ABSTRACT
Introduction
Opioid-induced constipation remains undertreated despite effective and safe treatment options exists. Previous guidelines have only been partially effective in improving management, possibly due to their complexity, and studies suggest that a simple setup of concise and behaviorally-orientated steps improves usability.
Areas covered
This article introduces the concept of opioid-induced constipation and provides an overview of existing guidelines in this field. We also propose simplified recommendations for managing opioid-induced constipation, derived from a synthesis of current guidelines and the principles of optimal guideline design theory.
Expert opinion
Despite standard treatment with laxatives and fluid intake in patients with opioid-induced constipation, escalation of treatment is often needed where μ-opioid receptor antagonists or newer medications such as lubiprostone, linaclotide, or prucalopride are used. Previous guidelines have not been used sufficiently and thus management of the condition is often insufficient. We therefore propose simplified recommendations to management, which we believe can come into broader use. It was validated in primary care for credibility, clarity, relevance, usability, and overall benefit. We believe that this initiative can lead to better management of the substantial proportion of patients suffering from side effects of opioids.
1 Introduction
With the global increase in chronic pain, opioid consumption is increasing, and it is estimated that 2–4% of the general population are treated with opioids [Citation1,Citation2]. Unfortunately, among other problems, opioid therapy is associated with debilitating and persistent gastrointestinal dysfunction, most commonly presenting as constipation [Citation3–5]. This is present in between 60–80% of patients dependent on the underlying disease, and has a major impact on quality of life [Citation6].
The analgesic effects of both endogenous and exogenous opioids are mediated through the activation of receptors in the central nervous system. However, the enteric nervous system, responsible for the regulation of gut motility and secretion, is likewise home to a vast number of opioid receptors. Activation of these receptors causes an overall inhibitory effect on the submucosal and myenteric neurons interfering with motility, fluid transport, and sphincter function [Citation7]. While important in the physiological regulation of gastrointestinal function, the excess activation of μ-receptors within the enteric nervous system by exogenous opioids is responsible for the development of opioid-induced bowel dysfunction (OIBD). Opioid-induced constipation (OIC) is the most common form of OIBD and may lead to suboptimal dosing or discontinuation of opioid therapy [Citation8,Citation9], thus resulting in insufficient pain management with both personal and socioeconomic consequences. OIC is underdiagnosed, and management of the condition is complicated by the delicate nature of the symptoms [Citation10].
As in chronic idiopathic constipation, standard laxative treatment strategies are considered first-line therapy [Citation11], but often fail due to the mechanistically different pathogenesis of OIC compared to other types of constipation [Citation7]. Consequently, a targeted pharmacological approach known as peripherally acting μ-opioid receptor antagonists (PAMORAs) has been developed specifically to counteract exogenous opioid-induced gastrointestinal adverse effects [Citation12,Citation13]. PAMORAs exert their effect by blocking μ-opioid receptors in the gastrointestinal tract, while their biochemical properties reduce the ability to cross the blood-brain barrier, thus preserving the desired analgesic effects [Citation14]. Experimental studies in healthy subjects have demonstrated how PAMORAs reduce gastrointestinal transit, improve anal sphincter relaxation, normalize stool consistency, and reduce gastrointestinal symptoms during opioid treatment [Citation15–18]. Despite evidence of the efficacy and safety of PAMORAs in OIC management [Citation19], the condition remains undertreated, and development of several guidelines on the topic () has partly failed to improve the implementation of an optimal treatment strategy [Citation7,Citation8,Citation20–24].
Table 1. Overview of current guidelines for management of opioid-induced constipation.
2 Rationale for development of simplified recommendations
The development of guidelines has grown exponentially in recent decades in many different medical areas. The purpose has been to standardize and improve quality of care by supporting clinical decisions to be based on evidence [Citation25]. While successful guideline implementation in the clinic has the potential to enhance patient care dramatically, several large-scale studies show that guideline compliance, in general, is low [Citation26,Citation27] also regarding management of gastrointestinal problems [Citation28]. An observational study from Italy has shown that factors such as the age of the patient and of the general practitioner influence the choice of therapeutic treatment of chronic constipation more than guidelines on the topic [Citation29]. This is backed up by a survey study from Germany that showed only about 55% of 511 general practitioners applied guidelines in their daily work. Reasons for not utilizing guidelines were reported to be a lack of knowledge of relevant guidelines (78%) and guidelines not being practical enough (21%) [Citation30]. This study clearly shows that in busy primary care, it is of high importance that key information is made easily available and applicable, and in that regard, guideline developers have a responsibility for facilitating implementation in daily clinical practice. Implementation strategies must also be considered to ensure that guidelines reach the end-users. The best way to achieve this may vary with local organizations and medical areas but could include presentation in relevant journals and forums.
Guideline content and wording are likewise crucial for successful implementation. A study from 2003 investigated which characteristics of a guideline were associated with high compliance. The most important facilitators were found to be evidence-based recommendations, simplicity, and compatibility with existing norms. On the other hand, barriers to successful compliance included complex decision trees and requirements for application of new knowledge and skills [Citation31]. This clearly demonstrates that guideline recommendations should be phrased with the end-user in mind rather than with the purpose of being an academic excise. Especially in figures and flowcharts, specific information that is only relevant for selected patients should be downscaled or shown only in footnotes. A simple and cost-effective way of facilitating guideline adherence in the clinic may be a simple rewriting of the recommendations into concise and behaviorally-orientated steps [Citation32]. A narrative literature review identified several such examples, demonstrating that simplification of existing guidelines can improve adherence and clinical outcomes in different medical areas () [Citation33–38].
Table 2. Overview of studies comparing proposed simple guidelines to standard guidelines.
In general, clinical guideline adherence is complex and difficult to achieve, but studies suggest that simplicity is a key component in successful implementation [Citation31]. Furthermore, clinical guidelines should be devised with the purpose of condensing evidence from randomized controlled trials and meta-analyses within a given topic into practical tools easily applicable in a busy clinical routine. Lastly, pilot testing and peer review of proposed guidelines raise the credibility and should be integrated into the developmental phase [Citation39].
3 Development of simplified recommendations
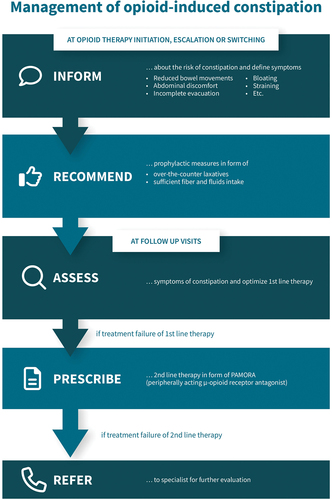
A panel of five European experts in pain medicine and gastroenterology was involved in the development of the simplified recommendations for the management of opioid-induced constipation. All members of the panel have extensive experience in opioid-induced bowel dysfunction. The recommendations were based on evidence-based literature, previous guidelines, and expert opinions of the panel. Moreover, the recommendations were devised with the purpose in mind of being a simple and practical tool for health care personnel in primary care dealing with chronic pain patients. Five easy steps of management were identified:
3.1 Step 1 – inform about opioid-induced constipation
The first step in successful management of OIC is effective patient-physician communication. Studies show that healthcare providers tend to focus solely on pain management when evaluating opioid therapy while underestimating the impact of constipation on the health-related quality of life [Citation7,Citation8]. The social and emotional burden of OIC, however, is substantial. Forty-five percent of patients with this condition reported difficulty following normal routines, while feelings of frustration, anxiety, and depression were experienced by 28%, 23%, and 21%, respectively [Citation19].
Patients may fail to report symptoms of constipation when not inquired directly for reasons such as embarrassment and lack of knowledge of the possible causal connection between symptoms and opioid therapy. Furthermore, chronic pain patients may have accepted constipation as a necessary evil of sufficient pain management due to unawareness of targeted treatment options. To overcome this communication gap, it is therefore essential that the prescribing physician address the issue firsthand. Patients should thus be informed about the risk of constipation when opioid therapy is initiated, escalated, or switched. Despite this recommendation, a study from Denmark showed that only 28% of 286 patients on opioid therapy recalled being informed about the risk of OIC [Citation20]. At follow-up visits, healthcare providers must also ask specifically about symptoms of constipation. Education on the correct definition of constipation is likewise important, as many patients only consider reduced frequency of bowel movements, while in fact, symptoms such as bloating, abdominal discomfort, straining, and incomplete evacuation are also included in the spectrum of symptoms seen in OIC [Citation8].
3.2 Step 2 – recommend first-line therapy
The initial information about the risk of OIC should be accompanied by recommendations for prevention and management. Although poorly documented, these include diet- and lifestyle-related actions such as sufficient intake of fibers and fluids, as well as exercise to induce intestinal motility. Standard over-the-counter laxatives should be advised as a first-line treatment strategy if symptoms of constipation occur, or they can be co-prescribed together with the opioid in risk patients such as those with immobilization. Osmotic agents or stimulants should be chosen over non-absorbable sugars as these may cause exacerbation of bloating due to fermentation in the colon [Citation21]. Often polyethylene glycol-based laxatives are needed. Enemas and suppositories may be also considered especially in the start of treatment, but the effect is largely undocumented.
3.3 Step 3 – assess symptoms of constipation
As mentioned, inquiry about gastrointestinal function and especially symptoms of constipation should always be included in the evaluation of opioid therapy. Benchmarking against baseline symptoms prior to initiating opioid analgesia is important. There are a number of different patient-reported outcome measures, which may help in the assessment of the severity of constipation as well as any improvement after interventional therapy. The Bristol Stool Form Scale is an easy and reliable tool to assess stool consistency, while the Bowel Function Index allows for evaluation of 1) ease of defecation, 2) feeling of incomplete evacuation, and 3) personal judgment of constipation. A Bristol Stool Form Scale score of 1–2 and a Bowel Function Index score above 29 suggest presence of constipation [Citation22,Citation23], while any changes in Bowel Function Index score above 12 are indicative of a clinically relevant change in symptoms [Citation24]. By applying a validated and quantitative approach in the evaluation of OIC, the quality of management can be improved by supporting correct and timely intervention.
It is important to emphasize that other causes of constipation must be considered and ruled out whenever OIC is suspected. Other medications, preexisting disease, or functional constipation are all possible contributors to symptoms of constipation and should be managed appropriately.
3.4 Step 4 – prescribe second-line therapy
In cases of OIC non-responding to standard laxatives, treatment with PAMORAs should be initiated. A convincing body of evidence exists regarding the efficacy and safety of these agents in the treatment of OIC [Citation16,Citation25,Citation26]. A recent meta-analysis found naldemedine and prolonged-release naloxone in combination with oxycodone to be superior in terms of efficacy [Citation16], but the choice of PAMORA should always be based on individual assessment of each patient. Relevant interactions with other medications or contraindications, such as possible gastrointestinal stenosis, must be considered in this regard. Effect and tolerance must be monitored regularly in the initial phase of PAMORA therapy. When a satisfactory result has been achieved, typically after 1–2 weeks, tapering of standard laxatives should be considered. Some reports suggest that PAMORAs may be more efficient in treating OIC compared to laxatives. However, considering the fact that PAMORAs are significantly more expensive and not accessible as over-the-counter medication, common laxative therapy are often more appropriate as a first-line choice. Likewise, co-prescription of PAMORAs upon opioid initiation can be considered in special circumstances, but in most cases, exploring other alternatives should be prioritized initially.
3.5 Step 5 – refer to specialist
When symptoms of constipation continue despite the above-mentioned initiatives, patients should be referred to gastrointestinal specialists for further evaluation, which might include anorectal manometry for assessment of pelvic floor function, etc. Specialized management with prokinetics and/or secretagogues agents can be needed when PAMORAs fail, or in cases with preexisting constipation.
The proposed simplified recommendations are presented in .
4 Validation of simplified recommendations
A cohort of 52 healthcare personnel from the United Kingdom participated in an online validation study of the simplified recommendations. Inclusion criteria for participation in the online survey included: at least three years of practicing, at least 70% of time working in direct patient care, and personal management of at least one OIC patient per month. The survey covered general awareness of OIC guidelines, the biggest challenges/unmet needs of current OIC guidelines, and the ability of the new simplified recommendations to improve those issues. Secondly, perception regarding credibility, clarity, relevance, and usability of an existing European OIC guideline [Citation8] and our new and simplified OIC recommendations were rated on a 7-point Likert scale ranging from ‘not at all’ to ‘very.’ Scores were compared statistically using a repeated mixed model with recommendations (existing, new) and domain (credibility, clarity, relevance, usability) as factors. Lastly, the overall benefit of the new simplified recommendations, over and above the current guideline, was rated on a 7-point Likert scale ranging from ‘no benefit at all’ to ‘a lot of benefit.’
In total, 42 general practitioners, eight general nurses, and two nurse practitioners completed the online survey. Of these, 62% had personally managed 1–50 patients with OIC during the past month, while 29% and 10% had managed above 50 and above 100 patients, respectively.
4.1 General awareness of guidelines for the management of opioid-induced constipation
A general awareness of guidelines for the management of OIC was reported by 62% of responders. Thirty-eight percent were not aware of any guidelines for the management of OIC.
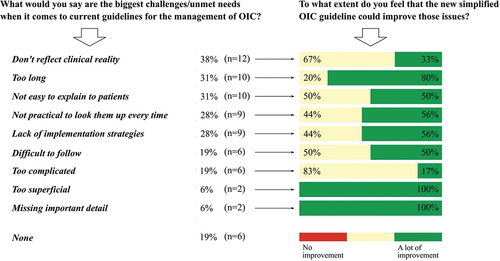
4.2 Biggest challenges and unmet needs of current guidelines
A need for simplification of current guidelines was reported by 48% of general practitioners and 60% of nurses. Furthermore, the biggest challenges and unmet needs of current guidelines reported by responders included the inability to reflect clinical reality, the length of the guideline, lack of implementation strategies, and complicity. In general, the new simplified recommendations were rated to improve most of the reported challenges and unmet needs of current OIC guidelines (). No challenges or unmet needs of current OIC guidelines were reported by 19%.
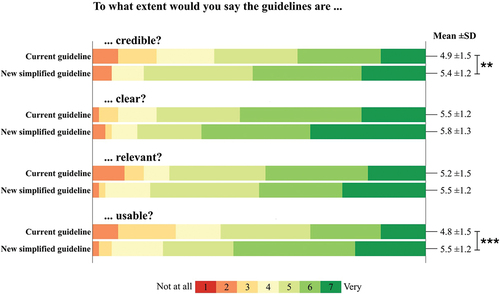
4.3 Credibility, clarity, relevance, and usability of current guidelines and new simplified recommendations
The new simplified recommendations were rated as being more credible (5.4 ± 1.2 vs. 4.9 ± 1.5, p = 0.008) and usable (5.5 ± 1.2 vs. 4.8 ± 1.5, p < 0.001) compared to the current guideline by Farmer et al. [Citation8] ().
Figure 3. Individual scores regarding the credibility, clarity, relevance, and usability of the current guideline and new simplified recommendations. Each domain was rated by healthcare practitioners (n = 52) on a 7-point Likert scale ranging from 1 (not at all) to 7 (very). Mean and standard deviation (SD) are shown to the right. **p < 0.01, ***p < 0.001.
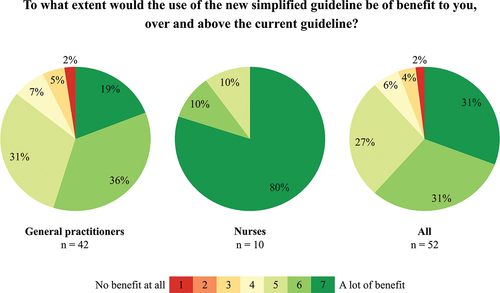
4.4 Overall benefit of new simplified recommendations
Of the 42 general practitioners, 19% rated the new simplified recommendations as providing ‘a lot of benefit’ in the management of OIC patients compared to the current guideline by Farmer et al. [Citation8]. Only 2% rated the new simplified recommendations as being of ‘no benefit at all.’ Of nurses, 80% regarded the new simplified recommendations as providing ‘a lot of benefit,’ while none regarded it as ‘no benefit at all’ ().
5 Discussion of the simplified recommendations
Our validation study showed that approximately 40% of the healthcare practitioners in our cohort regularly dealing with patients with OIC were unaware of any current guidelines for OIC management. Furthermore, half of those currently using OIC guidelines reported that a simplification of guidelines would be of benefit. These results clearly underline the need for an easy-to-use and easily applicable guideline for improving the management of OIC in primary care. When presented with our new simplified OIC recommendations, domains such as credibility and usability were rated higher as opposed to the current European guideline from Farmer et al. [Citation8], hereby demonstrating that simplification and user-friendliness are beneficial. Hence, the presentation of information in a clear and concise manner may increase credibility, as evidenced by the results of this validation study. For the validation study, we chose to include healthcare practitioners with strong clinical knowledge including experience in the management of OIC. This was done to ensure that the feedback was based on knowledge of daily clinical practice. However, this approach introduces a selection bias. Feedback from healthcare practitioners with less experience in OIC management might also provide important information regarding the usability of the recommendations. As the recommendations is intended for all healthcare practitioners regardless of clinical experience, it is important that it is considered usable in a broad sense and without prior knowledge within the area. Another limitation of the study is the relatively small sample size (n = 52). Larger validation studies in different countries would increase the reliability of our simplified recommendations.
6 Conclusion
Constipation is a common dose-limiting adverse effect of opioid therapy, responsible for reduced quality-of-life of affected individuals. There is great potential for improvement of the management, given that successful treatment options exist. In this study, we have provided recommendations for the management of OIC based on evidence, previous guidelines, and the opinions of a UK expert panel. The goal was to simplify existing guidelines while maintaining high quality in order to facilitate successful implementation in the clinic, ultimately leading to improved patient care for a condition known to be underdiagnosed and undertreated. We have arranged the recommendations in five easy-to-follow steps: 1) Inform, 2) Recommend, 3) Assess, 4) Prescribe and 5) Refer. With our simplified, escalating, user-friendly, and peer-reviewed recommendations, devised by a panel of experts within the field, we wish to facilitate implementation in the clinic, thereby improving quality of life of affected individuals in terms of both adequate pain management and gastrointestinal function.
7 Expert opinion
Opioid-induced constipation is the result of the effects of opioids on motility, secretion, and sphincter function. Although constipation is the predominant symptom, all segments of the alimentary tract can be involved and contribute to the clinical picture. Many patients have preexisting constipation that can worsen when opioids are prescribed, and in clinical practice, patients are often multimorbid and treated with many medications influencing motility and secretion. It is also important for the treating physician to note that OIC is common, and that number of bowel movements are a poor indication of the diagnosis. Other symptoms such as straining, lack of bowel emptying, and complaints about gas and abdominal distension are typical manifestations of OIC and must be detected and monitored. This is challenging and calls for a more structured approach to management.
Current guidelines for managing opioid-induced constipation have faced challenges when it comes to successful implementation in primary care settings. Consequently, this condition is often undertreated, and management may not prioritize the most efficient strategies. Drafting guidelines for use in healthcare is indeed a challenging task. On one hand, treatment should be tailored to individual patients, as a ‘one-size-fits-all’ approach may not be suitable. However, an overly intricate set of recommendations can reduce their utility in primary care. Similarly, guidelines may lose credibility and applicability if they appear too simplistic. The ideal approach in this context is to develop guidelines with recommendations grounded in solid scientific evidence, presented in a concise and user-friendly manner.
With the proposed simplified recommendations and better education, we believe that treatment can be facilitated in the right direction, especially in primary care. This will ensure that the difficult patients – not responding to sufficient standard treatment – are referred to specialists. Patients not responding to standard management should be examined for outlet obstruction and dyssynergic defecation etc. in the specialist settings. Additional management with medications such as lubiprostone, linaclotide, or prucalopride can be used, and in some cases, surgery is needed. Life expectancy, drug availability, and patient preference should also be considered when treatment is planned. However, it will be necessary to make a structure where primary care (starting opioid treatment in most cases) collaborates with secondary and tertiary care (including gastroenterologists, surgeons, and pain specialists). Such a framework will be multidisciplinary involving nurses, pharmacists, and pharmaceutical companies, where better communication and referral between the different entities will be mandatory for success. Unfortunately, in many regions, the different treatment offers are not coordinated, and an optimal organization is key to successful management.
In the future, identification of the segment and physiological functions of the alimentary tract that are mainly affected by side effects of opioids will be explored with advanced methods such as magnetic resonance imaging where motility and secretion can potentially be investigated. Sphincter function can be better explored with high-resolution manometry and new devices such as the Fecobionics. New classification systems based on molecular biology may also improve the field. As such, patients expected to develop major side effects from opioids can be identified in advance, allowing for the initiation of preventive measures and subsequent individualized treatment. This approach will improve pain management, prevent much suffering, and decrease the costs for society. Artificial intelligence methods will undoubtedly play a major role to dissect all the different variables from imaging, physiological studies, blood markers, and questionnaires into meaningful individual phenotypes representing patients responsive to the different treatment strategies. This is expected to enhance the field of medicine as a whole, including the field of opioid-induced constipation, by enabling the identification of numerous factors that influence its outcome. Even though these methods are until now only available in research settings, they will likely be available for clinical use in the near future. Until then, recommendations such as those presented here will facilitate the right treatment across disciplines involved in primary and secondary care.
Article highlights
Constipation is a common adverse effect of opioid therapy and is associated with decreased quality of life
The condition is often undertreated despite several guidelines on the topic exist.
Guideline implementation and adherence in general are challenging, but studies show that simplification may facilitate usability.
This article provides an overview of existing guidelines in the field and propose simplified recommendations for management of OIC in the form of a user-friendly flowchart.
A validation study including 52 healthcare professionals showed that credibility and usability were improved with our simplified recommendations compared to existing guidelines.
Declaration of interest
A Drewes participated in advisory boards sponsored by Shionogi and Kyowa Kirin and received a free grant from Shionogi to investigate the effects of naldemedine on the gut. A Emmanuel has participated in advisory boards sponsored by Shionogi and Kyowa Kirin.The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer receives grants and research support from Gilead, Mylan EPD, EA Pharma, Kowa, Taisho, and Biofermin and is a consulting adviser for Gilead, Boehringer Ingelheim, BMS, Kowa, Astellas, EA Pharma, and Mylan EPD. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
A Drewes, A Emmanuel, B Morlion, A Farmer, and G Varrassi developed the simplified guideline. A Drewes and T Okdahl were in charge of the validation study. T Okdahl performed the statistical analysis and wrote the first draft of the article. All authors critically reviewed the manuscript.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Additional information
Funding
References
- Sullivan MD, Edlund MJ, Fan MY, et al. Trends in use of opioids for non-cancer pain conditions 2000-2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008 Aug 31;138(2):440–449. doi: 10.1016/j.pain.2008.04.027
- Bosetti C, Santucci C, Radrezza S, et al. Trends in the consumption of opioids for the treatment of severe pain in Europe, 1990–2016. Eur J Pain (United Kingdom). 2019;23(4):697–707. doi: 10.1002/ejp.1337
- Argoff CE. Opioid-induced constipation: a review of health-related quality of life, patient burden, practical clinical considerations, and the impact of peripherally acting μ-opioid receptor antagonists. Clin J Pain. 2020 Sep 1;36(9):716–722. doi: 10.1097/AJP.0000000000000852
- Coyne KS, Margolis MK, Yeomans K, et al. Opioid-induced constipation among patients with chronic noncancer pain in the United States, Canada, Germany, and the United Kingdom: laxative use, response, and Symptom burden over time. Pain Med. 2015 Aug 1;16(8):1551–1565. doi: 10.1111/pme.12724
- Häuser W, Morlion B, Vowles KE, et al. European* clinical practice recommendations on opioids for chronic noncancer pain - part 1: role of opioids in the management of chronic noncancer pain. Eur J Pain. 2021 May 1;25(5):949–968. doi: 10.1002/ejp.1736
- Varrassi G, Banerji V, Gianni W, et al. Impact and consequences of opioid-induced constipation: a survey of patients. Pain Ther. 2021;10(2):1139–1153. doi: 10.1007/s40122-021-00271-y
- Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan 1;156(1):218–226. doi: 10.1053/j.gastro.2018.07.016
- Farmer AD, Drewes AM, Chiarioni G, et al. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. UEG J. 2019 Feb 1;7(1):7–20. doi: 10.1177/2050640618818305
- Keller MS, Jusufagic A, Spiegel BMR. Patient and provider differences in the treatment of opioid-induced constipation: a qualitative study. BMC Gastroenterol. 2019 Nov 12;19(1). doi: 10.1186/s12876-019-1097-7
- Vallerand AH, Hendry S, Baldys E, et al. Analysis of patient-provider interactions regarding the burden and treatment of opioid-induced constipation in adults with chronic noncancer pain. Pain Med. 2019 May 1;20(5):889–896. doi: 10.1093/pm/pny151
- Bassotti G, Satta PU, Bellini M. Chronic idiopathic constipation in adults: a review on current guidelines and emerging treatment options. Clin Exp Gastroenterol. 2021;14:413–428. doi: 10.2147/CEG.S256364
- Rekatsina M, Paladini A, Drewes AM, et al. Efficacy and safety of peripherally acting μ-opioid receptor antagonist (PAMORAs) for the management of patients with opioid-induced constipation: a systematic review. Cureus. 2021 Jul 6;13(7). doi: 10.7759/cureus.16201
- Hale ME, Wild JE, Yamada T, et al. Naldemedine is effective in the treatment of opioid-induced constipation in patients with chronic non-cancer pain who had a poor response to laxatives. Therap Adv Gastroenterol. 2021;14:14. doi: 10.1177/17562848211032320
- Gudin J, Vu L, Ceschim MR, et al. Peripherally acting mu-opioid receptor antagonists for the treatment of opioid-induced constipation. Aliment Pharmacol Ther. 2022 May 1;55(S2):S8–15. doi: 10.1111/apt.16864
- Olesen AE, Gronlund D, Mark EB, et al. Effects of naloxegol on gastrointestinal transit and colonic fecal volume in healthy participants receiving oxycodone. J Neurogastroenterol Motil. 2019 Oct 1;25(4):602–610. doi: 10.5056/jnm18079
- Grønlund D, Poulsen JL, Krogh K, et al. The impact of naloxegol on anal sphincter function - using a human experimental model of opioid-induced bowel dysfunction. Eur J Pharm Sci. 2018 May 30;117:187–192. doi: 10.1016/j.ejps.2018.02.008
- Poulsen JL, Mark EB, Brock C, et al. Colorectal transit and volume during treatment with prolonged-release oxycodone/Naloxone versus oxycodone plus macrogol 3350. J Neurogastroenterol Motil. 2018 Jan 1;24(1):119–127. doi: 10.5056/jnm17058
- Poulsen JL, Brock C, Grønlund D, et al. Prolonged-release oxycodone/Naloxone improves anal sphincter relaxation compared to oxycodone plus macrogol 3350. Dig Dis Sci. 2017 Nov 1;62(11):3156–3166. doi: 10.1007/s10620-017-4784-7
- Ouyang R, Li Z, Huang S, et al. Efficacy and safety of peripherally acting mu-opioid receptor antagonists for the treatment of opioid-induced constipation: a Bayesian Network meta-analysis. Pain Med. 2020;21(11):3224–3232. doi: 10.1093/pm/pnaa152
- Brenner DM, Chey WD. An evidence-based review of Novel and emerging therapies for constipation in patients taking opioid analgesics. Am J Gastroenterol Suppl. 2014;2(1):38–46. doi: 10.1038/ajgsup.2014.8
- Drewes AM, Munkholm P, Simrén M, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction–recommendations of the Nordic Working Group. Scand J Pain. 2016;11(1):111–122. doi: 10.1016/j.sjpain.2015.12.005
- O’Brien T, Christrup LL, Drewes AM, et al. European pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain (United Kingdom). 2017;21(1):3–19. doi: 10.1002/ejp.970
- Müller-Lissner S, Bassotti G, Coffin B, et al. Opioid-induced constipation and bowel dysfunction: a clinical guideline. Pain Med. 2017;18(10):1837–1863. doi: 10.1093/pm/pnw255
- Brenner DM, Stern E, Cash BD. Opioid-related constipation in patients with non-cancer pain syndromes: a review of evidence-based therapies and justification for a change in nomenclature. Curr Gastroenterol Rep. 2017;19(3):2–7. doi: 10.1007/s11894-017-0560-2
- Manchikanti L. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management, part I: introduction and general considerations. Pain Physician. 2008;11(2):161–186. doi: 10.36076/ppj.2008/11/161
- Gagliardi AR, Brouwers MC, Palda VA, et al. How can we improve guideline use? A conceptual framework of implementability. Implement Sci. 2011 Mar 22;6(1):1–11. doi: 10.1186/1748-5908-6-26
- Erchinger F, Tjora E, Nordaas IK, et al. Pancreatic enzyme treatment in chronic pancreatitis: quality of management and adherence to guidelines–A cross-sectional observational study. United Eur Gastroenterol J. 2022;10(8):844–853. doi: 10.1002/ueg2.12276
- Bellini M, Tosetti C, Costa F, et al. The general practitioner’s approach to irritable bowel syndrome: from intention to practice. Dig Liver Dis. 2005;37(12):934–939. doi: 10.1016/j.dld.2005.06.011
- Bellini M, Gambaccini D, Salvadori S, et al. Management of chronic constipation in general practice. Tech Coloproctol. 2014;18(6):543–549. doi: 10.1007/s10151-013-1093-9
- Butzlaff M, Kempkens D, Schnee M, et al. German ambulatory care physicians’ perspectives on clinical guidelines – a national survey. BMC Fam Pract. 2006;7(1):1–10. doi: 10.1186/1471-2296-7-47
- Burgers JS, Grol RPTM, Zaat JOM, et al. Characteristics of effective clinical guidelines for general practice. Br J Gen Pract. 2003 Jan 1;53(486):15.
- Michie S, Lester K. Words matter: increasing the implementation of clinical guidelines. BMJ Qual Saf. 2005 Oct 1;14(5):367–370. doi: 10.1136/qshc.2005.014100
- Avanzini F, Corsetti A, Maglione T, et al. Simple, shared guidelines raise the quality of antihypertensive treatment in routine care. Am Heart J. 2002 Oct;144(4):726–732. doi: 10.1016/S0002-8703(02)00149-7
- Ting S. Multicolored simplified asthma guideline reminder (MSAGR) for better adherence to national/global asthma guidelines. Ann Allergy Asthma Immunol. 2002;88(3):326–330. doi: 10.1016/S1081-1206(10)62016-9
- Wong E, Taylor Z, Thompson J, et al. A simplified gentamicin dosing chart is quicker and more accurate for nurse verification than the BNFc. Arch Dis Child. 2009 Jul;94(7):542–545. doi: 10.1136/adc.2007.137026
- Cabrera C, Casanova C, Martín Y, et al. Agreement between a simple dyspnea-guided treatment algorithm for stable COPD and the GOLD guidelines: a pilot study. Int J Chron Obstruct Pulmon Dis. 2016 Jun 8;11(1):1217–1222. doi: 10.2147/COPD.S100853
- Russell K, Addiman S, Grynszpan D, et al. The impact of new national guidance for the public health management of enteric fever in England. Public Health. 2018 Jan 1;154:79–86. doi: 10.1016/j.puhe.2017.10.018
- Bates AN, Ercolano E. Development and implementation of a simple wound care guideline for minor skin lesions: a quality improvement project. J Wound, Ostomy Cont Nurs. 2021;48(4):285–291. doi: 10.1097/WON.0000000000000778
- Dahm P, Yeung LL, Gallucci M, et al. How to use a clinical practice guideline. J Urol. 2009;181(2):472–479. doi: 10.1016/j.juro.2008.10.041