ABSTRACT
Introduction: Hepatocellular carcinoma (HCC) is a leading cause of death globally and is frequently seen following Hepatitis B virus (HBV) or Hepatitis C virus infection. Areas with high HBV infection rates, such as Asia and sub-Saharan Africa, are therefore also high-risk areas for HCC.
Areas covered: This review identifies and discusses the current evidence from robust clinical trials which have investigated the benefits of Nucleos(t)ide analogue (NA) antiviral therapy in HBV-related HCC patients, including HCC patients that underwent liver transplantation and HCC patients with or without curative treatment. In addition, we assess how this evidence has influenced current clinical practice, with a particular focus on those areas of high HBV infection rates.
Expert commentary: A number of studies have assessed whether NA antiviral treatment can improve the prognosis of HBV-related HCC patients. In this review we evaluate the current evidence, including that from trials in Asia, for antiviral NA treatments in HBV-related HCC patients. We also focus on those NAs with a high genetic barrier to resistance (i.e. ETV or TDF), on different therapeutic approaches, and on the future evidence that is required in this field.
1. Introduction
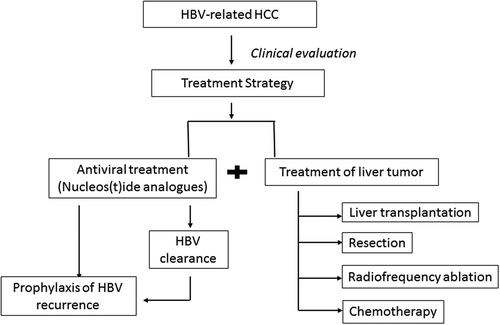
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death, with approximately 750,000 new cases worldwide reported each year [Citation1]. Currently, surgical resection, liver transplantation (LT), chemotherapy, and local tumor ablation are the four main treatment options for HCC patients. An estimated 78% of HCC cases result from chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection [Citation2], with areas of high HBV prevalence showing significant geographical correlation with HCC incidence and mortality worldwide [Citation3]. Approximately 50% of the total cases and almost all of childhood HCC is caused by chronic HBV (CHB) infection [Citation3]. In fact, HBV infection is the dominant risk factor in most areas of high HCC incidence in Asia and Sub-Saharan Africa, with the exception of Japan, where the major risk factor for HCC is chronic HCV infection [Citation3]. High HBV DNA levels have been found to be a critical risk factor for HCC development [Citation4,Citation5] as well as recurrence of HCC post-surgical resection [Citation6]. Nucleos(t)ide analogue (NA) antiviral therapy is recognized as an important treatment option to control HBV DNA load in patients and consequent HCC development () [Citation7–Citation10]. In HBV-infected patients already diagnosed with HCC, antiviral NA therapy can also improve the disease prognosis by reducing the risk of HCC recurrence and improving survival rate. In this review, we summarize the current evidence and practice for NA antiviral therapy in HBV-related HCC patients [Citation7,Citation11].
2. Clinical benefits/advantages of NA antiviral therapy in HBV-related HCC following diagnosis
2.1. Current antiviral therapy for CHB
Current global treatment strategies using standard antiviral therapy for HBV involve either a finite course of pegylated interferon (PegIFN) or an indefinite duration of treatment with NA’s [Citation12]. PegIFN therapy has limited clinical use due to an adverse safety profile and the risk of liver disease exacerbation. It is not recommended for patients who have hepatic decompensation, immunosuppression, or medical or psychiatric contraindications [Citation13].
Five oral NAs have previously been approved for the treatment of CHB [Citation14] in Asia, including lamivudine (LAM), adefovir dipivoxil (ADV), entecavir (ETV), telbivudine (LdT), and tenofovir disoproxil fumarate (TDF). In addition, a new NA (tenofovir alafenamide; TAF) has recently been approved in the USA. The low genetic barrier to resistance of agents such as LAM, ADV, and LdT has limited their administration in CHB patients. NAs such as ETV and TDF are potent inhibitors of HBV with a high genetic barrier to resistance. Superior virological response has been observed with ETV at 48 weeks, with 67% patients in the ETV group having undetectable serum HBV DNA levels compared to 36% in the LAM group (P < 0.001) [Citation15]. Treatment with TDF has also been shown to result in a stable response, with viral suppression of nearly 100% after 4 years of therapy [Citation16,Citation17]. Therefore, both agents have been recommended as first-line treatments for CHB patients [Citation12,Citation13,Citation18].
All available antiviral therapies for HBV have been proven to be associated with a reduced risk of HCC development [Citation8–Citation10,Citation12]. A nationwide cohort study from Taiwan revealed that NA-treated patients had a lower 7-year incidence of HCC (7.32; 95% CI, 6.77–7.87%) than untreated controls (22.7; 95% CI, 22.1–23.3%; P < 0.001) [Citation19]. In patients with HCC, antiviral NA therapy was found to enhance patients’ tolerance for HCC treatment and further improve prognosis [Citation19]. The most recent Asia Pacific Association for the Study of the Liver (APASL) guidelines recommend that NA treatment be given to patients with HBV-related HCC (before, during and after chemotherapy, resection or LT), if they have detectable serum HBV DNA [Citation13].
2.1.1. Anti-HBV treatment with NAs in patients awaiting liver transplantation
HCC patients with a single lesion ≥2 cm and <5 cm, or no more than three lesions (the largest of which is <3 cm in size), and no radiographic evidence of extrahepatic disease, are optimal candidates for LT [Citation20]. Antiviral therapy should commence in patients with HCC secondary to CHB who are awaiting liver transplantation [Citation21]. NAs are effective in suppressing HBV viral load pre-transplant and preventing HBV recurrence post-transplant, thus contributing to improved long-term prognosis [Citation13].
2.1.2. Anti-HBV treatment with NAs in patients listed for LT
Pre-transplant therapy with NAs aims to reduce HBV DNA at the time of LT and maintain undetectable serum HBV DNA post-transplant in order to prevent HBV recurrence [Citation21]. The use of NAs prior to LT in HCC patients is similar to that in patients who develop HBV-related hepatic decompensation [Citation22]. A summary of key clinical studies of NA use in this patient population are summarized in .
Table 1. Studies of pre-transplant anti-HBV treatment.
Studies have shown that LAM is generally safe and well tolerated in patients with decompensated cirrhosis, demonstrating clinical improvement compared with untreated controls [Citation29–Citation31]. Furthermore, a subset of patients with less advanced liver failure, as indicated by lower modified Child-Turcotte-Pugh (mCTP) scores, might also derive clinical benefit from LAM therapy, thus delaying the need for LT [Citation31]. Unfortunately, long-term use of LAM is associated with increasing rates of antiviral resistance [Citation13]. Virological breakthrough caused by antiviral resistance can induce hepatitis flares, worsen liver failure, and increase the risk of HBV recurrence post-LT, thus reducing survival rates [Citation32].
ADV has activity against both wild-type HBV and LAM-resistant HBV [Citation22,Citation33]. In a prospective, open-label, compassionate-use study, ADV was used in wait-listed patients with LAM-resistant HBV infection [Citation33]. After 48 weeks of treatment, serum HBV DNA was undetectable in 59% of patient with survival rates of 86%. Eleven patients (6%) in the wait-listed group experienced an increase of at least 0.5mg/dL from baseline in serum creatinine during ADV treatment [Citation33]. In a cohort of 128 pre-transplant patients with evidence of LAM-resistant HBV, 48 weeks of ADV therapy resulted in significant improvements in virological, biochemical, and clinical parameters [Citation33]. Although ADV is not recommended as a first-line therapy in patients with decompensated cirrhosis due to its slow viral suppression and risk of renal toxicity, it still presents a potential life-saving treatment option for pre-transplant patients with LAM-resistant HBV.
LdT treatment has shown greater antiviral and clinical efficacy than LAM in CHB patients with compensated cirrhosis [Citation24,Citation25] and also decompensated cirrhosis [Citation26]. A randomized, double-blind trial comparing the efficacy of LdT and LAM in patients with decompensated cirrhosis found that the proportion of patients achieving undetectable HBV DNA (<300 copies/mL) and ALT normalization was significantly higher in LdT-treated patients than in LAM-treated patients after 76 weeks of treatment (56.3% vs. 32.9%, P = 0.018) [Citation26]. However, up to 21.6% of HBeAg-positive and 8.6% of HBeAg-negative patients have been reported to show LdT resistance after 2 years of therapy [Citation34].
Long-term ETV treatment leads to substantial improvement and even regression of fibrosis or cirrhosis [Citation35]. Data from three large, randomized, multicenter, phase III studies involving patients with CHB confirmed that ETV provided a higher observed virologic efficacy than LAM in a subgroup of patients with advanced liver fibrosis/cirrhosis [Citation15,Citation27,Citation28]. A retrospective analysis from Korea showed that ETV was also associated with a significantly lower risk of death or transplantation than LAM in a matched pair of patients with decompensated cirrhosis (HR, 0.43; 95% CI: 0.30–0.63; P < 0.001) [Citation36]. In another multicenter, prospective cohort study of 707 patients with HBV-related decompensated cirrhosis [Citation37], antiviral-treated patients had significantly higher transplant-free survival than untreated patients (5-year survival rates of 59.7% vs. 46.0%, respectively). Considering that maintaining potent suppression of HBV led to improved long-term outcomes, the results of this research support the current recommendations of ETV as a preferred first-line treatment for patients with HBV-related decompensated cirrhosis [Citation12,Citation13]. However, for LAM-resistant patients, ETV monotherapy was not an effective rescue therapy [Citation38].
Tenofovir is a licensed drug for the treatment of CHB with potent activity against HBV [Citation12]; it is also a preferred option in patients with compensated or decompensated cirrhosis [Citation13]. A multinational, open-label, follow-up cohort study found that at least 5 year’s TDF treatment in CHB patients resulted in 87% of patients showing histological improvement and 51% demonstrating regression of fibrosis (P < 0.0001) [Citation39]. TDF with or without ETV also exhibited progressive improvement in the Model for End-Stage Liver Disease (MELD) scores in patients with decompensated cirrhosis in real-life clinical practice [Citation40]. TDF also produced a similar treatment response and clinical outcome to ETV in patients with severe acute HBV exacerbation [Citation41].
Based on these data, the European Association for the Study of the Liver (EASL) clinical practice guidelines recommend pre-transplant therapy using a potent NA with a high genetic barrier to resistance for all HBsAg-positive patients undergoing LT for HBV-related HCC or end-stage liver disease to achieve the lowest possible level of HBV DNA before LT [Citation12].
2.1.3. Post-transplant NA therapy to prevent HBV recurrence
HBV recurrence in post-transplant patients who have not received antiviral prophylaxis can be as high as 80%, and is associated with a severe course or failure of LT [Citation42]. Effective and safe antiviral therapy can contribute to additional survival benefits. Current prophylaxis strategies include Hepatitis B immune globulin (HBIg) alone, NA monotherapy, or combination therapy of HBIg, and NA with or without HBIg withdrawal after a certain period () [Citation21]. HBIg monotherapy requires indefinite treatment leading to prohibitively expensive costs post-transplant and was found to be less effective in candidates with detectable serum HBV DNA at the time of LT [Citation13]. With the introduction of NAs, HBIg monotherapy has been abandoned as an appropriate prophylactic strategy. Hence, here we focus primarily on the efficacy of NAs (monotherapy or combined with HBIg) in preventing post-transplant HBV recurrence ().
Table 2. Studies of post-transplant anti-HBV treatment.
Figure 2. Post-transplant treatment options for the prevention of HBV recurrence.
X: negative impact; ?: unclear impact; ✓: positive impact; HBV: hepatits B virus; HBIg: hepatitis B immune globulin; NA: nucleos(t)ide analogue; LAM: lamivudine; ETV: entecavir; TDF: tenofovir disoproxilfumarate; IM: intramuscular.
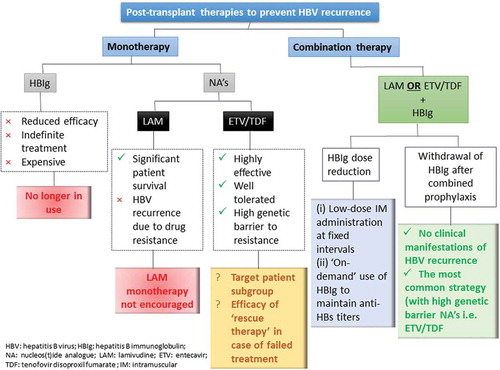
2.1.4. NA monotherapy
Malkan et al. [Citation52] investigated LAM as a single-agent treatment in LT recipients for the prevention of post-transplant HBV recurrence. Patient survival was 81% over a median of 24 months with 19% having evidence of breakthrough infection with the tyrosine-methionine-aspartate-aspartate (YMDD) mutant of HBV. Other studies have also identified YMDD variants post-LT, leading to death from graft failure [Citation53,Citation54]. A cohort study of LAM monotherapy before and after LT demonstrated that the 1- and 2-year cumulative post-transplant survival for patients with YMDD mutants was 83% and 28%, respectively [Citation55].
The recent approval of highly potent NAs with minimal risk of genetic resistance, such as ETV and TDF, has allowed physicians to re-visit NA (ETV and TDF) monotherapy as post-transplant prophylaxis. However, available data for ETV and/or TDF monotherapy as prophylaxis were limited and based on small retrospective studies. Ahn reported four patients receiving NA monotherapy for preventing post-LT HBV recurrence, among whom one patient received TDF monotherapy [Citation43]. The patient had no evidence of post-LT HBV recurrence during the follow-up [Citation43]. Fung et al. investigated the efficacy of ETV monotherapy as a treatment option in patients with CHB who received a liver transplant [Citation44]. It is still unclear which subgroup of patients is suitable for this regimen; the efficacy of a ’rescue therapy’ in patients who failed ETV/TDF monotherapy as post-transplant prophylaxis also remains to be elucidated.
2.1.5. Combination therapy with NA and HBIg
Combining NAs with HBIg as prophylaxis for CHB recurrence has produced general recurrence rates of <10%, which is substantially lower than HBIg or NAs as monotherapy [Citation56]. Therefore, the combination regimen is currently the best-practice approach recommended by the European Association for the Study of the Liver (EASL), Asian Pacific Association for the Study of the Liver (APASL) and American Association for the Study of Liver Disease (AASLD) guidelines [Citation12,Citation13,Citation18]. The efficacy of NA and HBIg in combination has mainly been investigated in patients receiving LAM and HBIg. Data from a study of 165 LT recipients (94 of 165 diagnosed as HCC) suggest that LAM and individualized low-dose intramuscular HBIg provided effective prophylaxis against HBV recurrence [Citation57]. A meta-analysis consisting of two prospective and four retrospective studies showed that the OR for risk reduction in HBV recurrence with HBIg and LAM versus HBIg alone was 0.08 (95% CI: 0.03–0.21) [Citation58].
Indefinite therapy is usually required due to the persistence of HBV in the liver or extrahepatic sites, which may trigger HBV recurrence post-transplant [Citation13]. Patients receiving long-term treatment with LAM are at high risk of developing LAM-resistant mutants. Therefore, a combination of HBIg and NA with a high genetic barrier to resistance (i.e. ETV or TDF) is increasingly in demand. A systematic review found that patients treated with HBIg and ETV/TDF experienced significantly lower rates of HBV recurrence than patients receiving HBIg and LAM (1.3% vs. 7.1%, P = 0.0005) [Citation59]. Despite these promising results, the optimal protocol for post-LT HBV prophylaxis has not been established, as HBIg has several limitations including high cost, need for parenteral administration, and poor safety profile. HBIg discontinuation after prior combination therapy or followed by NAs is the most common strategy currently utilized. A number of studies have assessed and demonstrated the efficacy of HBIg discontinuation in patients receiving NAs with a high genetic barrier to resistance [Citation45,Citation59,Citation60].
2.2. Patients eligible for surgical resection or local ablation therapy
Hepatic resection is the most efficient treatment available for HCC patients with tumors >5 cm, nodules >3 cm, or with tumor macroscopic vascular invasion, who are less likely to benefit from LT or local ablative therapy [Citation61]. Hepatic resection remains a radical therapeutic option that may offer a chance for disease-free survival in these patients [Citation62,Citation63]. For cases not suitable for liver resection, local tumor ablation is an alternative option for curative or palliative treatment of HCC, with a number of tumor ablative procedures being available such as radiofrequency ablation (RFA), transarterial chemoembolization (TACE), microwave (MW), and percutaneous ethanol injection (PCEI) [Citation64]. However, the prognosis for HCC patients remains discouraging because of the frequent incidence of intrahepatic metastasis and the high recurrence rate [Citation65]. In patients undergoing hepatic resection for HBV-related HCC, accumulating evidence suggests that viral load at the time of resection is an independent risk factor for HCC recurrence [Citation6,Citation63]. Liver resection itself could reactivate HBV replication, especially in patients who have not received prophylactic antiviral therapy [Citation65,Citation66].
2.3. Recurrence of HCC
Intrahepatic recurrence of HCC after hepatic resection has two distinct etiologies: recurrence via metastasis and new primary tumors [Citation67]. Administration of antiviral therapy after HCC surgery cannot prevent intrahepatic early recurrence, or recurrence outside of the liver, both of which account for at least 70 and 10% of HCC recurrences after resection, respectively [Citation68].
2.3.1. NAs against HCC recurrence after surgical resection or local ablation therapy
Higher serum HBV DNA level (> 106 copies/ml) has been shown to be associated with late tumor recurrence [Citation69]. The impact of postoperative antiviral treatment on survival in patients with HBV or HCV infection-related primary HCC after curative therapy was therefore evaluated in a number of studies [Citation6,Citation69–Citation77] (). A meta-analysis of these studies found that there was a trend of lower HCC recurrence in the treatment groups (33.3%) compared with controls (52.9%) (OR: 0.43, 95% CI: 0.17–1.07, P = 0.07) [Citation78]. Chan et al. also found that commencing antiviral therapy after hepatectomy improves the prognosis of HCC in preoperatively antiviral-naïve patients with HBV infection (5-year survival 71.2% vs. 43.5%, P = 0.005) [Citation72]. Similarly, a cohort study of patients receiving curative liver resection for HCC in Taiwan demonstrated a significant risk reduction of tumor recurrence in patients receiving NAs versus untreated patients (45.6% vs. 54.6%, P < 0.001) [Citation73]. This benefit was also seen following RFA (2-year recurrence rate: 41.8% vs. 54.3%; P < 0.05) [Citation74].
Table 3. Recurrence of HCC. Risk factors and survival rates.
2.3.2. Patients receiving chemotherapy for HBV-related HCC
Reactivation of HBV is common in HBsAg-positive individuals who are undergoing chemotherapy, especially in patients with hematologic diseases [Citation79,Citation80]. For patients with HBV-related HCC, HBV reactivation and subsequent liver failure or early termination of chemotherapy has a negative effect on HCC survival [Citation80]. It is believed that chemotherapy may enhance the replication of HBV in hepatocytes and suppress the cellular immune response at the same time [Citation81,Citation82]. All candidates for chemotherapy should be screened for HBsAg and anti-HBc prior to initiation of treatment [Citation13]. The risk and severity of HBV reactivation appears to be proportionate to the degree of immunosuppression and the intensity of cancer chemotherapy.
Meta-analyses have shown that LAM can reduce the risk of HBV reactivation by 4–7 fold during chemotherapy in patients with a positive HBsAg [Citation83]. In patients with solid tumors or lymphoma, prophylactic LAM could reduce the severity of HBV reactivation [Citation84,Citation85]. However, the optimal treatment duration of preventive LAM is still unclear, with studies ranging from 3 to 6 months [Citation86,Citation87]. A limitation of LAM is the development of resistance, ranging from 3 to 8% in published studies [Citation80]. Both ADV and ETV have lower resistance rates than LAM and studies have shown that both drugs are effective in preventing HBV reactivation in patients receiving chemotherapy [Citation88–Citation90], with one retrospective study demonstrating that ETV had significantly lower rates of HBV reactivation (0 vs. 12.4%, P = 0.024) [Citation90]. ETV also had significantly fewer hepatitis flares due to HBV reactivation (0.0% vs. 11.6%, P = 0.039) [Citation91].
2.4. Practical management strategies
Several organizations have published consensus guidelines on chronic HBV infection, including the EASL, AASLD, APASL and National Institute for Health and Clinical Excellence (NICE). Peg-IFN-α-2a, IFN-α-2b, LAM, ADV, ETV, LdT, TDF are the most commonly used approved antiviral drugs for the management of chronic HBV infection. For HBV patients, regardless of HBeAg-positive or negative, all guidelines recommend ETV, TDF and PEG-IFN-α-2a as the first-line treatment [Citation13,Citation18,Citation20].
However, antiviral therapy may not prove to be adequate in patients with very advanced liver disease who should be considered for liver transplantation simultaneously [Citation12]. In HBV-related HCC patients receiving curative surgical resection, one retrospective cohort study reported that adjuvant therapy with NAs could reduce the HCC recurrence rate and mortality by 9% and 13.4%, respectively [Citation73]. However, the study failed to compare the baseline tumor staging, surgical performances, and liver function or the development of antiviral resistance. Large randomized trials establishing the benefit of NA treatment in HBV-related HCC after curative resection are therefore necessary. As high HBV DNA levels are closely related to the recurrence of HBV-related HCC after surgical resection, studies have also compared the differential ability of NAs to suppress HBV viral load. ETV was reported to suppress HBV replication more rapidly and potently than LAM and ADV in compensated HBV patients [Citation27,Citation92,Citation93]. Fung et al. have however summarized various case reports and small studies of LAM that suggest an increased risk of hepatic failure, which could lead to death, following biochemical flares after virologic breakthrough [Citation94]; however, a meta-analysis of trials comparing ETV and LAM in CHB decompensated cirrhosis found no overall difference in mortality between the treatments [Citation92]. It is therefore reasonable to suggest that both ETV and TDF would have a superior treatment efficacy in HBV patients than other antiviral drugs, although further robust evidence for the long-term effect of TDF in the HBV-related HCC patient population is required. Data on the long-term safety of TDF in the HIV patient population have demonstrated that TDF therapy could lead to renal insufficiency and hypophosphatemic osteomalacia, although this has not yet been demonstrated in HCC patients [Citation95]. The EASL guidelines state that ETV is the most appropriate first-line agent in individuals with renal failure, whereas TDF is considered an appropriate alternative in LAM-resistant patients [Citation12]. However, the impact of either agent on survival in patients with HBV-related HCC remains to be fully elucidated.
3. Conclusion
The goal of antiviral therapy for HBV-related HCC is to suppress viral replication, reduce the recurrence rate of HCC, and increase patient overall survival. To date, the use of antiviral therapy in HBV-related HCC has been recommended by several global organizations. Both pre- and post-operative antiviral therapy could reduce the recurrence rate of HCC in patients, but little data are currently available comparing the efficacy of different NAs. Studies have recommended ETV and TDF as first-line antiviral agents for treatment-naïve patients with decompensated cirrhosis both pre- and post-transplantation, and using NAs with a low genetic barrier to drug resistance should be avoided. The use of antiviral agents should be combined with HBIg to further reduce HBV recurrence rates, while monotherapy of potent NAs with a high genetic barrier can be an alternative to combination therapy for post-transplant patients. For patients receiving chemotherapy treatment, LAM can reduce the risk and the severity of HBV reactivation but poses a drug resistance threat. Potent NAs with lower resistance rates than LAM, such as TVF and ETV, showed effectiveness in preventing HBV reactivation in patients receiving chemotherapy.
In future, large, multicenter randomized trials comparing the efficacy of different antiviral therapies in HBV-related HCC patients should be conducted.
4. Expert commentary
HCC is a leading cause of cancer-related death, with an estimated 750,000 new cases of HCC worldwide every year. It is further estimated that up to 80% of these cases are as a result of HBV or HCV infection, with evidence showing that HCC mortality and incidence is correlated with prevalence of HBV. Surgical resection, lT, chemotherapy, and local tumor ablation are currently the main treatment options available, however in cases related to HBV infection there should also be an antiviral component of treatment to prevent recurrence. Considering the continued high rates of Hepatitis B in sub-Saharan Africa and East Asia, there remains a high need for clear and effective antiviral treatment pathways in these regions for HBV patients that subsequently develop HCC. This need exists in three distinct but related patient populations, namely:
HBV-related HCC patients who have undergone lT. In these patients, a combination of HBIg and NAs with a high genetic barrier to resistance (i.e. ETV or TDF) prove to effectively prevent HBV recurrence posttransplant. It is still unclear whether HBIg discontinuation after prior combination therapy or NA monotherapy could provide equivalent long-term benefits, and this should be investigated in the future from both an efficacy and cost-effectiveness perspective.
HBV-related HCC patients with curative treatment. Accumulating evidence shows that NA antiviral treatment could reduce HCC recurrence and improve overall survival rate. This supports the use of NA antiviral therapy in the tertiary prevention of HCC.
HBV-related HCC patients without curative treatment. NAs have been shown to be effective in preventing HBV reactivation in patients receiving chemotherapy. However, future considerations should evaluate whether NAs should be administered in all or only a subset of late-stage HCC patients. This question should be considered from both a disease status perspective and an expected lifespan status.
An important next step would therefore be to conduct a series of clinical trials. Of particular interest would be the initiation of large multicenter trials with longer treatment duration from across Asia. This would enable specific sub-analyses and meta-analyses of the data to confirm the safety and efficacy of existing treatments in these high-risk populations, and would be of benefit in the generation of robust local guidelines. By understanding this issue more thoroughly, it will not only be possible to assess the best treatment option for each patient but will also provide data on the total healthcare cost associated with the long-term outcomes of the patients. This in turn will allow for more thorough total cost assessments, and potentially lead to an improved economic strategy for treatment of HBV-related HCC.
Although treatments are available for HBV-related HCC, future focus should also be on the prevention of HBV, thus avoiding HBV-related HCC. Safe and effective vaccines for HBV are available which have enabled infection rates to drop to less than 1% in Western Europe and North America. With improved prevention and clearer guidance for treatment of HBV-related HCC, we hope that the healthcare burden of this disease can be reduced across the region.
5. Five-year view
The current standard of antiviral therapy involves a finite course of pegIFN and an indefinite duration of NAs. Although ‘functional cure’, defined as HBsAg loss or seroconversion is rarely achieved with NAs, long-term treatment with NAs does reduce the incidence of cirrhosis, liver failure, and HCC. Therefore, NAs with potent antiviral effect and high genetic barrier are still likely to be a mainstay of long-term treatment in patients with HBV-related HCC.
In addition, a number of potential therapies are under preclinical and clinical investigation, and these have the potential to further improve treatment if approved. These include compounds that induce cccDNA degradation, compounds that inhibit HBV entry, compounds that inhibit the expression of viral proteins, and compounds that use immunotherapeutic approaches [Citation13,Citation96].
In addition to upcoming developments in treatment options, it is likely that there will be improvements in the identification of HBV patients at high-risk of HCC. A number of risk factors for HCC have already been identified [Citation97], and researchers have developed risk prediction models that can be used by doctors to stratify patients for their risk of HCC [Citation98]. This will allow preventative antiviral treatment for those patients at higher risk of HCC, thus reducing the overall burden of HBV-related HCC in a cost-effective manner. Future risk models should also take into account somatic and inherited biomarkers [Citation98].
Key issues
A high prevalence of HBV infection is associated with an increased incidence of HCC
Antiviral Nucleos(t)ide analogues (NA) treatment improves HBV-related HCC prognosis
Drug resistance can be an issue with some currently available NAs. NAs with a high genetic barrier to resistance are recommended by international guidelines.
More data are required that directly compare the safety and efficacy of current NAs
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
Notes on contributors
Yiqi Yu
Conceived and designed the manuscript: Wenhong Zhang Search the literature: Yiqi Yu and Jingwen Ai Wrote the manuscript: Yiqi Yu and Jingwen Ai Design the table and figure: Yiqi Yu and Jingwen Ai
References
- Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399.
- Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273.
- Yu MW, Yeh SH, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97:265–272.
- Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006;295:65–73.
- Hung IF, Poon RT, Lai CL, et al. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103:1663–1673.
- Lok AS, McMahon BJ, Brown RS, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016 Jan;63(1):284–306.
- Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531.
- Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107.
- Kim WR, Loomba R, Berg T, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121:3631–3638.
- Liu GM, Huang XY, Shen SL, et al. Adjuvant antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after curative treatment: A systematic review and meta-analysis. Hepatol Res. 2016;46:100–110.
- European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185.
- Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
- Ayoub WS, Keeffe EB. Review article: current antiviral therapy of chronic hepatitis B. Aliment Pharmacol Ther. 2011;34:1145–1158.
- Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010.
- Marcellin P, Buti M, Krastev Z, et al. Continued efficacy and safety through 4 years of tenofovir disoproxil fumarate (TDF) treatment in HBeAg negative patients with chronic hepatitis B (study 102): preliminary analysis. Hepatology. 2010;52(4 Suppl.):555A.
- Heathcote EJ, Gane EJ, De Man R, et al. Long term (4 years) efficacy and safety of tenofovir disoproxil fumarate (TDF) treatment in HBeAG-positive patients (HBeAg+) with chronic hepatitis B (Study 103): preliminary analysis. Hepatology. 2010;52(4 Suppl.):556A.
- Terrault NA, Bzowej NH, Chang KM, et al. American association for the study of liver diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283.
- Wu CY, Lin JT, Ho HJ, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147:143–151, e145.
- Murray KF, Carithers RL Jr, Aasld. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432.
- Papatheodoridis GV, Cholongitas E, Archimandritis AJ, et al. Current management of hepatitis B virus infection before and after liver transplantation. Liver Int. 2009;29:1294–1305.
- Papatheodoridis GV, Sevastianos V, Burroughs AK. Prevention of and treatment for hepatitis B virus infection after liver transplantation in the nucleoside analogues era. Am J Transplant. 2003;3:250–258.
- Schiff E, Lai CL, Hadziyannis S, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007;13:349–360.
- Hou J, Yin YK, Xu D, et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: results at 1 year of a randomized, double-blind trial. Hepatology. 2008;47:447–454.
- Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–2588.
- Chan HL, Chen YC, Gane EJ, et al. Randomized clinical trial: efficacy and safety of telbivudine and lamivudine in treatment-naive patients with HBV-related decompensated cirrhosis. J Viral Hepat. 2012;19:732–743.
- Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020.
- Sherman M, Yurdaydin C, Sollano J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039–2049.
- Manolakopoulos S, Karatapanis S, Elefsiniotis J, et al. Clinical course of lamivudine monotherapy in patients with decompensated cirrhosis due to HBeAg negative chronic HBV infection. Am J Gastroenterol. 2004;99:57–63.
- Perrillo RP, Wright T, Rakela J, et al. A multicenter United States-Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology. 2001;33:424–432.
- Fontana RJ, Keeffe EB, Carey W, et al. Effect of lamivudine treatment on survival of 309 North American patients awaiting liver transplantation for chronic hepatitis B. Liver Transpl. 2002;8:433–439.
- Lake JR. Do we really need long-term hepatitis B hyperimmune globulin? What are the alternatives? Liver Transpl. 2008;14(Suppl 2):S23–26.
- Gilson RJ, Chopra KB, Newell AM, et al. A placebo-controlled phase I/II study of adefovir dipivoxil in patients with chronic hepatitis B virus infection. J Viral Hepat. 1999;6:387–395.
- Sonneveld MJ, Janssen HL. Chronic hepatitis B: peginterferon or nucleos(t)ide analogues? Liver Int. 2011;31(Suppl 1):78–84.
- Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893.
- Lim YS, Han S, Heo NY, et al. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147:152–161.
- Jang JW, Choi JY, Kim YS, et al. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61:1809–1820.
- Tenny DJ, Pokornowski KA, Rose RE, et al. Entecavir at five years shows long-term maintenance of high genetic barrier to hepatitis B virus resistance. Zeitschrift Fur Gastroenterologie. 2008;46(9). DOI: 10.1055/s-0028-1089435.
- Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475.
- Miquel M, Nunez O, Trapero-Marugan M, et al. Efficacy and safety of entecavir and/or tenofovir in hepatitis B compensated and decompensated cirrhotic patients in clinical practice. Ann Hepatol. 2013;12:205–212.
- Hung CH, Hu TH, Lu SN, et al. Tenofovir versus entecavir in treatment of chronic hepatitis B virus with severe acute exacerbation. Antimicrob Agents Chemother. 2015;59:3168–3173.
- Wang ZX, Fu ZR, Ding GS, et al. Prevention of hepatitis B virus reinfection after orthotopic liver transplantation. Transplant Proc. 2004;36:2315–2317.
- Ahn J, Cohen SM. Prevention of hepatitis B recurrence in liver transplant patients using oral antiviral therapy without long-term hepatitis B immunoglobulin. Hepat Mon. 2011;11:638–645.
- Fung J, Cheung C, Chan SC, et al. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology. 2011;141:1212–1219.
- Cholongitas E, Vasiliadis T, Antoniadis N, et al. Hepatitis B prophylaxis post liver transplantation with newer nucleos(t)ide analogues after hepatitis B immunoglobulin discontinuation. Transpl Infect Dis. 2012;14:479–487.
- Perrillo R, Buti M, Durand F, et al. Entecavir and hepatitis B immune globulin in patients undergoing liver transplantation for chronic hepatitis B. Liver Transpl. 2013;19(8):887–895.
- Tanaka T, Renner EL, Selzner N, et al. One year of hepatitis B immunoglobulin plus tenofovir therapy is safe and effective in preventing recurrent hepatitis B infection post-liver transplantation. Can J Gastroenterol Hepatol. 2014;28(1):41–44.
- Ueda Y, Marusawa H, Kaido T, et al. Efficacy and safety of prophylaxis with entecavir and hepatitis B immunoglobulin in preventing hepatitis B recurrence after living-donor liver transplantation. Hepatol Res. 2013;43(1):67–71.
- Gao YJ, Zhang M, Jin B, et al. A clinicalpathological analysis of hepatitis B virus recurrence after liver transplantation in Chinese patients. J Gastroenterol Hepatol. 2014;29(3):554–560.
- Kim YK, Kim SH, Lee SD, et al. Clinical outcomes and risk factors of hepatitis B virus recurrence in patients who received prophylaxis with entecavir and hepatitis B immunoglobulin following liver transplantation. Transplant Proc. 2013;45(8):3052–3056.
- Lee S, Kwon CH, Moon HH, et al. Antiviral treatment for hepatitis B virus recurrence following liver transplantation. Clin Transplant. 2013;27(5):E597–604.
- Malkan G, Cattral MS, Humar A, et al. Lamivudine for hepatitis B in liver transplantation: a single-center experience. Transplantation. 2000;69:1403–1407.
- Mutimer D, Dusheiko G, Barrett C, et al. Lamivudine without HBIg for prevention of graft reinfection by hepatitis B: long-term follow-up. Transplantation. 2000;70:809–815.
- Mutimer D, Pillay D, Shields P, et al. Outcome of lamivudine resistant hepatitis B virus infection in the liver transplant recipient. Gut. 2000;46:107–113.
- Chan HL, Chui AK, Lau WY, et al. Outcome of lamivudine resistant hepatitis B virus mutant post-liver transplantation on lamivudine monoprophylaxis. Clin Transplant. 2004;18:295–300.
- Jimenez-Perez M, Gonzalez-Grande R, Mostazo Torres J, et al. Management of hepatitis B virus infection after liver transplantation. World J Gastroenterol. 2015;21:12083–12090.
- Jiang L, Yan L, Wen T, et al. Hepatitis B prophylaxis using lamivudine and individualized low-dose hepatitis B immunoglobulin in living donor liver transplantation. Transplant Proc. 2013;45:2326–2330.
- Loomba R, Rowley AK, Wesley R, et al. Hepatitis B immunoglobulin and Lamivudine improve hepatitis B-related outcomes after liver transplantation: meta-analysis. Clin Gastroenterol Hepatol. 2008;6:696–700.
- Cholongitas E, Papatheodoridis GV. High genetic barrier nucleos(t)ide analogue(s) for prophylaxis from hepatitis B virus recurrence after liver transplantation: a systematic review. Am J Transplant. 2013;13:353–362.
- Nath DS, Kalis A, Nelson S, et al. Hepatitis B prophylaxis post-liver transplant without maintenance hepatitis B immunoglobulin therapy. Clin Transplant. 2006;20:206–210.
- Bruix J, Sherman M. American association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022.
- Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137:403–410.
- Chau GY. Resection of hepatitis B virus-related hepatocellular carcinoma: evolving strategies and emerging therapies to improve outcome. World J Gastroenterol. 2014;20:12473–12484.
- Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1:144–158.
- Ke Y, Ma L, You XM, et al. Antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after radical hepatectomy. Cancer Biol Med. 2013;10:158–164.
- Guglielmi A, Ruzzenente A, Conci S, et al. Hepatocellular carcinoma: surgical perspectives beyond the barcelona clinic liver cancer recommendations. World J Gastroenterol. 2014;20:7525–7533.
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207.
- Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery. 2012;151:700–709.
- Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–897.
- Kuzuya T, Katano Y, Kumada T, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929–1935.
- Someya T, Ikeda K, Saitoh S, et al. Interferon lowers tumor recurrence rate after surgical resection or ablation of hepatocellular carcinoma: a pilot study of patients with hepatitis B virus-related cirrhosis. J Gastroenterol. 2006;41:1206–1213.
- Chan AC, Chok KS, Yuen WK, et al. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675–681.
- Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. Jama. 2012;308:1906–1914.
- Lee TY, Lin JT, Zeng YS, et al. Association between nucleos(t)ide analogue and tumor recurrence in HBV-related hepatocellular carcinoma after radiofrequency ablation. Hepatology. 2016;63(5):1517–1527.
- Hann HW, Coben R, Brown D, et al. A long-term study of the effects of antiviral therapy on survival of patients with HBV-associated hepatocellular carcinoma (HCC) following local tumor ablation. Cancer Med. 2014;3:390–396.
- Piao CY, Fujioka S, Iwasaki Y, et al. Lamivudine treatment in patients with HBV-related hepatocellular carcinoma–using an untreated, matched control cohort. Acta Med Okayama. 2005;59:217–224.
- Kim BK, Park JY, Kim DY, et al. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008;28:393–401.
- Miao RY, Zhao HT, Yang HY, et al. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2010;16:2931–2942.
- Yeo W, Chan PK, Hui P, et al. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol. 2003;70:553–561.
- Jang JW. Hepatitis B virus reactivation in patients with hepatocellular carcinoma undergoing anti-cancer therapy. World J Gastroenterol. 2014;20:7675–7685.
- Xunrong L, Yan AW, Liang R, et al. Hepatitis B virus (HBV) reactivation after cytotoxic or immunosuppressive therapy–pathogenesis and management. Rev Med Virol. 2001;11:287–299.
- Chung YL, Tsai TY. Promyelocytic leukemia nuclear bodies link the DNA damage repair pathway with hepatitis B virus replication: implications for hepatitis B virus exacerbation during chemotherapy and radiotherapy. Mol Cancer Res. 2009;7:1672–1685.
- Kohrt HE, Ouyang DL, Keeffe EB. Systematic review: lamivudine prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2006;24:1003–1016.
- Hsu C, Hsiung CA, Su IJ, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47:844–853.
- Yun J, Kim KH, Kang ES, et al. Prophylactic use of lamivudine for hepatitis B exacerbation in post-operative breast cancer patients receiving anthracycline-based adjuvant chemotherapy. Br J Cancer. 2011;104:559–563.
- Hui CK, Cheung WW, Au WY, et al. Hepatitis B reactivation after withdrawal of pre-emptive lamivudine in patients with haematological malignancy on completion of cytotoxic chemotherapy. Gut. 2005;54:1597–1603.
- Saab S, Dong MH, Joseph TA, et al. Hepatitis B prophylaxis in patients undergoing chemotherapy for lymphoma: a decision analysis model. Hepatology. 2007;46:1049–1056.
- Cortelezzi A, Vigano M, Zilioli VR, et al. Adefovir added to lamivudine for hepatitis B recurrent infection in refractory B-cell chronic lymphocytic leukemia on prolonged therapy with Campath-1H. J Clin Virol. 2006;35:467–469.
- Pelizzari AM, Motta M, Cariani E, et al. Frequency of hepatitis B virus mutant in asymptomatic hepatitis B virus carriers receiving prophylactic lamivudine during chemotherapy for hematologic malignancies. Hematol J. 2004;5:325–328.
- Li HR, Huang JJ, Guo HQ, et al. Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat. 2011;18:877–883.
- Li X, Zhong X, Chen ZH, et al. Efficacy of prophylactic entecavir for hepatitis B virus-related hepatocellular carcinoma receiving transcatheter arterial chemoembolization. Asian Pac J Cancer Prev. 2015;16:8665–8670.
- Ye XG, Su QM. Effects of entecavir and lamivudine for hepatitis B decompensated cirrhosis: meta-analysis. World J Gastroenterol. 2013;19:6665–6678.
- Jiang E, Shangguan AJ, Chen S, et al. The progress and prospects of routine prophylactic antiviral treatment in hepatitis B-related hepatocellular carcinoma. Cancer Lett. 2015;379(2):262–267.
- Fung J, Lai CL, Yuen MF. Management of chronic hepatitis B in severe liver disease. World J Gastroenterol. 2014;20:16053–16061.
- Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9:227–241.
- Zeisel MB, Lucifora J, Mason WS, et al. Towards an HBV cure: state-of-the-art and unresolved questions–report of the ANRS workshop on HBV cure. Gut. 2015;64:1314–1326.
- Nguyen VTT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16(7):453–463.
- Lee HW, Ahn SH. Prediction models of hepatocellular carcinoma development in chronic hepatitis B patients. World J Gastroenterol. 2016;22(37):8314–8321.