ABSTRACT
Introduction
Helicobacter pylori (Hp) is causal in benign and malignant gastrointestinal diseases. Accordingly, current guidelines recommend Hp eradication in patients with active infection. Unfortunately, treatment failure is common, exposing patients to complications associated with persistent Hp infection and consequences of repeated treatment, including promotion of antibiotic resistance. In the United States (US), data regarding eradication rates with available therapies are limited. Moreover, the clinical and economic burden of eradication treatment failure have not been thoroughly described.
Areas covered
We aimed to characterize Hp eradication rates and the clinical consequences and associated costs of persistent Hp infection among US adults. We conducted focused literature reviews using initial searches in Embase, MEDLINE, and Cochrane Database of Systematic Reviews via Ovid followed by manual searches to identify relevant publications.
Expert opinion
Hp eradication rates were suboptimal, with most studies reporting rates ≤80% with clarithromycin-based triple therapy and bismuth quadruple therapy. There was direct evidence supporting numerous benefits of successful Hp eradication, including decreased risk of recurrent or complicated peptic disease and non-cardia gastric cancer. Cost benefits of eradication were related to mitigation of conditions associated with persistent Hp infection, (e.g. complicated peptic ulcer disease, and gastric cancer) which altogether exceed US$5.3 billion.
1.0 Introduction
Helicobacter pylori (Hp) is a gram-negative bacterium estimated to infect more than 50% of the world’s population (4.4 billion people in 2015) and is the most common chronic bacterial infection. Hp is a human carcinogen that is responsible for 5.5% of all malignancies and 80–90% of the global burden of gastric cancer (GC) – the third leading cause of cancer-related death [Citation1]. Persistent Hp infection is therefore considered a major threat to public health, including by the World Health Organization (WHO) [Citation2,Citation3]. Hp prevalence varies geographically and on the basis of industrialization, with the highest rates observed in Asian Pacific regions, Eastern Europe, and Central/Latin America, while the lowest rates overall are reported in North America [Citation4]. However, there is also marked within-country variation, for example, based on race/ethnicity, immigrant status, geography, rural vs urban environment, and socioeconomic factors [Citation5–7]. While the United States (US) is considered a low-prevalence country overall, non-White races and ethnic groups may have Hp prevalence up to, and in certain groups in excess of, 50% [Citation8], compared to the non-Hispanic White population, among whom prevalence is estimated to be <15% [Citation4].
Based on data from endemic countries, Hp infection is most often acquired in childhood and, unless eradicated, generally persists for the lifetime of the host [Citation9,Citation10]. Hp infection invariably causes chronic gastric inflammation; most often, this does not result in major clinical complications although it can result in bothersome nonspecific GI symptoms, such as dyspepsia [Citation11]. Importantly, though, a minority of patients will develop serious and potentially life-threatening conditions, including complicated peptic ulcer disease (PUD), non-cardia GC (NCGC), and mucosa-associated lymphoid tissue (MALT) lymphoma [Citation12–14]. The potential economic cost of Hp-associated GI disease morbidity and mortality is enormous. In 2016, nearly $2 billion in health-care expenditures were for PUD, gastritis, and duodenitis alone [Citation15].
Because of the established role of Hp in malignant and benign disease, international guidelines, including the American College of Gastroenterology (ACG), recommend Hp eradication when active infection is diagnosed [Citation14,Citation16,Citation17]. Eradication treatment consists of one or more antibiotics in combination with a potent acid-suppressing regimen [Citation18–20]. In the US, common regimens are clarithromycin-based triple therapy consisting of a proton pump inhibitor (PPI) along with clarithromycin and amoxicillin (PCA) or metronidazole (PCM) or bismuth-quadruple therapy (i.e. PPI, bismuth, a nitroimidazole such as metronidazole, and tetracycline [PBMT]), though rifabutin-triple therapy (PPI plus high-dose amoxicillin and rifabutin), amoxicillin dual therapy (PPI plus high-dose amoxicillin), and other regimens are also used [Citation16,Citation17]. Recommended regimens for Hp eradication vary globally with the main differences being duration (i.e. in Asia Pacific many regimens are 7 days whereas most are 10–14 days in the US) and choice of acid suppressant (both PPIs and potassium-competitive acid blockers [PCABs] and are used in Asia and some other non-US geographies) [Citation14,Citation16,Citation17,Citation21].
Successful eradication of Hp is proven to decrease GC-related mortality and recurrent PUD complications. However, eradication failure is increasingly common and multifactorial. Rising rates of Hp antibiotic resistance are a leading reason, but other patient-related, genetic, and environmental factors certainly contribute [Citation17,Citation22]. Patients with Hp eradication failure should be prescribed an alternative first- or second-line treatment, although repeated attempts generally have a lower likelihood of success [Citation14,Citation16,Citation17]. Furthermore, Hp eradication failure subjects patients to ongoing risk of Hp-associated complications, not to mention the inconvenience, cost, and complications associated with repeated treatment.
Outside the US, monitoring programs such as the European Registry on Helicobacter pylori Management provide surveillance of Hp resistance and management patterns [Citation23,Citation24]. The US had a country-wide surveillance program known as H. pylori Antimicrobial Resistance Monitoring Program (HARP); however, surveillance data have not been available post-2002 [Citation25]. While global data demonstrate Hp eradication rates are declining [Citation14,Citation16,Citation26–28], there is a dearth of epidemiological data from the US, particularly in recent years. A network meta-analysis of eradication therapies by Rokkas et al. demonstrated a pooled overall cure rate of 81.3% (79.5–83.1) with bismuth quadruple therapy and 75.7% (74.9–76.4) with clarithromycin-based triple therapy. Though analyses of grouped Western, Eastern Asian, and West Asian countries were conducted, evidence was insufficient to conduct a US analysis [Citation29].
To address these clear knowledge gaps in the US and inform future research directions, we conducted a series of focused literature reviews to identify and synthesize evidence on Hp eradication success rates using US-guideline recommended Hp eradication regimens. We secondarily aimed to describe the clinical and economic impact of failed Hp eradication in the US; for example, the resulting rates, risk, resource utilization, and costs of PUD complications, gastric precancerous conditions (e.g. gastric intestinal metaplasia), and NCGC.
1. Methods
We developed three key research questions based on an initial landscape assessment of the literature in Hp to understand the existing evidence and identify gaps. The research questions were vetted by clinical experts in gastroenterology and Hp treatment and outcomes. Once the initial questions were finalized, we selected outcomes of interest that would provide relevant data to help inform our search strategy and aid in study selection ().
Table 1. Search question and outcomes of interest
1.1. Search strategy and study selection
For each research question, we performed a comprehensive, but targeted literature search using an iterative hybrid of ‘pearl growing’ and ‘snowball’ search methods [Citation30–32]. Briefly, consistent with this methodologic approach, we conducted an initial broad, structured search to identify publications providing data relevant to the specific outcomes of interest for each research question, followed by ‘pearl-growing’ and ‘snowball’ citation chasing methods as described below. The initial search strategies included a combination of indexed and free-text terms for H. pylori, eradication therapy, and outcomes of interest (see Supplementary Tables 1–3 for initial search strategies). We conducted these initial searches in Embase, MEDLINE, MEDLINE In-Process, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews via Ovid.
Studies were selected based on their relevance to the specific research question. We focused on studies reporting data from adults in the US. Initial searches were limited to articles in English published in the last 10 years (January 2011–April 2021); however, in instances where there was a paucity of high-quality data from the US in the last 10 years (i.e. Question 1), we expanded our search to include publications from prior years (2000 onward). While we were most interested in real-world evidence (RWE), we also included other sources of high-quality data including published randomized controlled trials (RCTs) and meta-analyses when available evidence was limited. In addition, free-text terms were used to narrow the focus of the searches to studies conducted in the US.
We next applied ‘pearl-growing’ and ‘snowball’ citation chasing methods to the studies that were identified in the initial search. Such methods involve performing prospective searches (e.g. identify articles that cite the selected study using the ‘cited by’ search listing in PubMed) as well as retrospective bibliographic searches of key publications (e.g. identified clinical guidelines, meta-analyses) to ensure that all key studies from the US were captured. Additionally, we also identified new index terms and used them to conduct targeted manual searches on PubMed. This multi-step process ensures maximal capture of relevant studies [Citation30–32].
Each search and study selection was conducted by a single reviewer (EH or LV) and relevant outcomes for each research question were captured. Study population size, in addition to other relevant study details, was captured, but no exclusions were made based on study sample size.
1.2. Data analysis
To evaluate whether eradication rates changed significantly over time, we conducted a linear regression model using reported eradication rates weighted by sample size with treatment timeframe as the predictor variable. We assessed for fit via ANOVA using JMP®, Version 16.1. SAS Institute Inc., Cary, NC, 1989–2021. All graphs were created in Microsoft Excel®.
2. Results
2.1. Research Question 1: What are overall and regimen-specific Hp eradication rates in the US? How have they varied over time and geography?
A total of 20 publications for 18 distinct study cohorts that reported eradication rates in the US were identified (see ) [Citation33–50]. Overall, these studies included 7075 people; 4414 (62.4%) were treated with clarithromycin-based triple therapy, 1450 (20.5%) bismuth quadruple therapy, 236 (3.3%) received rifabutin triple therapy, 227 (3.2%) received high-dose amoxicillin dual therapy, 309 (4.4%) received another treatment regimen. Treatment cohorts in these studies were generally small, with a median of 122 patients (range: 2–1228).
Figure 1. Eradication rates in identified US clinical trials and real-world studies (a) Eradication rates (crude) from identified clinical and observational studies. (b) Eradication rates reported in identified clinical trials (weighted by sample size). (c) Eradication rates reported in observational ‘real-world’ studies (weighted by sample size). (d) US map of reported eradication rates from 2000 to 2021CT, clinical trial; RWE, real-world evidence; US, United States.
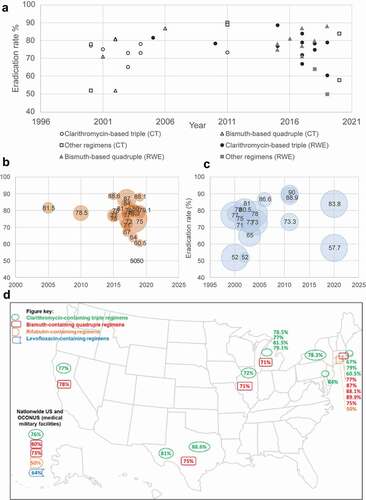
Among identified US observational studies and clinical trials of clarithromycin-based triple therapy, bismuth quadruple therapy, rifabutin triple therapy, and levofloxacin-based therapy none reported eradication rates above 90%. In the US, over the past two decades, 10 out of 14 study arms reported real-world eradication rates with clarithromycin-based triple therapy below 80% see (). According to a retrospective analysis of patients with treatment-naïve Hp infection treated with PCA or PCM for 10–14 days between 2001 and 2019 at a single center in Michigan (N = 1058), the overall eradication rate was 78.1% (range: 81.5% [2001–2005] to 79.1% [2016–2019]) [Citation41]. In a large, multicenter retrospective analysis (N = 1966) of patients diagnosed and treated for Hp infection at US military medical facilities nationwide between 2015 and 2018, the eradication rate with clarithromycin-based triple therapy was 76% [Citation34,Citation45]. Across 14 total studies of 10-day or 14-day clarithromycin-based triple therapies, eradication rates ranged from 72.0% to 88.0% and 58.1% to 88.6%, respectively. Notably, the proportion of patients treated with clarithromycin-based triple therapy varied, ranging from 25% (n/N: 262/1101) to 84.6% (88/104) between 2010 and 2018 [Citation33,Citation44,Citation45,Citation49]. In 2017, the ACG guideline was updated to recommend that clarithromycin-based triple therapy only be used when local resistance to clarithromycin is <15% or the patient is confirmed to be colonized with clarithromycin-sensitive Hp. Only one study included in the present analysis was conducted after the 2017 ACG guidelines publication; in this study (2018–2019), 42.8% (80/187) were prescribed clarithromycin-based triple therapy [Citation34].
Table 2. Observational studies describing Hp regimen-specific eradication rates in the US
Studies of other regimens, including bismuth quadruple therapy (doxycycline substituted for tetracycline), rifabutin triple therapy, levofloxacin-based triple therapy, and bismuth-based triple therapies with tetracycline or doxycycline were also identified () [Citation34,Citation36–38,Citation42,Citation43,Citation45]. In observational studies, bismuth quadruple therapy was associated with eradication rates ranging from 71% to 88% (see ) [Citation33,Citation34,Citation44,Citation45,Citation48,Citation49]. Among identified clinical trials employing PBMT, eradication rates ranged from 71% to 89% [Citation36,Citation37,Citation39,Citation52]. Rifabutin triple therapy (amoxicillin 3 g, omeprazole 120 mg, and rifabutin delayed-release 150 mg, every 8 h for 14 days), which, prior to its approval as first-line treatment in November 2019, was used predominantly as salvage, was associated with an 83.8% eradication rate vs 57.7% in the comparison arm (amoxicillin 3 g and omeprazole 120 mg, every 8 h for 14 days) in the phase 3 ERADICATE Hp2 trial of treatment-naïve patients [Citation43]. Rifabutin-triple therapy use was reported in two retrospective observational studies: one of two hospitals in Rhode Island (2 out of 187 patients) and at various military medical facilities throughout the US and OCONUS (6 patients out of 1966). Both studies, which recruited patients between 2015 and 2019, reported eradication rates of 50% with rifabutin triple therapy () [Citation34,Citation45]. Levofloxacin-based regimens were reported in two studies, with Hp eradication success rates ranging from a high of 90% in a randomized single-center, open-label trial in 2011, to a low of 64% in a retrospective observational study of patients treated between 2015 and 2018 [Citation42,Citation45].
Table 3. Other Hp eradication regimens
Few studies included patients who had refractory Hp infection after first-line or subsequent eradication treatment. In a recent US-based study by Argueta et al., 65.6% of Hp isolates were resistant to at least one antimicrobial used for treatment, including metronidazole (33.3%), clarithromycin (30.0%), and levofloxacin (29.6%) [Citation34]. This contrasts a 2004 report citing a resistance rate of 29.1% to one antibiotic used for eradication therapy, either metronidazole, clarithromycin, or metronidazole [Citation53]. Resistance rates are even higher among patients with at least one prior episode of Hp eradication treatment failure. In one single-center study in Pennsylvania of patients with refractory Hp infection, 89% of patients had confirmed resistance to at least one antibiotic commonly used for Hp eradication, including levofloxacin (69.2%), metronidazole (41.5%), clarithromycin (43.3%); not surprisingly, only 38% of refractory patients had confirmed eradication success with subsequent treatment [Citation54].
2.1.1. Factors influencing eradication treatment outcomes
Details of included studies that analyzed or described factors associated with eradication treatment outcomes (i.e. success or failure) are reported in . In identified studies, patients lost to follow-up or not tested for eradication were common. Based on the few studies that actually reported the proportion of patients who underwent confirmatory testing, frequencies of Hp eradication confirmation testing ranged from 33.9% to 85.6% [Citation44,Citation46,Citation48,Citation49,Citation55].
2.1.2. Temporal trends
Identified eradication rates (crude data) in the US are illustrated in . Studies conducted prior to 2015 were predominantly clinical trials, whereas more recent evidence tended to be observational. No clear trends were identified when visualizing raw eradication rates for individual studies over time by study type. According to a linear regression model using reported eradication rates weighted by sample size with treatment timeframe as the predictor, there was no statistically significant temporal trend (p = 0.23).
2.2. Research Question 2: Hp disease-related outcomes of eradication treatment failure
2.2.1. PUD and complications
We identified no US-based studies that reported on the association between Hp eradication and risk of PUD or PUD complications. As such, to inform this research question, we extended the search to include international data, and prioritized systematic literature reviews (SLRs) with meta-analyses. We identified three meta-analyses, all of which suggested that Hp eradication therapy is associated with a higher likelihood of duodenal ulcer healing and reduced likelihood of duodenal and gastric ulcer recurrence vs no treatment () [Citation56]. Compared to no Hp eradication treatment, confirmed Hp eradication was associated with a significantly lower likelihood of rebleeding with or without long-term acid suppression maintenance therapy [Citation57], as well as reduced risk of recurrent perforation following surgical repair at 1 year [Citation58]. Among a cohort of patients in the US, 16% and 25% of the patients with gastric and duodenal ulcers, respectively, were Hp positive. The proportion of Hp-positive ulcers East Asians and Hispanics was 37% and 35%, respectively [Citation59].
Table 4. Hp-associated PUD complications, precancerous gastric mucosal changes, and risk of GC
2.2.2. Precancerous gastric mucosal changes
Persistent Hp gastritis may progress to precancerous gastric mucosal changes, including atrophic gastritis and gastrointestinal metaplasia (GIM), with a minority having neoplastic transformation to dysplasia and ultimately noncardia gastric adenocarcinoma. Based on a multivariable analysis of a single VA medical center in Texas from 2009 to 2014, patients with Hp-positive gastritis were significantly more likely to be Black than non-Hispanic White [Citation60]. According to a large cohort study in Utah from 2011 to 2016, the prevalence of GIM was 2.6% and approximately 26% of cases of GIM had active Hp infection based on histology [Citation61]. Two retrospective studies of patients (N = 1140; 17,710) undergoing endoscopy with gastric biopsy reported significant associations between Hp infection and GIM prevalence [Citation62,Citation63]. In addition, GIM or higher-grade pathology was more prevalent in non-White populations in both studies with Asian/Pacific Islanders having the highest prevalence, followed by Hispanic and African American persons [Citation62,Citation63]. Prevalence of precancerous changes increased with age; moreover, this observation was more marked in non-White populations [Citation63].
2.2.3. NCGC
We identified six studies that analyzed the association of confirmed Hp eradication and risk of NCGC, representing a total of 43,807 patients (range: 91 to 38,535) from 1993 to 2018 [Citation64–69]. Five reported reduced risk of NCGC with Hp eradication [Citation64–66,Citation68,Citation69], whereas one study by Dhingra et al. did not demonstrate a significant relationship over a median of 3.5 years of follow-up (interquartile range of 1.8–6.6 years) up to 18 years () [Citation67]. According to a retrospective cohort study of 934 patients with diverse racial/ethnic backgrounds (68.2% non-White race or ethnicity) and confirmed GIM with a median duration of follow-up of 4.6 years (interquartile range, 3.0–6.7 years), 2.7% were diagnosed with GC on follow-up between 2000 and 2011 [Citation70]. While extensive vs limited GIM (OR 9.4 [95% CI 1.8–50.4]) and positive family history of GC were significant independent risk factors of GC, a history of Hp was not independently associated with GC risk [Citation70].
We did not identify any US-based studies of Hp eradication and risk of metachronous GC following GC resection, nor of Hp eradication in patients who are high risk for GC based on a positive family history.
2.2.3.1. NCGC risk – ethnicity and race
All studies reporting on Hp eradication status and NCGC also identified race and/or ethnicity as risk factors for NCGC [Citation64–68]. Therefore, we examined additional studies reporting on the association of patient characteristics, including race and ethnicity, and incidence of NCGC.
Vulnerable populations demonstrate increased rates of NCGC and Hp positivity, with studies suggesting twofold higher prevalence. Reported seroprevalence among non-Hispanic Blacks and Hispanics ranged from 53% to over 60% compared to 26% for non-Hispanic Whites in samples from the National Health and Nutritional Examination Survey (1988–1991) [Citation71]. Prevalence in a contemporary study (2009–2018) of over one million patients undergoing esophagogastroduodenoscopy was approximately 9% to 11% for the overall population, 22.0% to 23.6% among Hispanics, and 15.1% to 21.4% among East Asians; rates declined with time and prevalence in non-Hispanic Blacks was not assessed [Citation59]. Compared to non-Hispanic Whites, rates of NCGC for non-Hispanic Blacks and Hispanics are 3- to 4-fold higher and up to 13.3-fold higher for Korean individuals in the US [Citation72].
According to an analysis of California Cancer Registry (CCR) data between 2011 and 2015, non-White populations ≥50 years had higher age- and sex-adjusted incidence rates of NCGC adenocarcinoma [Citation71]. Incidence rate ratios (IRRs) were highest for Asian individuals, particularly Korean (13.3), Vietnamese (6.46), Southeast Asian (5.71), Japanese (5.18), and Chinese (4.77) individuals. Hispanics and non-Hispanic Black persons had 3.79 and 3.03 higher rates than non-Hispanic White persons [Citation72].
An analysis of NCGC in the North American Association of Central Cancer Registries database from 1995 to 2013 yielded age-standardized rates (ASRs) of 2.16 for non-Hispanic Whites, 6.43 for non-Hispanic Blacks, and 6.15 for Hispanics [Citation73]. The ASR for high vs low county-level poverty percentage was 4.38 vs 2.67, respectively [Citation73]. According to an analysis of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program data from from 2000 to 2014luding 25,825 cases of NCGC, incidence rates were higher among non-Hispanic Blacks and Hispanic persons (2.8-fold), Asians and Pacific Islanders (3.9-fold), and indigenous US populations (1.7-fold) compared to non-Hispanic Whites [Citation74]. Increased risk of NCGC was also noted with decreasing neighborhood socioeconomic status (nSES); the effect of nSES on age-adjusted incidence rates was more marked for Black and Hispanic populations compared to non-Hispanic Whites [Citation74]. Of note, incidence patterns observed in SEER and CCR were unique to NCGC and were essentially the opposite of those seen with cardia GC, a cancer that is not attributed to H. pylori [Citation72,Citation74].
Overall, NCGC rates have declined and remained stable after 2007; however, annual percent change in incidence rates were less favorable for non-White populations [Citation74]. Of note, Asian/Pacific Islanders are more likely to be diagnosed with localized NCGC, whereas Hispanic patients’ distant-stage NCGC rates are increasing in Hispanic persons under age 50 years (annual percent change 1.78; 95% CI 0.66, 2.91) [Citation75–77].
2.2.3.2. NCGC mortality
The search yielded no US-based studies identified that reported on Hp eradication vs no eradication and GC-related mortality. A number of studies suggested worse NCGC survival outcomes for non-White populations [Citation75,Citation76,Citation78–81]. According to an analysis of the SEER database from 2004 to 2016 conducted by Laszkowska (2020), NCGC mortality rates were two to three times higher for non-Hispanic Blacks, Hispanics, and Asians/Pacific Islanders compared to non-Hispanic Whites regardless of age or disease stage [Citation78]. However, an analysis of the CCR from 2006 to 2015 reported similar mortality risks for non-Hispanic Blacks, Hispanics, and non-Hispanic Whites with NCGC, and 20% reduced risk for Asians/Pacific Islanders [Citation79].
In a single-center retrospective analysis of 638 patients with GC, ethnicity was an independent predictor of survival (multivariable OR: 1.69; 95% CI 1.07–2.55, p < 0.02). Non-Hispanic Whites had a median overall survival (OS) of 99 months vs 51 months for Hispanics who tended to be younger at diagnosis and present with more advanced disease [Citation76]. Similarly, in a study of young-onset NCGC using SEER program data from 2007 to 2015, Hispanics had increased risk of diagnosis at higher grade (III/IV) compared with non-Hispanic Whites (OR 1.93; 95% CI 1.32–2.84, p = 0.001), but were 38% less likely to undergo surgery [Citation75].
A retrospective cohort study of patients with resectable gastric adenocarcinoma identified from SEER data (N = 15,991; 2007 to 2015) found that Black patients were more likely to receive a recommendation against surgical resection than non-Hispanic White, Asian, or Native American patients (adjusted ORs for surgery recommendation were 0.86, 0.55, and 0.50, respectively) [Citation80]. Non-Hispanic Whites were more likely to receive preoperative chemotherapy than other racial or ethnic groups, according to a study by Ikoma et al.. Use of preoperative chemotherapy mitigated racial/ethnic disparities in OS [Citation81].
2.3. Research Question 3: economic impact of failed eradication
We did not identify direct evidence of the costs of Hp eradication treatment failure. Herein we present indirect evidence through estimated costs of Hp-associated diseases. Note that these costs also do not account for the cost of repeated Hp eradication treatment courses and downstream multifaceted consequences.
2.3.1. PUD and acute complications
PUD was associated with a total expense of US$777 million in 2015 (see ) [Citation15]. While PUD has its own cost of management, according to the studies identified in our search, complications are key drivers of the economic impact. Cost is primarily driven by hospitalizations (43.5% of total expense), medications (24.4%), and outpatient (19.2%) and emergency department (ED) visits (11.4%) [Citation15]. Complicated PUD, including bleeding and perforation, is associated with high costs and healthcare resource utilization, including ED visits, admissions, and surgery [Citation82]. Identified costs of complicated PUD ranged from $1,883 to $25,444 per patient prior to 2010 [Citation82]; adjusted for inflation, this amounts to at least roughly $2,201 and $29,745 in 2021.
Table 5. Costs associated with PUD, PUD-associated complications, and GC in the US
Bleeding is the most common complication of PUD, observed in 15–20% of patients and accounting for roughly 40–60% of acute GI bleeding () [Citation83]. Among 119,684 admissions between 2003 and 2012 in the Healthcare Cost and Utilization Project’s National Inpatient Sample (NIS), 6,571 (5.4%) had GI bleeding [Citation84]. According to the 2014 National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, GI bleeding led to 2,147,949 office visits and 606,970 emergency department visits [Citation15]. Three studies reported Hp status and GI bleeding rates; among them, Hp was observed in 42–69% of patients with GI bleeds [Citation85–87].
Three studies reported PUD complication rates between 2000 and 2015 () [Citation84,Citation88,Citation89]. GI hemorrhage accounted for 84–87% of complications, whereas perforation was less common (10.6–12%) [Citation84,Citation88,Citation89]. GI hemorrhage was responsible for 231,567 ED visits in 2015, with a 78.4% admission rate [Citation15]. Median inpatient stay was 3 days, leading to a total of 896,224 hospital days, and generating total expenses of $2.14 to $2.74 billion; moreover, the observed 30-day all-cause readmission rate for patients with an index hospitalization for GI hemorrhage was 14.0%, with a median charge of $26,019 and $27,717 for the index and readmission stay, respectively [Citation15].
PUD-related perforations were associated with higher costs than GI hemorrhage. According to a study using data from the NIS from 2007 to 2010, 5,361 patients with PUD-related perforations were identified [Citation90]. The majority of PUD perforations required surgical intervention and were associated with longer inpatient stays (median 7 days for laparoscopic and 8 days for open repair), with median total charges ranging from $27,908 to $93,604 [Citation90].
According to a retrospective cohort study of the NIS between 2012 and 2013 that included all patients with an admission due to PUD (N = 52,396), 76% of patients underwent surgery or endoscopy [Citation88]. An analysis of the NIS from 2005 to 2014 found surgery reported in 1.9%, 67.4%, 20.2% of PUD-associated hemorrhage, perforation, and obstruction cases, respectively [Citation89]. Among Medicare recipients who underwent emergency general surgery between 2008 and 2014 (N = 481,417), PUD surgery was the second most common type, accounting for 28.2% of admissions [Citation92].
In complicated PUD, sepsis was a substantial cost driver [Citation91]. Reported aggregate treatment costs for hospitalized patients with GI bleeding and septicemia in the US in 2008 were $15 billion in 2008 with an average annual increase of 12% [Citation92,Citation93]. Patients with GI bleeding and septic shock required longer stays (20.56 days vs 15.76; p < 0.001) and had higher mean hospital admission costs ($192,524.89 vs $142,688.55; p < 0.001) [Citation94]. Based on an estimated rate of 17% Hp positivity among patients with PUD, Hp associated costs are still substantial [Citation59].
2.3.2. Gastric cancer
In the US, there were an estimated 27,600 new cases of GC in 2020, amounting to 1.5% of all new cancer diagnoses [Citation95]. While mortality decreased over time, hospitalizations remained stable and costs rose significantly from 2000 to 2013 [Citation96–98]. Overall, the projected cost of GC in the US in 2020 was $2.31 billion [Citation99], largely driven by costs of treatment and supportive care as well as hospitalizations ().
In the absence of guidelines for GC screening in the US, the majority of patients are diagnosed with late-stage GC, which is typically when symptoms are present and prompt diagnostic workup. Based on SEER data, an estimated 36% of GC cases are expected to be diagnosed at stage 4 in 2021 [Citation95]. In a retrospective analysis of the National Cancer Database (2004–2013; N = 93,734), approximately 49% of all patients with GC received chemotherapy [Citation100]. According to a retrospective SEER-Medicare claims analysis (2000 to 2009), total GC-related cost per patient was $70,808 ± $56,6206; 55% received additional GC treatment after first-line therapy ($14,668 ± $17,501) and 45% received supportive care only ($12,236 ± $18,251) [Citation99]. Costs drivers include chemotherapy-related costs and inpatient care [Citation99,Citation101]. First-line treatment costs ranged from $15,066 to $40,811, second-line ranged from $12,699 to $26,588, and third-line was $7,199 [Citation101,Citation102]. In a retrospective cohort study of GC and non-GC patients between 2007 and 2012, healthcare costs for patients with GC were over 10 times that of matched non-GC [Citation103]. While patients with GC have high healthcare resource utilization and costs overall, advanced disease is associated with the highest costs, primarily driven by hospitalizations.
Despite higher rates of diagnosis during more expensive stages of GC, the mean cost of care for Hispanic patients was less than a quarter of the average for White patients in the NIS from 2001 to 2011 [Citation96].
3. Discussion
Untreated Hp has major clinical and economic consequences. The success rates of Hp eradication treatment are decreasing globally as Hp antibiotic resistance rises; however, the limited published evidence in the US hinders our ability to completely evaluate trends in the US. The data for regimen-specific eradication rates in US cohorts were limited and heterogeneous. The overall quality of data across studies was also inconsistent; of the seven studies that reported eradication rates with regimens other than clarithromycin-based triple or bismuth-based quadruple therapies, three were RCTs and one was a randomized prospective study. Most studies reported predominantly on clarithromycin-based triple therapy and/or bismuth quadruple therapy, with rifabutin triple therapy and levofloxacin-based therapy reported in two non-randomized studies each. We did not identify recent evidence of eradication rates with high-dose amoxicillin dual therapy apart from RCTs in the US, although this regimen is commonly used outside of the US. Studies from non-US regions appear conflicting, especially for high-dose amoxicillin dual therapy, which requires appropriate acid suppression for treatment success. This treatment is enticing though, given the consistently low rates of amoxicillin-resistant Hp strains, increased patient tolerability, relative simplicity, and parsimony of the regimen compared to other Hp treatment regimens, and the alignment with antibiotic stewardship goals. Of note, two studies of non-amoxicillin-based dual regimens were identified but excluded because they are not FDA approved or recommended by current clinical guidelines; one reported a 46% eradication success rate with a 7-day pantoprazole-clarithromycin dual regimen and the other reported 46% eradication success rate with a 10-day esomeprazole-clarithromycin dual regimen [Citation35,Citation40].
Eradication rates are inherently difficult to compare or generalize because of the heterogenous nature of the studies, as regimens vary by PPI type and dose used, antibiotics studied and formulations, treatment duration, patient demographics, comorbidities, and other characteristics (e.g. known PUD). Study findings may also differ from true rates given the possibility of publication biases. Moreover, eradication rates reported in clinical trials may be higher than ‘real-world’ practice; one reason perhaps being higher adherence rates in trial participants and participant bias. There is also a possibility of bias for patients who are tested vs not tested for successful Hp eradication in clinical practice. For example, patients who complete post-treatment testing may disproportionately represent patients who are at higher risk for complications related to persistent Hp infection (e.g. history of complicated PUD or family history of GC) or perhaps may represent a group of higher-adherence patients or patients with greater resources vs those who do not complete Hp eradication confirmatory testing. As such, our analysis was only descriptive and we were not able to conduct pooled analyses of the data from real-world evidence and RCTs.
Across studies, Hp antibiotic sensitivity profile was variably associated with eradication success vs failure [Citation34,Citation53,Citation54]. Clarithromycin-based triple therapy was used for a substantial proportion of patients, even though its use is not recommended in regions with known or suspected clarithromycin resistance rates >15% or in patients with a history of macrolide use [Citation16,Citation17]. While identified studies did not provide insight as to why physicians may be selecting a potentially suboptimal regimen, limited information regarding patterns of antibiotic resistance in the US and lack of routine susceptibility testing to inform treatment decisions may be contributing factors. According to a survey by Murakami et al., physicians did not demonstrate knowledge of resistance rates and tended to over- and underestimate resistance rates [Citation104].
Because eradication therapy typically consists of complex multidrug regimens that are associated with side effects, patients often miss doses or discontinue treatment, which may contribute to treatment failure [Citation16,Citation17]. While there is concern that adherence rates are generally higher in trials than in the real world, creating the potential for lower rates in practice, the scarcity of adherence data in the RWE we identified limited our ability to draw conclusions. The vast majority of studies we identified utilized 10 or 14-day regimens. A previous study demonstrated that adherence above 60% was not associated with treatment failure [Citation105]; however, the recent ERADICATE Hp2 trial of rifabutin reported a nearly 7-percentage point difference among the whole population and the confirmed adherent population [Citation43]. That said, even in studies with high adherence, eradication treatment failure still occurs. This suggests that the underlying causes of failure are multifactorial and include host, microbial, and system factors.
With respect to patient characteristics, in the studies identified for the primary research questions, age, sex, race, and ethnicity were not consistently associated with eradication success vs failure. Genetic polymorphisms that affect host gastric acid suppression (e.g. CYP2C19, IL-1B polymorphisms may influence the success of Hp eradication treatment. This is because effective, durable acid suppression is needed to achieve the intragastric pH range necessary to ensure Hp are replicating and therefore susceptible to antibiotics [Citation20] and to improve bioavailability and activity of certain growth-dependent antibiotics, such as amoxicillin and clarithromycin [Citation19]. While higher rates of Hp eradication have been noted among patients with poor vs rapid metabolizer phenotypes for first-generation PPIs in Asian studies [Citation106], the impact of racial and ethnic phenotypic differences on eradication outcomes in the US has not been extensively explored [Citation107].
Overall, the reported costs of conditions attributable to Hp infection in identified studies were substantial. However, we did not identify direct evidence associating failed Hp eradication with economic impact of conditions related to persistent Hp infection (e.g. dyspepsia, PUD, PUD complications, NCGC) in the US. Moreover, it was not possible to determine with certainty the proportion of the clinical conditions of interest that were attributable to failed Hp eradication and further investigations, such as modeling studies, would be of value. We were also not able to quantify indirect downstream costs related to retreatment of Hp after failed a failed episode of treatment, such as promotion of antibiotic resistance. Notably, though, such costs have been suggested to be as high as $33 billion in the US alone [Citation108].
Strengths of this study include the comprehensive literature search and the focus on contemporary US studies. There are limitations that merit mentioning, in addition to those alluded to above such as publication and participant biases. We did not focus on costs related to adverse outcomes related to the treatment itself, including GI side effects. We will note, however, that two large US cohort studies have at least reported the lack of serious adverse effects associated with Hp treatment, including cardiovascular mortality and Clostridium difficile infection [Citation109,Citation110]. We also did not evaluate rarer Hp associated conditions, including MALT lymphoma, and extragastric manifestations such as immune thrombocytopenia purpura.
4. Conclusion
Hp poses an inarguable threat to human health with the potential for severe clinical consequences and high costs; our findings suggest a substantial proportion of people in the US with chronic Hp infection may not experience the benefits of eradication due to suboptimal rates of eradication and rising antibiotic resistance.
More effective eradication regimens would offer the potential for reducing both acute and long-term clinical consequences of Hp as well as reductions in costs of treatment and healthcare resource utilization for Hp-associated complications
5. Expert opinion
Hp eradication treatment failure is common and exposes patients to complications associated with persistent Hp infection, not to mention downstream consequences of repeated treatment, including promotion of antibiotic resistance. In the US, the impact of eradication treatment failure on clinical outcomes, including PUD and progression to GC, and the concomitant economic burden, have not been thoroughly described nor quantified.
According to the findings of this review, eradication rates in the US are poor, with most studies reporting rates ≤80% with current guideline-approved first-line regimens. Clarithromycin-based triple therapy was used for a substantial proportion of patients, despite its limited utility in patients with prior macrolide use or in regions with clarithromycin resistance rates >15% [Citation16,Citation17]. The absence of systematic regional and national registries for monitoring and recording Hp resistance patterns along with a lack of routine use of commercially available susceptibility testing to inform treatment decisions may contribute to the paucity of US data and impedes detailed understanding of Hp antibiotic resistance profiles. This is in stark contrast to the European consortium [Citation23] where the data generated have already translated to updated clinical practice recommendations regarding selection of Hp eradication regimens. Another contributing factor is that in the US, despite current guideline recommendations [Citation16], testing to confirm successful Hp eradication is not regularly performed in clinical practice following Hp treatment, which reduces both the volume and generalizability of RWE.
Future studies to establish the prevalence of specific strains of Hp and rates of associated clinical conditions such as PUD, precancerous lesions, and GC among vulnerable populations would be important to support improvements in monitoring, treatment, and outcomes. Potential initiatives include addressing the need for better antibiotic resistance surveillance and Hp registries in the US, streamlining culture and sensitivity testing, as well as encouraging widespread adoption of best practices for eradication treatment selection guided by clinical guidelines and publicly available local aggregate data [Citation17].
Efforts to individualize eradication therapy based on genetic factors (IL-1B, CYP2C19, and non-genetic clinical factors [e.g. smoking, diet, obesity]) may improve treatment decision-making by identifying patients most at-risk for negative sequelae vs those who may not benefit from eradication [Citation111]. In addition, the adoption of programs to support maximal adherence and studies investigating alternative regimens that consistently achieve eradication rates >90% with initial therapy will presumably not only improve patient outcomes and experience but would also reduce waste in the healthcare system.
For vulnerable populations who experience increased risk of Hp and GC, screening may be beneficial and cost-effective. Interestingly, a recent study in a Korean population demonstrated that for individuals with a family history of GC (first-degree relative) and Hp infection, the risk of GC was reduced by eradication treatment [Citation112]. While early eradication seems to be important to provide the greatest protective benefit, Korean patients with endoscopically resected early GC who received Hp eradication therapy exhibited reduced rates of metachronous GC compared to patients in the placebo arm [Citation113.
Investigation of both new and repurposed antibiotic agents as well as potentially promising adjunctive therapies is sorely needed. Excitingly, the increase in availability of molecular modalities to test for Hp antibiotic susceptibility (either on biopsies or noninvasively) has very strong potential to optimize our treatment approach. Moreover, individualized Hp eradication therapy that is further optimized for both host and Hp-specific factors, including the most appropriate antibiotics combined with potent acid suppression, is critical to achieve high rates of successful eradication and ensure that the protective benefits are experienced by patients in need.
Article highlights
Overall, our search results demonstrated that current guideline-approved first-line regimens achieve poor eradication rates in the US, with most studies reporting rates ≤80% with current guideline-approved first-line regimens (10/14 studies of clarithromycin triple therapy and 5/7 studies of bismuth quadruple therapy). The most common reasons for Hp eradication failure are antibiotic resistance and patient nonadherence. There are limited data on clinical factors associated with Hp eradication treatment failure. Higher BMI and clarithromycin resistance were identified factors based on limited literature.
No recent US studies have evaluated Hp eradication outcomes and risk of peptic ulcer disease (PUD) and associated complications. Based on non-US studies, successful eradication of Hp was associated with increased likelihood of ulcer healing and decreased risk of ulcer recurrence, rebleeding, and reperforation.
Eradication of Hp is associated with reduced likelihood of precancerous mucosal changes compared to no Hp treatment. Hp infection was positively associated with incidence of precancerous lesions, which were more prevalent in non-White groups .
Based on a handful of US studies, Hp eradication is associated with reduced risk of non-cardia gastric cancer (NCGC).
Based on indirect evidence, the cost of Hp eradication treatment failure in the US is enormous. Reported costs associated with complicated PUD and NCGC were high, with aggregate costs over $5.3 billion driven primarily by hospitalizations, medication, and outpatient visits.
Declaration of interests
R Yadlapati is supported by NIH K23 DK125266 (PI: Yadlapati); SC Shah is supported by a 2019 American Gastroenterological Association Research Scholar Award (PI: Shah), and Veterans Affairs Career Development Award under award number ICX002027A01 (PI: Shah) The authors disclose the following relationships: R Yadlap: Institutional Consulting Agreement: Medtronic, Ironwood Pharmaceuticals; StatLinkMD; Consultant: Phathom Pharmaceuticals; Research support: Ironwood Pharmaceuticals; Advisory Board with Stock Options: RJS Mediagnostix; S Shah: Consultant, Phathom Pharmaceuticals; C Pelletier and R Jacob: Employment: Phathom Pharmaceuticals; E Hubscher and L Vinals: Employment: Cytel, Inc, which was a paid consultant on the project. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. DOI:https://doi.org/10.1016/S1470-2045(12)70137-7.
- World Health Organization. GLOBAL PRIORITY LIST OF ANTIBIOTIC-RESISTANT BACTERIA TO GUIDE RESEARCH, DISCOVERY, AND DEVELOPMENT OF NEW ANTIBIOTICS [Internet]. 2017. [cited 2021 Apr 27]. Available from: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
- Herrero R, Park JY, Forman D. The fight against gastric cancer - the IARC Working Group report. Best Pract Res Clin Gastroenterol. 2014;28(6):1107–1114.
- Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. DOI:https://doi.org/10.1053/j.gastro.2017.04.022.
- Krueger WS, Hilborn ED, Converse RR, et al. Environmental risk factors associated with Helicobacter pylori seroprevalence in the United States: a cross-sectional analysis of NHANES data. Epidemiol Infect. 2015;143(12):2520–2531. DOI:https://doi.org/10.1017/S0950268814003938.
- Torres J, Correa P, Ferreccio C, et al. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control. 2013;24(2):249–256. DOI:https://doi.org/10.1007/s10552-012-0114-8.
- Subsomwong P, Miftahussurur M, Uchida T, et al. Prevalence, risk factors, and virulence genes of Helicobacter pylori among dyspeptic patients in two different gastric cancer risk regions of Thailand. PLoS One. 2017;12(10):e0187113. DOI:https://doi.org/10.1371/journal.pone.0187113.
- Nguyen T, Ramsey D, Graham D, et al., The prevalence of Helicobacter pylori remains high in African American and Hispanic veterans. Helicobacter. 2015;20(4):305–315 . DOI:https://doi.org/10.1111/hel.12199.
- Harris PR, Smythies LE, Smith PD, et al. Role of childhood infection in the sequelae of H. pylori disease. Gut Microbes. 2013;4(6):426–438. DOI:https://doi.org/10.4161/gmic.26943.
- Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11(6):385–399.
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–1186.
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–1315.
- IARC. Heliobacter Pylori [Internet]. World Health Organization; 2018 [cited 2021 Jul 27]. Available from: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100B-15.pdf.
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht v/Florence consensus report. Gut. 2017;66(1):6–30. DOI:https://doi.org/10.1136/gutjnl-2016-312288.
- Peery AF, Crockett SD, Murphy CC, et al., Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 156(1): 254–272.e11. 2019. . https://doi.org/10.1053/j.gastro.2018.08.063.
- Chey WD, Leontiadis GI, Howden CW, et al. ACG Clinical Guideline: treatment of Helicobacter pylori Infection. Official journal of the American College of Gastroenterology [Internet]. 2017;112: https://journals.lww.com/ajg/Fulltext/2017/02000/ACG_Clinical_Guideline__Treatment_of_Helicobacter.12.aspx.
- Shah SC, Iyer PG, Moss SF. AGA clinical practice update on the management of refractory Helicobacter pylori Infection. Expert Review. Gastroenterology. 2021;160(5):1831–1841.https://doi.org/10.1053/j.gastro.2020.11.059
- Sachs G, Scott DR, Wen Y. Gastric infection by Helicobacter pylori. Curr Gastroenterol Rep. 2011;13(6):540–546.
- Erah PO, Goddard AF, Barrett DA, et al. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother. 1997;39(1):5–12. DOI:https://doi.org/10.1093/jac/39.1.5.
- Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am. 2010;39(3):465–480.
- Huang -C-C, Tsai K-W, Tsai T-J, et al. Update on the first-line treatment for Helicobacter pylori infection - a continuing challenge from an old enemy. Biomark Res. 2017;5(1):23. DOI:https://doi.org/10.1186/s40364-017-0103-x.
- Miftahussurur M, Pratama Putra B, Yamaoka Y. The potential benefits of vonoprazan as Helicobacter pylori infection therapy. Pharmaceuticals (Basel). 2020;14(1):13.
- McNicholl AG, O’Morain CA, Megraud F, et al. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). Helicobacter. 2019;24(5):e12630. DOI:https://doi.org/10.1111/hel.12630.
- Megraud F, Bruyndonckx R, Coenen S, et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021;70(10):1815–1822. DOI:https://doi.org/10.1136/gutjnl-2021-324032.
- Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori–infected persons. Emerg Infect Dis. 2004;10(6):1088–1094. DOI:https://doi.org/10.3201/eid1006.030744.
- Kim SE, Park MI, Park SJ, et al. Trends in Helicobacter pylori eradication rates by first-line triple therapy and related factors in eradication therapy. Korean J Intern Med. 2015;30(6):801–807. DOI:https://doi.org/10.3904/kjim.2015.30.6.801.
- Sasaki M, Ogasawara N, Utsumi K, et al. Changes in 12-year first-line eradication rate of Helicobacter pylori based on triple therapy with proton pump inhibitor, amoxicillin and clarithromycin. J Clin Biochem Nutr. 2010;47(1):53–58. DOI:https://doi.org/10.3164/jcbn.10-10.
- Lahaie RG, Gaudreau C. Helicobacter pylori antibiotic resistance: trends over time. Can J Gastroenterol. 2000;14(10):895–899.
- Rokkas T, Gisbert JP, Malfertheiner P, et al. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: a network meta-analysis. Gastroenterology. 2021;161(2):495–507.e4. DOI:https://doi.org/10.1053/j.gastro.2021.04.012.
- Jonkers J. LibGuides: information Literacy History: search methods [Internet]. [cited 2020 Oct 21]. Available from: https://libguides.rug.nl/c.php?g=470628&p=3218096.
- Ramer SL. Site-ation pearl growing: methods and librarianship history and theory. J Med Libr Assoc. 2005;93(3):397–400.
- Zwakman M, Verberne LM, Kars MC, et al. Introducing PALETTE: an iterative method for conducting a literature search for a review in palliative care. BMC Palliat Care. 2018;17(1):82. DOI:https://doi.org/10.1186/s12904-018-0335-z.
- Liu RP, Romero R, Sarosiek J, et al., Eradication rate of Helicobacter pylori on the US-Mexico border using the urea breath test. South Med J. 111(1): 51–55. 2018. . https://doi.org/10.14423/SMJ.0000000000000747.
- Argueta EA, Alsamman MA, Moss SF, et al., Impact of antimicrobial resistance rates on eradication of Helicobacter pylori in a US population. Gastroenterology. 160(6): 2181–2183.e1. 2021. . https://doi.org/10.1053/j.gastro.2021.02.014.
- Laine L, Fennerty MB, Osato M, et al. Esomeprazole-based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: results of three US multicenter, double-blind trials. Am J Gastroenterol. 2000;95(12):3393–3398. DOI:https://doi.org/10.1111/j.1572-0241.2000.03349.x.
- Magaret N, Burm M, Faigel D, et al. A randomized trial of lansoprazole, amoxycillin, and clarithromycin versus lansoprazole, bismuth, metronidazole and tetracycline in the retreatment of patients failing initial Helicobacter pylori therapy. Dig Dis. 2001;19(2):174–178. DOI:https://doi.org/10.1159/000050674.
- Stevens VJ, Shneidman RJ, Johnson RE, et al. Helicobacter pylori eradication in dyspeptic primary care patients: a randomized controlled trial of a pharmacy intervention. West J Med. 2002;176(2):92–96.
- Sullivan B, Coyle W, Nemec R, et al. Comparison of azithromycin and clarithromycin in triple therapy regimens for the eradication of Helicobacter pylori. Am J Gastroenterol. 2002;97(10):2536–2539. DOI:https://doi.org/10.1111/j.1572-0241.2002.06036.x.
- Chen Y, Jajodia P, DeGuzman L, et al. Randomized controlled trial comparing proton pump inhibitor based eradication regimen versus low-cost eradication regimen for patients with Helicobacter pylori with uninvestigated dyspepsia. J Appl Res. 2006;6(3):214–222.
- Bochenek WJ, Peters S, Fraga PD, et al. Eradication of Helicobacter pylori by 7-day triple-therapy regimens combining pantoprazole with clarithromycin, metronidazole, or amoxicillin in patients with peptic ulcer disease: results of two double-blind, randomized studies. Helicobacter. 2003;8(6):626–642. DOI:https://doi.org/10.1111/j.1523-5378.2003.00179.x.
- Ginnebaugh BD, Baker J, Watts L, et al. S1348 triple therapy for primary treatment of Helicobacter pylori: a 19-year U.S. single center experience. Official journal of the American College of Gastroenterology | ACG. 2020;115(1):S680. DOI:https://doi.org/10.14309/01.ajg.0000707440.64691.0e
- Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106(11):1970–1975. DOI:https://doi.org/10.1038/ajg.2011.306.
- Graham DY, Canaan Y, Maher J, et al. Rifabutin-based triple therapy (RHB-105) for Helicobacter pylori eradication. Ann Intern Med. 2020;172(12):795–802. DOI:https://doi.org/10.7326/M19-3734.
- Alsamman MA, Vecchio EC, Shawwa K, et al. Retrospective analysis confirms tetracycline quadruple as best Helicobacter pylori regimen in the USA. Dig Dis Sci. 2019;64(10):2893–2898. DOI:https://doi.org/10.1007/s10620-019-05694-4.
- Mertz A. Helicobacter pylori Treatment & Eradication Rates in Department of Defense Patients from 2016-2018ACG Annual Meeting [Internet]. 2020 [cited 2021 Jun 4]. Available from: https://www.eventscribe.com/2020/ACG/fsPopup.asp?Mode=presInfo&PresentationID=766539.
- Camero A, Haris M, Valadez D, et al. Su1275 – Helicobacter pylori in South Texas: eradication rates and practice trends in a university setting. Gastroenterology. 2019;156(6):S. DOI:https://doi.org/10.1016/S0016-5085(19)38200-9.
- Nayar DS. Current eradication rate of Helicobacter pylori with clarithromycin-based triple therapy in a gastroenterology practice in the New York metropolitan area. Infect Drug Resist. 2018;11:205–211.
- Barrett-Englert M, Stern E, Keswani RN. Su1277 – the management of H. pylori is of low quality in practice due to low rates of eradication testing. Gastroenterology. 2019;156(6):S.
- Rubin J, Lai A, Dulai P, et al. Su1210 - Low Rates of H. Pylori Eradication Testing and Cure Rates in Usual Care. Gastroenterology. 2018;154. S-S-504.
- Vakil N, Lanza F, Schwartz H, et al. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20(1):99–107. DOI:https://doi.org/10.1111/j.1365-2036.2004.02029.x.
- Tariq H, Patel H, Kamal MU, et al. Reevaluation of the efficacy of first line regimen for Helicobacter pylori. Clin Exp Gastroenterol. 2020;13:25–33.
- Graham DY, Lew GM, Klein PD, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. Ann Intern Med. 1992;116(9):705–708. DOI:https://doi.org/10.7326/0003-4819-116-9-705.
- Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial Resistance Incidence and Risk Factors among Helicobacter pylori–Infected Persons, United States Volume Volume 10, 6June 2004 - Emerging Infectious Diseases journal - CDC. [cited 2021 Mar 16]; Available from: https://wwwnc.cdc.gov/eid/article/10/6/03-0744_article.
- Kumar S, Sangitha R, Nachamkin I, et al. Resistance patterns of refractory Helicobacter pylori infection in a referral center in the Delaware Valley. GastroHep. 2020;2(1):6–12. DOI:https://doi.org/10.1002/ygh2.382.
- Dong E, Chang JI, Chen Q, et al. Su1268 – Helicobacter pylori infection: practice patterns in a community-based US healthcare system. Gastroenterology. 2019;156(6):S. DOI:https://doi.org/10.1016/S0016-5085(19)38193-4.
- Ford AC, Gurusamy KS, Delaney B, et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst Rev. 2016 *Meta-analysisshowing benefits of Hp eradication for patients with PUD;4:CD003840. DOI:https://doi.org/10.1002/14651858.CD003840.pub5.
- Gisbert JP, Khorrami S, Carballo F, et al. Helicobacter pylori eradication therapy vs. antisecretory non‐eradication therapy (with or without long‐term maintenance antisecretory therapy) for the prevention of recurrent bleeding from peptic ulcer. Cochrane Database of Systematic Reviews [Internet]. 2004 [cited 2021 Sep 4]; Available from: https://www.cochranelibrary.com/cdsr/doi/https://doi.org/10.1002/14651858.CD004062.pub2/full?cookiesEnabled.
- Tomtitchong P, Siribumrungwong B, Vilaichone R-K, et al. Systematic review and meta-analysis: helicobacter pylori eradication therapy after simple closure of perforated duodenal ulcer. Helicobacter. 2012;17(2):148–152. DOI:https://doi.org/10.1111/j.1523-5378.2011.00928.x.
- Sonnenberg A, Turner KO, Genta RM. Low prevalence of Helicobacter pylori-positive peptic ulcers in private outpatient endoscopy centers in the United States. Am J Gastroenterol. 2020;115(2):244–250.
- Shiota S, Thrift AP, Green L, et al. Clinical manifestations of Helicobacter pylori-negative gastritis. Clin Gastroenterol Hepatol. 2017;15(7):1037–1046.e3. DOI:https://doi.org/10.1016/j.cgh.2017.01.006.
- Parbhu SK, Cole GG, Fang JC, et al. Su1252 – incident diagnoses of gastric intestinal metaplasia in the us: patient characteristics, egd findings, and clinical practice patterns at a large us tertiary care center. Gastroenterology. 2019;156(6):S. DOI:https://doi.org/10.1016/S0016-5085(19)38178-8.
- Kligman G, Szafron D, Ali H, et al. Su1247 – screening for gastric intestinal metaplasia: what to consider and whom to screen. Gastroenterology. 2019;156(6):S. DOI:https://doi.org/10.1016/S0016-5085(19)38173-9.
- Huang RJ, Ende AR, Singla A, et al., Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc. 91(1): 70–77. 2020. . https://doi.org/10.1016/j.gie.2019.07.038.
- Kumar S, Metz DC, Ellenberg S, et al. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: a large cohort study. Gastroenterology. 2020;158(3):527–536.e7. DOI:https://doi.org/10.1053/j.gastro.2019.10.019.
- Fansiwala K, Liang PS. S1353 risk factors for gastric cancer in a veteran population. Official journal of the American College of Gastroenterology. 2020;115(1):S682.https://doi.org/10.14309/01.ajg.0000707460.70708.51
- Li D, Jiang S, Postlethwaite D, et al. Incidence of noncardia gastric adenocarcinoma after Helicobacter pylori eradication in a large diverse US population. Gastroenterology. 2020;158:S-185-S-186.
- Dhingra R, Natov NS, Daaboul Y, et al. Increased risk of progression to gastric adenocarcinoma in patients with non-dysplastic gastric intestinal metaplasia versus a control population. Dig Dis Sci. 2020;65(11):3316–3323. DOI:https://doi.org/10.1007/s10620-019-06031-5.
- Nguyen TH, Mallepally N, Hammad T, et al. Prevalence of Helicobacter pylori positive non-cardia gastric adenocarcinoma is low and decreasing in a US population. Dig Dis Sci. 2020;65(8):2403–2411. DOI:https://doi.org/10.1007/s10620-019-05955-2.
- Li D, Bautista MC, Jiang S-F, et al. Risks and predictors of gastric adenocarcinoma in patients with gastric intestinal metaplasia and dysplasia: a population-based study. Am J Gastroenterol. 2016;111(8):1104–1113. DOI:https://doi.org/10.1038/ajg.2016.188.
- Reddy KM, Chang JI, Shi JM, et al. Risk of gastric cancer among patients with intestinal metaplasia of the stomach in a US. Integrated Health Care System. Clinical Gastroenterology and Hepatology. 2016;14(10):1420–1425. DOI:https://doi.org/10.1016/j.cgh.2016.05.045
- Everhart JE, Kruszon-Moran D, Perez-Perez GI, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181(4):1359–1363. DOI:https://doi.org/10.1086/315384.
- Shah SC, McKinley M, Gupta S, et al., Population-based analysis of differences in gastric cancer incidence among races and ethnicities in individuals age 50 years and older. Gastroenterology. 159(5): 1705–1714. 2020. . https://doi.org/10.1053/j.gastro.2020.07.049.
- Anderson WF, Rabkin CS, Turner N, et al. The changing face of noncardia gastric cancer incidence among us non-Hispanic whites. J Natl Cancer Inst. 2018;110(6):608–615. DOI:https://doi.org/10.1093/jnci/djx262.
- Gupta S, Tao L, Murphy JD, et al. Race/ethnicity, socioeconomic status-, and anatomic subsite-specific risks for gastric cancer. Gastroenterology. 2019;156(1):59–62.e4. DOI:https://doi.org/10.1053/j.gastro.2018.09.045.
- Holowatyj AN, Ulrich CM, Lewis MA. Racial/ethnic patterns of young-onset noncardia gastric cancer. Cancer Prev Res (Phila). 2019;12(11):771–780.
- Duma N, Sanchez LJ, Castro YS, et al. Gastric adenocarcinoma: clinicopathologic differences among Hispanics and non-Hispanic whites. A single Institution’s experience over 14 years. Ann Gastroenterol. 2016;29(3):325–331. DOI:https://doi.org/10.20524/aog.2016.0030.
- Wang Z, El-Serag HB, Thrift AP. Increasing incidence of advanced non-cardia gastric cancers among younger hispanics in the USA. Dig Dis Sci. 2021;66(5):1669–1672.
- Laszkowska M, Tramontano AC, Kim J, et al. Racial and ethnic disparities in mortality from gastric and esophageal adenocarcinoma. Cancer Med. 2020;9(15):5678–5686. DOI:https://doi.org/10.1002/cam4.3063.
- Klapheke AK, Carvajal-Carmona LG, Cress RD. Racial/ethnic differences in survival among gastric cancer patients in California. Cancer Causes Control. 2019;30(7):687–696.
- Ulanja MB, Beutler BD, Rishi M, et al. Influence of race and geographic setting on the management of gastric adenocarcinoma. J Surg Oncol. 2019;120(2):270–279. DOI:https://doi.org/10.1002/jso.25503.
- Ikoma N, Cormier JN, Feig B, et al. Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: analysis of the National Cancer Data Base, 2006-2014. Cancer. 2018;124(5):998–1007. DOI:https://doi.org/10.1002/cncr.31155.
- Barkun A, Leontiadis G. Systematic review of the symptom burden, quality of life impairment and costs associated with peptic ulcer disease. Am J Med. 2010;123(4):358–366.e2.
- Lanas A, García-Rodríguez LA, Polo-Tomás M, et al. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33(5):585–591. DOI:https://doi.org/10.1111/j.1365-2036.2010.04563.x.
- Kanotra R, Ahmed M, Patel N, et al. Seasonal variations and trends in hospitalization for peptic ulcer disease in the United States: a 12-year analysis of the nationwide inpatient sample. Cureus [Internet]. [cited 2021 May 26];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5130352/.
- Al-Jashaami L, Al-Zubaidi YA, Boddu S, et al. Helicobacter pylori-associated ulcers and related hospitalizations are common at a safety net hospital in Arizona: 937. Official journal of the American College of Gastroenterology | ACG. 2016; 111: S406. https://doi.org/10.14309/00000434-201610001-00937
- Viviane A, Alan BN. Estimates of costs of hospital stay for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health. 2008;11(1):1–3.
- Bernica J, Cole R, Flores A, et al. HP QI abstract: improving helicobacter pylori testing in patients with acute upper gi bleeding due to peptic ulcer disease. Gastroenterology. 2020;159(2):e22–e23. DOI:https://doi.org/10.1053/j.gastro.2020.06.060.
- Havens JM, Castillo-Angeles M, Nitzschke SL, et al. Disparities in peptic ulcer disease: a nationwide study. Am J Surg. 2018;216(6):1127–1128. DOI:https://doi.org/10.1016/j.amjsurg.2018.08.025.
- Olufajo OA, Wilson A, Yehayes B, et al. Trends in the surgical management and outcomes of complicated peptic ulcer disease. Am Surg. 2020;86(7):856–864. DOI:https://doi.org/10.1177/0003134820939929.
- Wright GP, Davis AT, Koehler TJ, et al. Cost-efficiency and outcomes in the treatment of perforated peptic ulcer disease: laparoscopic versus open approach. Surgery. 2014;156(4):1003–1007. DOI:https://doi.org/10.1016/j.surg.2014.06.047.
- Søreide K. Sepsis drives the cost in perforated peptic ulcer. Surgery. 2015;158(1):312–313.
- Lee KC, Sturgeon D, Lipsitz S, et al. Mortality and health care utilization among medicare patients undergoing emergency general surgery vs those with acute medical conditions. JAMA Surg. 2020;155(3):216–223. DOI:https://doi.org/10.1001/jamasurg.2019.5087.
- Hall MJ, Williams SN, DeFrances CJ, et al. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;62:1–8.
- Siddiqui AH, Ahmed M, Khan TMA, et al. Trends and outcomes of gastrointestinal bleeding among septic shock patients of the United States: a 10-Year Analysis of a Nationwide Inpatient Sample. Cureus. 2020;12(5):e8029. DOI:https://doi.org/10.7759/cureus.8029.
- Cancer of the Stomach - Cancer Stat Facts. [Internet]SEER. [cited 2021 Mar 12]. Available from: https://seer.cancer.gov/statfacts/html/stomach.html.
- Solanki S, Chakinala RC, Haq KF, et al. Inpatient burden of gastric cancer in the United States. Ann Transl Med Internet]. 2019 [cited 2021 Jun 15];7. https://doi.org/10.21037/atm.2019.11.54
- Sarvepalli S, Garg SK, Sarvepalli SS, et al. Hospital utilization in patients with gastric cancer and factors affecting in-hospital mortality, length of stay, and costs. J Clin Gastroenterol. 2019;53(4):e157–e163. DOI:https://doi.org/10.1097/MCG.0000000000001016.
- Liu D, Mehta D, Kaur S, et al. Decreasing mortality and hospitalizations with rising costs related to gastric cancer in the USA: an epidemiological perspective. J Hematol Oncol. 2018;11(1):138. DOI:https://doi.org/10.1186/s13045-018-0682-5.
- Karve S, Lorenzo M, Liepa AM, et al. Treatment patterns, costs, and survival among medicare-enrolled elderly patients diagnosed with advanced stage gastric cancer: analysis of a linked population-based cancer registry and administrative claims database. J Gastric Cancer. 2015;15(2):87–104. DOI:https://doi.org/10.5230/jgc.2015.15.2.87.
- Trumbull D, Lemini R, Elli F, et al. Age-based trends of gastric adenocarcinoma in the United States. Am Surg. 2020;86(5):407–414. DOI:https://doi.org/10.1177/0003134820918250.
- Hess LM, Michael D, Mytelka DS, et al. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer. 2016;19(2):607–615. DOI:https://doi.org/10.1007/s10120-015-0486-z.
- Sherman KL, Merkow RP, Shah AM, et al. Assessment of advanced gastric cancer management in the United States. Ann Surg Oncol. 2013;20(7):2124–2131. DOI:https://doi.org/10.1245/s10434-013-2953-2.
- Hirst C, Ryan J, Tunceli O, et al. Cost profile of patients with gastric cancer using United States Administrative Claims Data. Value Health. 2014;17(3):A78. DOI:https://doi.org/10.1016/j.jval.2014.03.457.
- Murakami TT, Scranton RA, Brown HE, et al. Management of helicobacter pylori in the United States: results from a national survey of gastroenterology physicians. Prev Med. 2017;100:216–222.
- Graham DY, Lew GM, Malaty HM, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 1992;102(2):493–496. DOI:https://doi.org/10.1016/0016-5085(92)90095-G.
- Kuo C-H, C-y L, Shih H-Y, et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 2014;20(43):16029–16036. DOI:https://doi.org/10.3748/wjg.v20.i43.16029.
- Martis S, Peter I, Hulot J-S, et al. Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharmacogenomics J. 2013;13(4):369–377. DOI:https://doi.org/10.1038/tpj.2012.10.
- Malnick SDH, Melzer E, Attali M, et al. Helicobacter pylori: friend or foe? World J Gastroenterol. 2014;20(27):8979–8985. DOI:https://doi.org/10.3748/wjg.v20.i27.8979.
- Shah S, Shah S, Halvorson A, et al. Helicobacter pylori eradication therapy is not associated with increased cardiovascular mortality: a propensity score weighted analysis of a national veteran cohort. Gastro Hep Adv. 2021;1:25–28. DOI:https://doi.org/10.1016/j.gastha.2021.10.003.
- Kumar S, Metz DC, Kaplan DE, et al. Treatment of Helicobacter pylori is not associated with future Clostridium difficile infection. Am J Gastroenterol. 2020;115(5):716–722. DOI:https://doi.org/10.14309/ajg.0000000000000626.
- Shah SC, Tepler A, Chung CP, et al. Host genetic determinants associated with Helicobacter pylori eradication treatment failure: a systematic review and meta-analysis. Gastroenterology. 2021;161(5):1443–1459. DOI:https://doi.org/10.1053/j.gastro.2021.07.043.
- Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382(5):427–436. DOI:https://doi.org/10.1056/NEJMoa1909666.
- Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378(12):1085–1095. DOI:https://doi.org/10.1056/NEJMoa1708423.
