ABSTRACT
Introduction: The purpose of this review is to highlight the role of biosimilars in early treatment in IBD and introduce ways to facilitate a patient-centric switching process through multidisciplinary approach.
Areas covered: We summarize existing scientific literature related to the role of biosimilars in inflammatory bowel disease in terms of early treatment and cost-saving and implementing switching process.
Expert opinion: Use of anti-TNF biosimilars in patients has the potential for large drug-acquisition cost-saving, which can be reinvested into early treatment. Managed switched programs for adalimumab can add further benefits in the future.
1. Introduction
Crohn’s disease and ulcerative colitis are the two main clinicopathological subtypes of inflammatory bowel diseases (IBDs), which are chronic, relapsing conditions with unclear etiology that require effective early and sustained treatment to induce and maintain remission, and prevent functional disability [Citation1,Citation2]. Over the past two decades, biologic therapies, beginning with the anti-tumor necrosis factor (TNF) monoclonal antibodies, have been instrumental in improving outcomes for patients with IBD [Citation3,Citation4]. In this review, we discuss the need for early anti-TNF therapy in patients with IBD, and optimal integration of biosimilars into gastroenterology practice using a patient-centric interdisciplinary approach. The content of the review is based on the proceedings of a symposium held during the 26th United European Gastroenterology Week in Vienna, Austria in October 2018.
2. Expanding treatment armamentarium in IBD
The pharmacological management of IBD involves different classes of drugs with distinct but anti-inflammatory mechanisms of action, including anti-TNF, anti-integrin and anti-interleukin biologic agents () [Citation5]. As the number of treatment options available for patients with IBD expands, the IBD treatment paradigm is evolving from an approach aimed at control of symptoms toward a strategy that aims to prevent progressive bowel damage and subsequent disability, which is associated with earlier use of biologics [Citation2,Citation5,Citation6]. A rapid step-up approach is used in most patients, beginning with corticosteroids followed by traditional immunomodulators, such as azathioprine and methotrexate, before moving to biologics () [Citation7].
Table 1. Biologics and biosimilars approved in Europe or the United States for the treatment of patients with IBD (correct as of January 2019) [Citation1,Citation5,Citation8,Citation9,Citation11].
Figure 1. Step-up treatment choices for inflammatory bowel disease based on disease severity, patient responsiveness and drug toxicity [Citation7].
![Figure 1. Step-up treatment choices for inflammatory bowel disease based on disease severity, patient responsiveness and drug toxicity [Citation7].](/cms/asset/c5caba31-5c60-4ca6-baae-46dbeb051a2c/ierh_a_1645595_f0001_b.gif)
Compared with the pre-biologics era, when hospitalization and surgery were the major cost drivers in IBD, a European cost-of-illness study conducted after the introduction of the anti-TNF biologics infliximab (Remicade) and adalimumab (Humira) showed that IBD healthcare costs are now mainly driven by medication costs, particularly by expensive biologic therapy [Citation10]. However, the recent expiry of infliximab and adalimumab patents in Europe (2015 and 2018, respectively) has opened the market to lower-cost biosimilar agents [Citation1], which contain equivalent versions of the active substance of the authorized originator or reference agent [Citation11]. Unlike generic copies of small-molecule drugs that are widely used as alternatives to originator small molecules, the complex molecular structure and cell culture production methods of biologic agents mean that they cannot be copied exactly, but they must be similar to the reference agent in terms of quality characteristics, biologic activity, efficacy and safety [Citation1,Citation11–Citation13].
2.1. Biosimilars in IBD
Several adalimumab and infliximab biosimilars have been approved by the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) for use in IBD, but clinical testing of most of these agents was performed in other indications, such as rheumatoid arthritis [Citation1]. For example, CT-P13 was the first biosimilar to receive regulatory approval for all therapeutic indications of infliximab based on clinical studies in patients with rheumatoid arthritis or ankylosing spondylitis [Citation1]. This process of extrapolation reduces the need for duplicative clinical studies and expedites the developmental process [Citation1]. Moreover, extrapolation is accepted by regulatory bodies including the EMA and FDA when it is scientifically justified and based on the overall evidence, including extensive physicochemical and biological characterization of the biosimilar relative to the reference product [Citation11,Citation14]. For example, the physicochemical and biological characteristics of the infliximab biosimilar SB2, which in 2016 received European marketing authorization for the full range of indications of the reference product, were shown to be highly similar to reference infliximab [Citation15], and predictions of equivalent long-term clinical efficacy, safety and immunogenicity based on these comparability exercises were borne out in a randomized, double-blind phase III study in patients with rheumatoid arthritis [Citation16–Citation18]. Similar findings have now also been reported for the adalimumab biosimilars ABP501, BI695501, GP2017 and SB5, which have received European marketing authorization for all indications of reference adalimumab [Citation19].
3. Early anti-TNF therapy
Crohn’s disease progresses from a preclinical phase with subclinical inflammation to early-stage disease with inflammation and no complications (fistula, abscess or stricture) to late-stage disease with bowel damage and impairment of gastrointestinal function () [Citation20,Citation21]. Ideally, diagnosis of complicated disease should be made at an early-stage and followed by early and sustained immunosuppressive treatment to control inflammation and prevent irreversible bowel damage [Citation2,Citation20].
Figure 2. Progression of digestive disease damage and inflammation in a theoretical patient with Crohn’s disease [Citation20].
CDAI, Crohn’s disease activity index; CDEIS, Crohn’s disease endoscopic index of severity; CRP, C-reactive protein
![Figure 2. Progression of digestive disease damage and inflammation in a theoretical patient with Crohn’s disease [Citation20].CDAI, Crohn’s disease activity index; CDEIS, Crohn’s disease endoscopic index of severity; CRP, C-reactive protein](/cms/asset/c5750278-9878-4026-a624-ea8229181d34/ierh_a_1645595_f0002_b.gif)
3.1. Early treatment with anti-TNF agents
Studies in patients with Crohn’s disease indicate that anti-TNF agents have greater efficacy in earlier Crohn’s disease than more established disease [Citation22–Citation27]. For example, corticosteroid-free remission rates of up to 40–50% were achieved at 48 to 50 weeks in immunomodulatory- and biologic-naive patients treated with infliximab in the SONIC study (median disease duration 2.3 years) [Citation22,Citation28], or adalimumab in the CALM study (mean disease duration 1 year) [Citation23], compared with corticosteroid-free remission of 20–29% with adalimumab and infliximab in the CHARM and ACCENT I populations, respectively (median disease duration of approximately 8 years) [Citation24,Citation26]. The availability of relatively low-cost anti-TNF biosimilars may facilitate earlier use of anti-TNF agents in IBD patients, and more of a top-down treatment approach [Citation29], involving early anti-TNF therapy alone or in combination with traditional immunomodulators [Citation25,Citation30].
3.2. Diagnostic delays
A definition of early Crohn’s disease as disease duration ≤18 months after diagnosis and without previous exposure to immunomodulators or biologic therapies has been proposed [Citation31]. However, studies show that complications are already present in 20–40% of Crohn’s disease patients at time of diagnosis, reflecting a diagnostic delay [Citation32,Citation33]. This is a common issue in IBD, with more than 20% of patients in the pan-European IMPACT survey reporting that they experienced symptoms for more than 5 years before seeing a gastroenterologist [Citation34].
Delayed diagnosis is a particular problem in Crohn’s disease because symptoms may initially be non-specific and overlap with those of irritable bowel syndrome. In patients with Crohn’s disease, a long diagnostic delay was associated with a complicated disease course and more surgeries [Citation35], and also missed the therapeutic window to intervene with anti-TNF agents before complications occurred [Citation36]. Early referral and close collaboration between GPs and gastroenterologists play a key role in improving early diagnosis, and a Red Flags index has been developed to detect signs and symptoms suggestive of Crohn’s disease that should prompt referral to a gastroenterologist for further evaluation and appropriate therapy [Citation37].
4. Patient-centric pre-switch scenarios in daily clinical practice
Patients are often unfamiliar with biosimilar medicines, and express concerns about the safety and efficacy of treatment when asked to switch to a biosimilar from a reference biologic; it is therefore important to provide them with evidence-based information to support such a switch [Citation1,Citation38]. Therapeutic drug monitoring (TDM), immunogenicity and real-world switching data for biosimilars of infliximab in IBD patients are becoming increasingly available, and can be used to help to reassure patients of the safety and efficacy of biosimilars when switching from a reference biologic [Citation1,Citation39].
4.1. Therapeutic drug monitoring and immunogenicity
TDM is a cost-effective tool that allows the dose of a biologic agent to be adjusted on the basis of serum drug and anti-drug antibody (ADA) concentrations in order to establish and maintain response [Citation39,Citation40]. Several enzyme-linked immunosorbent assay (ELISA) methods are available to allow for easy and efficient quantification of infliximab serum levels and adjustment of dose during TDM [Citation41]. Studies have shown that commercially available assays used in patients receiving reference infliximab can also be used with confidence to monitor drug levels in patients treated with the infliximab biosimilars CT-P13 or SB2 [Citation41–Citation44]. Assays such as the Promonitor-ANTI-IFX kit can also be used to monitor ADAs in patients receiving reference infliximab, CT-P13 or SB2 [Citation42]. Comparable cross-reactivity between these drugs was demonstrated in patients with IBD, the clinical implication being that switching is not likely to result in any new immunogenic reactions [Citation42,Citation45,Citation46].
4.2. Real-world data
Real-world data showing that a switch from reference infliximab to CT-P13 (the first infliximab biosimilar to be granted European marketing authorization) is effective and safe in patients with IBD are accumulating from various European countries [Citation47–Citation52]. The first results from prospective observational studies in IBD patients switched from reference infliximab to the more recently introduced infliximab biosimilar SB2, demonstrating no loss of efficacy or increase in immunogenicity over a period of 6 months after the switch, have also been reported [Citation53,Citation54].
5. Implementing gain share models in the UK
The primary motivation for using anti-TNF biosimilars in patients with IBD is the potential for large drug-acquisition cost savings, which can be reinvested into other aspects of patient care [Citation51]. For maximal cost savings to be achieved, IBD patients already receiving maintenance therapy with reference adalimumab or infliximab should be switched to a biosimilar in a carefully managed environment, with shared informed decision-making between patients and healthcare providers [Citation38,Citation51,Citation55].
In the UK, University Hospital Southampton (UHS) and the Royal Free Hospital (RFH) used managed switching programs to support IBD patients through a switch from reference infliximab (Remicade) to biosimilar infliximab CT-P13 (Inflectra at UHS and Remsima at RFH) [Citation51,Citation55]. The programs, which included risk-management plans with robust pharmacovigilance and drug traceability (brand name-only prescribing) procedures, were developed by UHS and designed with input from all key stakeholders, including patients, IBD nurses, gastroenterologists and pharmacists. Patient panels (small groups of highly engaged patients) played a central role in the development of the switching programs. After discussions with the IBD team, these patients developed an understanding of the science, regulatory processes and evidence behind biosimilars, and recognized the opportunity to secure investment in the IBD service caring for them, so they supported the switch from reference to biosimilar infliximab [Citation51].
The extent of cost savings achieved with biosimilars in IBD depends not only on local pricing and procurement policies, but also on the willingness of healthcare providers and patients to start or switch to biosimilar [Citation29]. UHS and the RFH used gain-share agreements, whereby costs savings associated with more efficient use of high-cost drugs were distributed between stakeholders [Citation56] to fund their managed switching programs and to reinvest a proportion of the savings associated with the use of biosimilar infliximab into improving IBD services [Citation51,Citation55]. Such gain-share agreements provide direct incentive for healthcare providers or patients to make the switch to a biosimilar [Citation51]. At UHS, the patient panel specified that they would like to see expansion of the specialist nursing team and improved dietician support, both of which were included in the gain-share agreement [Citation51].
5.1. Clinical findings
More than 190 patients at the RFH are now receiving biosimilar infliximab, and 143 patients at UHS were switched to biosimilar infliximab. The increased monitoring and IBD specialist-nursing support patients received during the UHS switching program may have contributed to a significant improvement in the IBD Control Patient-Reported Outcome Measures (PROM) score observed after the switch [Citation51]. There were no changes in adverse effects, immunogenicity or infliximab trough levels after the switch [Citation51]. There was also no statistically significant difference in drug persistence between the cohort of UHS patients who switched to biosimilar infliximab versus the cohort of all IBD patients treated with reference infliximab in the year before the switch () [Citation51].
Figure 3. Survival curve showing drug persistence in relation to reference infliximab in all patients treated with reference infliximab (from April 2014 to March 2015), and in relation to biosimilar infliximab CT-P13 in patients switched from reference infliximab (between April 2014 and March 2015) at University Hospital Southampton [Citation51].
![Figure 3. Survival curve showing drug persistence in relation to reference infliximab in all patients treated with reference infliximab (from April 2014 to March 2015), and in relation to biosimilar infliximab CT-P13 in patients switched from reference infliximab (between April 2014 and March 2015) at University Hospital Southampton [Citation51].](/cms/asset/b8c70c5e-7cd6-4ac8-9da4-85a3a90f5d67/ierh_a_1645595_f0003_b.gif)
Interestingly, patients at UHS had the option to switch back to reference infliximab, and two patients requested to switch back: one patient with non-specific flu-like symptoms, which may have been a nocebo effect, and one patient with abnormal liver enzymes, which was likely not related to the switch [Citation51]. When a nocebo response, such as perceived side effects or lack of efficacy, occurs in response to active therapy it can have a negative impact on treatment adherence and outcomes [Citation13]. At baseline before the switch and after every CT-P13 infusion, UHS patients were given a simple questionnaire asking if they had any side effects since their last infusion, which was important in terms of managing any possible nocebo effects. At the RFH, only five patients reported that they felt their symptoms were worse after the switch, but after checking trough infliximab and antibody levels, no intervention was required and the symptoms eventually subsided.
5.2. Economic benefits
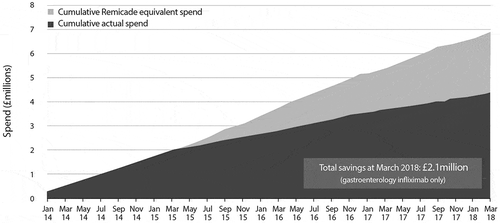
The UHS and UFH switching programs have delivered substantial savings to the local health economy: approximately £2.1 million of savings over 3 years at UHS (), and £500,000 over 12 months at the RFH. These savings have contributed to development of IBD services in both hospitals, funding additional nursing, pharmacy, dietician and clerical support. Net of the investment in the IBD services, savings were shared between the hospitals and their clinical commissioning groups.
5.3. Connecting with patients: the importance of IBD nurses
Nurses are pivotal in the day-to-day care of patients with IBD and are ideally positioned to educate and inform patients about biosimilars, and to manage any concerns that patients may have about switching [Citation55,Citation57]. As a trusted source of information for patients, nurses were integral to patient acceptance of the switch to biosimilar infliximab during the UHS and RFH switching programs. Before the switch at UHS, patients were given an information sheet on biosimilars while receiving their reference infliximab (Remicade) infusion and offered the opportunity to discuss this with their nurse practitioner [Citation51]. At their next infusion, 141 of 143 patients agreed to switch to biosimilar infliximab. The remaining two patients needed more time to think about it and made the switch at the following infusion. Before the switch at the RFH, patients received an information leaflet containing frequently asked questions developed by nurses with help from the patient panel. Patients were advised when the switch would happen and to contact an IBD clinical nurse specialist if they had any concerns (consent was implied if there was no contact). Overall, no patient at either service refused to switch, which is testament to the effectiveness of nurse-led education to aid patients’ understanding of biosimilars and the reasons for a switch to a biosimilar.
5.4. Looking ahead
As biosimilars of adalimumb enter the market, switching programs will also need to be developed for patients currently receiving reference adalimumab (Humira, >540 patients in total at UHS and the RFH). Information given to patients before a switch to biosimilar adalimumab will need to account for the fact that the adalimumab switch will be much more visible than the infliximab switch (home-based subcutaneous administration versus clinic-based intravenous infusion). This may lead to different patient concerns and the need for reassurance that adequate training and support will be provided to facilitate the switch to biosimilars from reference adalimumab. With input from panels of patients currently receiving reference adalimumab as part of their care, nurses should be at the forefront of all adalimumab switch plans and pathways.
6. Conclusions
Early diagnosis of Crohn’s disease and use of anti-TNF therapy before the onset of complications leads to better outcomes. By easing the economic burden of anti-TNF biologic treatment for IBD on healthcare systems, the availability of adalimumab and infliximab biosimilars is expected to facilitate early access to anti-TNF therapy, thereby potentially reducing the complications and functional disability associated with IBD. For maximal cost savings to be achieved with these drugs, biologic-naive patients should begin therapy with a biosimilar, and patients already receiving reference biologics should be switched to a biosimilar. The availability of assays for measuring drug concentrations and ADAs of biologics and biosimilars alike facilitates the implementation of TDM to optimize patient care when performing such a switch. When possible, gain-share agreements should be arranged to distribute the cost-saving benefits from using biosimilars between key stakeholders, and reinvestment opportunities in IBD services identified to provide incentives for IBD teams and patients to switch to adalimumab and infliximab biosimilars. Education and effective communication between IBD nurses and patients as part of managed switching programs are essential to ensure that patients have confidence in biosimilar agents, and realistic expectations of therapy, thereby avoiding nocebo effects and facilitating long-term adherence to the biosimilar.
7. Expert opinion
Anti-TNF biosimilar agents that have been approved by the EMA and FDA for use in IBD are safe and effective treatment options that are cost effective, and an extremely important part of the future of IBD treatment. Routinely starting biologic-naive patients on treatment with an anti-TNF biosimilar, rather than a reference product, and switching patients from a reference anti-TNF agent to a biosimilar, has the potential to improve quality of care as gastroenterology departments capitalize on substantial drug acquisition cost savings to fund earlier access to biologic treatment for more patients, and improve IBD clinic resources and services.
Now, as biosimilars of adalimumab are beginning to enter the market, we have a lot more experience with biosimilars in general. Increasing real-world data regarding infliximab biosimilars in IBD is leading to better acceptance of biosimilar agents in this field. However, many patients with IBD who are doing well on reference adalimumab may have concerns about switching to biosimilar therapy and large-scale switching to adalimumab may be a major challenge. Before starting an adalimumab biosimilar switch program, it is important that gastroenterology services ensure that they have the capacity to properly manage the switch and support patients. Establishing nurse-led education and structured communication strategies before the switch will be key to dispelling any pre-existing negative perceptions patients may have about a cost-driven switch from reference adalimumab to a biosimilar. Ensuring patients have a good understanding of what biosimilars are, confidence in their treatment plan and managing expectations of adverse events or loss of response will also help to reassure patients, avoid nocebo effects and boost long-term adherence rates. Putting robust risk-management programs in place to collect and audit data on fecal calprotectin, drug levels, drug antibody levels, adverse events, loss of response and any other changes that occur after switching will help to build further confidence in anti-TNF biosimilars among physicians. Efficient patient monitoring and pharmacovigilance will depend on effective traceability involving batch documentation and brand-name prescribing to reduce the risk of inadvertent interchangeability of biosimilar and reference products. There are obviously significant costs associated with managed switch and risk-management programs, but these can be offset by the drug acquisition cost savings generated by the switch to the biosimilar.
The extent of the cost savings realized with anti-TNF biosimilars for the treatment of IBD ultimately depends on the willingness of healthcare professionals (HCPs) to start biologic-naive patients on biosimilars and support managed switch programs. To successfully convey the concept of biosimilars to their patients and aid the transition to biosimilars, all members of the healthcare team must be educated about biosimilars and deliver the same message so as not to confuse patients. It is therefore important to ensure that there is effective communication within the team before a switch to biosimilars. It is important that patients understand that the same adverse events may occur with a biosimilar as for the reference product, but based on experience with the reference product can be effectively taken care of. For example, an infusion reaction with a biosimilar is treated the same way as that of an infusion reaction that occurs during infusion of a reference product. Patients can be reassured that this adverse event is expected in a proportion of patients, whether or not they receive a reference product or a biosimilar. This is also the case for loss of response in relation to the natural history of long-term treatment with anti-TNF monoclonal antibodies. Confidence in biosimilars among HCPs caring for patients with IBD will grow as long as real-world evidence from patient registries and data from prospective observational studies and pharmacovigilance programs in patients with IBD continue to support the efficacy and safety of biosimilars, and show that switching long-term IBD patients from a reference product to a biosimilar is a safe option without increased immunogenicity. Additional controlled clinical studies of biosimilars performed specifically in patients with IBD would help to boost HCP confidence in biosimilars for the treatment of IBD. In the future, cost-effectiveness from biosimilar use can be translated into early, aggressive biologic treatment in patients who might not have been able to receive care in the past. As a result, irreversible, structural changes can be prevented from inflammatory bowel disease, which will lead to a better quality of life and further reduced healthcare cost.
Article highlights
The recent expiry of infliximab and adalimumab patents in Europe has opened the market to lower-cost biosimilar agents, which contain equivalent versions of the active substance of the authorized originator or reference agent
By easing the economic burden of anti-TNF biologic treatment for IBD on healthcare systems, the availability of biosimilars is expected to facilitate early access to anti-TNF therapy, thereby potentially reducing the complications and functional disability associated with IBD.
The availability of assays for measuring drug concentrations and ADAs of biologics and biosimilars alike facilitates the implementation of TDM to optimize patient care when performing such a switch
When possible, gain-share agreements should be arranged to distribute the cost-saving benefits from using biosimilars between key stakeholders, and reinvestment opportunities in IBD services identified to provide incentives for IBD teams and patients to switch to adalimumab and infliximab biosimilars
Multidisciplinary approach is pivotal in the day-to-day care of patients with IBD and is ideally positioned to educate and inform patients about biosimilars, and to manage any concerns that patients may have about switching
Declaration of interest
L. Peyrin-Biroulet discloses professional fees from Tillots, Celltrion, Allergan, Biogen, MSD, Genentech, Index Pharmaceuticals, Ferring, Roche, Arena, Sterna, Gilead, Nestlé, Boerhinger Ingelheim, Sandoz, Celgene, Enterome, Pfizer, Samsung, Abbvie, Takeda, Pharmacosmos, Janssen, Hikma, Alma, Amgen.
S. Danese discloses consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring Pharmaceuticals Inc., Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Pfizer, Roche, Sandoz, Takeda, TiGenix, UCB Inc., Vifor.
R. Atreya discloses consultancy, grants or personal fees from AbbVie, Biogen, Boehringer Ingelheim GmbH & Co KG, DrFalk Pharma GmbH, Ferring GmbH, GlaxoSmithKline plc, InDex Pharmaceuticals AG, Janssen-Cilag GmbH, MSD Sharp & Dome GmbH, Pfizer Inc., Roche Pharma, Samsung Bioepsis, Stelic Institute, Takeda GmbH & Co. KG, Tillotts Pharma AG.
F. Cummings discloses grants and personal fees from Samsung Bioepis, grants and personal fees from Biogen, grants and personal fees from Amgen, personal fees from MSD, personal fees and non-financial support from Janssen, grants, personal fees and non-financial support from Takeda, grants and personal fees from Pfizer, personal fees and non-financial support from Abbvie, personal fees from Sandoz, personal fees from Celltrion, and personal fees from NAPP.
K. Greveson discloses Personal fees from Abbott Laboratories, and consultancy fees from Takeda, Abbvie, Janssen, Tillotts Pharma, MSD, Samsung Bioepis.
B. Pieper discloses being an employee of Biogen International GmbH.
T. Kang is an employee of Samsung Bioepis Co., Ltd.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors acknowledge Weber Shandwick, Hong Kong for editorial support in the preparation of this manuscript.
Additional information
Funding
References
- Danese S, Bonovas S, Peyrin-Biroulet L. Biosimilars in IBD: from theory to practice. Nat Rev Gastroenterol Hepatol. 2017;14:22–31.
- Allen PB, Gower-Rousseau C, Danese S, et al. Preventing disability in inflammatory bowel disease. Therap Adv Gastroenterol. 2017;10:865–876.
- Mao EJ, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3–13.
- Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45:1291–1302.
- Hindryckx P, Vande Casteele N, Novak G, et al. The expanding therapeutic armamentarium for inflammatory bowel disease: how to choose the right drugs for our patients? J Crohns Colitis. 2018;12:105–119.
- Rogler G. Top-down or step-up treatment in Crohn’s disease? Dig Dis. 2013;31:83–90.
- McQuaid RR. Chapter 62. Drugs used in the treatment of gastrointestinal diseases. In: Katzung BG, Trevor AJ, editors. Basic and Clinical Pharmacology; 2015.
- Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215–226.
- US Food and Drug Administration. Biosimilar product information [Internet]. Silver Spring, MD, USA; [Updated 18 Jan 2019; cited Feb 2019]. Available from: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm580432.htm
- van der Valk ME, Mangen M-J-J, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72–79.
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues [Internet]. London, UK; 18 December 2014 [cited Dec 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf.
- Declerck P, Farouk Rezk M. The road from development to approval: evaluating the body of evidence to confirm biosimilarity. Rheumatology (Oxford). 2017;56:iv4–iv13.
- Rezk MF, Pieper B. Treatment outcomes with biosimilars: be aware of the nocebo effect. Rheumatol Ther. 2017;4:209–218.
- US Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. [Internet]. Silver Spring, MD, USA; April 2015 [cited Dec 2018]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291128.pdf.
- Hong J, Lee Y, Lee C, et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade® (infliximab). MAbs. 2017;9:364–382.
- Choe J-Y, Prodanovic N, Niebrzydowski J, et al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76:58–64.
- Smolen JS, Choe J-Y, Prodanovic N, et al. Comparing biosimilar SB2 with reference infliximab after 54 weeks of a double-blind trial: clinical, structural and safety results. Rheumatology (Oxford). 2017;56:1771–1779.
- Smolen JS, Choe J-Y, Prodanovic N, et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: results of a randomised, double-blind, phase III transition study. Ann Rheum Dis. 2018;77:234–240.
- Fiorino G, Gilardi D, Correale C, et al. Biosimilars of adalimumab: the upcoming challenge in IBD. Expert Opin Biol Ther. 2019. Epub ahead of print. doi:10.1080/14712598.2019.1564033.
- Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415–1422.
- Peyrin-Biroulet L, Loftus EV, Colombel J-F, et al. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut. 2010;59:141–147.
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395.
- Colombel J-F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789.
- Colombel J-F, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
- D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
- Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863–873. quiz 1165-6
- Colombel J-F, Rutgeerts P, Reinisch W, et al. One year data from the SONIC study: a randomized, double-blind trial comparing infliximab and infliximab plus azathioprine to azathioprine in patients with Crohn’s disease naive to immunomodulators and biologic therapy. Gut. 2009;58:A69.
- Severs M, Oldenburg B, van Bodegraven AA, et al. initiative of Crohn’s and Colitis. The economic impact of the introduction of biosimilars in inflammatory bowel disease. J Crohns Colitis. 2017;11:289–296.
- Hirschmann S, Neurath MF. Top-down approach to biological therapy of Crohn’s disease. Expert Opin Biol Ther. 2017;17:285–293.
- Peyrin-Biroulet L, Billioud V, D’Haens G, et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am J Gastroenterol. 2012;107:1770–1776.
- Fiorino G, Morin M, Bonovas S, et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J Crohns Colitis. 2017;11:274–280.
- Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250.
- Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1:10–20.
- Schoepfer AM, Dehlavi M-A, Fournier N, et al. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013;108:1744–1753. quiz 1754
- Danese S, Fiorino G, Fernandes C, et al. Catching the therapeutic window of opportunity in early Crohn’s disease. Curr Drug Targets. 2014;15:1056–1063.
- Danese S, Fiorino G, Mary J-Y, et al. Development of Red Flags Index for early referral of adults with symptoms and signs suggestive of Crohn’s disease: an IOIBD initiative. J Crohns Colitis. 2015;9:601–606.
- Boone NW, Liu L, Romberg-Camps MJ, et al. The nocebo effect challenges the non-medical infliximab switch in practice. Eur J Clin Pharmacol. 2018;74:655–661.
- Gils A. Combining therapeutic drug monitoring with biosimilars, a strategy to improve the efficacy of biologicals for treating inflammatory bowel diseases at an affordable cost. Dig Dis. 2017;35:61–68.
- Martelli L, Olivera P, Roblin X, et al. Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. J Gastroenterol. 2017;52:19–25.
- Afonso J, de Sousa HT, Rosa I, et al. Therapeutic drug monitoring of CT-P13: a comparison of four different immunoassays. Therap Adv Gastroenterol. 2017;10:661–671.
- Fiorino G, Ruiz-Agüello MB, Maguregui A, et al. P633 Antibodies to infliximab in patients treated with either the reference biologic or the biosimilar CT-P13 show identical reactivity towards biosimilars CT-P13 and SB2 in inflammatory bowel disease. Eur Crohn’s Colitis Organ Congr Abstr. 2017; 11(S1):S403–S404. doi:10.1093/ecco-jcc/jjx002.757.
- Ruiz-Agüello MB, Maguregui A, Martínez A, et al. P387 Infliximab therapeutic drug monitoring test validated for measuring CT-P13 and SB2 biosimilar. Eur Crohn’s Colitis Organ Congr Abstr. 2018;12(S1):S299. doi:10.1093/ecco-jcc/jjx180.514.
- Magro F, Rocha C, Vieira AI, et al. P229 The new biosimilar of infliximab SB2 can be quantified by IFX-optimised therapeutic drug monitoring assays. Eur Crohn’s Colitis Organ Congr Abstr. 2018;12(S1):S216. doi:10.1093/ecco-jcc/jjx180.356.
- Ben-Horin S, Yavzori M, Benhar I, et al. Cross-immunogenicity: antibodies to infliximab in Remicade-treated patients with IBD similarly recognise the biosimilar Remsima. Gut. 2016;65:1132–1138.
- Goncalves J, Santos M, Acurcio R, et al. Antigenic response to CT-P13 and infliximab originator in inflammatory bowel disease patients shows similar epitope recognition. Aliment Pharmacol Ther. 2018;48:507– 522.
- Argüelles-Arias F, Guerra Veloz MF, Perea Amarillo R, et al. Switching from reference infliximab to CT-P13 in patients with inflammatory bowel disease: 12 months results. Eur J Gastroenterol Hepatol. 2017;29:1290–1295.
- Eberl A, Huoponen S, Pahikkala T, et al. Switching maintenance infliximab therapy to biosimilar infliximab in inflammatory bowel disease patients. Scand J Gastroenterol. 2017;52:1348–1353.
- Høivik ML, Buer LCT, Cvancarova M, et al. Switching from originator to biosimilar infliximab - real world data of a prospective 18 months follow-up of a single-centre IBD population. Scand J Gastroenterol. 2018;53:692–699.
- Kolar M, Duricova D, Bortlik M, et al. Infliximab biosimilar (Remsima™) in therapy of inflammatory bowel diseases patients: experience from one tertiary inflammatory bowel diseases centre. Dig Dis. 2017;35:91–100.
- Razanskaite V, Bettey M, Downey L, et al. Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis. 2017;11:690–696.
- Smits LJT, Grelack A, Derikx LAAP, et al. Long-term clinical outcomes after switching from Remicade® to biosimilar CT-P13 in inflammatory bowel disease. Dig Dis Sci. 2017;62:3117–3122.
- Macaluso FS, Cappello M, Giuffrida E, et al. Letter: SPOSIB SB2-a Sicilian prospective observational study of IBD patients treated with infliximab biosimilar SB2. Aliment Pharmacol Ther. 2019;49:234–236.
- Fischer S, Klenske E, Schmitt H, et al. P1592 Effectiveness, immunogenicity, safety and pharmacoeconomic aspects following a switch from reference infliximab to the biosimilar SB2 in inflammatory bowel disease patients: a 6 months prospective cohort study. United European Gastroenterol J. 2018;6(8_suppl).
- Armuzzi A, Avedano L, Greveson K, et al. Nurses are critical in aiding patients transitioning to biosimilars in inflammatory bowel disease: education and communication strategies. J Crohns Colitis. 2019;13:259–266.
- England NHS. Principles for sharing the benefits associated with more efficient use of medicines not reimbursed through national prices [Internet]. England; 2014 [cited Jan 2018]. Available from: https://www.england.nhs.uk/wp-content/uploads/2014/01/princ-shar-benefits.pdf.
- Kemp K, Dibley L, Chauhan U, et al. Second N-ECCO consensus statements on the European nursing roles in caring for patients with Crohn’s disease or ulcerative colitis. J Crohns Colitis. 2018;12:760–776.