and agree to be accountable for all aspects of the work. GFP, RGC, and ZM led writing of the Plain Language Summary and LEE Perspective sections.
Presentation of content
MVR, JAS and GY presented highlights of the deliberations of Working Group 6 at the National Hemophilia Foundation State of the Science (virtual) Research Summit (SOSRS), September12–15, 2021. Summit discussions informed the Discussion and Conclusions of this paper.
Supplemental Material
Download Zip (109.6 KB)Acknowledgments
The Executive Committee of the National Hemophilia Foundation National Research Blueprint initiative were actively engaged in the conception, design, preparation, and oversight of each of the State of the Science manuscripts in this supplement. Maria E. Santaella actively engaged with the lived experience expert (LEE) WG members throughout the process, empowering their inclusion and participation. The Executive Committee consisted of: Kevin Mills, Michael Recht, Michelle L. Witkop, Maria E. Santaella, Donna DiMichele, Keri L. Norris, Esmeralda Vázquez and Brett Spitale.
The authors thank Keri L. Norris, PhD, JM, MPH, MCHES for her review of the manuscript.
The authors acknowledge Fiona Robinson, PhD, for providing professional medical writing support during manuscript development, paid for by NHF, and Matt Evans for the creation of professional illustrations, paid for by NHF.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17474086.2023.2181781
Additional information
Funding
References
- Baker JR, Riske B, Drake JH, et al. US hemophilia treatment center population trends 1990-2010: patient diagnoses, demographics, health services utilization. Haemophilia. 2013;19(1):21–26.
- Valentino L, Baker J, Butler R, et al. Integrated hemophilia patient care via a national network of care centers in the United States: a model for rare coagulation disorders. J Blood Med. 2021;12:897–911.
- Centers for Disease Control and Prevention. HTC population profile patient characteristics March 2022 - technical notes 2022. [ cited 2022 Nov 4]. Available from: https://www.cdc.gov/ncbddd/hemophilia/communitycounts/data-reports/2022-03/index.html
- Karazivan P, Dumez V, Flora L, et al. The patient-as-partner approach in health care: a conceptual framework for a necessary transition. Acad Med. 2015;90(4):437–441.
- Baker JR, Crudder SO, Riske B, et al. A model for a regional system of care to promote the health and well-being of people with rare chronic genetic disorders. Am J Public Health. 2005;95(11):1910–1916.
- Centers for Disease Control and Prevention. Hemophila treatment centers. [ cited 2022 Aug 17]. Available from: https://www.cdc.gov/ncbddd/hemophilia/documents/a-simmons_htc-brochure-508c.pdf.
- Skinner MW, Soucie JM, McLaughlin K. The national haemophilia program standards, evaluation and oversight systems in the United States of America. Blood Transfus. 2014;12(Suppl 3):e542–8.
- National Hemophilia Program Coordinating Center. Regional hemophilia networks. [ cited 2022 Jul 18]. Available from: https://nhpcc.org/regional-hemophilia-networks/
- Resources H, Services A. 340B drug pricing program. [ cited 2022 Jun 19]. Available from: https://www.hrsa.gov/opa/index.html
- Malouin RA, McKernan L, Forsberg A, et al. Impact of the 340B pharmacy program on services and supports for persons served by hemophilia treatment centers in the United States. Matern Child Health J. 2018;22(9):1240–1246.
- American Thrombosis and Hemostasis Network. ATHN affiliate directory. [ cited 2022 Oct 1]. Available from: https://athn.org/affiliate-directory.html
- Centers for Disease Control and Prevention. About community counts. [ cited 2022 Jun 16]. Available from: https://www.cdc.gov/ncbddd/hemophilia/communitycounts/about.html
- American Thrombosis and Hemostasis Network. ATHN projects and research studies—an overview. [ cited 2022 Apr 12]. Available from: https://www.athn.org/what-we-do/national-projects/athn-projects-and-research-studies.html
- National Hemophilia Foundation. Mission & history. [ cited 2022 Jan 5]. Available from: https://www.hemophilia.org/who-we-are/our-story/mission-history
- Valentino LA, Witkop ML, Santaella ME, et al. Building the blueprint: formulating a community-generated national plan for future research in inherited bleeding disorders. Haemophilia. 2022;16(S1):1–5. DOI: 10.1080/17474086.2023.2178412.
- Valentino LA, Witkop ML, Santaella ME, et al. The National Hemophilia Foundation State of the Science Research Summit initiative: executive summary. Expert Rev Hematol. 2023;16(S1):127–132. DOI: 10.1080/17474086.2023.2181782.
- Vázquez E, Kim M, Santaella ME. Lived Experience Experts: a name created by us for us. Expert Rev Hematol. 2023;16(S1):7–11. DOI: 10.1080/17474086.2023.2178410.
- Rauch A, Valentino LA, Mills K, et al. Big picture initiatives in bleeding disorders. Haemophilia. 2022;28(Suppl 4):53–60.
- Baldwin MK, Ahmadzia HK, Bartlett DL, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research to advance the health of people with inherited bleeding disorders with the potential to menstruate. Expert Rev Hematol. 2023;16(S1):71–86. DOI: 10.1080/17474086.2023.2175660.
- Byams VR, Baker JR, Bailey C, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities in health services; diversity, equity, and inclusion; and implementation science. Expert Rev Hematol. 2023;16(S1):87–106. DOI: 10.1080/17474086.2023.2183836.
- Nugent D, Acharya SS, Baumann KJ, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities for ultra-rare inherited bleeding disorders. Expert Rev Hematol. 2023;16(S1):55–70. DOI: 10.1080/17474086.2023.2175661.
- Sidonio RF Jr., Bryant PC, Di Paola J, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities for mucocutaneous bleeding disorders. Expert Rev Hematol. 2023;16(S1):39–54. DOI: 10.1080/17474086.2023.2171983.
- Tran DQ, Benson CC, Boice JA, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities to transform the care of people with hemophilia. Expert Rev Hematol. 2023;16(S1):19–37. DOI: 10.1080/17474086.2023.2171981.
- Valentino LA, Witkop ML, Santaella ME, et al. The National Hemophilia Foundation’s State of the Science Research Summit initiative: the foundation of an inherited bleeding disorders national research blueprint. Expert Rev Hematol. 2023;16(S1):1-5. DOI: 10.1080/17474086.2023.2178412.
- Merriam-Webster. Infrastructure – definition. [ cited 2022 Sept 14]. Available from: https://www.merriam-webster.com/dictionary/infrastructure
- Croteau SE, Wang M, Wheeler AP. 2021 clinical trials update: innovations in hemophilia therapy. Am J Hematol. 2021;96(1):128–144.
- Hermans C, Makris M. Disruptive technology and hemophilia care: the multiple impacts of emicizumab. Res Pract Thromb Haemost. 2021;5(4):e12508.
- O’Mahony B, Wong O, Eichler H, et al. Preparing for tomorrow: defining a future agenda. Haemophilia. 2022;28(Suppl 2):35–41.
- Regling K, Callaghan MU, Sidonio R Jr. Managing severe hemophilia A in children: pharmacotherapeutic options. Pediatric Health Med Ther. 2022;13:27–35.
- Hoots WK, Abkowitz JL, Coller BS, et al. Planning for the future workforce in hematology research. Blood. 2015;125(18):2745–2752.
- Masselink LE, Erikson CE, Connell NT, et al. Associations between hematology/oncology fellows’ training and mentorship experiences and hematology-only career plans. Blood Adv. 2019;3(21):3278–3286.
- Sharma D, Wallace N, Levinsohn EA, et al. Trends and factors affecting the US adult hematology workforce: a mixed methods study. Blood Adv. 2019;3(22):3550–3561.
- Arya S, Wilton P, Page D, et al. “Everything was blood when it comes to me”: understanding the lived experiences of women with inherited bleeding disorders. J Thromb Haemost. 2020;18(12):3211–3221.
- Arya S, Wilton P, Page D, et al. “They don’t really take my bleeds seriously”: barriers to care for women with inherited bleeding disorders. J Thromb Haemost. 2021;19(6):1506–1514.
- Weyand AC, James PD. Sexism in the management of bleeding disorders. Res Pract Thromb Haemost. 2021;5(1):51–54.
- Arya S, Wilton P, Page D, et al. Healthcare provider perspectives on inequities in access to care for patients with inherited bleeding disorders. PLoS One. 2020;15(2):e0229099.
- Lopez K, Norris K, Hardy M, et al. Defining the impact of social drivers on health outcomes for people with inherited bleeding disorders. J Clin Med. 2022;11:15.
- Racism: science and tools for the public health professional. Washington (DC): APHA Press. 2019. Available fromhttps://ajph.aphapublications.org/doi/book/10.2105/9780875533049
- Connors JM, Middeldorp S. Transgender patients and the role of the coagulation clinician. J Thromb Haemost. 2019;17(11):1790–1797.
- U.S. Food and Drug Administration. 2015-2019 drug trials snapshots summary report. [ cited 2022 Sept 16]. Available from: https://www.fda.gov/media/143592/download
- Statler MC, Wall BM, Richardson JW, et al. African American perceptions of participating in health research despite historical mistrust. ANS Adv Nurs Sci. 2023;46(1):41–58.
- Glandon D, Paina L, Alonge O, et al. 10 best resources for community engagement in implementation research. Health Policy Plan. 2017;32(10):1457–1465.
- Squitieri L, Chung KC. Funding research in the twenty-first century: current opinions and future directions. Hand Clin. 2014;30(3):367–376.
- Israel BA, Schulz AJ, Parker EA et al. Community-based participatory research: policy recommendations for promoting a partnership approach in health research. Educ Health. (Abingdon). 2001;14(2):182–197.
- National Institutes of Health. NIH clinical center patient handbook. [ cited 2022 Dec 11]. Available from: https://clinicalcenter.nih.gov/sites/nihinternet/files/internet-files/participate/_pdf/pthandbook.pdf
- American Thrombosis and Hemostasis Network. ATHN grants & awards. [ cited 2022 Dec 11]. Available from: https://www.athn.org/grants/overview.html
- American Thrombosis and Hemostasis Network. Advancing research with better data through the ATHNdataset. [ cited 2021 Nov 12]. Available from: https://athn.org/what-we-do/national-projects/athndataset.html
- American Thrombosis and Hemostasis Network. ATHN Data quality counts is helping ATHN affiliates enrich the ATHNdataset. [ cited 2022 Sept 1].
- National Institutes of Health. Research career development awards: K24 midcareer investigator award in patient-oriented research. [ cited 2022 Jul 16]. Available from: https://researchtraining.nih.gov/programs/career-development/K24
- U.S. News. FAQ: how and why we rank and rate hospitals. [ cited 2022 Jul 16]. Available from: https://health.usnews.com/health-care/best-hospitals/articles/faq-how-and-why-we-rank-and-rate-hospitals#best-hospitals
- Foundation for Women & Girls with Blood Disorders. About - foundation for women and girls with blood disorders. [ cited 2022 Jul 16]. Available from: https://www.fwgbd.org/about
- American Thrombosis and Hemostasis Network. ATHN community partners. [ cited 2022 Jul 16]. Available from: https://athn.org/who-we-are/about/community-partners.html
- Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301–325.
- James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5(1):280–300.
- Escobar MA, Beijlevelt M, Loney A, et al. World federation of hemophilia international hemophilia training fellowship program: 50 years of enhancing global care. Haemophilia. 2022;28(5):S129–S31.
- Kempf L, Goldsmith JC, Temple R. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A. 2018;176(4):773–783 .
- Moore J, Goodson N, Wicks P, et al. What role can decentralized trial designs play to improve rare disease studies?. Orphanet J Rare Dis. 2022;17(1):240 .
- Khozin S, Coravos A. Decentralized trials in the age of real-world evidence and inclusivity in clinical investigations. Clin Pharmacol Ther. 2019;106(1):25–27.
- Adaptive Platform Trials Coalition. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18(10):797–807.
- Koerper MA, Frick N, Kessler CM, et al. impediments to conducting clinical research in persons with haemophilia, von Willebrand’s disease and rare bleeding disorders. Haemophilia. 2013;19(2):188–193.
- Kakkis ED, O’Donovan M, Cox G, et al. Recommendations for the development of rare disease drugs using the accelerated approval pathway and for qualifying biomarkers as primary endpoints. Orphanet J Rare Dis. 2015;10:16.
- Marks P, Witten C. Toward a new framework for the development of individualized therapies. Gene Ther. 2021;28(10–11):615–617.
- National Hemophilia Foundation. NHF policy statement: hemophilia treatment center participation in the 340B drug discount program. [ cited 2022 Aug 23]. Available from: https://www.hemophilia.org/sites/default/files/document/files/NHF-Position-Statement-Regarding-Hemophilia-Treatment-Center-Participation-in-the-340B-Drug-Discount-Program.pdf
- National Institutes of Health. Research career development awards. [ cited 2022 Sept 17]. Available from: https://researchtraining.nih.gov/programs/career-development
- National Institutes of Health. Institutional career development (K12) programs. [ cited 2022 Sept 17]. Available from: https://www.nichd.nih.gov/grants-contracts/training-careers/extramural/FAQs/K12
- National Hemophilia Foundation. Fund your research. [ cited 2022Sept 17]. Available from: https://www.hemophilia.org/research/fund-your-research
- American Society of Hematology. About the ASH-AMFDP award. [ cited 2022 Sept 17]. Available from: https://www.hematology.org/awards/career-enhancement-and-training/amos-medical-faculty-development-program/about
- American Society of Hematology. ASH minority student award program. [ cited 2022 Sept 17]. Available from: https://www.hematology.org/awards/medical-student/minority-medical-student-award-program
- National Hemophilia Foundation. Jeanne marie lusher diversity fellowship. [ cited Sept 18 2022]. Available from: https://www.hemophilia.org/research/fund-your-research/jeanne-marie-lusher-diversity-fellowship
- National Medical Fellowships. Programs portfolio overiew. [ cited 2022 Sept 17]. Available from: https://nmfonline.org/scholarships-programs/programs-portfolio-overview/
- National Hemophilia Foundation. What is CVR? [ cited 2022 Jan 5]. Available from: https://www.hemophilia.org/research/community-voices-in-research/what-is-cvr
- Indiana Hemophilia & Thrombosis Center. Partners in bleeding disorders education. [ cited 2022 Sept 17]. Available from: https://www.partnersprn.org/
- National Hemophilia Foundation. Bringing resources to the people on the front lines of bleeding disorders care [ cited 2022 Sept 17]. Available from: https://www.hemophilia.org/healthcare-professionals
- National Hemophilia Foundation. NHF-takeda clinical fellowship program. [ cited 2022 Sept 17]. Available from: https://www.hemophilia.org/healthcare-professionals/education-resources/nhf-takeda-clinical-fellowship-program
- National Medical Fellowships. Bristol myers squibb foundation diversity in clinical trials career development program announces first group of physicians to be trained. [ cited 2022 Sept 17]. Available from: https://nmfonline.org/bristol-myers-squibb-foundation-diversity-in-clinical-trials-career-development-program-announces-first-group-of-physicians-to-be-trained/
- Boivin A, Dumez V, Castonguay G, et al. The Ecology of Engagement: Fostering cooperative efforts in health with patients and communities. Health expect. 2022;25(5):2314–27.
- Zahradka N, Pugmire J, Lever Taylor J, et al. Deployment of an end-to-end remote, digitalized clinical study protocol in COVID-19: process evaluation. JMIR Form Res. 2022;6(7):e37832.
- Mackwood M, Butcher R, Vaclavik D, et al. Adoption of telemedicine in a rural US cancer center amid the COVID-19 pandemic: qualitative study. JMIR Cancer. 2022;8(3):e33768.
- Saito M, Tsuzaki T, Takeda Y. Evaluation of postgraduate rural medical training programs and implications for rural workforce development: a systematic review. Rural Remote Health. 2022;22(2):7118.
- Lin JL, Huber B, Amir O, et al. Barriers and facilitators to the implementation of family-centered technology in complex care: feasibility study. J Med Internet Res. 2022;24(8):e30902.
- Pew Research Center. Internet/broadband fact sheet. [ cited 2022 Aug 26]. Available from: https://www.pewresearch.org/internet/fact-sheet/internet-broadband/?menuItem=3109350c-8dba-4b7f-ad52-a3e976ab8c8f
- Huang H, Aschettino S, Lari N, et al. A versatile and scalable platform that streamlines data collection for patient-centered studies: usability and feasibility study. JMIR Form Res. 2022;6(9):e38579.
- Visibelli A, Cicaloni V, Spiga O, et al. Computational approaches integrated in a digital ecosystem platform for a rare disease. Front Mol Biosci. 2022;9:827340.
- Wang H, I-SPY YD. 2: a neoadjuvant adaptive clinical trial designed to improve outcomes in high-risk breast cancer. Curr Breast Cancer Rep. 2019;11(4):303–310.
- Lin PC, Tsai YS, Yeh YM, et al. Cutting-edge ai technologies meet precision medicine to improve cancer care. Biomolecules. 2022;12:8.
- Khare R, Kappelman MD, Samson C, et al. Development and evaluation of an EHR-based computable phenotype for identification of pediatric crohn’s disease patients in a national pediatric learning health system. Learn Health Syst. 2020;4(4):e10243.
- Bottini S, Emmert-Streib F, Editorial: FL. AI and multi-omics for rare diseases: challenges, advances and perspectives, volume II. Front Mol Biosci. 2022;9:986749.
- Nguyen QH, Nguyen T, Le DH. Identification and validation of a novel three hub long noncoding rnas with m6a modification signature in low-grade gliomas. Front Mol Biosci. 2022;9:801931.
- Yang X, Wang C, Lin Y, et al. Identification of crucial hub genes and differential t cell infiltration in idiopathic pulmonary arterial hypertension using bioinformatics strategies. Front Mol Biosci. 2022;9:800888.
- Deprez M, Moreira J, Sermesant M, et al. Decoding genetic markers of multiple phenotypic layers through biologically constrained genome-to-phenome bayesian sparse regression. Front Mol Biosci. 2022;9:830956.
- Amann J, Blasimme A, Vayena E, et al. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inform Decis Mak. 2020;20(1):310.
- Delgado J, de Manuel A, Parra I, et al. Bias in algorithms of AI systems developed for COVID-19: A scoping review. J Bioeth Inq. 2022;19(3):407–19.