1. High dose chemotherapy-autologous stem cell transplant (HDT-ASCT), when compared to chemotherapy alone, has been associated with improvement in both progression-free survival (PFS) and overall survival (OS) for patients with newly diagnosed multiple myeloma [Citation1,Citation2]. However, timing of HDT-ASCT, whether early (after induction chemotherapy) or delayed (upon relapse), remains controversial. Presented at the American Society of Clinical Oncology 2022 meeting and published in the New England Journal of Medicine, the DETERMINATION trial is an important phase III randomized controlled trial in the United States exploring the role of early versus delayed HDT-ASCT for patients with newly diagnosed multiple myeloma () [Citation3]. With a primary end point of PFS, at a median follow-up of 76 months, Lenalidomide, Velcade, and dexamethasone (RVd) followed by HDT-ASCT was associated with a superior PFS of 67.5 versus (vs) 46.2 months with RVd-alone (hazard ratio (HR) = 1.53, 95% confidence interval (CI) 1.23–1.91) without a difference in OS (~80% at 5 years in each arm). While the overall response rates were similar between the two arms (~95%), the incidence of minimal residual disease (MRD) negativity by next generation sequencing (10–5) was higher with RVd+ASCT (55.9% vs 40%), driving the difference in PFS. There was no difference in PFS between patients who achieved MRD negativity, regardless of having received RVD-alone or RVd+ASCT.
Figure 1. DETERMINATION study treatment schema.
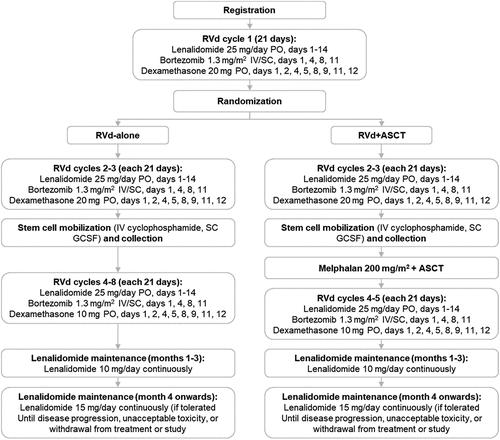
1.1 In 2017, the Intergroupe Francophone du Myelome (IFM) group published their version of a similar trial showing that for patients with newly diagnosed multiple myeloma, PFS is longer with RVd+ASCT vs RVd-alone without an OS benefit [Citation4]. At the 2021 American Society of Hematology meeting, 8-year follow-up showed similar OS between the two groups (~60%) [Citation5]. Again, the prolonged PFS benefit seen with RVd followed by HDT-ASCT was largely driven by higher incidence of MRD negativity (30% vs 20% at 10–5).
1.2 Although the DETERMINATION trial is fairly similar to the IFM study, there are few key differences between the two trials. In the U.S. DETERMINATION trial, lenalidomide maintenance was given until progression or intolerable toxicities as compared to only for 1 year in IFM 2009 trial. This difference in duration of maintenance chemotherapy led to a clear benefit in PFS in both RVd+ASCT (67.5 vs 48 months) and RVd-alone (46.2 vs 36 months) arms of DETERMINATION trial when compared to the IFM 2009 trial. The rate of HDT-ASCT at relapse in the RVd-alone arm was significantly lower in the DETERMINATION trial (27.7%) vs in the IFM study (76.7%). Whether this is related to worsening performance status or comorbidity burden of an aging patient population or physician discretion due to availability of very effective salvage therapies (i.e. anti-CD38 monoclonal antibodies) remains unknown. These differences are highly relevant and reflect the treatment landscape of newly diagnosed multiple myeloma in the U.S. Despite these differences, both trials showed similar results where there is improvement in MRD negativity and PFS but not OS with early transplant.
2. It is important to highlight certain aspects of this landmark trial and potential impact on everyday clinical practice. The DETERMINATION trial only included patients aged 65 years or less, and hence, these results may not be applicable for patients above 65 years of age, which represent around a third of real-world patients with myeloma. Median age of diagnosis for multiple myeloma is 69 years, and with advancing age, patients remain at risk of increasing comorbidity burden and worsening performance status that may make them ineligible for high-dose chemotherapy later in life. This was perhaps one of the reasons why only 27% of the patients in the delayed transplant group were able to undergo HDT-ASCT upon relapse. While patients in the RVd-alone arm were able to collect and store hematopoietic stem cells after three cycles of induction chemotherapy, this is not always feasible in the real-world setting due to the limitations on collection and storage for certain insurances and additional burden on cell storage services for a transplant center. While the DETERMINATION trial showed a superior PFS with long-term lenalidomide maintenance compared to 1-year maintenance in the IFM trial, there is growing interest in limited duration maintenance in several clinical trials (NCT04071457). Incorporation of anti-CD38 monoclonal antibody in the induction, consolidation, and maintenance regimens for newly diagnosed multiple myeloma has led to significantly deeper and perhaps more durable responses [Citation6,Citation7], raising the question of whether ASCT is necessary in the era of quadruplet regimens. Although no OS benefit was seen, this could be related to limited follow-up (median OS not reached) and a difference may emerge with longer follow-up. It is relevant to state that the majority of patients in the trial had standard risk disease (standard risk cytogenetics: 80%, International Staging System Stage 1: 50%) which is associated with prolonged overall survival.
2.1 Approximately 20% of the patients enrolled in the trial were non-Hispanic Black. Median PFS was not reached with RVd-alone arm vs 61.4 months with RVd-ASCT (HR = 1.07, 95% CI 0.61–1.89). Although this is one of the largest minority populations enrolled in a clinical trial for multiple myeloma, the absolute numbers are not large enough to explain the observed lack of benefit from HDT-ASCT in this group. Similarly, an ad hoc analysis showed significantly superior PFS (55.5 vs 17.1 months, HR = 1.99, 95% CI 1.21–3.26) with RVd-ASCT for high-risk cytogenetic abnormalities. Additionally, ASCT and anti-CD38 are not available in many regions with limited resources which may disproportionally affect minorities [Citation8–11]. Again, these data need to be interpreted cautiously due to the small subgroup size and number of events. Although the incidence of secondary primary malignancies was similar between the two arms (~10%), 13 (3.6%) and 9 (2.5%) of the patients developed secondary hematologic malignancies in the RVd-alone vs RVd-ASCT arms, respectively. Acute myeloid leukemia/myelodysplastic syndrome was reported only in the RVd-ASCT arm. Development of secondary hematologic malignancies after ASCT is associated with poor outcomes and remains a major complication that needs better strategies for early detection and mitigation, perhaps through testing for clonal hematopoiesis of indeterminate potential in this patient population. Nevertheless, the most common cause of mortality in patients with multiple myeloma continues to be underlying hematologic malignancy. While transplant appears to be associated with a transient decline in the quality of life, overall quality of life remains very comparable between RVd-alone vs RVd+ASCT arms. What needs to be determined is the long-term impact on quality of life, especially the impact of salvage chemotherapy in case of disease relapse in the non-transplant arm. In resource-limited settings, longer duration of induction chemotherapy and, more importantly, salvage chemotherapy with novel agents including anti-CD38 monoclonal antibody may be financially prohibitive, and hence, early ASCT may be a more viable option for long-term remission.
3. In summary, the DETERMINATION trial met its primary end point of superior PFS with early transplant, and hence, induction chemotherapy followed by HDT-ASCT and maintenance chemotherapy until disease progression or intolerable toxicities continues to be the standard of care for a vast majority of patients with newly diagnosed multiple myeloma. For a young and fit patient with standard risk disease who prefers to delay ASCT until disease progression, RVd-alone followed by lenalidomide maintenance remains a reasonable option.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Palumbo A, Cavallo F, Gay F, et al., Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014 Sep 4;371(10):895–905.
- Gay F, Oliva S, Petrucci MT, et al., Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16(16):1617–1629. doi: 10.1016/S1470-2045(15)00389-7
- Richardson PG, Jacobus SJ, Weller EA, et al., Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022 Jul 14;387(2):132–147.
- Attal M, Lauwers-Cances V, Hulin C, et al., Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311–1320. doi: 10.1056/NEJMoa1611750
- Perrot A, Lauwers-Cances V, Cazaubiel T, et al., Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: long-term follow-up analysis of the IFM 2009 Trial. Blood. 2020;136(Supplement 1):39. doi: 10.1182/blood-2020-134538
- Voorhees PM, Kaufman JL, Laubach J, et al., Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–945. doi: 10.1182/blood.2020005288
- Moreau P, Attal M, Hulin C, et al., Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;394(10192):29–38. doi: 10.1016/S0140-6736(19)31240-1
- Ruiz-Argüelles GJ, Gómez-Almaguer D. Lessons learned treating patients with multiple myeloma in resource-constrained settings. Curr Hematol Malig Rep. 2021 Feb;16(1):40–44. doi: 10.1007/s11899-021-00616-6
- Gómez-Almaguer D, de Moraes Hungria VT. Multiple myeloma in Latin America. Hematology. 2022 Dec;27(1):928–931. doi: 10.1080/16078454.2022.2112643
- Hong YD, Mullins CD, Onukwugha E, et al., Association of individual low-income status and area deprivation with mortality in multiple myeloma. J Geriatr Oncol. 2023 Mar;14(2):101415.
- Fatoki RA, Koehn K, Kelkar A, et al., Global myeloma trial participation and drug access in the era of novel therapies. JCO Glob Oncol. 2022 Aug;8:e2200119.
