ABSTRACT
Objectives
Cannabis-based medicinal products (CBMPs) have shown promising preclinical activity in inflammatory bowel disease (IBD). However, clinical trials have not demonstrated effects on inflammation. This study aims to analyze changes in health-related quality of life (HRQoL) and adverse events in IBD patients prescribed CBMPs.
Methods
A case series from the UK Medical Cannabis Registry was performed. Primary outcomes included changes from baseline in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ), Generalized Anxiety Disorder-7 (GAD-7), Single-Item Sleep Quality Scale (SQS), and EQ-5D-5L Index score at 1 and 3 months. Statistical significance was defined using p < 0.050.
Results
Seventy-six patients with Crohn’s disease (n = 51; 67.11%) and ulcerative colitis (n = 25; 32.89%) were included. The median baseline SIBDQ score improved at 1 and 3 months. EQ-5D-5L index values, GAD-7, and SQS also improved after 3 months (p < 0.050). Sixteen (21.05%) patients reported adverse events with the majority being classified as mild to moderate in severity.
Conclusion
Patients treated with CBMPs for refractory symptoms of Crohn’s disease and ulcerative colitis demonstrated a short-term improvement in IBD-specific and general HRQoL. Prior cannabis consumers reported greater improvement compared to cannabis-naïve individuals.
1. Introduction
The global prevalence of inflammatory bowel disease (IBD) continues to rise and now affects up to 1 in 200 individuals in Western countries [Citation1]. In addition, there is a rapidly increasing incidence in newly industrialized countries within Asia, Africa, and South America [Citation1,Citation2]. IBD encompasses two distinct disorders, Crohn’s disease (CD) and ulcerative colitis (UC), which differ in pathophysiology, affected parts of the gastrointestinal tract, clinical presentation, complications, disease course, and management. The predominant symptoms in IBD are persistent diarrhea, abdominal pain, weight loss, malnutrition, and fatigue, commonly complicated by extra-intestinal manifestations. These features adversely affect daily productivity and inflict a sizable psychosocial burden [Citation3,Citation4]. Moreover, IBD inflicts a financial burden limiting career options and lifetime expenses being comparable to other major chronic diseases [Citation5]. The debilitating nature of symptoms, lack of cure, and alternating pattern of disease relapse and remission negatively affects a patient’s health-related quality of life (HRQoL) [Citation3,Citation6,Citation7].
Clinically, the aims of IBD treatment are to induce and maintain disease remission, promote mucosal healing, and enhance patients’ quality of life [Citation8]. The therapeutic approach to the management of IBD largely depends on the severity of the disease, the extent of inflammation, and its evolution over time. There have been revolutionary advances in the medical and surgical treatment of each condition, particularly with the advent of biologic therapies for refractory disease. This has led to significant improvements in the rates of remission and the need for surgical intervention [Citation9]. However, there are a portion of patients who are still refractory to maximal medical therapy. Even in individuals who demonstrate an initial clinical response, subsequent secondary loss of response affects up to 40% of patients [Citation10]. Moreover, many patients are not able to tolerate medications for the treatment of IBD due to adverse events; an important concern considering IBD often requires long-term treatment [Citation11]. Hence, there remains an unmet need in currently licensed treatment options to meet the demands of many IBD patients. As such, it is necessary to develop further therapies both for the treatment of IBD and its sequelae.
Cannabis-based medicinal products (CBMPs) have received growing interest as a therapeutic option for IBD. (−)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are the active pharmaceutical cannabinoids found in the highest abundance within the cannabis plant [Citation12]. Cannabinoids predominantly exert their effects by interacting with the endocannabinoid system (ECS), particularly cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptors, which are both G-protein coupled receptors [Citation12]. Δ9-THC is a partial agonist of CB1 and CB2, whereas CBD has more complex pharmacodynamics without clear affinity for CB1 or CB2 [Citation12]. CBD’s primary mechanism of action is through increasing the concentration of anandamide, an endogenous CB1 agonist, through inhibiting its breakdown [Citation13]. CB1 is distributed densely throughout the central nervous system, and CB2 predominantly resides within peripheral immune cells [Citation14]. CB1 stimulation is responsible for the psychotropic effects associated with Δ9-THC [Citation12]. Both CB1 and CB2 are located in the enteric nervous system; hence, the ECS is critical for regulation of multiple aspects of gastrointestinal function [Citation14,Citation15].
Pre-clinical studies have hinted at the therapeutic potential of targeting the ECS for treating IBD-related symptoms [Citation16]. Multiple in vitro IBD models have shown the ability of CB1 and CB2 agonists to ameliorate intestinal inflammation in murine models, whilst antagonists increase the inflammatory response [Citation17–19]. CB2 stimulation is associated with reductions in cytokine production and inhibition of neutrophil migration [Citation20–22]. CBD in isolation and when combined with Δ9-THC has subsequently been shown to initiate an anti-inflammatory response in vivo [Citation23]. In addition, a number of in vivo and in vitro studies have elucidated local immunosuppressive effects of CB2 agonists through induction of cellular apoptosis [Citation24,Citation25]. Moreover, CB1 and CB2 agonists have both been implicated in reducing intestinal hypermotility in rodent models during inflammatory states and hence has been proposed for symptomatic management of diarrhea [Citation26–28]. In rodent models with colorectal distension, activation of CB1 and CB2 has been linked with the ability to modulate visceral sensitivity, suggesting its potential as a molecular target for relief of chronic abdominal pain associated with IBD [Citation29–33]. The antiemetic activity of CB1 and CB2 activation has also been demonstrated in several animal models [Citation34]. There is also evidence that CB1 stimulation increases appetite and conservation of energy, both useful properties in the context of IBD, where symptoms of poor appetite and malnutrition are prevalent [Citation35].
There is sufficient evidence from experimental studies demonstrating a rationale for cannabinoids to have positive outcomes in IBD patients. However, there has been a paucity of studies evaluating the clinical efficacy of cannabinoids in this group. A recent meta-analysis appraised the small number of existing clinical studies in this field, including 15 observational studies and 5 randomized controlled trials (RCTs) [Citation36]. However, this meta-analysis was limited due to included studies being underpowered, possessing clinical heterogeneity, and having a substantial risk of bias, although a number of clinically relevant effects of cannabinoids were asserted [Citation36]. Cannabinoids did not significantly improve objective parameters of active inflammation, in contrast to those effects shown in preclinical models of disease. Moreover, cannabinoids were not effective in inducing clinical remission as measured by several disease activity indices including the commonly used Crohn’s Disease Activity Index score (CDAI). However, these disease activity indices have limitations as measurement tools, such as certain parameters of CDAI, are dependent on subjective responses of patients on wellbeing and symptoms [Citation37]. Other studies have found that improvement in CDAI after administration of cannabinoids in IBD was secondary to improvement in general well-being and abdominal pain [Citation38]. The implication that cannabinoids do not induce remission is clinically and socioeconomically significant, as it is a key goal of IBD treatment and the annual cost of treating IBD relapses in the UK is up to six times greater than the cost of treating patients in clinical remission [Citation39].
Although the clinical efficacy of cannabinoids in directly controlling IBD remains inconclusive, cannabinoids have demonstrated a role as palliative therapy for patients. Significant improvements in HRQoL of IBD patients have been noted in meta-analyses; symptoms of abdominal pain, diarrhea, nausea, and poor appetite were all perceived to improve after cannabinoid administration [Citation36]. Naftali et al. also demonstrated a significant improvement in patient well-being and within a shorter timeline compared to placebo [Citation38]. The benefits on HRQoL and patient-reported symptoms provide a reasoning to the high rates of illicit cannabis use in IBD patients, especially in patients refractory to conventional treatment and/or seeking complementary or alternative medical therapies [Citation40,Citation41]. Several self-reported studies indicate that up to 27% of IBD patients are active cannabis users [Citation40–45]. Although existing literature suggests an association between cannabinoids and improved HRQoL, there remains concern over CBMP prescriptions long-term due to lack of safety data and risk of complications from causing relief of IBD symptoms without resolving underlying intestinal inflammation.
This study describes an analysis of a case series of IBD patients who were prescribed CBMPs and registered in the UK Medical Cannabis Registry (UKMCR), which was established to prospectively collect outcomes data in patients prescribed CBMPs. The primary aim of the study was to assess multiple domains of HRQoL outcomes using patient-reported outcome measures (PROMs). A secondary aim was to assess the incidence of adverse effects to characterize the safety profile of CBMPs in IBD patients.
2. Materials and methods
2.1. Study design and participants
This study is an uncontrolled clinical case series of IBD patients identified from the UKMCR. The UKMCR was established in December 2019 and is managed by Sapphire Medical Clinics. It is the first patient registry with the main objective of prospectively collecting longitudinal pseudonymized data from patients treated with CBMPs in the UK. It is designed for remote data collection via an online web-based platform using automated reminders. It captures clinical data across the UK and Channel Islands. Following NHS Health Research Authority and Research Ethics Committee guidance, this study was considered not to require formal ethical approval. The study is reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [Citation46]. Written and informed consent was provided by all participants, prior to enrollment.
Unlicensed CBMPs were rescheduled in the UK in November 2018 and can be initiated by attending-grade doctors for individuals who have not had a satisfactory response to licensed therapies [Citation47]. The decision to prescribe must be confirmed by a multidisciplinary team of similar level physicians. For IBD, CBMPs are only prescribed for symptomatic management, rather than with the intent of disease control. The effects of prescribed CBMP products were investigated in patients treated for auxiliary symptoms of CD or UC who were enrolled in the UKMCR. The inclusion criteria for the study were individuals aged over 18 years with a primary diagnosis of CD or UC, for whom CBMP therapy was adjudged to be appropriate for refractory symptoms by a specialist in IBD and following discussion with a multidisciplinary team. Patients were excluded from analysis if they did not complete baseline PROMs or had not been treated for more than 1 month. All CBMP products prescribed were in line with Good Manufacturing Practice guidelines. The strains of CBMP were derived from either Cannabis sativa L., Cannabis indica Lam., or a hybrid species. The formulations of CBMPs included either dried flower or oil. Dried flower CBMPs were consumed by vaping. The oils were consumed either orally or sublingually.
2.2. Outcomes of interest
Patients who gave fully informed written consent were prompted to complete questionnaires electronically at baseline, 1 month, and 3 months. Patients were sent electronic reminders to complete the questionnaires if they had not completed these questionnaires three times per week until they were complete.
The baseline questionnaires captured data regarding demographic details, including age, gender, and occupation. The Body Mass Index (BMI) and comorbidities of participants were also recorded. Smoking, alcohol, and illicit cannabis use data were extracted including smoking status, smoking pack years, weekly alcohol consumption (units), cannabis use status, frequency of cannabis use for current users, and current quantity of cannabis consumption (g). To quantify the individual quantity of illicit cannabis consumption in a patient, ‘cannabis gram years’ were calculated using a novel metric that has been previously described by our group [Citation48].
Data regarding CBMP prescriptions were recorded and analyzed including Δ9-THC dose per 24 hours (mg), CBD dose per 24 hours (mg), route of administration, and most commonly prescribed products. The overall dose of oil preparations was determined by multiplication of the concentration (mg/ml) and the daily dose prescribed (ml/day). The overall dose of dried flower preparations was similarly calculated by the product of the concentration (mg/g) and daily dose prescribed (g/day). It should be noted that for both concentration and daily dose, there may be a range, e.g. 180–220 mg/g for concentration and 0.5–1 g/day. Where this is present, the halfway value was taken for each, i.e. 200 mg/g and 0.75 g/day in the above example.
Participants were asked to complete validated PROMs to assess quality of life. The primary outcome of the study was the change from baseline at 1 month and 3 months in the following self-reported PROMS: Short Inflammatory Bowel Disease Questionnaire (SIBDQ), General Anxiety Disorder 7 (GAD-7), EQ-5D-5L, Single-Item Sleep Quality Scale (SQS), and Patient Global Impression of Change (PGIC).
The SIBDQ is a validated assessment for measuring clinically significant changes in health-related quality of life (HRQoL) among IBD patients [Citation49–51]. The SIBDQ consists of a total of 10 questions grouped into 4 different domains of health and function: bowel symptoms, systemic symptoms, social function, and emotional function. Each question is rated by a 7-point Likert scale hence the total SIBDQ score ranges from 10 to 70. Lower total SIBDQ scores are indicative of poorer HRQoL, whereas higher S-IBDQ reflects more optimal HRQOL.
The GAD-7 score is designed to effectively screen and characterize the severity of anxiety symptoms in the general population [Citation52]. It consists of 7 questions asking how often they have been bothered by the core symptoms of generalized anxiety disorder (‘0ʹ = ‘not at all’ to ‘3ʹ = ‘nearly every day’) hence the total GAD-7 score ranges from 0 to 21. Scores of ≥5, ≥10, ≥15 are used as the cutoffs to categories mild, moderate, and severe anxiety symptoms, respectively.
EQ-5D-5L is a tool that assesses general HRQoL [Citation53]. It consists of five domains (mobility, pain, or discomfort, self-care, usual activities, anxiety, or depression) and participants are asked to rate the level of severity from 1 to 5 (‘1’ = ‘no problems’ to ‘5’ = ’extreme problems’). The score on each domain and level can be mapped to create an overall EQ-5D-5L index value, using a technique described by van Hout et al. [Citation54]. An EQ-5D-5L index value of <0 signifies a perceived HRQoL worse than death, whereas a score of 1 indicates ‘optimal’ HRQoL.
The SQS score is a widely used tool to assess the quality of sleep [Citation55]. It consists of a single question asking patients to rate their sleep quality over the past 10 days on a scale of 1–10 (‘0ʹ = ‘terrible’ to ‘10ʹ = ‘excellent’). Sleep quality was considered as an outcome measure due to the considerable evidence of CBMPs on regulation of circadian rhythm and sleep, however despite its inclusion as an outcome measure in studies on chronic pain and CBMPs, it is unclear in clinical studies whether there are associations with positive or negative effects on sleep [Citation56–58].
PGIC is used to assess the patient’s perception of the efficacy of CBMP treatment [Citation59]. It consists of two domains: PGIC 1 and PGIC 2. PGIC 1 asks patients to rate their perception of improvement in activity, limitations, symptoms, emotions, and overall quality of life on a scale from 1 to 7 (‘0ʹ = ‘No change or condition has got worse’ to ‘7ʹ = ‘A great deal better, and a considerable improvement that has made all the difference). PGIC 2 asks patients to rate their overall perception of the degree of change (‘0ʹ = ‘Much better’ to ‘10ʹ = ‘Much worse’) since beginning care at the clinic.
A secondary outcome of the study was to report the incidence of adverse events. Adverse events data was collected by patients self-reporting alongside completion of PROMs, or during routine clinician follow-up. Adverse events were recorded in accordance with the common terminology criteria for adverse events version 4.0 [Citation60].
Another secondary outcome was to assess the change in prescribed opiate medications during treatment. Patient medications were recorded at baseline and changes recorded via patient self-reporting or during clinical assessment. All medications were recorded utilizing the SNOMED-CT nomenclature [Citation61]. Opiate medication doses were converted to oral morphine equivalent (OME) doses for comparison between baseline and end of follow-up. OME doses were calculated using the conversion factors quoted by the British National Formulary [Citation62].
A subgroup analysis was also performed on changes to PROMs and incidence of adverse events in current/ex-users and cannabis naïve patients, respectively.
2.3. Statistical analysis
Patient data was extracted from the UKMCR according to the recorded primary diagnosis of CD or UC. Demographic data including patient conditions, cannabis status, tobacco and alcohol use, medication data, and adverse events were analyzed using descriptive statistics. Incidence of adverse events is presented as a percentage of the total number of participants.
Data from PROMs were analyzed at 1 and 3 months compared to baseline. The normality of the data distribution was tested using a Shapiro–Wilk test. Parametric data were presented as mean ± standard deviation, while nonparametric data were presented as median (interquartile range [IQR]). Baseline PROM data and change scores (calculated as a difference between baseline and follow-up data) were compared. Statistical analysis was performed with a t-test or Wilcoxon rank-sum test depending on whether the data were parametric or nonparametric, respectively.
Statistical significance was defined using p-value <0.050. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) [IBM Statistics version 28 SPSS Inc]. Figures were constructed using GraphPad Prism Version 9.3.1.
3. Results
3.1. Patient data
Seventy-six patients with CD (n = 51; 67.11%) and UC (n = 25; 32.89%) were included in this study. The systematic process of how patients registered in the UKMCR were included in this study is illustrated in .
Figure 1. CONSORT diagram showing the process of inclusion and exclusion of patients for analysis in this study.
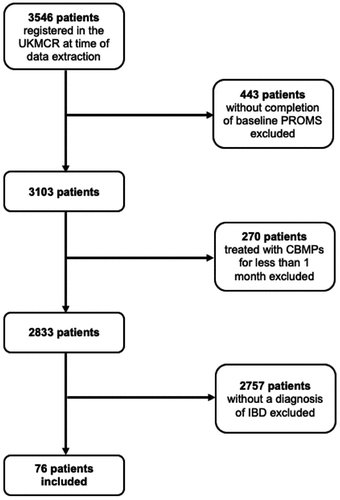
The demographic details of all participants at baseline are presented in . Most participants were male (n = 63, 82.89%). The mean age of participants was 38.46 (± 9.29), and the mean BMI was 25.07 kg/m2 (± 6.02). The most frequent occupation recorded was ‘Professional’ (n = 30; 39.47%).
Table 1. Demographic details of study participants at baseline assessment.
The tobacco, alcohol, and illicit cannabis status of study participants at baseline are presented in . Most participants were current cannabis users (n = 52, 68.42%) at baseline assessment. Most of these participants were daily cannabis users (n = 48; 92.31%), and their median daily quantity of cannabis consumption was 1.00 g/day (IQR: 0.50–1.50). The median lifetime cannabis consumption of participants who were current cannabis users was 4.00 gram years (IQR: 2.13–10.00). The remaining participants were either ex-users (n = 10; 13.16%) or cannabis-naive (n = 14; 18.42%).
Table 2. Tobacco, alcohol, and cannabis status of study participants.
3.2. CBMP dosing and route of administration
Prescribed CBMPs were recorded in the UKMCR for 73 (96.05%) patients at last recorded follow-up. The majority of participants (n = 62; 84.93%) were prescribed both Δ9-THC and CBD. Of the remaining participants, four patients (5.48%) were prescribed CBD only and seven patients (9.59%) were prescribed Δ9-THC only. The overall median Δ9-THC dose per day prescribed was 120.00 mg (IQR: 20.00–205.00), and the overall median CBD dose per day prescribed was 20.50 mg (IQR: 20.00–50.00).
Most participants (n = 39; 53.42%) were prescribed both dried flower and oil preparation (via either oral or sublingual routes). Nineteen participants (26.03%) were prescribed an oil preparation only and fifteen participants (20.55%) were prescribed dried flower only. For participants using oil preparations, the median Δ9-THC dose per day prescribed was 10.00 mg (7.50–20.00), and the median CBD dose per day prescribed was 20.00 mg (20.00–50.00). The cannabinoid doses prescribed were higher for participants using dried flower; the median Δ9-THC dose per day prescribed was 186.25 mg (100.00–216.25), and the median CBD dose per day prescribed was 5.00 mg (0.00–10.00).
3.3. Patient reported outcome measures
The full paired results comparing PROMs at baseline to 1 month and 3 months are outlined in . Thirteen (17.11%) patients were excluded from PROM analysis due to lack of data at 1 month.
Table 3. Paired baseline and follow-up scores for all patient reported outcome measures for all participants included after 1 month and 3 months of follow-up. n = number of patients.
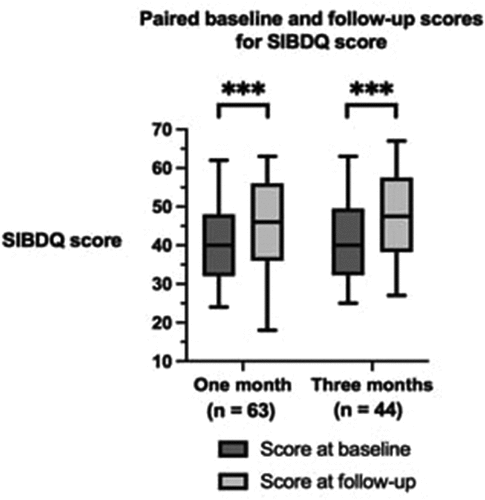
There was a statistically significant improvement in the IBD-specific HRQoL, measured by the SIBDQ score, after 1 month and 3 months of follow-up (, p < 0.001). For patients included in the analysis following 1 month of CBMP therapy, the median baseline SIBDQ score was 40.00 (IQR: 32.00–48.00) and the severity improved at follow-up (median: 46.00; IQR: 36.00–56.00; p < 0.001). For 3 months of CBMP therapy, the median baseline SIBDQ score was 40.00 (IQR: 32.25–49.50) and the SIBDQ score similarly increased at follow-up (median: 47.50; IQR: 38.25–57.50; p < 0.001).
Figure 2. Paired baseline and follow-up scores for the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) score after 1 month and 3 months of follow-up. The boxes represent the interquartile range. The horizontal line within the box represents the median value. The whiskers represent the minimum and maximum values. n = number of patients. ***p < 0.001.
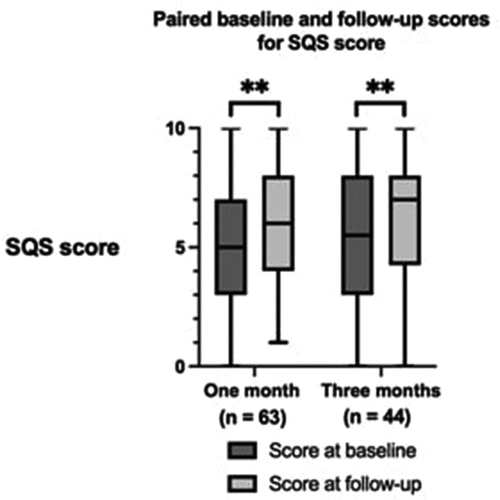
The paired baseline and follow-up scores for the GAD-7 score and SQS scores are shown in respectively. Significant improvements in generalized anxiety and depression symptoms, as measured by GAD-7, were observed after 3 months of treatment (p < 0.001). Improvements in sleep quality were also observed after 1 month and 3 months of treatment, demonstrated by improvements in the SQS score (p < 0.010).
Figure 3. Paired baseline and follow-up scores for the General Anxiety Disorder 7 (GAD-7 score) after 1 month and 3 months of follow-up. The boxes represent the interquartile range. The horizontal line within the box represents the median value. The whiskers represent the minimum and maximum values. n = number of patients. *p < 0.050; ***p < 0.001.
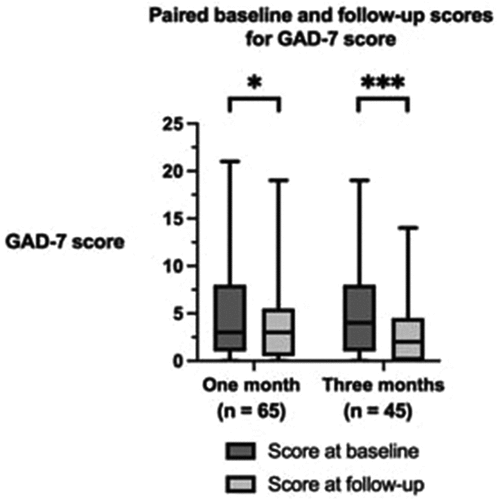
Figure 4. Paired baseline and follow-up scores for the Single-Item Sleep Quality Scale (SQS score) after 1 month and 3 months of follow-up. The boxes represent the interquartile range. The horizontal line within the box represents the median value. The whiskers represent the minimum and maximum values. n = number of patients. **p < 0.010, ***p < 0.001.
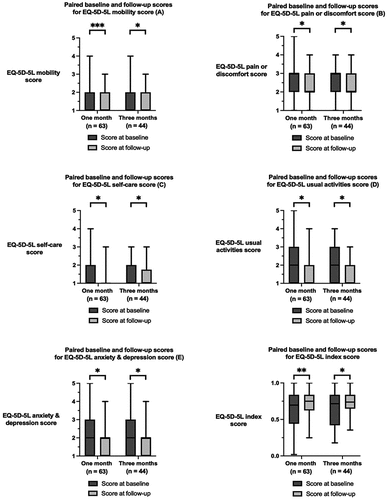
The paired baseline and follow-up scores for the general HRQoL outcomes, measured by the individual EQ-5D-5L domains and index values, are shown in . Statistically significant improvements were noted after 1 month and 3 months compared to the baseline data as measured by the EQ-5D-5L self-care score, EQ-5D-5L usual activities score, EQ-5D-5 L anxiety and depression score, and EQ-5D-5L index score (p < 0.050).
Figure 5. Paired baseline and follow-up scores for the EQ-5D-5L questionnaire scores after 1 month and 3 months of follow-up: (A) EQ-5D-5L mobility score, (B) EQ-5D-5L pain or discomfort score, (C) EQ-5D-5L self-care score, (D) EQ-5D-5L usual activities score, (E) EQ-5D-5L anxiety & depression score, (F) EQ-5D-5L index score. The boxes represent the interquartile range. *p<0.05; ** p<0.010; *** p<0.001.
The median PGIC 1 score at 1 month was 5.50 (IQR: 4.25–6.00) and at 3 months was 6.00 (IQR: 5.00–6.00). The median PGIC 2 score after 1 month was 3.00 (IQR: 2.00–4.00) and after 3 months was 2.00 (IQR: 1.00–3.00).
A subgroup analysis on PROMs comparing current/ex-users with cannabis naïve patients at baseline was performed (). Supplementary Figures S1 and S2 show the SIBDQ scores for current/ex-users and cannabis naïve patients at 1 month and 3 months, respectively. There was a greater increase in the SIBDQ score in current/ex-users compared to cannabis naïve patients at 1 and 3 months (p < 0.001). Another subgroup analysis on PROMs comparing CD and UC patients is shown in Supplementary Table S1.
Table 4. Paired baseline and follow-up scores for patient reported outcome measures for current/ex-users and cannabis naive patients after 1 month and 3 months of follow-up. n = number of patients.
3.4. Oral morphine equivalent
At baseline, 15 (19.74%) patients had recorded opioid prescriptions; however, the dosage of opioids prescribed was recorded for 12 (15.79%) patients. At baseline, the median OME of those prescribed opioid medication was 11.00 mg (IQR: 5.25–26.25). There was no significant change in OME at the end of follow-up (median = 9.00 mg; IQR: 5.25–23.00; p = 0.317).
3.5. Adverse events
The incidence of adverse events reported is outlined in and reported in brackets (%) where appropriate. A total of 122 (160.53%) adverse events were reported by 16 (21.05%) patients. The most common adverse event reported was fatigue (n = 9, 11.84%). There were 53 (69.74%) mild adverse events, 56 (73.68%) moderate adverse events, and 13 (17.11%) severe adverse events. There were no life-threatening adverse events reported by any of the study participants. The adverse events according to the cannabis status of participants at baseline are shown in Supplementary Table S2.
Table 5. Adverse events reported by study participants (n = 16). Adverse events were divided into mild, moderate, severe, and life-threatening. n = number of patients. The adverse event incidence is reported in brackets.
4. Discussion
This study evaluated the effects of CBMPs on HRQOL outcomes, frequency of adverse events and effects on reducing opioid burden in a case series of IBD patients identified from the UKMCR. The findings suggest that there was an associated improvement in IBD-specific HRQoL over a short-term evaluation period, in addition to general, anxiety-specific, and sleep-specific outcomes. CBMPs were well tolerated over the course of follow-up, with most patients (78.95%) not reporting any adverse events, whilst most adverse events were either mild or moderate. There was no change in the concomitant opioids used by the cohort over this period.
Improvements were notably seen in IBD-specific HRQoL outcomes compared to baseline, as measured by the SIBDQ scores at 1-month and 3-month follow-up (p < 0.050). In addition to an improvement in IBD-specific HRQoL, this study demonstrated significant improvements in general nonspecific HRQoL measures including the EQ-5D-5L Index, EQ-5D-5L self-care, and EQ-5D-5L usual activities scores at each follow-up (p < 0.050). This short-term benefit of CBMPs is supported by several studies demonstrating similar improvements in HRQoL [Citation36]. Whilst it is not possible to confirm causation from this observational study, recent RCTs in CD and UC patients have similarly demonstrated short-term improvements in HRQoL [Citation38,Citation63]. Moreover, in an RCT of CD patients, the CBD dose prescribed was four times higher than the THC dose [Citation38]. This contrasts the median THC dose prescribed in this study, which was over six times higher than the median CBD dose. This high THC:CBD ratio is likely due to existing evidence indicating THC is the major contributor in controlling IBD symptoms, albeit without potentially targeting intestinal inflammation specifically. CBD has been strongly attributed to the anti-inflammatory effects of CBMPs due to its effects in CB2 receptors and downstream cytokine signaling [Citation64]. However, THC has complementary effects on pain receptors [Citation65]. Although the SIBDQ score correlates with inflammatory biomarkers and is sensitive to changes in disease activity, the UKMCR does not collect objective information regarding underlying inflammation including biochemical, endoscopic, or histological data [Citation51]. Hence, the effects of CBMPs on active inflammation remain clinically inconclusive. However, this does support the further evaluation of additional preparations of CBMPs in RCTs to identify the optimum therapeutic ratio and route of administration of CBD and THC according to disease status and symptoms. Those patients who had previously consumed cannabis had a greater improvement in SIBDQ scores compared to cannabis-naïve individuals. This suggests that these patients receive supplementary benefits through accessing CBMPs, which meet regulatory standards and medical oversight compared to illicitly obtained cannabis. Moreover, the median PGIC 1 score was greater than 5 at both 1 and 3 months. A response of 5 suggests that patients felt ‘moderately better, and a slight but noticeable change [Citation59]. This adds further weight to the outcomes as assessed through the SIBDQ and EQ-5D-5L that patients experienced an increase in health-related quality of life.
The increase seen in EQ-5D-5L self-care and usual activities subscales indicates CBMP therapy was associated with a short-term functional benefit; another promising finding considering the impact of IBD on work productivity and activities of daily living [Citation66]. This contrasts with a recent study where CBMPs were given for pain relief and no improvement was observed in both subscales after 6 weeks [Citation67]. Although these clinical benefits on function were only demonstrated in the short term, this is a clinically relevant finding in patients with quiescent disease who are refractory to medical treatment; hence, these findings underline the potential utility of CBMPs for alleviation of IBD-associated symptoms not controlled by available therapies [Citation36].
Significant improvements were also observed in anxiety symptoms and sleep quality after initiation of CBMP therapy, demonstrated by improvements after 3-month follow-up in the GAD-7 and EQ-5D-5L anxiety and depression for anxiety symptoms and improved SQS score for sleep quality (p < 0.050). The improvement in anxiety symptoms is contextualized by a breadth of experimental and human studies, which have signified the anxiolytic properties of cannabinoids, especially CBD [Citation68,Citation69]. Poor sleep quality is reflective of reduced HRQOL and has a prognostic value due to its association with increased risk of disease flares and complications [Citation70,Citation71]. The improvement in sleep quality corroborates with the findings of a recent cohort study of CBMP therapy in IBD patients where similar effects on sleep quality were observed [Citation72]. Notably, this study had a longer follow-up and was based in Israel, however the somniferous effects of CBMPs were further indicated by a recent RCT in patients with chronic pain, albeit only a small benefit compared to placebo [Citation73]. The upcoming CANSLEEP trial will likely further characterize CBMP effects on sleep and daytime function [Citation74].
Based on this study and existing literature, a concern remains that the overall improvement in HRQoL associated with CBMP therapy is primarily due to the alleviation of psychiatric co-morbidities [Citation41,Citation75–77]. This emphasizes the need to objectively evaluate the effects of CBMPs on underlying IBD pathophysiology, as the relatively high dose of THC prescribed can exert strong psychotropic effects that may be the true mechanism for the observed improvements in HRQoL. However, most patients received oral/sublingual preparations, which are associated with reduced psychotropic effects compared to vaporizing cannabis as well as increasing absorption time and increased direct interaction of cannabinoids with the target site [Citation38]. The lack of clarity in this area is emphasized by Naftali et al., where significant clinical improvements were observed in UC patients enrolled in a RCT following high doses of THC therapy, however no improvements were noted in any endoscopic measures of inflammation [Citation63]. Further evidence must be garnered, as although HRQoL benefits can be observed, there are risks of complications from uncorrected prolonged intestinal inflammation, including colorectal cancer [Citation78].
The use of long-term opioids in chronic pain is increasingly controversial due to their side effects, risk of abuse, and studies not demonstrating any meaningful functional benefit [Citation79]. IBD is also an independent risk factor for heavy opioid use, with around 5% of IBD patients becoming heavy opioid users within 10 years of diagnosis [Citation80]. Despite these issues, there has been an increase in opioid prescriptions in IBD patients, which necessitates development of effective tapering interventions [Citation81]. In this study, there was no significant median oral morphine equivalent reductions following 3 months of CBMP therapy. However, the lack of reduction in OME does correlate with the lack of improvement in the EQ-5D-5L Pain and Discomfort score. Considering the short follow-up and small sample size, it was likely not feasible to detect any reduction in OME within the defined period. Moreover, the associated risk of biases in this analysis is a consistent theme for studies evaluating efficacy of interventions to reduce opioid burden; hence, the utility of CBMPs requires further investigation in this context [Citation82].
Adverse events were only reported by 21.05% of participants, and the majority of adverse events were mild to moderate in severity, with no disabling or life-threatening adverse events reported. A similarly low proportion of participants reported adverse events in a large cross-sectional Australian study of IBD patients, where there were few severe or intolerable adverse events reported after cannabis use [Citation41]. There were 122 (160.53%) adverse events reported in total. This adverse event incidence is higher compared to the findings recorded in previous analyses of the UKMCR, which has ranged from 8.8% to 39.5% [Citation48,Citation83–86]. The likely explanation for the higher incidence is due to more frequent prompting to report AEs within the UKMCR. Moreover, symptoms due to underlying disease may have been inaccurately reported as adverse events of CBMPs. This is corroborated by the most frequent adverse events being fatigue (11.84%), abdominal pain (10.53%), nausea (9.21%), and lethargy (9.21%), which are common clinical manifestations of IBD. Additionally, optic neuritis is not a known adverse event associated with CBMPs but has been shown to be more prevalent in those with IBD [Citation87]. The adverse event burden of concurrent IBD treatments may have also been captured in this analysis.
4.1. Strengths and limitations
There were several inherent limitations to the study. The lack of a placebo control group means a causality relationship cannot be demonstrated between improved HRQoL outcomes and initiation of CBMP therapy. Moreover, heterogeneity in baseline characteristics of underlying intestinal disease and medications that were simultaneously being taken increases the risk of confounding. All included participants self-funded access to CBMP therapy from the same private clinic, which introduces selection bias. However, it should be noted that a large proportion of included participants were unemployed (23.68%) indicating this selection bias was not restricted to patients with higher disposable income. However, this cohort is significantly over-represented by current/ex-cannabis users. Although illicit cannabis is common among IBD patients, 68.42% of participants were current consumers at baseline, which is higher compared to previous descriptive studies of cannabis use in the IBD population [Citation40–44]. These patients therefore may have been self-identified as responders to therapy with cannabis. Whilst all patients would be required to meet national criteria to be prescribed CBMPs, having failed to gain sufficient benefit from licensed therapies, it is possible that prior response may bias the decision of clinicians to prescribe to patients who meet these criteria [Citation47]. Moreover, as the stress of obtaining illicit cannabis is reduced by obtaining CBMPs, this also may have contributed to the greater improvement in HRQoL for current users, in addition to the improved clinical guidance and pharmaceutical quality of CBMPs. Conversely, these patients may have already developed pharmacological tolerance to the effects of THC and/or CBD, as well as experiencing a ceiling effect of the PROMs. Subgroup analysis showed that current/ex-consumers of CBMPs had a greater improvement in SIBDQ scores indicating that these patients likely had a supplementary improvement in their HRQoL after starting CBMPs under medical supervision. Further analysis of cannabis naïve populations will help to identify the true effects in this population. In addition, there is an over-representation of male participants in this analysis compared to the normal prevalence of IBD in the population. It has been shown in other studies from the UK Medical Cannabis Registry that there is a higher proportion of males who commence treatment with CBMPs for all chronic conditions, even in conditions where there is typically a higher incidence in females, such as anxiety [Citation83,Citation84]. There have previously been suggestions that there are sex-dependent effects of cannabinoids, which may have implications on therapeutic drug potency and adverse events, which may mean that the results are less applicable to female patients [Citation88]. Moreover, the limitations in sample size prevented additional subgroup analyses beyond whether patients had previous exposure to cannabis. It has been demonstrated that both patient- and medication-specific factors affect the pharmacokinetics, pharmacodynamics, and reported effects of CBMPs, which must be studied in future analyses of patients with IBD [Citation89,Citation90]. The retrospective design of the study also lends itself to recall bias due to inaccurate reporting. Another limitation was missing baseline and follow-up data leading to patients being excluded from analysis. At the time of data extraction, there was insufficient data for patients beyond three-month follow-up; hence, this study is unfortunately not able to showcase long-term effects of CBMP therapy. The number of patients at each follow-up point is noticeably lower compared to baseline; hence, there is a risk of attrition bias. Moreover, reasons for discontinuing CBMP therapy were not captured in the UKMCR. The limited sample size and follow-up also limited the examination of rare adverse events or those which develop after long-term chronic cannabis consumption, such as cannabinoid hyperemesis syndrome [Citation91].
Contrarily, the study design had notable strengths. Clinical cannabis research is still in its infancy, and this analysis has contributed to the limited evidence base assessing clinical outcomes in IBD patients. The UKMCR captures outcome data for patients across the UK and Channel Islands, making this patient population geographically diverse. The inherent heterogeneity of including both CD and UC patients in the same cohort was addressed by performing a subgroup analysis, which generally demonstrated similar improvements in both conditions. Moreover, the observational design is economical and provided real-world data collected as part of standard care.
4.2. Future directions
Future studies investigating CBMPs in IBD should ideally be conducted through robust RCTs comparing different doses of CBD and THC, as well as routes of administration. Moreover, continued longitudinal assessment of CBMPs through the UKMCR is an important facet of pharmacovigilance as prescribing becomes more common. These results, along with the discussed limitations, should hopefully inform the design of future RCTs, particularly in terms of statistical power, length of follow-up, and identifying key sources of bias. In addition to clinical and endoscopic parameters, histological remission should be assessed to effectively evaluate the effects of CBMPs on disease progression.
5. Conclusion
Initiation of CBMPs was associated with an improvement in HRQoL in the short term, with statistically significant improvements in IBD-specific and general HRQoL outcomes at 1 and 3 months after initiating treatment. Participants who previously consumed cannabis had greater improvements in HRQoL and fewer adverse events compared to naïve individuals. These findings highlight the potential utility of CBMPs as an adjunctive therapeutic option in the short term, especially in patients who continue to experience debilitating symptoms despite maximal medical therapy. However, despite statistical significance, the limitations of the study design make it difficult to draw definite conclusions to support widespread utilization. Further high-quality RCTs are needed to precisely evaluate the therapeutic efficacy and long-term safety profile of CBMPs.
Declaration of interest
S Erridge, C Holvey, R Coomber, JJ Rucker, J Hoare & MH Sodergren are the founding clinicians and current employees of Sapphire Medical Clinics, which is the first clinic registered with the CQC to evaluate patients for medical cannabis in England. MH Sodergren is also Chief Medical Officer at Curaleaf International. JJ Rucker is funded by a fellowship (CS-2017-17-007) from the National Institute for Health Research (NIHR). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conception and design of the study – N Dalavaye, S Erridge, JJ Rucker, J Hoare, MH Sodergren. Acquisition of data - N Dalavaye, S Erridge, M Nicholas, M Pillai, L Bapir, C Holvey, J Hoare. Analysis and interpretation of the data - N Dalavaye, S Erridge, J Hoare, MH Sodergren. Drafting of the paper - N Dalavaye, S Erridge, J Hoare, MH Sodergren. Critical revisions for intellectual content – N Dalavaye, S Erridge, M Nicholas, M Pillai, L Bapir, C Holvey, R Coomber, JJ Rucker, J Hoare, MH Sodergren. All authors have approved the final version to be published and agreed to be accountable for all aspects of the work.
Data availability
The data underlying this study are available from the UK Medical Cannabis Registry. Restrictions apply to the availability of these data. Data specifications and applications are available from the corresponding author.
Supplemental Material
Download MS Word (35.4 KB)Supplemental Material
Download MS Word (40.4 KB)Supplemental Material
Download JPEG Image (67.8 KB)Supplemental Material
Download JPEG Image (66.6 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17474124.2022.2161046
Additional information
Funding
References
- Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020 Jan;5(1):17–30.
- Park SH. Update on the epidemiology of inflammatory bowel disease in Asia: where are we now? Intest Res. 2022 Apr 30;20(2):159–164.
- Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007 Sep 1;1(1):10–20. Internet
- de Boer AGEM, Bennebroek Evertsz’ F, Stokkers PC, et al. Employment status, difficulties at work and quality of life in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2016 Oct;28(10):1130–1136.
- Luces C, Bodger K. Economic burden of inflammatory bowel disease: a UK perspective. Expert Rev Pharmacoecon Outcomes Res. 2006 Aug 9;6(4):471–482.
- Naegeli AN, Balkaran BL, Shan M, et al. The impact of symptom severity on the humanistic and economic burden of inflammatory bowel disease: a real-world data linkage study. Curr Med Res Opin. 2022Apr3;38(4):541–551.
- Armuzzi A, Tarallo M, Lucas J, et al. The association between disease activity and patient-reported outcomes in patients with moderate-to-severe ulcerative colitis in the United States and Europe. BMC Gastroenterol. 2020 Dec 21;20(1):18.
- Cai Z, Wang S, Li J. Treatment of inflammatory bowel disease: a comprehensive review. Front Med (Lausanne). 2021 Dec 20;8:2681.
- Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol. 2021Oct1;19(10):2031–2045.e11.
- Peyrin-Biroulet L, Danese S, Argollo M, et al. Loss of response to vedolizumab and ability of dose intensification to restore response in patients with crohn’s disease or ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019 Apr;17(5):838–846.e2.
- Barberio B, Savarino EV, Card T, et al. Incidence comparison of adverse events in patients with inflammatory bowel disease receiving different biologic agents: retrospective long-term evaluation. Intest Res. 2022 Jan 30;20(1):114–123.
- Shahbazi F, Grandi V, Banerjee A, et al. Cannabinoids and cannabinoid receptors: the story so far. iScience. 2020 Jul;23(7):101301.
- Elmes MW, Kaczocha M, Berger WT, et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. 2015 Apr;290(14):8711–8721.
- Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015 May;36(5):277–296.
- Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med. 2005 Dec 26;83(12):944–954.
- Hryhorowicz S, Kaczmarek-Ryś M, Zielińska A, et al. Endocannabinoid system as a promising therapeutic target in inflammatory bowel disease – a systematic review. Front Immunol. 2021 Dec 22;12. 10.3389/fimmu.2021.790803
- Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Investig. 2004 Apr 15;113(8):1202–1209.
- Kimball ES, Schneider CR, Wallace NH, et al. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol. 2006 Aug;291(2):G364–71.
- Storr MA, Keenan CM, Zhang H, et al. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009 Nov;15(11):1678–1685.
- Kapellos TS, Taylor L, Feuerborn A, et al. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB J. 2019 May 25;33(5):6154–6167.
- Klein TW, Newton CA, Nakachi N, et al. Δ9-tetrahydrocannabinol treatment suppresses immunity and early IFN-γ, IL-12, and IL-12 receptor β2 responses to legionella pneumophila infection. J Immunol Internet]. 2000;164(12):6461–6466 [cited 2022 Aug 1]. https://www.jimmunol.org/content/164/12/6461.
- Jean-Gilles L, Gran B, Constantinescu CS. Interaction between cytokines, cannabinoids and the nervous system. Immunobiology. 2010 Aug;215(8):606–610.
- Henshaw FR, Dewsbury LS, Lim CK, et al. The effects of cannabinoids on pro- and anti-inflammatory cytokines: a systematic review of in vivo studies. Cannabis Cannabinoid Res. 2021 Jun 1;6(3):177–195.
- Hegde VL, Hegde S, Cravatt BF, et al. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory t cells. Mol Pharmacol. 2008 Jul;74(1):20–33.
- Rieder SA, Chauhan A, Singh U, et al. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. 2010 Aug;215(8):598–605.
- Aviello G, Romano B, Izzo A. Cannabinoids and gastrointestinal motility: animal and human studies. Eur Rev Med Pharmacol Sci. 2008 Sep 1;12 Suppl 1:81–93.
- Izzo AA, Fezza F, Capasso R, et al. Cannabinoid CB 1 -receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001 Oct;134(3):563–570.
- Izzo AA. Cannabinoids and intestinal motility: welcome to CB2 receptors. Br J Pharmacol. 2004 Aug 1;142(8):1201–1202. Internet
- Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motility. 2006 Oct 1;18(10):949–956. Internet
- Kikuchi A, Ohashi K, Sugie Y, et al. Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF-03550096, In vitro and in vivo by using a rat model of visceral hypersensitivity. J Pharmacol Sci. 2008;106(2):219–224.
- Manzanares J, Julian M, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006 Jul 1;4(3):239–257.
- Lötsch J, Weyer-Menkhoff I, Tegeder I. Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur J Pain. 2018 Mar;22(3):471–484.
- O’Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016 Jun;173(12):1899–1910.
- Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol. 2011 Aug;163(7):1411–1422.
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005 May 26;8(5):585–589.
- Doeve BH, van de Meeberg MM, van Schaik FDM, et al. A systematic review with meta-analysis of the efficacy of cannabis and cannabinoids for inflammatory bowel disease: what can we learn from randomized and nonrandomized studies? J Clin Gastroenterol. 2021;55(9):798–809. [cited 2022 Aug 1]. Available from: https://journals.lww.com/jcge/Fulltext/2021/10000/A_Systematic_Review_With_Meta_Analysis_of_the.12.aspx
- Best WR, Becktel JM, Singleton JW, et al. Development of a crohn’s disease activity index. Gastroenterology. 1976 Mar;70(3):439–444.
- Naftali T, Bar-Lev Schleider L, Almog S, et al. Oral CBD-rich cannabis induces clinical but not endoscopic response in patients with crohn’s disease, a randomised controlled trial. J Crohns Colitis. 2021 Nov 8;15(11):1799–1806.
- Ghosh N, Premchand P. A UK cost of care model for inflammatory bowel disease. Frontline Gastroenterol. Internet]. 2015 Feb 24. 2015Jul;6(3):169–174. https://pubmed.ncbi.nlm.nih.gov/28839807
- Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011 Oct;23(10):891–896.
- Benson MJ, Abelev SV, Connor SJ, et al. Medicinal cannabis for inflammatory bowel disease: a survey of perspectives, experiences, and current use in Australian patients. Crohns Colitis 360. 2020 Apr 1;2(2):1–25.
- Merker AM, Riaz M, Friedman S, et al. Legalization of medicinal marijuana has minimal impact on use patterns in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2018 Oct 12;24(11):2309–2314.
- Ravikoff Allegretti J, Courtwright A, Lucci M, et al. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013 Dec;19(13):2809–2814.
- Cruz-Cruz J, Muñiz L, Negron F, et al. 702 knowledge, perception, and use of cannabis therapy in patients with inflammatory bowel disease. Am J Gastroenterol. 2019 Oct;114(1):S413–S413.
- Appleton K, Whittaker E, Cohen Z, et al. Attitudes towards and use of cannabis in New Zealand patients with inflammatory bowel disease: an exploratory study. N Z Med J. 2021 Feb 19;134:38–47.
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) Statement: guidelines for reporting observational studies. Ann Intern Med. 2007 Oct 16;147(8):573.
- Case P. The NICE guideline on medicinal cannabis: keeping pandora’s box shut tight? Med Law Rev. 2020 May 1;28(2):401–411.
- Erridge S, Salazar O, Kawka M, et al. An initial analysis of the UK medical cannabis registry: outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. Internet]. 2021 14 May. 2021Sep;41(3):362–370. Available from: https://pubmed.ncbi.nlm.nih.gov/33988306
- Irvine EJ, Zhou Q, Thompson AK. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn’s relapse prevention trial. Am J Gastroenterol. 1996;91(8):1571–1578.
- Jowett SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001 Oct;96(10):2921–2928.
- Reggev S, Goran G, Sarid O, et al. P267 Validation of the short inflammatory bowel disease questionnaire as an outcome measure in Crohn’s disease patients. J Crohns Colitis. 2020 Jan 15;14:S283–S283.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006 May 22;166(10):1092.
- Devlin NJ, Brooks R. EQ-5D and the euroqol group: past, present and future. Appl Health Econ Health Policy. 2017 Apr 13;15(2):127–137.
- van Hout B, Janssen MF, Feng YS, et al. Interim Scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L Value Sets. Value Health. 2012 Jul;15(5):708–715.
- Snyder E, Cai B, DeMuro C, et al. A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018 Nov 15;14(11):1849–1857.
- Wang L, Hong PJ, May C, et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic review and meta-analysis of randomised clinical trials. BMJ. 2021 Sep 8;n1034.
- Kuhathasan N, Dufort A, MacKillop J, et al. The use of cannabinoids for sleep: a critical review on clinical trials. Exp Clin Psychopharmacol. 2019 Aug;27(4):383–401.
- Sznitman SR, Vulfsons S, Meiri D, et al. Medical cannabis and insomnia in older adults with chronic pain: a cross-sectional study. BMJ Support Palliat Care. 2020 Dec;10(4):415–420.
- Scott W, McCracken LM. Patients’ impression of change following treatment for chronic pain: global, specific, a single dimension, or many? J Pain. 2015 Jun;16(6):518–526.
- US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0. Natl Cancer Inst. 2009 May 28;4(3)
- Donnelly K. SNOMED-CT: the advanced terminology and coding system for eHealth. Stud Health Technol Inform. 2006;121:279–290.
- BNF. Prescribing in palliative care [cited 2022 Aug 1]. https://bnf.nice.org.uk/guidance/prescribing-in-palliative-care.html.
- Naftali T, Bar-Lev Schleider L, Scklerovsky Benjaminov F, et al. Cannabis is associated with clinical but not endoscopic remission in ulcerative colitis: a randomized controlled trial. PLoS One. 2021 Feb 11;16(2):e0246871.
- Nagarkatti P, Pandey R, Rieder SA, et al. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009 Oct;1(7):1333–1349.
- Anand U, Oldfield C, Pacchetti B, et al. Dose-related inhibition of capsaicin responses by cannabinoids CBG, CBD, THC and their combination in cultured sensory neurons. J Pain Res. 2021 Nov;14:3603–3614.
- Parra RS, Chebli JM, Amarante HM, et al. Quality of life, work productivity impairment and healthcare resources in inflammatory bowel diseases in Brazil. World J Gastroenterol. 2019 Oct 14;25(38):5862–5882.
- Peterson AM, Le C, Dautrich T. Measuring the change in health-related quality of life in patients using marijuana for pain relief. Med Cannabis Cannabinoids. 2021 Aug 12;4(2):114–120.
- Silczuk A, Smułek D, Kołodziej M, et al. The construct of medical and non-medical marijuana-critical review. Int J Environ Res Public Health. Internet]. 2022 Feb 27;19(5):2769. https://pubmed.ncbi.nlm.nih.gov/35270462
- Wright M, Di Ciano P, Brands B. Use of cannabidiol for the treatment of anxiety: a short synthesis of pre-clinical and clinical evidence. Cannabis Cannabinoid Res. 2020 Sep 1;5(3):191–196.
- Sofia MA, Lipowska AM, Zmeter N, et al. Poor sleep quality in crohn’s disease is associated with disease activity and risk for hospitalization or surgery. Inflamm Bowel Dis. 2019 Dec 10. 10.1093/ibd/izz258.
- Ballesio A, Zagaria A, Baccini F, et al. A meta-analysis on sleep quality in inflammatory bowel disease. Sleep Med Rev. 2021 Dec;60:101518.
- Hirsch A, Seidenberg C, Fliss Isakov N, et al. P559 cannabis improves sleep quality in patients with inflammatory bowel diseases in a prospective observational study. J Crohns Colitis. 2022 Jan 21;16(Supplement_1):i503–i503.
- AminiLari M, Wang L, Neumark S, et al. Medical cannabis and cannabinoids for impaired sleep: a systematic review and meta-analysis of randomized clinical trials. Sleep. 2022 Feb 14;45(2). 10.1093/sleep/zsab234.
- Suraev A, Grunstein RR, Marshall NS, et al. Cannabidiol (CBD) and Δ9 -tetrahydrocannabinol (THC) for chronic insomnia disorder (‘CANSLEEP’ trial): protocol for a randomised, placebo-controlled, double-blinded, proof-of-concept trial. BMJ Open. 2020 May 18;10(5):e034421.
- Farbod Y, Popov J, Armstrong D, et al. Reduction in anxiety and depression scores associated with improvement in quality of life in patients with inflammatory bowel disease. J Can Assoc Gastroenterol. 2022 Feb 1;5(1):12–17.
- Sweeney L, Moss-Morris R, Czuber-Dochan W, et al. Pain management in inflammatory bowel disease: feasibility of an online therapist-supported CBT-based self-management intervention. Pilot Feasibility Stud. 2021 Dec 16;7(1):95.
- Blågestad T, Pallesen S, Grønli J, et al. How perceived pain influence sleep and mood more than the reverse: a novel, exploratory study with patients awaiting total hip arthroplasty. Front Psychol. 2016 Oct 28;7. 10.3389/fpsyg.2016.01689
- Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:3.
- Zielińska A, Sałaga M, Włodarczyk M, et al. Focus on current and future management possibilities in inflammatory bowel disease-related chronic pain. Int J Colorectal Dis. 2019 Feb 19;34(2):217–227.
- Targownik LE, Nugent Z, Singh H, et al. The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am J Gastroenterol. 2014 Oct;109(10):1613–1620.
- Burr NE, Smith C, West R, et al. Increasing prescription of opiates and mortality in patients with inflammatory bowel diseases in England. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534–41.e6.
- Avery N, McNeilage AG, Stanaway F, et al. Efficacy of interventions to reduce long term opioid treatment for chronic non-cancer pain: systematic review and meta-analysis. BMJ. 2022 Apr 4:e066375. doi10.1136/bmj-2021-066375.
- Ergisi M, Erridge S, Harris M, et al. An updated analysis of clinical outcome measures across patients from the UK medical cannabis registry. Cannabis Cannabinoid Res. 2022 Jan 24; 10.1089/can.2021.0145
- Ergisi M, Erridge S, Harris M, et al. UK medical cannabis registry: an analysis of clinical outcomes of medicinal cannabis therapy for generalized anxiety disorder. Expert Rev Clin Pharmacol. 2022 Jan 18;15(4):487–495.
- Harris M, Erridge S, Ergisi M, et al. UK medical cannabis registry: an analysis of clinical outcomes of medicinal cannabis therapy for chronic pain conditions. Expert Rev Clin Pharmacol. 2021 Dec 31;14:1–13.
- Nimalan D, Kawka M, Erridge S, et al. UK Medical cannabis registry palliative care patients cohort: initial experience and outcomes. J Cannabis Res. 2022 Dec 4;4(1):3.
- Hsieh YH, Chung CH, Sun CA, et al. Association between optic neuritis and inflammatory bowel disease: a population-based study. J Clin Med. 2021 Feb 10;10(4):688.
- Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology. 2018 Jan 17;43(1):34–51.
- Zamarripa CA, Vandrey R, Spindle TR. Factors that impact the pharmacokinetic and pharmacodynamic effects of cannabis: a review of human laboratory studies. Curr Addict Rep. 2022 Jul 26;9:608–621.
- de la Fuente A, Zamberlan F, Sánchez Ferrán A, et al. Relationship among subjective responses, flavor, and chemical composition across more than 800 commercial cannabis varieties. J Cannabis Res. 2020 Dec 17;2(1):21.
- Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome. South Med J. 2011 Sep;104(9):659–664.
