ABSTRACT
Introduction
Capsule endoscopy has had significant development since it was introduced into the field of medicine in 2000. It is non-invasive, well tolerated, does not require sedation and is a first-line small bowel investigative modality. As it transits through the entire gastrointestinal (GI) tract, it has the potential to provide a pan-enteric examination.
Areas covered
In this review we will discuss the new diagnostic modalities along with traditional methods which have been used for examination of the gastro intestinal (GI) tract. The main focus of this review will be on the use of capsule endoscopy for pan-enteric examination.
Expert opinion
Capsule endoscopy is an accepted first-line investigation for the small bowel. Diagnostic sensitivity of the colon capsule is comparable to colonoscopy in controlled trials and is being evaluated in high-risk patients in routine clinical practice in national programs. Preliminary data suggest that a magnetic-controlled examination of the upper GI tract could be developed to enable a complete upper GI examination.
1. Introduction
The GI tract can be examined by flexible fiber-optic endoscopy, radiological imaging, and capsule endoscopy since its introduction in 2000. Conventional endoscopy of the upper GI tract and the colon although considered the gold standard, can be uncomfortable, often requires sedation and comes with the inherent risks of perforation and bleeding. Cross sectional imaging is superseding barium contrast radiology, at least in the colon and magnetic resonance imaging has the advantage of not involving any radiation. Capsule endoscopy has rapidly been established as a first-line investigation for small bowel examination particularly for the indication of obscure gastrointestinal bleeding. A prototype to image the colon has since been developed and more recently a capsule for the upper GI tract, thus providing clinicians with three investigative modalities for assessing GI symptoms.
2. Upper GI tract investigations
2.1. Gastroscopy
Gastroscopy is the gold standard investigation for examination of the upper GI tract, however gastroscopy is an uncomfortable procedure. It has been shown that patient satisfaction is significantly better with sedation, although it increases the risk of hypoxia 6% (95% CI 4–7%) and hypotension 7% (95% CI 5–10%) during the procedure [Citation1]. A recent cohort study showed that the unexpected hospital admission after an elective day case gastroscopy was 5.1% within 30 days of the procedure [Citation2]. Pneumonia was the most frequent cardiovascular or respiratory diagnosis in an emergency hospital admission after an endoscopy [Citation2]. The demand for gastroscopies is growing worldwide. In 2019, there were 866,844 gastroscopies performed in National Health Services (NHS) in the UK with a 6% increase in demand since 2017 [Citation3]. The vast majority of gastroscopies performed for dyspepsia are normal and gastroesophageal malignancy is identified in less than 0.5% of the patients [Citation4]. It does allow direct mucosal visualization, and also the ability for tissue sampling and therapy when required. Whilst there is a three-fold increase in the diagnosis of gastric cancer and dysplasia when the procedure time is at least 7 min, many are performed more quickly than this. A study by Tai and colleagues show a 7.7% missed cancer rate with fibreoptic gastroscopy [Citation5]. A study by Paul Moayyedi shows that the number of gastroenterologists per 100,000 of the population was 3.9 in the United States, 3.48 in France, 2.1 in Australia, 1.83 in Canada, and 1.41 in the U.K. [Citation6]. To meet the growing demand and also to ease pressure on endoscopy units, noninvasive techniques warrant consideration as an alternative.
2.2. Radiology
In patients who are unwilling or unable to undergo gastroscopy, radiology might be considered. However, the detection rate of early gastric cancer, using axial computerized tomography (CT) is low (53–65%) [Citation7,Citation8]. Barium contrast is used as an alternative examination for patients presenting with dysphagia. Double contrast barium has been shown to have sensitivity of >95% in detecting esophageal cancer [Citation9,Citation10]. A meta-analysis that compared barium contrast with gastroscopy for detection of gastric cancer in a screening population showed that gastroscopy is significantly better than barium contrast for gastric cancer detection [Citation11]. The multidetector row CT (MDCT) gastrography is an imaging technique, which can generate 3D images of the stomach and this has enhanced the detection rate of early gastric cancers on imaging [Citation12,Citation13]. MDCT is mainly used for staging prior to endoscopic resection of gastric cancers. Whilst imaging does have a role, it also has some limitations; there is exposure to radiation and more so with MDCT. Patients with suspicious findings on imaging need a gastroscopy for direct visualization and biopsies if required. MDCT is also a more time-consuming procedure and requires a longer reading time by the radiologist.
2.3. Capsule endoscopy of the upper gastrointestinal (GI) tract
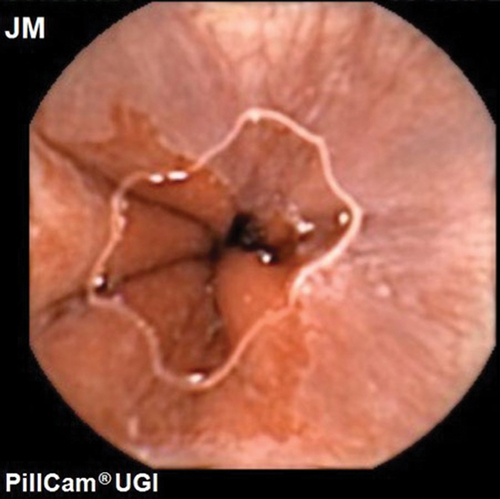
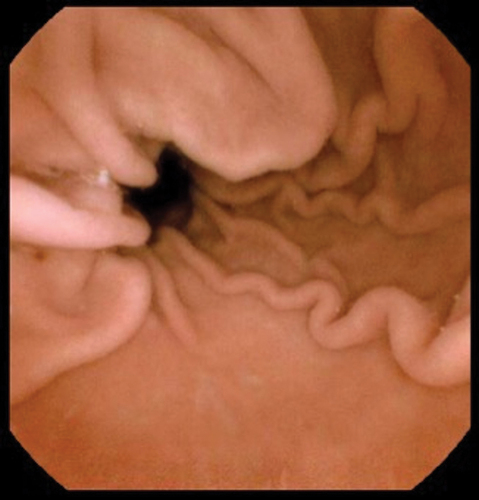
Capsule endoscopy is better tolerated than fibreoptic endoscopy [Citation14,Citation15]. Capsule endoscopy of the esophagus was first approved by the Food and Drug Administration in U.S. in 2004 [Citation16]. A meta-analysis of the first- and second-generation esophageal capsule showed a moderate sensitivity of 77% for the diagnosis of Barrett’s esophagus [Citation17]. There were concerns about inadequate visualization of the gastro-esophageal junction (GOJ). Due to rapid transit of the capsule through the esophagus it was recognized that increasing image capture rate was necessary. The most recent technological advance in this field is the Upper GI capsule (Medtronic Ltd, Dublin, Ireland; originally Given imaging) which was approved in 2014 (, ). This dual headed capsule can capture as many as 35 fps for 10 min followed by 18fps for a further 80 min with a field of view of 174 degrees for each head. A study by Ching et al. using the upper GI capsule visualized the GOJ in its entirety in 92.5% of patients, it was also possible to examine a water filled stomach using a sequence of position changes [Citation15]. In 40%, the recording ceased before the capsule entered the duodenum and reading the videos was time consuming [Citation15]. To enable the routine use of capsule endoscopy in the upper GI tract, the capsule needs to overcome a few challenges; the transit time in the esophagus and the duodenum is very rapid and the stomach is capacious and non-uniform. Steerable capsules with tails, fins, and rotating blades have been developed to overcome the structural challenges of the stomach, but more progress has been made with external magnetic control in capsule endoscopy [Citation18,Citation19]. The use of magnets in capsule endoscopy was initially described by Carpi in 2006 [Citation20]. The MiroCam-Navi was a hand-held magnet developed by Intromedic Ltd (Seoul, South Korea). Although it had advantages like portability and excellent patient tolerance, it was difficult to maneuver a 1 kg magnetic device over a supine patient, also the views obtained of the fundus were suboptimal [Citation21–23]. In France, an electromagnetic coil system was developed in a joint project by the Olympus Medial Systems Corporation (Tokyo, Japan) and Siemens Healthcare (Erlangen, Germany). A blinded study which compared this system to conventional gastroscopy showed that it had a sensitivity of 62% when compared with conventional gastroscopy in identifying major lesions [Citation24] but it was massive and likely expensive system. Ankon technologies Co LTD developed a system in Shanghai, China, where the capsule movement was controlled with the help of magnetic attraction generated by the robotic arm ( and ). The capsule contained a magnet in its dome which allowed the capsule to be steered around the stomach with the help of () two joysticks. A multicentre study of 350 patients showed that magnetically controlled capsule endoscopy (MACE) had a sensitivity of >90% when compared with gastroscopy at detecting focal gastric lesions [Citation25].
Table 1. Current upper gastrointestinal capsules.
The main aim of upper GI tract examination is to detect treatable neoplasia. A major concern is whether or not this new technology is capable of identifying early, treatable neoplasia. MACE identified neoplasia in all patients of a cohort awaiting ESD and accurately localized the lesion and size when compared to gastroscopy [Citation26]. It missed one of the two lesions in one patient. Capsule endoscopy is widely used for population screening for early gastric cancer in China. A review of over 3000 patients who were investigated for screening purposes found 0.22% with cancer and 17.8% with focal lesions (polyps, ulcers, or submucosal tumor). All cancers identified were adenocarcinomas and occurred in individuals of over 50 years of age. Moreover, it demonstrated that the procedure was safe, with no long-term capsule retentions occurring [Citation27]. However, its uptake in the rest of the world has been slower.
Histological samples including those for H. Pylori testing cannot be obtained using capsule endoscopy. However, information often provided by histology can be obtained using alternative, noninvasive methods, such as urea breath test or stool antigen test for H. Pylori or serum pepsinogen levels as a marker of gastric atrophy. Furthermore, whilst most endoscopists have a low threshold for taking biopsies, our previous study suggested that it affected patient management in only 16% [Citation28]. Currently, the capsule is also for single use only which increases the costs. A cost comparison of the two modalities is also needed.
3. Small bowel investigations
3.1. Small bowel radiology
Prior to the advent of capsule endoscopy, radiology was the main stay of investigation for the small bowel. Traditionally, this included cross-sectional imaging and small bowel follow through [Citation29]. Ultrasound has also been used for small bowel examination and has the advantages of being free of ionizing radiation and easily available, however accuracy of findings is operator dependent [Citation30]. Magnetic resonance enterography (MRE) has become a useful modality for assessing disease activity in small bowel Crohns disease. In a large meta-analysis, the sensitivity and specificity were 0.88 (95% CI 0.86 to 0.91) and 0.88 (95% CI 0.84 to 0.91), respectively, when compared with surgery, ileocolonoscopy and/or histolopathology, confirming a high sensitivity and specificity of MRE at identifying small bowel Crohns disease [Citation31].
3.2. Small bowel capsule endoscopy (SBCE)
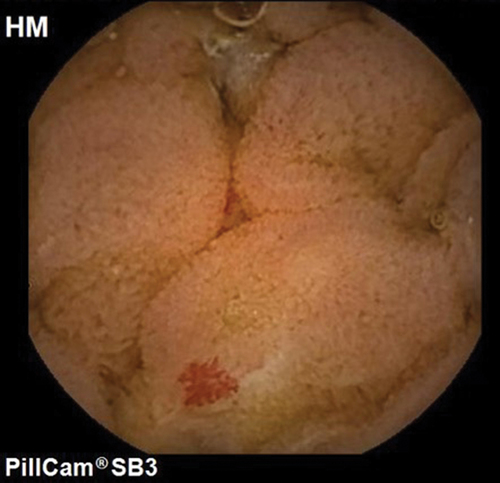
Capsule endoscopy was first introduced in the field of medicine in 2000. The tubular shape and a small diameter of the small bowel makes it an ideal organ to be examined with the capsule. It provides a non-invasive method for complete visualization of the small bowel mucosa. There are a few different manufacturers for SBCE (PillCam, Medtronic, Dublin, Ireland; Endocapsule, Olympus Optical Co, Tokyo, Japan; Miro-Cam, IntroMedic, Seoul, Korea; OMOM capsule, Jinshan Science and Technology Group, Chongqing, China, Navicam, Ankon, Shanghai, China). These models have three parts: a capsule, a sensor belt with receiver, and a work station to download and read the images. The images are transmitted to a data recorder from which a video is created following download on to a computer. The capsocam (CapsoVision, Cupertino, California, USA) provides panoramic views of the small bowel. This capsule does not have a data recorder and the capsule needs to be collected after expulsion from the stool for retrieval of data [Citation32]. The capsocam retains imaging data in-situ and this is subsequently transferred onto a computer using bluetooth following capsule retrieval. All the capsules are for single use only. Head to head comparison in majority of the studies have shown similar diagnostic yields, image quality, and completion rates for all the commercially available small bowel capsules [Citation33–36]. Majority of the studies have been done using the PillCam capsule (). Studies have shown the overall diagnostic yield of SBCE in patients with iron deficiency anemia to be between 41% and 65% [Citation32,Citation37–39]. A systematic review by Liao et al. has also shown that the diagnostic yield for SBCE is up to 50% for all indication [Citation40].
Whilst bowel preparation is imperative prior to colonic investigations, the benefit of laxative-based preparation protocols prior to small bowel capsule endoscopy is less clear. A systematic review and meta-analysis on the use of bowel prep for small bowel capsule endoscopy showed that bowel preparation did not increase the diagnostic yield of small bowel findings, however the small bowel visualization quality was improved [Citation41]. Bowel preparation is recommended for identification of subtle lesions. Small bowel cleanliness is one of the key factors for a good quality study with a high diagnostic yield. Currently, 2 liters of polyethylene glycol (PEG) based laxative the day before the procedure is the most commonly used regime in clinical practice [Citation42]. European Society of Gastrointestinal Endoscopy (ESGE) recommends an early SBCE in obscure GI bleeding as the yield is significantly higher compared to a delayed examination [Citation43–45]. This is the most common indication for SBCE worldwide [Citation46]. In patients with iron deficiency anemia who have negative bidirectional endoscopy ESGE also recommends a SBCE [Citation32].
CE is a safe procedure. It is imperative to screen for risk factors such as obstructive symptoms, Crohn’s disease and long-term use of non-steroidal anti-inflammatory drugs (NSAID). The only procedure-related complication which is associated with SBCE is capsule retention, which can occur in 1–2% of the cases for all indications [Citation40,Citation47]. The risk of capsule retention, however, is notably higher in patients with Crohn’s disease. A recent meta-analysis by Pasha et al. showed that the capsule retention rates were 4.63% in established disease (95% CI, 3.42–6.25%) and 2.35% (95% CI, 1.31–4.19%) in suspected small bowel Crohns disease, respectively [Citation48]. A patency capsule (PC) (Given Imaging, Yoqneam Israel) which is similar in size to a standard CE, is useful in patients with suspected small bowel stenosis to minimize the risk of capsule retention. It is a radiopaque and dissolvable capsule which has a radiofrequency tag. The two timer plugs at either end dissolve at approximately 30 hours due to contact with luminal fluid [Citation49]. A study by Rozenderen et al. showed that MRE has a low positive predictive value (40%) and low specificity (59%) for predicting small bowel patency. Hence, patients with a positive MRE or those with a high suspicion for small bowel strictures should undergo a patency capsule prior to capsule endoscopy [Citation50]
Apart from the risk of retention, capsule endoscopy does have some limitations. Due to a rapid transit through the duodenum focal lesions in the proximal small bowel can be missed [Citation51]. It is also unable to take histological samples or provide therapy when disease is identified. summaries the current small bowel capsules used.
Table 2. Current small bowel capsules.
3.3. Device assisted enteroscopy (DAE)
There are three techniques which are being utilized for deep enteroscopy. Namely, double balloon enteroscopy (DBE), single balloon enteroscopy (SBE) and spiral enteroscopy. DBE is the most commonly used DAE procedure which lends a therapeutic arm to capsule endoscopy [Citation32]. It was first described in 2001 and has made it possible to perform diagnostic and therapeutic procedures during the same examination. It is also useful for obtaining histological samples and placing tattoos prior to surgical intervention. In recent years, DBE has largely replaced push enteroscopy as well as intra operative enteroscopy as DBE achieves greater depth of insertion in the small bowel and is less invasive compared to an intra-operative enteroscopy. However, a SBCE has higher rates of completion enteroscopy with a much lower complication rate and is less invasive. Hence, DBE is often used as a confirmatory tool when other less invasive techniques like SBCE or imaging identify a lesion which needs therapy or histological sampling. The Single balloon enteroscopy (SBE) system (Olympus Optical Co, Tokyo, Japan) uses only one latex-free balloon, which is attached to the distal end of the overtube. A recent meta-analysis has shown no difference in the diagnostic yield or therapeutic yield of DBE or SBE [Citation52]. In spiral enteroscopy (Spirus Medical LLC, West Bridgewater, Massachusetts, USA), an enteroscope is passed through a disposable specialized over tube that has a spiral raised element at its distal end, which aids in the advancement of the enteroscope through the small bowel. This is the most recent prototype for deep enteroscopy and has been developed with encouraging results [Citation53].
4. Colonic investigations
4.1. Colonoscopy
Currently, colonoscopy is the gold standard investigation for the large bowel. It can be challenging and associated with infrequent but serious complications. A population-based study in the US, which looked at 165,527 colonoscopies, documented a perforation in (n = 101), and splenic injury in (n = 12) [Citation54]. A study which looked at post colonoscopy infection rates from six states in the U.S. showed that 30-day infection-related unplanned visits per 1000 procedures were 4.0 for screening colonoscopy, 5.4 for non-screening colonoscopy compared with 2.9 for screening mammography [Citation55]. There is also the added risk of sedation which is used to improve patient tolerance [Citation1]. The demand for colonoscopies is increasing worldwide. There were 704,125 colonoscopies performed in UK in 2019, with a 13% increase in standard colonoscopies and 30% increase in screening for bowel compared to 2017 [Citation3]. However, colonoscopy can miss significant lesions. A systematic review and meta-analysis of tandem colonoscopies ((in which patients undergo a second colonoscopy by endoscopists who are both blinded to each other’s findings) has shown miss rates of 26% for all adenomas, 9% for advanced adenomas, and 27% for serrated polyps [Citation56]. A stool test is used for screening for colorectal cancer in some countries in Europe. The uptake of this test for screening for colorectal cancer is still very low (66%) [Citation57]. A qualitative study has shown that one of the reasons for the poor uptake is because of unwillingness of the participants to undergo a colonoscopy in case of a positive result [Citation58]. The diagnostic yield of colonoscopy in the symptomatic population is low, with 46–75% of patients having a normal examination [Citation59,Citation60]. However, colonoscopy has an advantage that it allows active intervention such as suction, position change, and inflation which improves the views and colonoscopy completion rates. In the future, with improved noninvasive technology, colonoscopy may be reserved for only those patients who need a biopsy or a polypectomy.
4.2. Computerized tomographic colonography (CTC)
CTC is considered to be the gold standard radiological examination for the colon. A recent meta-analysis, which compared CTC and colon capsule (CC) in a group of patients with incomplete colonoscopies, showed that the completion rate of CTC was higher compared to CC (86–100% vs 65–93%), respectively. However, the diagnostic yield of polyps was lower for CTC compared to CC (4–22% vs 8–41% for polyps >5 mm) and (0–11% vs 3–22% for polyps >9 mm), respectively [Citation61]. A potential advantage of CTC is its ability to evaluate for any extra colonic pathology during colonic examination. This can provide reassurance to adults and also early detection of potentially significant pathology. A systematic review and meta-analysis which evaluated the extracolonic findings at CTC showed a 4% rate for identifying potentially important extracolonic findings. The rate of identifying unsuspected extracolonic malignancy ranging from 0.3% to 2.5% [Citation62]. Currently, CTC is offered as an alternative test for suspected CRC and also for completion for incomplete/inadequate colonoscopy. It has been proposed as an alternative imaging modality for examination of the colonic mucosa by the European Society of Gastrointestinal Endoscopy [Citation63].
4.3. Colon capsule
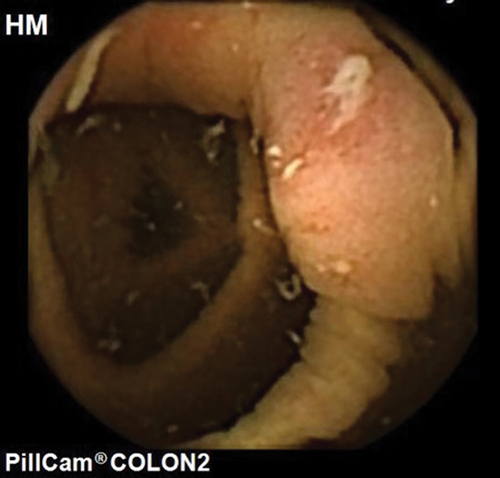
Colon capsule is a noninvasive technique used to examine the colon without the need for air insufflation or sedation (, ). Colon capsule (PillCam given imaging, Yokneam, Israel) was first introduced in 2006. A second-generation colon capsule is currently in use with a variable image capture rate of 4–35 fps based on the speed of peristalsis and almost a 360-degree field of view (). A colon capsule captures images from esophagus and stomach for 3 minutes before entering the sleep mode for 1 h and 45 min to conserve battery life. Systematic reviews which looked at the use of the second-generation capsule in screening for colorectal cancer showed that for polyps >6 mm, sensitivity was 79–96% and specificity was 66–97% [Citation64,Citation65]. For polyps ≥10 mm, sensitivity of CC-2 was 84–97%, which was superior to computed tomographic colonography (CTC) [Citation64,Citation65]. The colorectal cancer detection rate for completed capsules was 93% (25/27) [Citation64]. It is an innovative technique as it can be carried out in the community minimizing hospital attendance. The procedure involves administrating the oral capsule and fitting a receiver belt after which the patient is sent home. This can be done in a simple clinic room. After completion of the test, the patient returns the receiver from which the images can be downloaded and read [Citation66].
Table 3. Current colon capsule.
A study which looked at patient acceptability of colon capsule versus colonoscopy showed that 77.5% (n = 31/40) of patients would prefer CCE if they required a further lower GI investigation in the future. Of these, 77.4% (n = 24/31) still preferred CCE despite the potential need for a follow-up colonoscopy [Citation67]. A multicentre prospective study comparing Crohns capsule, magnetic resonance enterography (MRE) and colonoscopy in detecting active Crohns disease in the small bowel and the colon showed that the overall sensitivity of Crohns capsule was 94% compared to 100% of MRE and/or colonoscopy. The specificity of Crohns Capsule was significantly higher (74%) when compared with MRE (22%) and/or colonoscopy in small bowel Crohns disease [Citation68].
The European Society of Gastrointestinal endoscopy guidelines recommended a colon capsule in cases of incomplete traditional colonoscopy and also in patients who refuse a colonoscopy [Citation69,Citation70]. The use of CC in high-risk individuals is being established. In the UK, a national pilot is underway to evaluate its use in those with a positive FIT test (10–100 micrograms hemoglobin/gm). Patients referred on the suspected cancer pathway are being offered a colon capsule as a first-line diagnostic test [Citation71].
Most patients with inflammatory bowel disease require several endoscopic and radiological procedures in their life time to assess for disease activity and response to therapy along with surveillance for bowel cancer. A colon capsule allows for a noninvasive pan-enteric examination of the entire gastrointestinal tract. Up to 30% of patients with Crohns disease can have isolated small bowel involvement [Citation72]. In a population-based cohort study, the cumulative probability of stricturing in CD after long-term follow-up was 12.4% at 5 years, 15.2% at 10 years and 21.6% at 20 years, respectively [Citation73]. The role of colon capsule has been studied in patients with inflammatory bowel disease to evaluate its role in assessing for mucosal healing and/or response to therapy [Citation74–76]. The PillCam Crohn’s system (Medtronic, Yoqneam, Israel) was approved by the US FDA in 2017. It is similar to the colon capsule as it is a double headed capsule with a field of view of 336 degrees. It also has an adaptive image capture rate of 4–35 fps based on the speed of transit. This capsule starts photographing as soon as it is ingested and has an extended battery life of up to 12 h, hence obtaining pan-enteric images. A multicentre feasibility study which included 41 patients (n = 29 established CD; n = 5 established UC; n = 7 suspected CD) showed a high functionality and no adverse events for the Crohns capsule (CC) [Citation77]. The bowel preparation regime for colon capsule and Pillcam Crohn’s capsule is more rigorous compared to a routine colonoscopy as bowel cleanliness has to be excellent or at least good for adequate sensitivity of the examination. The European Society of Gastrointestinal Endoscopy guidelines recommend a liquid diet the day before the procedure and a split preparation of 4 lts polyethylene glycol electrolyte solution the day before and on the day of the examination. An oral promotility agent is administered if gastric emptying is longer than 1 h, and two further boosters of low-dose sodium phosphate may be given to propel the capsule through the small bowel into the colon [Citation69]. Data from the ScotCAP study show that Colon CE reduced colonoscopy by 37% in the symptomatic patients. Polyps identified needing therapy was the main reason for requiring a repeat colonoscopy in this cohort (92%) and the completion rate was 72% [Citation78]. A study which looked at the role of prucalopride in improving completion rates has shown that completion rates were higher in the prucalopride group with 74.9% complete investigations compared to 56.7%in the control group. Bowel preparation quality was also better in the prucalopride group (75.9%) compared to the control (57.1%) [Citation79]. There are some challenges that a colon capsule needs to overcome; with traditional colonoscopy mucosal visualization can be improved if the bowel prep is not perfect with the help of air insufflation and suction whereas good bowel prep is essential for a successful colon capsule.
5. Green endoscopy
Climate change is a serious and sustained threat that needs to be addressed urgently by every part of society. Endoscopy is the third highest carbon emitting hospital department generating 3.09 kg of waste per bed per day [Citation80]. The endoscopy decontamination process requires large amounts of water and disinfectants and has a major impact on the environment [Citation81]. Reducing the number of endoscopic procedures would have the greatest impact in reducing the waste generated. Capsule endoscopy has huge potential for becoming a low carbon alternative to traditional endoscopy. Currently, all capsules are for single use only and unfortunately there is no clear disposal pathway. More research is required in this field to make capsule endoscopy a greener alternative technology.
6. Conclusion
In conclusion, traditional endoscopic procedures have a firm role in the investigation and management of the GI tract. However, with the expanding role of capsule endoscopy, in the future, these procedures will likely be favored for patients who need tissue sampling or a therapeutic procedure. More studies need to be conducted to compare costs between the traditional endoscopy and capsule endoscopy.
7. Expert opinion
Capsule endoscopy is slowly proving its potential as a noninvasive pan-enteric examination technique. Magnetic control capsule endoscopy of the upper gastrointestinal tract is very well tolerated. Studies using the magnetic capsule have shown very good diagnostic yield. Feasibility studies on the use of fully automated magnetic control capsule endoscopy have shown promising results. Whilst there remain issues in relation to the lack of suction or the ability to wash and clear debris, further studies addressing the gastric preparation are required. Cost comparison between the two modalities is also needed. Small bowel capsule endoscopy is an established technique used for visualizing the small bowel and also the accepted gold standard for indications such as obscure gastrointestinal bleeding. There are several prototypes of SBCE now available with comparable diagnostic yields. SBCE helps to direct patients that may require device assisted enteroscopy. Capsule endoscopy is a safe procedure with capsule retention risk of 1–2% being its only procedure-related complication. Colon capsule is rapidly becoming an important alternative diagnostic tool for the lower gastrointestinal tract. It is being offered to patients who refuse a traditional colonoscopy or in cases of incomplete colonoscopy. It is now also being evaluated in patients who are Fecal Immunochemical Test positive. This has been pertinent during the COVID 19 pandemic but likely to remain as an important modality within the diagnostic algorithm of symptomatic patients. The bowel preparation required is greater than conventional colonoscopy and is a limitation of colon capsule, however it is painless and can be performed out of the hospital setting, making it an attractive option to patients We envisage that colon capsule will soon be routinely offered as an initial diagnostic modality particularly to lower risk groups and conventional colonoscopy being reserved for only those patients who require biopsy or therapy. Most patients with inflammatory bowel disease require several endoscopic procedures in their life time to assess for disease activity and response to therapy along with surveillance for bowel cancer. With the extended battery life of the PillCam Crohns system, it is now possible to have a complete pan enteric noninvasive examination. Further large studies with cost comparisons are needed to bring this technology into routine practice. One of the drawbacks of capsule endoscopy is the increased reading time which is encountered with the increasing image capture rate of all modern capsules. The majority of the software for CE have the ability to remove duplicate images and reduce reading times. Artificial intelligence has already been developed in a few of the software algorithms. Initial studies have shown promising results with sensitivity of picking up pathology of over 99% and reading time of under 6 minutes. We envisage that this will be a routine feature of all CE reading platforms within the next 2 years.
Article highlights
Endoscopy is an aerosol generating procedure with an increasing demand.
Capsule endoscopy of the upper GI tract is well tolerated and could help reduce pressures on endoscopy services.
Although MDCT gastrography has high sensitivity in detecting gastric cancer, it exposes patients to higher levels of radiation and is time consuming.
SBCE is a safe and reliable method for examination of the small bowel.
DBE is the most commonly used technique for small bowel sampling and therapy.
With the expansion of the bowel cancer screening program the demand for colonoscopy is rising.
ESGE recommends a colon capsule as an alternative for those patients who cannot have a colonoscopy.
Pan-enteric capsule endoscopy is a safe and reliable method to assess disease activity in patients with non stricturing Crohns disease.
A patency capsule should be used prior in patients with suspected stricturing Crohn’s to minimize the risk of capsule retention.
Abbreviations
GI (gastro intestinal), MACE (magnetically controlled capsule endoscopy), SBCE (small bowel capsule endoscopy), DBE (double balloon enteroscopy), NHS (national health services), FIT (fecal immunochemical testing)
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
R. Sidhu designed the review. R. Sidhu, M. McAlindon and P Oka wrote the initial draft and all authors approved the final draft of the manuscript.
Additional information
Funding
References
- McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67(6):910–923.
- Crooks CJ, Card TR, West J. The risk of unexpected hospital admissions and primary care visits after an elective day-case gastroscopy: a cohort study within England. Aliment Pharmacol Ther. 2022. DOI:10.1111/apt.16946.
- Ravindran S, Bassett P, Shaw T, et al. National census of UK endoscopy services in 2019. Frontline Gastroenterol. 2021;12(6):451–460.
- Ford AC, Marwaha A, Lim A, et al. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8(10):830–7, 7.e1–2.
- Tai FWD, Wray N, Sidhu R, et al. Factors associated with oesophagogastric cancers missed by gastroscopy: a case-control study. Frontline Gastroenterol. 2020;11(3):194–201.
- Moayyedi P, Tepper J, Hilsden R, et al. International comparisons of manpower in gastroenterology. Am J Gastroenterol. 2007;102(3):478–481.
- Hori S, Tsuda K, Murayama S, et al. CT of gastric carcinoma: preliminary results with a new scanning technique. Radiographics. 1992;12(2):257–268.
- Minami M, Kawauchi N, Itai Y, et al. Gastric tumors: radiologic-pathologic correlation and accuracy of T staging with dynamic CT. Radiology. 1992;185(1):173–178.
- Low VH, Levine MS, Rubesin SE, et al. Diagnosis of gastric carcinoma: sensitivity of double-contrast barium studies. AJR Am J Roentgenol. 1994;162(2):329–334.
- Levine MS, Chu P, Furth EE, et al. Carcinoma of the esophagus and esophagogastric junction: sensitivity of radiographic diagnosis. AJR Am J Roentgenol. 1997;168(6):1423–1426.
- Khanderia E, Markar SR, Acharya A, et al. The influence of gastric cancer screening on the stage at diagnosis and survival: a meta-analysis of comparative studies in the far east. J Clin Gastroenterol. 2016;50(3):190–197.
- Kim JW, Shin SS, Heo SH, et al. The role of three-dimensional multidetector CT gastrography in the preoperative imaging of stomach cancer: emphasis on detection and localization of the tumor. Korean J Radiol. 2015;16(1):80–89.
- Kim JH, Park SH, Hong HS, et al. CT gastrography. Abdom Imaging. 2005;30(5):509–517.
- Bouchard S, Ibrahim M, Van Gossum A. Video capsule endoscopy: perspectives of a revolutionary technique. World J Gastroenterol. 2014;20(46):17330–17344.
- Ching HL, Healy A, Thurston V, et al. Upper gastrointestinal tract capsule endoscopy using a nurse-led protocol: first reported experience. World J Gastroenterol. 2018;24(26):2893–2901.
- Park J, Cho YK, Kim JH. Current and future use of esophageal capsule endoscopy. Clin Endosc. 2018;51(4):317–322.
- Bhardwaj A, Hollenbeak CS, Pooran N, et al. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett’s esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2009;104(6):1533–1539.
- Liu L, Towfighian S, Hila A. A review of locomotion systems for capsule endoscopy. IEEE Rev Biomed Eng. 2015;8:138–151.
- Koulaouzidis A, Iakovidis DK, Karargyris A, et al. Wireless endoscopy in 2020: will it still be a capsule? World J Gastroenterol. 2015;21(17):5119–5130.
- Carpi F, Galbiati S, Carpi A. Magnetic shells for gastrointestinal endoscopic capsules as a means to control their motion. Biomed Pharmacother. 2006;60(8):370–374.
- Ching HL, Hale MF, Sidhu R, et al. Magnetically assisted capsule endoscopy in suspected acute upper GI bleeding versus esophagogastroduodenoscopy in detecting focal lesions. Gastrointest Endosc. 2019;90(3):430–439.
- Rahman I, Pioche M, Shim CS, et al. Magnetic-assisted capsule endoscopy in the upper GI tract by using a novel navigation system (with video). Gastrointest Endosc. 2016;83(5):889–95.e1.
- Ching HL, Hale MF, Kurien M, et al. Diagnostic yield of magnetically assisted capsule endoscopy versus gastroscopy in recurrent and refractory iron deficiency anemia. Endoscopy. 2019;51(5):409–418.
- Denzer UW, Rösch T, Hoytat B, et al. Magnetically guided capsule versus conventional gastroscopy for upper abdominal complaints: a prospective blinded study. J Clin Gastroenterol. 2015;49(2):101–107.
- Liao Z, Hou X, Lin-Hu EQ, et al. Accuracy of magnetically controlled capsule endoscopy, compared with conventional gastroscopy, in detection of gastric diseases. Clin Gastroenterol Hepatol. 2016;14(9):1266–73.e1.
- Qian YY, Zhu SG, Hou X, et al., Preliminary study of magnetically controlled capsule gastroscopy for diagnosing superficial gastric neoplasia. Dig Liver Dis. 2018;50(10):1041–1046.
- Zhao AJ, Qian YY, Sun H, et al. Screening for gastric cancer with magnetically controlled capsule gastroscopy in asymptomatic individuals. Gastrointest Endosc. 2018;88(3):466–74.e1.
- Ching HL, Hale MF, Sidhu R, et al. Reassessing the value of gastroscopy for the investigation of dyspepsia. Frontline Gastroenterol. 2018;9(1):62–66.
- Kelvin FM, Maglinte DD. Enteroclysis or small bowel follow-through in Crohn’ s diseases? Gastroenterology. 1998;114(6):1349–1351.
- Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7(7):556–585.
- Ahmed O, Rodrigues DM, Nguyen GC. Magnetic resonance imaging of the small bowel in crohn’s disease: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. 2016;2016:7857352.
- Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European society of gastrointestinal endoscopy (ESGE) clinical guideline. Endoscopy. 2015;47(4):352–376.
- Dolak W, Kulnigg-Dabsch S, Evstatiev R, et al. A randomized head-to-head study of small-bowel imaging comparing mirocam and endocapsule. Endoscopy. 2012;44(11):1012–1020.
- Hartmann D, Eickhoff A, Damian U, et al. Diagnosis of small-bowel pathology using paired capsule endoscopy with two different devices: a randomized study. Endoscopy. 2007;39(12):1041–1045.
- Cave DR, Fleischer DE, Leighton JA, et al. A multicenter randomized comparison of the endocapsule and the pillcam SB. Gastrointest Endosc. 2008;68(3):487–494.
- Pioche M, Gaudin JL, Filoche B, et al. Prospective, randomized comparison of two small-bowel capsule endoscopy systems in patients with obscure GI bleeding. Gastrointest Endosc. 2011;73(6):1181–1188.
- Riccioni ME, Urgesi R, Spada C, et al. Unexplained iron deficiency anaemia: is it worthwhile to perform capsule endoscopy? Dig Liver Dis. 2010;42(8):560–566.
- Milano A, Balatsinou C, Filippone A, et al. A prospective evaluation of iron deficiency anemia in the GI endoscopy setting: role of standard endoscopy, videocapsule endoscopy, and CT-enteroclysis. Gastrointest Endosc. 2011;73(5):1002–1008.
- Koulaouzidis A, Rondonotti E, Giannakou A, et al. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: a systematic review. Gastrointest Endosc. 2012;76(5):983–992.
- Liao Z, Gao R, Xu C, et al. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71(2):280–286.
- Yung DE, Rondonotti E, Sykes C, et al. Systematic review and meta-analysis: is bowel preparation still necessary in small bowel capsule endoscopy? Expert Rev Gastroenterol Hepatol. 2017;11(10):979–993.
- Rokkas T, Papaxoinis K, Triantafyllou K, et al. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: a meta-analysis. Am J Gastroenterol. 2009;104(1):219–227.
- Katsinelos P, Fasoylas K, Chatzimavroudis G, et al. Diagnostic yield and clinical management after capsule endoscopy in daily clinical practice: a single-center experience. Hippokratia. 2010;14(4):271–276.
- Ramirez FC, Shaukat MS, Young MA, et al. Feasibility and safety of string, wireless capsule endoscopy in the diagnosis of Barrett’s esophagus. Gastrointest Endosc. 2005;61(6):741–746.
- Singh A, Marshall C, Chaudhuri B, et al. Timing of video capsule endoscopy relative to overt obscure GI bleeding: implications from a retrospective study. Gastrointest Endosc. 2013;77(5):761–766.
- Rondonotti E, Soncini M, Girelli C, et al. Small bowel capsule endoscopy in clinical practice: a multicenter 7-year survey. Eur J Gastroenterol Hepatol. 2010;22(11):1380–1386.
- Cheung DY, Kim JS, Shim KN, et al., Group KGIS. The usefulness of capsule endoscopy for small bowel tumors. Clin Endosc. 2016;49(1):21–25.
- Pasha SF, Pennazio M, Rondonotti E, et al. Capsule retention in crohn’s disease: a meta-analysis. Inflamm Bowel Dis. 2020;26(1):33–42.
- Caunedo-Alvarez A, Romero-Vazquez J, Herrerias-Gutierrez JM. Patency and Agile capsules. World J Gastroenterol. 2008;14(34):5269–5273.
- Rozendorn N, Klang E, Lahat A, et al. Prediction of patency capsule retention in known Crohn’s disease patients by using magnetic resonance imaging. Gastrointest Endosc. 2016;83(1):182–187.
- Zagorowicz ES, Pietrzak AM, Wronska E, et al. Small bowel tumors detected and missed during capsule endoscopy: single center experience. World J Gastroenterol. 2013;19(47):9043–9048.
- Kim TJ, Kim ER, Chang DK, et al. Comparison of the efficacy and safety of single- versus double-balloon enteroscopy performed by endoscopist experts in single-balloon enteroscopy: a single-center experience and meta-analysis. Gut Liver. 2017;11(4):520–527.
- Morgan D, Upchurch B, Draganov P, et al. Spiral enteroscopy: prospective U.S. multicenter study in patients with small-bowel disorders. Gastrointest Endosc. 2010;72(5):992–998.
- Cooper GS, Kou TD, Rex DK. Complications following colonoscopy with anesthesia assistance: a population-based analysis. JAMA Intern Med. 2013;173(7):551–556.
- Wang P, Xu T, Ngamruengphong S, et al. Rates of infection after colonoscopy and osophagogastroduodenoscopy in ambulatory surgery centres in the USA. Gut. 2018;67(9):1626–1636.
- Zhao S, Wang S, Pan P, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology. 2019;156(6):1661–74.e11.
- Moss S, Mathews C, Day TJ, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut. 2017;66(9):1631–1644.
- Palmer CK, Thomas MC, von Wagner C, et al. Reasons for non-uptake and subsequent participation in the NHS bowel cancer screening programme: a qualitative study. Br J Cancer. 2014;110(7):1705–1711.
- Al-Najami I, Rancinger CP, Larsen MK, et al. The diagnostic yield of colonoscopy stratified by indications. Gastroenterol Res Pract. 2017;2017:4910143.
- Gavin DR, Valori RM, Anderson JT, et al. The national colonoscopy audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut. 2013;62(2):242–249.
- Deding U, Kaalby L, Bøggild H, et al. Colon capsule endoscopy vs. CT colonography following incomplete colonoscopy: a systematic review with meta-analysis. Cancers (Basel). 2020;12(11):3367.
- Pickhardt PJ, Correale L, Morra L, et al. JOURNAL CLUB: extracolonic findings at CT colonography: systematic review and meta-analysis. AJR Am J Roentgenol. 2018;211(1):25–39.
- Spada C, Hassan C, Bellini D, et al. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European society of gastrointestinal endoscopy (ESGE) and European society of gastrointestinal and abdominal radiology (ESGAR) guideline - update 2020. Eur Radiol. 2021;31(5):2967–2982.
- Vuik FER, Nieuwenburg SAV, Moen S, et al. Colon capsule endoscopy in colorectal cancer screening: a systematic review. Endoscopy. 2021;53(8):815–824.
- Möllers T, Schwab M, Gildein L, et al. Second-generation colon capsule endoscopy for detection of colorectal polyps: systematic review and meta-analysis of clinical trials. Endosc Int Open. 2021;9(4):E562–E71.
- Spada C, Barbaro F, Andrisani G, et al. Colon capsule endoscopy: what we know and what we would like to know. World J Gastroenterol. 2014;20(45):16948–16955.
- Ismail MS, Murphy G, Semenov S, et al. Comparing colon capsule endoscopy to colonoscopy; a symptomatic patient’s perspective. BMC Gastroenterol. 2022;22(1):31.
- Bruining DH, Oliva S, Fleisher MR, et al. Panenteric capsule endoscopy versus ileocolonoscopy plus magnetic resonance enterography in Crohn’s disease: a multicentre, prospective study. BMJ Open Gastroenterol. 2020;7:1.
- Spada C, Hassan C, Galmiche JP, et al. Colon capsule endoscopy: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2012;44(5):527–536.
- Baltes P, Bota M, Albert J, et al. PillCamColon2 after incomplete colonoscopy - A prospective multicenter study. World J Gastroenterol. 2018;24(31):3556–3566.
- NHS. Clinical guide for using colon capsule endoscopy in the lower gastrointestinal pathway. November 2020. cited 2021 Mar 31. https://www.nice.org.uk/Media/Default/About/COVID-19/Specialty-guides/triaging-patients-with-lower-gi-symptoms.pdf
- Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794.
- Yamamoto T, Watanabe T. Surgery for luminal Crohn’s disease. World J Gastroenterol. 2014;20(1):78–90.
- Meister T, Heinzow HS, Domagk D, et al. Colon capsule endoscopy versus standard colonoscopy in assessing disease activity of ulcerative colitis: a prospective trial. Tech Coloproctol. 2013;17(6):641–646.
- Sung J, Ho KY, Chiu HM, et al. The use of pillcam colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy. 2012;44(8):754–758.
- Mehdizadeh S, Chen GC, Barkodar L, et al. Capsule endoscopy in patients with Crohn’s disease: diagnostic yield and safety. Gastrointest Endosc. 2010;71(1):121–127.
- Eliakim R, Spada C, Lapidus A, et al. Evaluation of a new pan-enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease - feasibility study. Endosc Int Open. 2018;6(10):E1235–E46.
- MacLeod C, Hudson J, Brogan M, et al. ScotCap – a large observational cohort study. Colorectal Dis. 2022;24(4):411–421.
- Deding U, Kaalby L, Baatrup G, et al. The effect of prucalopride on the completion rate and polyp detection rate of colon capsule endoscopies. Clin Epidemiol. 2022;14:437–444.
- Vaccari M, Tudor T, Perteghella A. Costs associated with the management of waste from healthcare facilities: an analysis at national and site level. Waste Manag Res. 2018;36(1):39–47.
- Maurice JB, Siau K, Sebastian S, et al. Green endoscopy: a call for sustainability in the midst of COVID-19. Lancet Gastroenterol Hepatol. 2020;5(7):636–638.