ABSTRACT
Introduction
Children who require enteral nutrition often report gastrointestinal symptoms. There is a growing interest in nutrition formulas that meet nutritional requirements and also maintain gut ecology and function. Fiber-containing enteral formulas can improve bowel function, promote the growth of healthy gut microbiota, and improve immune homeostasis. Nonetheless, guidance in clinical practice is lacking.
Areas covered
This expert opinion article summarizes the available literature and collects the opinion of eight experts on the importance and use of fiber-containing enteral formulas in pediatrics. The present review was supported by a bibliographical literature search on Medline via PubMed to collect the most relevant articles.
Expert opinion
The current evidence supports using fibers in enteral formulas as first-line nutrition therapy. Dietary fibers should be considered for all patients receiving enteral nutrition and can be slowly introduced from six months of age. Fiber properties that define the functional/physiological properties of the fiber must be considered. Clinicians should balance the dose of fiber with tolerability and feasibility. Introducing fiber-containing enteral formulas should be considered when initiating tube feeding. Dietary fiber should be introduced gradually, especially in fiber-naïve children, with an individualized symptom-based approach. Patients should continue with the fiber-containing enteral formulas they tolerate best.
1. Introduction
Dietary fiber is an essential component of the human diet and a major determinant of digestive health [Citation1]. Beyond the bulking capacity of fiber which facilitates bowel movements, fermentation of dietary fiber by the gut microbiota produces a wide range of compounds, such as short-chain fatty acids (SCFA), which can have benefits beyond the gastrointestinal (GI) system, including metabolic and immune functions [Citation2–7]. Thus, dietary fiber has gained much attention over the past few decades as an important component of nutritional support, including enteral nutrition (EN). Fiber is generally considered to be highly beneficial, but there are some specific settings where it may be detrimental, so its use in any individual requires judgment.
EN is a commonly utilized method of nutrition support in infants and children who cannot meet their nutritional requirements orally. It is used in in-patient and outpatient settings in various disease states. In pediatric patients, it is important to ensure that EN meets dietary requirements for growth and development with a tolerable safety profile [Citation8]. Nonetheless, adverse events are common with EN, including diarrhea and constipation, which can cause distress, intolerance, undernutrition, and fluid/electrolyte imbalance [Citation9–11]. Therefore, fiber-containing formulas have been investigated for their potential positive effects on gut microbiota and intestinal function. There is growing evidence of the nutritional benefits of fiber-containing formulas and their tolerance in children receiving EN [Citation12].
While the health benefits of dietary fiber are well established, concerns remain regarding the tolerance of pediatric patients when adding fiber to EN formulas [Citation13,Citation14]. Long-term use of fiber-containing enteral formulas has not yet been well-studied in the literature, and the role of fiber in improving GI symptoms in tube-fed patients is not well-characterized [Citation10]. The result is a lack of guidance on the appropriate use of fiber-containing enteral formulas in pediatrics, with variability between international guidelines [Citation10,Citation15,Citation16]. Thus, this article aims to collect the opinions of experts on the importance of dietary fiber in pediatric EN based on available literature and clinical and research experience. The experts share their insights and opinions on the target patients for fiber-containing enteral formulas, types, and amounts of fiber to be considered for pediatric EN, and the appropriate nutritional approaches in EN therapy.
2. Review development
Eight experts attended an expert meeting ahead of the 54th Annual Meeting of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) in Copenhagen to discuss the benefits and clinical applications of fiber-containing formulas in pediatric EN. Comments and feedback were collected from the experts to develop the present expert review and were supported by a bibliographical literature search on Medline via PubMed to collect the most relevant articles. The keywords used in the literature search were: (fiber-fortified OR fiber-fortified OR fibrous) AND (enteral nutrition OR tube feeding) AND (pediatric OR children). The manual screening of relevant references complemented the online bibliographic search.
3. Benefits of dietary fiber and dietary recommendations for children
The definition of ‘fiber’ varies across countries and international organizations, and it has been challenging to get a consensus on a single definition due to differences in biological, chemical, and physiological characteristics. The Institute of Medicine (IOM) defines ‘dietary fiber’ as ‘nondigestible carbohydrates and lignin that are intrinsic and intact in plants.’ At the same time, ‘functional fiber’ consists of ‘isolated, nondigestible carbohydrates that have beneficial physiological effects in humans,’ with total fiber being the sum of nonfunctional fiber and functional fiber [Citation17]. Such definition encompasses natural and synthetic fibers, generally demonstrating positive human physiological benefits. In 2009, the codex Alimentarius commission defined dietary fiber as ‘carbohydrate polymers with three or more monomeric units, which are neither digested nor absorbed in the small intestine.’ This includes edible carbohydrate polymers naturally occurring in the food, carbohydrate polymers obtained from food raw material by physical, enzymatic, or chemical means, and synthetic carbohydrate polymers which have been shown to have a physiological effect of benefit to health [Citation18]. The European Food Safety Authority (EFSA) defines the dietary fiber as ‘nondigestible carbohydrates plus lignin’ [Citation19].
shows the potential physiological benefits of dietary fiber in humans. Increasing fecal bulk and promoting defecation frequency are two of the main advantages of insoluble fibers [Citation20]. Some fermentable fibers work as prebiotics, boosting populations of ‘beneficial bacteria like bifidobacteria and lactobacilli in the colon [Citation21]. Dietary fibers have been shown to influence insulin secretion and satiety via their effects on a broad range of gut hormones known as incretins [Citation22]. Certain fibers can bind bile acids and prevent their development into micelles, increasing the excretion of bile acids and cholesterol in the stool [Citation23]. Improved cognition in prepubertal children, weight control, and prevention of constipation were also reported as potential long-term health benefits of dietary fiber [Citation6,Citation24]. Patients with constipation can benefit from fiber supplements, high-fiber cereals, and wheat bran that help normalize bowel function [Citation25].
Figure 1. The potential physiological benefits of dietary fiber in humans. SCFA, Short-chain fatty acid.
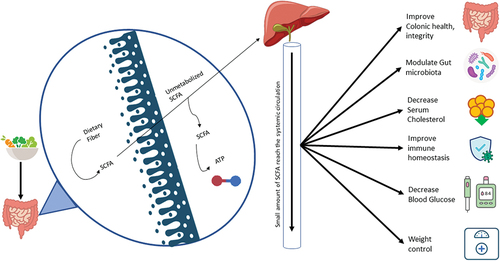
The density, variety, structure and metabolic activity of the gut microbiota are all significantly affected by the consumed diet. Microbiota composition is linked to long-term dietary habits, particularly the consumption of protein, animal fat, carbohydrates, or plant-based meals [Citation26]. High fiber intake and low refined food consumption may enhance microbiota diversity. As seen in the Western world, reduced fiber intake over the long term may lead to the irreversible elimination of essential microbial species, as shown in rodents [Citation27]. In humans, dietary habit modifications in the course of urbanization play a role in shaping gut microbiota, and fiber-degrading bacteria are at risk of being eliminated by the fast-paced globalization of foods and by the advent of a westernized lifestyle [Citation28–30]. Gut microbiota populations adapt within 24 hours to substantial alterations in macronutrient intake, although more sustained changes in diet are needed to sustain changes in composition [Citation31].
Lack of carbohydrates over time significantly decreased the amount of fiber-degrading bacteria, while the quantity of Streptococcus, Eggerrthella, and Lactococcus rose, resulting in reduced levels of SCFAs [Citation32]. SCFAs are of crucial physiological importance within the gut providing energy requirements for enterocytes and regulating intestinal permeability and peristalsis [Citation33]. In addition, SCFAs reaching the circulation exerts favorable systemic effects on the immune system, including boosting T cell development and associated immunity and immunological tolerance [Citation34,Citation35] and having potential advantages in preventing or reducing autoimmune diseases, including allergic disease, inflammatory gastrointestinal disorders, inflammatory arthritis, and diabetes [Citation36,Citation37].
For example, fiber supplements or high-fiber foods are suggested for many gut disorders, including in adult hemorrhoids, constipation, diverticular disease, irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), duodenal ulcers, and gastroesophageal reflux disease (GERD) [Citation38,Citation39]. Patients with constipation or hemorrhoids can benefit from fiber supplements, high-fiber cereals, wheat bran, and increased dietary fiber intake. Hemorrhoid treatment and prevention may be aided by a diet higher in fiber [Citation40]. Consuming high amounts of dietary fiber has been linked to preventative, ameliorative, and protective effects against the recurrence of diverticular disease [Citation41,Citation42]. For IBD, ulcerative colitis and Crohn’s disease (CD), fiber diet was associated with reduced risk of IBD of CD [Citation43], improved quality of life, reduced Serum amyloid A, and reduced serum level of C-reactive protein in UC patients [Citation44]. Previous reports suggested that dietary fiber in meals or supplements, together with the sympathetic support of a primary care practitioner, may effectively relieve symptoms of IBS [Citation45]. Although the evidence is scant, GERD and duodenal ulcer disease may be prevented by consuming guar gum and other soluble fibers [Citation46]. A previous report demonstrated that a fiber-enriched diet led to a significant decrease in heartburn frequency per week, a decrease in the number of GERs, and an increase in minimal lower esophageal sphincter resting pressure [Citation47].
In children fiber-containing EN formulas are not routinely used for patients who need nutritional support and have a normal gut function. In contrast, they are frequently reserved for managing some specific GI conditions, including functional disorders (FGIDs); however, there is a lack of large, controlled trials. Studies showed that fibers benefit children with FGIDs with no distinction between fermentable and/or bulking fiber [Citation1]. In functional constipation, the ESPGHAN and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) recommend a normal fiber intake with no indication of the type of fiber that might benefit these children [Citation48]. A systematic review of 10 studies, including 728 children, and one follow-up study, including 80 children, compared the effect of seven different mixtures of fibers, prebiotics, and/or infant formulas with placebo or control treatment. The results showed evidence that specific fibers or prebiotic supplements may be more effective than placebo or as effective as a laxative treatment [Citation49]. In pediatric short bowel syndrome (SBS) and in critical clinical conditions, such as oncological patients who need chemotherapy, adding fiber to enteral feedings is a treatment method to manage increased stool output. However, there are no standardized recommendations on using fiber in these settings, including type, dosage, titration strategies, etc [Citation50].
In children with a normal gut function who require nutritional support both orally or by tube feeding, benefits of fibers in enteral formulas have been shown in clinical studies, including decreased diarrhea, lower stool pH, improved bowel frequency and improved fecal microbiota profile [Citation1].
Beyond the digestive systems, a meta-analysis found that an increase in the dietary fiber of 7 g per day was associated with a significant reduction in the risk of diabetes (6% risk reduction; p = 0.001), rectal cancer (9% risk reduction; p = 0.007), colorectal cancer (8% risk reduction; p = 0.02), ischemic and hemorrhagic stroke (7% risk reduction; p = 0.002), and cardiovascular disease (9% risk reduction; p < 0.001) [Citation51]. Daily fiber consumption between 25 g and 29 g was linked with a 15% risk reduction in all-cause mortality, according to a 2019 meta-analysis [Citation52].
Recommendations for daily fiber intake for healthy children vary across international societies. They have been expressed in various ways, either as a function of energy intake (g/1000 Kcal), in grams per day, or in grams per kilogram of body weight. Most of these intake recommendations are based on scientific evidence of the relationship between dietary fiber intake and adult health outcomes. Williams et al. (1995) suggested that children older than two years should consume at least the amount of fiber equivalent to their age plus 5 g/day, up to a maximum of 10 g/day [Citation53]. The Institute of Medicine (IOM) has advocated that healthy adults receive 14 g of fiber for every 1,000 calories they consume [Citation17]. Extrapolating this recommendation to children would mean that children aged 1–3 years should receive an intake of fiber of 19 g/day, while children aged 4–8 years should receive 25 g/day [Citation54]. The EFSA has set lower recommended daily fiber intake for children, ranging between 8.4 to 10.4 g of fiber per 1000 kcal (2 to 2.5 g per MJ) [Citation19]. In Australia, fiber intake has been derived from National Dietary Surveys for different age groups [Citation55]. summarizes the dietary fiber reference values established by the main international societies. However, it is worth noting that the current guidelines for daily fiber intake refer to total fiber regardless of the source or fiber quality provided. This is critical since consuming fiber from various types and sources results in various functional and physiological impacts on the human body, as not all fibers are the same [Citation56].
Table 1. Dietary fiber recommendations for children.
4. Fiber-contained enteral formulas and different types of fiber used in enteral products
4.1. Physiochemical properties of different types of dietary fiber used in enteral products
The chemical and physical properties of fibers can vary depending on their origin ().
Table 2. Physicochemical Characteristics of different types of fibers used in EN products.
Historically, fiber was classified into a soluble and insoluble fiber. Although solubility was previously used to categorize dietary fiber, viscosity and fermentability may be more important concerning specific physiological effects, and so nutrition and health [Citation57]. Fibers that are fermentable by colonic bacteria can be broken down into energy, while fibers with gel-forming properties are known as viscous fibers. Fiber fermentability depends on how much it can be metabolized by gut microbiota. This is determined by the physical and chemical characteristics of fiber that affect bacteria accessibility. Compared to insoluble fiber, soluble fiber is often more fermented and has a higher viscosity. Bulking fibers are predominantly insoluble and poorly fermentable, while certain soluble fibers (such as partially hydrolyzed guar gum [PHGG] and acacia gum) are not viscous [Citation58]. However, there are some exceptions to this; for example, insoluble soy polysaccharides may be well fermented, and soluble fibers such as oat bran and psyllium can increase stool mass [Citation59]. Of note, nutrition labeling still distinguishes between insoluble fiber and soluble fiber.
More recently, it has been suggested that binding, structural, and transport barriers are more important classifiers of fiber and should be used to discriminate between different fiber types due to their direct association with dietary fiber outcomes [Citation41]. In this classification, it is suggested that fiber mass structure may play a role in determining the rate of food digestion and absorption. In addition, the molecular binding of fiber with other micronutrients, enzymes, or bacteria can affect fiber digestion, passage, and fermentation. At the same time, transport barriers can restrict molecular transport. In turn, the authors proposed these three physicochemical properties – binding, structuring, and transport barriers – as the most important determinants of dietary fiber functionality [Citation60].
Several types of dietary fiber are used in enteral products. Soy polysaccharides are a fiber source obtained from soy cotyledon and consist of several fiber components, including cellulose, hemicelluloses, lignin, and pectin-like molecules. PHGG is a nonviscous soluble fiber that is obtained from guar gum through partial enzymatic hydrolysis. Although the viscosity of PHGG is minimal compared to its source, it appears to retain the lowering effect on glucose and insulin levels in healthy and diabetic subjects [Citation61,Citation62]. Inulin-type fructans, which include fructooligosaccharides (FOS), oligofructose (OF), and inulin, are frequently included in enteral formulas with GI and immunological benefits but have also been linked to pro-inflammatory effects in pediatric inflammatory bowel diseases [Citation63]. Acacia gum (AG) is a soluble fiber that has gained popularity due to its prebiotic properties and high tolerance levels. Human studies have demonstrated that 3 g of AG per day is sufficient to promote the development of bifidobacteria when paired with the same amount of FOS [Citation64].
Starch and starch breakdown products, which are not absorbed in healthy people’s small bowel and move to the colon, are called resistant starch (RS) [Citation65]. During fermentation, RS produces more butyrate and less acetate than most other fibers, enhancing the proliferation of bifidobacteria [Citation66]. Cellulose is another insoluble fiber composed of glucose polymers and effectively produces bulk stool and suppresses osmotic diarrhea [Citation67]. Field pea hulls are the source of insoluble outer pea fiber, which comprises hemicellulose, cellulose, and pectic materials. Pea fiber is mainly used to increase the amount of fiber in products without changing their functional or technical features, and it makes healthy adults have heavier stools [Citation68].
4.2. Commercially available fiber-containing formulas
shows several commercially available fiber-containing formulas with different types and sources of fiber. Several technical points should be considered when adding fiber into formulas:
Viscosity: Fiber may add viscosity to the formula, which can affect the formula’s flowability. These considerations are especially important in the pediatric population whose feeding tube size is small.
Dose: The nutritional composition of formulas aims to address the needs of children of different ages. Often, the different nutritional needs of children at different ages are addressed by providing different volumes of the same formula. This practice may affect the amount of fibers added to the formula to limit tolerance issues in higher volume consumption.
Personalization: Some healthcare providers may prefer to choose the type and amount of fiber according to each child’s specific needs. It is possible to add fiber as needed, but mixing an external source of fiber into a formula may cause clumps or affect the formula’s characteristics, such as viscosity.
Table 3. Examples of fibers in commercially available fiber-containing formulas.
4.3. Use of fiber blends in pediatric EN
Current enteral formulas include different types and doses of fiber in polymeric and semi-elemental formulas, which makes comparing their effect difficult. Furthermore, the fiber content of some currently available enteral formulas may be too low compared to estimated fiber requirements resulting in low fiber intake [Citation69]. While some formulas provide a mixture of fibers, others include soluble fiber. In a regular diet, most fiber-containing foods contain around one-third soluble and two-thirds insoluble fiber. In recent years, there has been a growing interest in using blenderized feeds and commercial real food-based formulas. These formulas include a mixture of food ingredients (such as peas, green beans, peaches, etc.), which provide a fiber blend that mimics a normal diet [Citation70].
Despite the beneficial effects of fiber overall, fiber-containing formulas are not routinely used in clinical practice. According to a home enteral nutrition survey in 2005, only 7% of the commercially available enteral preparations used were fiber-supplemented in the pediatric population [Citation71]. In another home enteral nutrition survey conducted in Poland in 2014, including 456 patients (142 children and 314 adults), fiber-rich diets were used in only 9% of cases [Citation72]. A recent Polish nationwide home enteral nutrition survey performed on adults in 2018 found that about 17% of enteral formulas used were supplemented with fiber [Citation55].
4.4. Side effects of dietary fiber for children receiving EN
Overall, fiber-containing formulas are generally well-tolerated; however, dietary fiber may be associated with some side effects in specific cases. High fiber intake can cause flatulence and abdominal distension, especially when introduced rapidly in subjects naïve or consuming low amounts of fiber [Citation73]. Previous reports showed that higher fiber intake may lead to constipation, similar to what is observed with lower intake [Citation74]. However, such findings may be attributed to the various underlying etiology of constipation rather than an actual impact of high fiber intake [Citation75]. Certain types of fiber can have a pro-inflammatory role during active inflammation. In a recent report, β-fructan induced pro-inflammatory response in some patients with active inflammatory bowel disease, highlighting a potential detrimental effect of some fibers during inflammation [Citation63].
5. Clinical evidence on the use of fiber-containing enteral formulas in children
The benefits of fiber-containing EN formulas on gut function have been the focus of several clinical studies. Some studies tested isolated fibers, such as PHGG, while others tested specific blends of fiber for a few weeks to six months. Clinical studies on fiber covered a range of pediatric disorders, including children with neurological impairment, cystic fibrosis, cardiac disease, liver transplant, bone marrow transplant, cancer, and growth failure. However, the sample size was generally small in most studies. Overall, the evidence supports the safety and tolerability of fiber across different age groups [Citation59,Citation69,Citation76,Citation77]. Although more evidence is available in adults, extrapolating findings from adult studies in children with developing gastrointestinal systems is difficult, as fiber may have different actions on the gut and health in children compared to adults.
In a randomized crossover trial, a fiber blend of FOS (3.5 g/day) and pea fiber (3.8 g/day) for two weeks improved stool consistency and reduced the proportion of hard and watery stool in children with compromised gut function [Citation78]. Evans et al. studied the effects of an enteral formula including six fibers (soy polysaccharide, cellulose, AG, FOS, inulin, and RS; a total of 11.2 g/L) during six months in 25 tube-fed children with a range of medical conditions. They found evidence of reduced constipation, less need for laxatives, and decreased abdominal pain on the fiber-containing formula compared to the fiber-free formula [Citation69]. A similar finding was reported in 45 children with chronic illness who received fiber supplementation in pediatric sip feeds at 20 g/L for 12 weeks [Citation79]. Compared to fiber-free sip feeds, laxative usage decreased while GI tolerance, anthropometry, and nutritional biochemistry were comparable for both groups. A fiber blend of five fibers (oat, soy polysaccharide, acacia gum, carboxymethylcellulose, and FOS; 25% soluble) was investigated in a randomized controlled trial in tube-fed children for three weeks. Significant improvement in GI symptoms scores was found despite the non-significant changes in stool consistency [Citation80]. Data on the use of fiber in critically ill children is lacking. A previous randomized trial on critically ill children showed that a synbiotic blend-containing enteral formula was well tolerated and increased the beneficial fecal bacterial groups [Citation9].
Concerning the effect of dietary fiber on gut microbiota, a prospective study on 67 pediatric cancer patients showed that the 70:30 blend of FOS and inulin led to a significant increase in the fecal Lactobacilli level after one month of supplementation of 1.2 g/day [Citation81]. Supplementation of the same formula at 2.5 g/day for three weeks significantly increased the fecal bifidobacterial levels, with a trend toward improved fecal Lactobacilli levels [Citation82]. In a randomized crossover design, a six-fiber formula for three months increased fecal Bifidobacterium levels for 12 weeks compared to the fiber-free formula [Citation77]. Notably, a prospective study over ten weeks found that the 70:30 blend (1.7 g/day) supplementation was associated with improved IgG antibody response and growth [Citation83]. In an observational study by Kansu et al., high-fiber EN formulas significantly improved the anthropometric parameters, with a well-tolerable safety profile, in children with growth failure [Citation12]. Finally, previous studies suggested that fiber may affect the bioavailability of some micronutrients, such as zinc [Citation84]; however other studies do not support these findings [Citation69,Citation79].
Although the clinical benefits and tolerability of fiber-containing EN formulas are consistently supported in the literature, well-designed studies with larger sample sizes are still needed in specific patient populations of children receiving EN. In adults, higher intakes of dietary fiber have been linked to a reduced risk of chronic diseases such as cardiovascular disease, type 2 diabetes, obesity, and cancer [Citation1]. The evidence is lacking to confirm these benefits in childhood; however, it seems reasonable to recommend fiber in children for their future adult health.
6. Conclusion
Although there is clinical evidence for the use of some fibers such as PHGG, FOS, and/or inulin, future research is warranted to tailor the fiber choice according to the patient’s needs. There is a need for primary research to guide the selection of different fibers and their effects, as the current recommendations rely on expert opinion. This can aid in the development of personalized diets, which can also be supported by the use of microbial profiles to guide diet composition. Future research should also assess the impact of introducing a high amount of fiber in constipated children.
7. Expert opinion (Box 1)
7.1. The targeted population for fiber-containing formulas
Recommendations on the use of fiber-containing formulas in specific patient populations are lacking. The 2010 ESPGHAN committee on nutrition states that a fiber-containing formula is appropriate for most pediatric patients requiring EN [Citation85]. Clinical evidence supports the use of fiber in children receiving EN support in various clinical conditions [Citation78,Citation86]. Data show that fiber-containing EN formulas can modulate gut microbiota, improve GI tolerance, and decrease blood glucose levels in glucose-intolerant patients. Therefore, the expert panel recommends that dietary fiber should be included in the diet of all pediatric patients and that fiber-containing formula should be considered in all tube-fed children unless when contraindicated in specific situations or poorly tolerated. Children who receive EN can benefit from fiber-enriched whole food to improve GI function, anti-inflammatory effects, enhance glycemic profile, and increase satiety. Future studies in specific patient groups with a paucity of clinical data (such as cardiac and ICU patients) are needed to provide supportive data on the safety, tolerability, and benefits of fiber-containing formula and avoid unnecessary restrictions.
Previously, fiber-containing formulas were recommended for children over the age of two. However, it is now clear that enteral feeding should reflect the natural transition from breast milk to complementary foods as much as possible. During the first few months, a breastfed child will not have fiber, nor should a tube-fed child. From about six months of age, fiber can be introduced in most children.
Clinical conditions in which fiber may be contraindicated include bowel obstruction or stenosis, acute inflammation, and ileostomy. However, there are no specifically defined contraindications, and the introduction of fiber is set and assessed according to a follow-up of tolerance of patients.
7.2. Fiber selection in different clinical settings
Various fiber levels and sources are used in enteral products (see ). The ideal fiber profile for enteral formula products is unknown, and there is no guidance on the ratio of soluble and insoluble fibers that children should consume. In addition to solubility, other fiber properties such as viscosity and fermentability must also be considered, which define the functional/physiological properties of the fiber. Recent findings show that there can also be complex interactions between different types or subsets of fibers. We need to distinguish between different fiber types and recognize that they may play a complex role. Certain fibers can be harmful during periods of active inflammation [Citation87].
Although individual fibers may reduce the incidence of diarrhea and constipation, the use of a mixture of bulking and fermentable fiber has been suggested as a preferable approach particularly for long-term feeding. Similar physiological effects to those of a regular mixed diet may be seen with the consumption of mixed fibers or fiber blends, a prima facie plausible finding since they will more closely reflect the composition of the average diet [Citation64]. Despite the lack of universal agreement, it has been proposed that soluble fiber should account for nearly 30% of the fiber blends, which mimics the ratio found in normal food [Citation64]. Fiber blends can combine the benefits of fermentable prebiotic and non-fermentable fibers and can be used in oral nutrition supplements, and tube feeds. Fermentable prebiotic fibers promote the growth of healthy gut microbiota [Citation64]. On the other hand, non-fermentable fiber within the fiber blend can improve stool consistency and mass [Citation57]. It was suggested that homemade blended or commercial ‘real-food’ formulas have beneficial effects on GI symptoms, such as vomiting and abnormal bowel habit [Citation88,Citation89], which can be explained by the fact that they are loaded with a mix of fibers that improve GI tolerance. Although there are a variety of fiber blends on the market, further research is required to find the optimal fiber blend combinations and doses.
While constipation and diarrhea are easy to assess clinically, the assessment of gut microbiota is not part of routine practice in most centers.
7.3. Age- and condition-specific dosing for tube-fed children
The recommended daily fiber intake for healthy children is summarized in . However, there are no recommendations on the dose of fiber to use in tube-fed children with acute and chronic illnesses due to a lack of clinical data. It has been suggested to use a daily fiber intake comparable to that recommended for healthy children [Citation1,Citation69]; however, evidence to support this is lacking. It is unknown whether sick children require similar, higher, or lower fiber doses than healthy children. It is also unclear whether fiber requirements and tolerance may differ depending on the underlying condition and clinical status of the child.
Furthermore, the fiber content of most current enteral formulas does not match the fiber intake recommendations of healthy children and is also likely low for the potential needs of patients. The fixed amount of fiber present in enteral formulas does not allow for adjustment of the dose of fiber unless extra fiber is added separately. Dietary fiber should be introduced gradually on symptom-based approach. Based on clinical experience an estimated 10 g/day <3 years and >20 g/day for ≥14-year-old adolescents might be considered.
A multidisciplinary approach can help optimize nutritional care in complex patients. Further studies are required to investigate the ideal fiber dose in children requiring nutrition support.
7.4. Fiber-containing enteral formula as first-line nutritional therapy
The current evidence supports the use of dietary fiber in enteral feeding formulas as a first-line therapy for children who need nutritional support to prevent the occurrence of diarrhea or constipation and support gut microbiota. For children on a low-fiber diet, the fiber content of the enteral formula may need to be increased gradually to the target dose to allow a progressive gastrointestinal adaptation and reduce the risk of gastrointestinal intolerance symptoms.
7.5. Recommended feeding approach
Introducing a fiber-containing formula should be considered when initiating tube feeding. A stepwise process, especially in fiber-naïve patients, is advised. Children, including those receiving nutritional support, will benefit from consuming fiber from various sources. Previous studies suggest a potential benefit of adding soluble fiber in children receiving high-energy enteral feed [Citation86]. Still, more studies are needed to discover more about the effect of the amount and mix of fiber, including soluble versus insoluble fiber, and the contribution of oral fiber intake. Patients should continue on the fiber-containing formula that they tolerate best. Because tolerance can change over time, monitoring child tolerance to the fiber-containing formula is recommended. Dietary fiber in children receiving enteral nutrition can be provided through a fiber-containing formula or by adding dietary fiber supplements. Fiber-containing formulas can prevent a recurrence, so continuation should be considered even after achieving clinical goals.
Box 1 Summary of Experts’ Recommendations
Article highlights
Dietary fiber plays an important role in pediatric nutrition by supporting gut health and microbiome and promoting normal laxation. Nonetheless, practical guidance on the use of fiber containing EN in the pediatric population is still lacking.
Current evidence supports the use of dietary fiber in enteral feeding formulas as a first line nutritional therapy.
Fiber should be considered for all patients requiring enteral nutrition and can be gradually introduced from 6 months of age.
The use of a mixture of bulking and fermentable fiber is suggested as a preferable approach, particularly for longterm feeding.
There is no universal consensus on the dose of fiber to use in tubefed children with acute and chronic illness. However, based on clinical experience an estimated 10 g/day <3 years and >20 g/day for ≥14 year old adolescents might be considered.
Dietary fiber should be introduced gradually, especially in fibernaïve children, with an individualized symptombased approach.
Patients should continue on the fiber containing formula they tolerate best, with fiber intake adapted to their tolerance. Longterm fiber intake might be recommended to prevent the recurrence of gastrointestinal (GI) problems.
Declaration of interests
P Lionetti received in the past 36 months consulting fees from Nestlé Institute of Health Sciences, Takeda, Firma Menarini, Dr Falk, and Sandoz. C Romano received honoraria from Nestlé Institute of Health Sciences for the development of this manuscript. He received a grant from Nestlé Institute of Health Sciences in the past 36 months. He also received consulting fees and honoraria from Nestlé Institute of Health Sciences in the past 36 months. G Minor received consulting fees and honoraria from Nestlé Institute of Health Sciences in the past 36 months. E Wine received honoraria from Nestlé Institute of Health Sciences for the development of this manuscript. He received Payment or honoraria from Nestlé Institute of Health Sciences, AbbVie, Janssen, BioJamp, Pfizer, and Mead Johnson Nutrition in the past 36 months. F Gottrand received honoraria from Nestlé Institute of Health Sciences for the development of this manuscript. G Major is an employee of Société des Produits Nestlé. He has received grants from the Medical Research Council and National Institute for Health Research in the UK, Cystic Fibrosis Foundation, Cystic Fibrosis Trust, Sanofi, Vertex and speaker fees from Vertex in the past 36 months. R Ran Ressler and B Zemrani are employees of Nestlé Health Sciences. The funders had no role in the interpretation of experts’ opinion. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Hojsak I, Benninga MA, Hauser B, et al. Benefits of dietary fibre for children in health and disease. Arch Dis Child. 2022 [cited 2022 Aug 23];0. Internetarchdischild2021323571. Available from: . Internetarchdischild2021323571. Available from: https://adc.bmj.com/content/early/2022/03/10/archdischild-2021-323571
- Yang G, Wu X-T, Zhou Y, et al. Application of dietary fiber in clinical enteral nutrition: a meta-analysis of randomized controlled trials. World J Gastroenterol. 2005;11(25):3935–3938. doi: 10.3748/wjg.v11.i25.3935
- Cronin P, Joyce SA, O’toole PW, et al. Dietary Fibre Modulates the Gut Microbiota. Nutr 2021. 2021;13(5):1655. [Internet] [cited 2022 Sep 22];13:1655. Available from: https://www.mdpi.com/2072-6643/13/5/1655/htm
- Korczak R, Kamil A, Fleige L, et al. Dietary fiber and digestive health in children. Nutr Rev. 2017;754:241–259. [Internet]. 2017 [cited 2022 Sep 22];75:241–259. Available from: https://academic.oup.com/nutritionreviews/article/75/4/241/3747768.
- Dayib M, Larson J, Slavin J. Dietary fibers reduce obesity-related disorders: mechanisms of action. Curr Opin Clin Nutr Metab Care. 2020;23(6):445–450. Internet[cited 2022 Sep 22]Available from: https://journals.lww.com/co-clinicalnutrition/Fulltext/2020/11000/Dietary_fibers_reduce_obesity_related_disorders_.12.aspx
- Naveed S, Venäläinen T, Eloranta A-M, et al. Associations of dietary carbohydrate and fatty acid intakes with cognition among children. Public Health Nutr. 2020;23(9):1657–1663. doi: 10.1017/S1368980019003860
- Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A Prospective Study of Long-term Intake of Dietary Fiber and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology. 2013;145(5):970–977. Internet[cited 2022 Nov 23]Available from: https://pubmed.ncbi.nlm.nih.gov/23912083/
- Yi DY. Enteral Nutrition in Pediatric Patients. 2018. [[cited 2022 Aug 23]]. InternetAvailable from:/pmc/articles/PMC5788946/ Pediatr Gastroenterol Hepatol Nutr. 21(1):12. doi: 10.5223/pghn.2018.21.1.12
- Simakachorn N, Bibiloni R, Yimyaem P, et al. Tolerance, safety, and effect on the faecal microbiota of an enteral formula supplemented with pre- and probiotics in critically ill children. J Pediatr Gastroenterol Nutr. [cited 2022 Sep 22]. 2011;53:174–181. InternetAvailable from. 2. https://pubmed.ncbi.nlm.nih.gov/21788759/
- Tarleton SM, Kraft CA, Dibaise JK, et al. Fiber-enriched enteral formulae: advantageous or adding fuel to the fire? In: Balint G, Antala B Carty C, editors. Pract Gastroenterol [Internet]. 2013 [[cited 2022 Aug 23]]Vol. 37. p. 11–22. Available from: https://mayoclinic.pure.elsevier.com/en/publications/fiber-enriched-enteral-formulae-advantageous-or-adding-fuel-to-th
- Gacouin A, Camus C, Gros A, et al. Constipation in long-term ventilated patients: associated factors and impact on intensive care unit outcomes. Crit Care Med. [cited 2022 Sep 22]. 2010;38:1933–1938. InternetAvailable from. 10. https://pubmed.ncbi.nlm.nih.gov/20639749/
- Kansu A, Durmaz Ugurcan O, Arslan D, et al. High-fibre enteral feeding results in improved anthropometrics and favourable gastrointestinal tolerance in malnourished children with growth failure. Acta Paediatr. [cited 2022 Aug 23]. 2018;107:1036–1042. InternetAvailable from. 6. https://pubmed.ncbi.nlm.nih.gov/29364537/
- Turza KC, Krenitsky J, Sawyer RG. Enteral feeding and vasoactive agents: suggested guidelines for clinicians. Pract Gastroenterol. 2009;33:11–22.
- Cresci G, Cúe J. The patient with circulatory shock: to feed or not to feed? Nutr Clin Pract. [cited 2022 Sep 22] 2008;23:501–509. InternetAvailable from https://pubmed.ncbi.nlm.nih.gov/18849555/
- Cara KC, Beauchesne AR, Wallace TC, et al. Safety of Using Enteral Nutrition Formulations Containing Dietary Fiber in Hospitalized Critical Care Patients: a Systematic Review and Meta-Analysis. J Parenter Enter Nutr. [cited 2022 Nov 24]. 2021;45:882–906. InternetAvailable from. 5. https://onlinelibrary.wiley.com/doi/full/10.1002/jpen.2210
- Romano C, Van Wynckel M, Hulst J, et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children with Neurological Impairment. J Pediatr Gastroenterol Nutr. [cited 2023 Jan 9]. 2017;65:242–264. InternetAvailable from. 2. https://pubmed.ncbi.nlm.nih.gov/28737572/
- Trumbo, P. , Schlicker, S. , Yates, A. A. , Poos, M; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002 Nov;102(11):1621–30.
- Codex Committee on Nutrition and Foods for Special Dietary Uses. 30th SESSION of the CODEX COMMITTEE on NUTRITION and FOODS for SPECIAL DIETARY USES [Internet]. 2008 [cited 2023 Jan 9]. Available from: https://www.naturalproductsinsider.com/regulatory/report-30th-session-ccnfsdu.
- European Food Safety Authority (EFSA). Dietary Reference Values for the European Union [Internet]. Diet Ref Values Eur Union. 2019. Available from: https://multimedia.efsa.europa.eu/drvs/index.htm
- Cummings J. H. The effect of dietary fiber on fecal weight and composition. In G. A. Spiller, editor. CRC Handbook of Dietary Fiber in Human Nutrition. 2nd ed. Boca Raton, Florida, USA: CRC Press; 1993. p. 183–252.
- Roberfroid MB. Introducing inulin-type fructans. Br J Nutr. 2005;93(Suppl 1):S13–25.
- Anderson JW. Dietary Fiber and Associated Phytochemicals in Prevention and Reversal of Diabetes. Nutraceuticals, Glycemic Heal Type 2 Diabetes. Nutraceuticals, glycemic health and type 2 diabetes. 2008;97–125.
- Kirby RW, Anderson JW, Sieling B, et al. Oat-bran intake selectively lowers serum low-density lipoprotein cholesterol concentrations of hypercholesterolemic men. Am J Clin Nutr. 1981;34(5):824–829. doi: 10.1093/ajcn/34.5.824
- Khan NA, Raine LB, Drollette ES, et al. Dietary Fiber is Positively Associated with Cognitive Control among Prepubertal Children. J Nutr. 2015;145(1):143–149. doi: 10.3945/jn.114.198457
- Chao HC, Lai MW, Kong MS, et al. Cutoff volume of dietary fiber to ameliorate constipation in children. J Pediatr. [cited 2023 Jan 9]. 2008;153:45–49.e1. InternetAvailable from. 1. https://pubmed.ncbi.nlm.nih.gov/18571534/
- Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;80(6052):105–108. doi: 10.1126/science.1208344
- Sonnenburg ED, Smits SA, Tikhonov M, et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–215. doi: 10.1038/nature16504
- De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107
- De Filippo C, Di Paola M, Ramazzotti M, et al. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front Microbiol. 2017;8 [[cited 2023 Jan 9]]. InternetAvailable from: https://pubmed.ncbi.nlm.nih.gov/29081768/
- Casari S, Di Paola M, Banci E, et al. Changing Dietary Habits: the Impact of Urbanization and Rising Socio-Economic Status in Families from Burkina Faso in Sub-Saharan Africa. Nutrients. 2022;14(9):1782. InternetAvailable from:/pmc/articles/PMC9104313/. [cited 2023 Jan 9]. doi: 10.3390/nu14091782
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820
- Mardinoglu A, Wu H, Bjornson E, et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018;27(3):559–571.e5. doi: 10.1016/j.cmet.2018.01.005
- Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. [cited 2023 Jan 9] 2012;95:50–60. InternetAvailable from https://pubmed.ncbi.nlm.nih.gov/22468341/
- Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015;8(1):80–93. doi: 10.1038/mi.2014.44
- Cummings J, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227. doi: 10.1136/gut.28.10.1221
- Campos-Perez W, Martinez-Lopez E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim Biophys Acta, Mol Cell Biol Lipids. 2021;1866(5):158900.
- Roduit C, Frei R, Ferstl R, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74(4):799–809. doi: 10.1111/all.13660
- Anderson JW, Baird P, Davis RH, et al. Health benefits of dietary fiber. Nutr Rev. [cited 2022 Aug 23]. 2009;67:188–205. InternetAvailable from. 4. https://pubmed.ncbi.nlm.nih.gov/19335713/
- Massironi S, Viganò C, Palermo A, et al. Inflammation and malnutrition in inflammatory bowel disease. lancet Gastroenterol Hepatol [Internet]. 2023 [cited 2023 Apr 30];Available from: https://pubmed.ncbi.nlm.nih.gov/36933563/.
- Ho YH, Tan M, Seow-Choen F. Micronized purified flavonidic fraction compared favorably with rubber band ligation and fiber alone in the management of bleeding hemorrhoids: randomized controlled trial. Dis Colon Rectum. 2000;43(1):66–69.
- Lahner E, Esposito G, Zullo A, et al. High-fibre diet and Lactobacillus paracasei B21060 in symptomatic uncomplicated diverticular disease. World J Gastroenterol. 2012;18(41):5918–5924. doi: 10.3748/wjg.v18.i41.5918
- Chen K, Chen H, Faas MM, et al. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol Nutr Food Res. 2017;61(8):61. doi: 10.1002/mnfr.201601006
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. [cited 2023 Apr 30] 2011;106:563–573. InternetAvailable from https://pubmed.ncbi.nlm.nih.gov/21468064/
- Fritsch J, Garces L, Quintero MA, et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2021;19(6):1189–1199.e30. doi: 10.1016/j.cgh.2020.05.026
- Giannini EG, Mansi C, Dulbecco P, et al. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition. 2006;22(3):334–342. doi: 10.1016/j.nut.2005.10.003
- Smits BJ. The irritable bowel syndrome. Practitioner. 1974;213(1273):37–46.
- Morozov S, Isakov V, Konovalova M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J Gastroenterol. 2018;24(21):2291–2299.
- Tabbers MM, Dilorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258–274. doi: 10.1097/MPG.0000000000000266
- Wegh CAM, Baaleman DF, Tabbers MM, et al. Nonpharmacologic Treatment for Children with Functional Constipation: a Systematic Review and Meta-analysis. J Pediatr. 2022;240:136–149.e5.
- Harvie ML, Norris MAT, Sevilla WMA. Soluble Fiber Use in Pediatric Short Bowel Syndrome: a Survey on Prevailing Practices. Nutr Clin Pract. 2018;33(4):539–544.
- Scientific Advisory Committee on Nutrition. Carbohydrates and health. TSO (The Stationery Office). 2015.
- Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet (London, England). 2019;393(10170):434–445. doi: 10.1016/S0140-6736(18)31809-9
- Williams CL, Bollella M, Wynder EL A new recommendation for dietary fiber in childhood. Pediatrics [Internet]. American Academy of Pediatrics; 1995 [cited 2022 Aug 23]. p. 985–988. Available from:/pediatrics/article/96/5/985/59911/A-New-Recommendation-for-Dietary-Fiber-in.
- Stephen AM, Champ MMJ, Cloran SJ, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. [cited 2022 Aug 23]. 2017;30:149–190. InternetAvailable from. 2. https://pubmed.ncbi.nlm.nih.gov/28676135/
- National Health and Medical Research Council. Nutrient reference values for Australia and New Zealand [Internet]. Food Aus 2006. Available from: https://www.nhmrc.gov.au/sites/default/files/images/nutrient-refererence-dietary-intakes.pdf.
- Armstrong H, Mander I, Zhang Z, et al. Not All Fibers are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front Pediatr. 2021;8 [[cited 2023 Jan 9]]. InternetAvailable from: https://pubmed.ncbi.nlm.nih.gov/33520902/
- Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2008;108:1716–1731.
- Slavin JL, Savarino V, Paredes-Diaz A, et al. A review of the role of soluble fiber in health with specific reference to wheat dextrin. J Int Med Res. 2009;37(1):1–17. doi: 10.1177/147323000903700101
- Elia M, Engfer MB, Green CJ, et al. Systematic review and meta-analysis: the clinical and physiological effects of fibre-containing enteral formulae. Aliment Pharmacol Ther. [cited 2022 Aug 23]. 2008;27:120–145. InternetAvailable from. 2. https://pubmed.ncbi.nlm.nih.gov/17922802/
- Gidley MJ, Yakubov GE. Functional categorisation of dietary fibre in foods: beyond “soluble” vs “insoluble. Trends Food Sci Technol. 2019;86:563–568.
- Tokunaga M, Yasukawa Z, Ozeki M, et al. Effect of partially hydrolyzed guar gum on postprandial hyperglycemia a randomized double-blind, placebo-controlled crossover-study. Japanese Pharmacol Ther. 2016;44:85–91.
- Trinidad T, Perez E, Loyola A, et al. Glycemic index of Sunfibre (Cyamoposis tetragonolobus) products in normal and diabetic subjects. Int J Food Sci Technol. [cited 2023 Jan 9]. 2004;39:1093–1098. InternetAvailable from. 10. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2621.2004.00880.x
- Armstrong HK, Bording-Jorgensen M, Santer DM, et al. Unfermented β-fructan Fibers Fuel Inflammation in Select Inflammatory Bowel Disease Patients. Gastroenterology. 2022 [[cited 2022 Nov 23]];0. InternetAvailable from: http://www.gastrojournal.org/article/S0016508522011507/fulltext
- Klosterbuer A, Roughead ZF, Slavin J. Benefits of dietary fiber in clinical nutrition. Nutr Clin Pract. [cited 2022 Aug 23] 2011;26:625–635. InternetAvailable from https://pubmed.ncbi.nlm.nih.gov/21947646/
- Topping DL, Fukushima M, Bird AR. Resistant starch as a prebiotic and synbiotic: state of the art. Proc Nutr Soc. 2003;62(1):171–176.
- Lesmes U, Beards EJ, Gibson GR, et al. Effects of resistant starch type III polymorphs on human colon microbiota and short chain fatty acids in human gut models. J Agric Food Chem. 2008;56(13):5415–5421. doi: 10.1021/jf800284d
- Oku T, Hongo R, Nakamura S. Suppressive effect of cellulose on osmotic diarrhea caused by maltitol in healthy female subjects. J Nutr Sci Vitaminol (Tokyo). [cited 2022 Sep 23] 2008;54:309–314. InternetAvailable from https://pubmed.ncbi.nlm.nih.gov/18797153/
- Leterme P, Andr S, van Leeuwen P, et al. Chemical Composition of Pea Fibre Isolates and their Effect on ihe Endogenous Amino Acid Flow at the Ileum of the Pig*. J Sci Food Agric. 1996;72(1):127–134. doi: 10.1002/(SICI)1097-0010(199609)72:1<127:AID-JSFA637>3.0.CO;2-C
- Evans S, Daly A, Davies P, et al. Fibre content of enteral feeds for the older child. J Hum Nutr Diet Off J Br Diet Assoc. 2009;22(5):414–421. doi: 10.1111/j.1365-277X.2009.00991.x
- O’Connor G, Watson M, Van Der Linde M, et al. Monitor gastrointestinal tolerance in children who have switched to an “enteral formula with food-derived ingredients”: a national, multicenter retrospective chart review (RICIMIX study). Nutr Clin Pract. [cited 2023 Jan 9]. 2022;37:929–934. InternetAvailable from. 4. https://pubmed.ncbi.nlm.nih.gov/34935190/
- Daveluy W, Guimber D, Mention K, et al. Home enteral nutrition in children: an 11-year experience with 416 patients. Clin Nutr. [cited 2023 Jan 9]. 2005;24:48–54. InternetAvailable from. 1. https://pubmed.ncbi.nlm.nih.gov/15681101/
- Klek S, Hermanowicz A, Dziwiszek G, et al. Home enteral nutrition reduces complications, length of stay, and health care costs: results from a multicenter study. Am J Clin Nutr. [cited 2023 Jan 9]. 2014;100:609–615. InternetAvailable from. 2. https://academic.oup.com/ajcn/article/100/2/609/4576541
- Chutkan R, Fahey G, Wright WL, et al. Viscous versus nonviscous soluble fiber supplements: mechanisms and evidence for fiber-specific health benefits. J Am Acad Nurse Pract. 2012;24(8):476–487. doi: 10.1111/j.1745-7599.2012.00758.x
- Speridião PGL, Tahan S, Fagundes-Neto U, et al. Dietary fiber, energy intake and nutritional status during the treatment of children with chronic constipation. Braz J Med Biol Res. [cited 2022 Nov 23]. 2003;36:753–759. InternetAvailable from. 6. http://www.scielo.br/j/bjmbr/a/r7pzpY9WcyFKHsLkcdcKcFb/?lang=en
- Kranz S, Brauchla M, Slavin JL, et al. What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv Nutr. 2012;3(1):47–53. doi: 10.3945/an.111.001362
- Costalos C, Kapiki A, Apostolou M, et al. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum Dev. 2008;84(1):45–49. doi: 10.1016/j.earlhumdev.2007.03.001
- Guimber D, Bourgois B, Beghin L, et al. Effect of multifibre mixture with prebiotic components on bifidobacteria and stool pH in tube-fed children. Br J Nutr. 2010;104(10):1514–1522. doi: 10.1017/S0007114510002461
- Khoshoo V, Sun SS, Storm H. Tolerance of an enteral formula with insoluble and prebiotic fiber in children with compromised gastrointestinal function. J Am Diet Assoc InternetAvailable from. [cited 2022 Sep 23] 2010;110(11):1728–1733. https://pubmed.ncbi.nlm.nih.gov/21034888/.*
- Daly A, Johnson T, MacDonald A. Is fibre supplementation in paediatric sip feeds beneficial? J Hum Nutr Diet InternetAvailable from. [cited 2022 Sep 23] 2004;17(4):365–370. https://pubmed.ncbi.nlm.nih.gov/15250846/.*
- Van Aerde J, Alarcon P, Lam W, et al. Tolerance and Safety of Energy-Dense Enteral Formulae for Young Children. Int Pediatr. 2003;18:95–99.
- Zheng S, Steenhout P, Kuiran D, et al. Nutritional support of pediatric patients with cancer consuming an enteral formula with fructooligosaccharides. Nutr Res. 2006;26(4):154–162. doi: 10.1016/j.nutres.2006.04.001
- Brunser O, Gotteland M, Cruchet S, et al. Effect of a Milk Formula with Prebiotics on the Intestinal Microbiota of Infants After an Antibiotic Treatment. Pediat Res. [cited 2022 Sep 23]. 2006;59:451–456. Pediatr Res 2006 593 [Internet] Available from. 3. https://www.nature.com/articles/pr200686
- Haschke F, Firmansyah A, Meng M, et al. Functional food for infants and children. Monatsschr Kinderheilkd. 2001;149(0):S66–70. doi: 10.1007/s001120170011
- Gottrand M, Muyshont L, Couttenier F, et al. Micronutrient status of children receiving prolonged enteral nutrition. Ann Nutr Metab. [cited 2023 Jan 9]. 2013;63:152–158. InternetAvailable from. 1–2. https://pubmed.ncbi.nlm.nih.gov/24008240/
- Braegger C, Decsi T, Dias JA, et al. Practical approach to paediatric enteral nutrition: a comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. [cited 2022 Aug 23]. 2010;51:110–122. InternetAvailable from. 1. https://pubmed.ncbi.nlm.nih.gov/20453670/
- Hoekstra JH, Szajewska H, Zikri MA, et al. Oral rehydration solution containing a mixture of non-digestible carbohydrates in the treatment of acute diarrhea: a multicenter randomized placebo controlled study on behalf of the espghan working group on intestinal infections. J Pediatr Gastroenterol Nutr. [cited 2022 Sep 23]. 2004;39:239–245. InternetAvailable from. 3. https://pubmed.ncbi.nlm.nih.gov/15319622/
- Trumbo P, Schlicker S, Yates AA, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. [cited 2022 Nov 23]. 2002;102:1621–1630. InternetAvailable from. 11. https://pubmed.ncbi.nlm.nih.gov/12449285/
- Thomas S, Marchesi J, Sadlier C, et al. G478(P) to blend or not to blend? the benefits/risks of a blended diet in gastrostomy-fed children. Arch Dis Child. 2019 [[cited 2023 Jan 9]];104:A192. InternetAvailable from https://adc.bmj.com/content/104/Suppl_2/A192.3
- Gallagher K, Flint A, Mouzaki M, et al. Blenderized Enteral Nutrition Diet Study: feasibility, Clinical, and Microbiome Outcomes of Providing Blenderized Feeds Through a Gastric Tube in a Medically Complex Pediatric Population. JPEN J Parenter Enteral Nutr. [cited 2023 Jan 9]. 2018;42:1046–1060. InternetAvailable from. 6. https://pubmed.ncbi.nlm.nih.gov/29338077/
