ABSTRACT
Introduction
New evidence supports the benefits of bolus feeding for children receiving home enteral feeding (HEN). Current home methods of bolus feeding have certain limitations, particularly in mobile or restless patients. Therefore, innovative delivery methods have been introduced to provide more flexible methods of reducing feeding time and formula handling.
Areas covered
This manuscript presents an expert review of the updates in HEN for children and the results of an online user experience questionnaire about an innovative new cap-based bolus feeding system. A literature bibliographic search was conducted on Medline via PubMed up to September 2023 to collect relevant studies. We presented recent evidence demonstrating a dramatic increase in HEN use among children requiring EN and its benefits on patients’ nutritional status and quality of life. In addition, the article examined the clinical and social benefits of bolus feeding and current challenges in delivery methods. We described the benefits of the new system and its user experience.
Expert opinion
The uses and indications for bolus feeding in HEN are increasing among children. However, there are still some unmet needs regarding traditional delivery methods. Innovative techniques can improve flexibility, reduce feeding time, and improve user experience and quality of life.
1. Introduction
Enteral nutrition (EN) is the preferred route of nutritional support in children with a functioning gastrointestinal (GI) tract who cannot meet their nutritional needs through oral intake [Citation1]. The benefits of EN in children are well documented in terms of providing adequate and balanced nutrition for optimal growth, development, and overall patient outcomes [Citation2,Citation3].
Due to recent advances in percutaneous endoscopic gastrostomy techniques and the shift to more cost-effective community-based care, more and more patients are candidates for long-term EN at home, known as Home Enteral Nutrition (HEN) therapy [Citation4]. Despite the paucity of epidemiological studies, recent data suggest a significant increase in the number of children receiving HEN. For instance, a report from Poland showed that the use of HEN increased from 104.1 cases in 2010 to 270.3 per million children in 2018 [Citation5]. HEN is an easy-to-administer, quantifiable, and safe nutritional support for a broad spectrum of debilitating conditions, including neurological disorders, head and neck cancer, upper GI malignancies, malnutrition, and failure to thrive [Citation6].
Continuous and intermittent feedings are common delivery methods for commercially available EN formula. Continuous feeding provides a steady flow of pediatric enteral formula over an extended period of time, often 16 to 24 hours a day, and is commonly used in critical care facilities. On the other hand, intermittent feeding involves the cyclic delivery of smaller amounts of feed, typically every few hours of the day or night, via an automatic pump [Citation7,Citation8]. Both continuous and intermittent feeding showed beneficial effects in terms of optimal nutritional support, energy efficiency, and mucosal stimulation for adequate absorption [Citation7,Citation9].
However, even if portable pumps allow to continue home protracted EN programs, the lack of flexibility – particularly in mobile children, the complexity of home administration, and the growing interest in mixed feeding has shifted the practice of HEN toward a more flexible bolus feeding approach [Citation4].
Bolus feeding allows the administration of a given amount of food at specific intervals (usually 3–6 times per day) for short periods (each 4–10 minutes) [Citation7]. This method is similar to physiological eating habits and can be easily performed at home, promoting patient mobility and independence. Previous reports indicated that bolus feeding also stimulated a physiological pattern of GI hormone release, GI development, and protein accumulation [Citation10,Citation11] and positively impacted biological rhythms, body composition, and metabolic response [Citation12].
It was also found that bolus feeding had comparable aspiration risk and food tolerance to continuous feeding [Citation13–15]. In addition, bolus tube feeding is characterized by ease of administration via syringe or, less commonly, pump [Citation16]. However, current home methods of bolus feeding have certain limitations, particularly in mobile or restless patients with excessive movement. According to the European Society for Clinical Nutrition and Metabolism (ESPEN) consensus on HEN, it is crucial to consider activity, social interactions, and other aspects of the patient’s quality of life when deciding on HEN administration methods [Citation17]. Therefore, innovative delivery methods such as the new cap-based bolus feeding system SimpLinkTM (Nestle Health Science, Switzerland) have been introduced to provide more flexible delivery methods, thereby reducing feeding time and formula handling.
This expert opinion article provides an overview of the latest trends and updates in HEN for children with functioning GI requiring nutritional support. We also examined the current challenges in delivery methods and the benefits of new techniques.
1.1. Review development
A bibliographical literature search on Medline via PubMed from its inception to September 2023 to collect the most relevant articles and support the present expert opinion article. The following keywords were used in the literature search: (Enteral nutrition [Mesh] OR tube nutrition[Mesh] OR enteral nutrition) AND (Home Care Services[Mesh] OR home OR Home Care) AND (Pediatrics[Mesh] OR Child[Mesh] OR children OR children). Advanced search strategies were used to retrieve relevant literature, including using Boolean operators, filters for language (English), and article types (peer-reviewed, clinical studies). A manual check of the relevant references supplemented the bibliographic online search.
Additionally, this review presented the results of a two-step Delphi-based questionnaire that was distributed among the authors to evaluate users’ experience with the new cap-based bolus-feeding technology SimpLinkTM (Nestlé Health Science, Switzerland). An online questionnaire was distributed from January to March 2023 to evaluate the general perception of the authors, as pediatric specialists, toward the new SimpLink device and user experience and to characterize a patient profile that could most benefit from the SimpLink.
2. HEN for children
2.1. Current trends in the use and indications of HEN in children
The use of EN in children mainly depends on the age, the underlying acute or chronic diseases, and the condition of the patient [Citation3]. In general, EN is the preferred route of nutritional support for children with a functioning GI tract who cannot meet their nutritional needs through oral intake or for whom oral intake is contraindicated [Citation18]. Multiple patient cohorts are indicated for EN. Children who have GI disorders that affect nutrient absorption or cause severe vomiting or diarrhea, such as inflammatory bowel disease, celiac disease, or short bowel syndrome, account for the majority of patients who need EN to meet their nutritional needs [Citation19]. EN has also been shown to be useful in children with food intolerance or allergies [Citation20]. EN is often indicated in children who are unable to thrive or in critically ill patients with a functioning GI tract [Citation19]. Other common indications for EN in children are neuromuscular disorders associated with dysphagia or risk of aspiration, malignant malnutrition, and pre- and postoperative care [Citation21].
Interest in HEN has increased over the past few decades, particularly among patients who require long-term EN. Epidemiological studies from the U.S.A. indicate that the use of HEN doubled between 1989 and 1992 [Citation22]. Recent data among adults showed a dramatic increase in the prevalence of HEN in the US, United Kingdom (UK), Poland, Italy, and Spain, and other European countries [Citation23–28]. Likewise, a growing number of studies showed an increased trends of HEN use in children (). Diamanti et al [Citation29]. retrieved data from children who received HEN in four Italian centers between 1996 and 2009 and showed a dramatic increase in children who received HEN over those 14 years. The main indications for HEN were neurogenetic disorders and digestive disorders, followed by congenital heart and lung diseases, with the median age at the start of HEN therapy being two years. Another report from southern Italy showed that the number of children with HEN increased from 55 cases in 2006 to 101 in 2008. The vast majority of patients had neurological disorders, followed by chronic bowel failure and cancer [Citation30]. A study from France on 4196 children receiving HEN found that the most common indications were digestive disorders (35%) and neuromuscular diseases (35%), followed by cancer (11%) and failure to thrive (8%) [Citation21]. Another report from France showed that the number of children with HEN increased from 16 in 1990 to 200 patients in 2000, with 65 new patients observed annually since 1999 [Citation32].
Table 1. A summary of studies reporting the overall HEN prevalence and indications in children.
A recent report from Poland retrieved data from the National Health Fund to assess the characteristics of children using commercial enteral feeding at home (HAN). Over a nine-year follow-up, the number of children using HEN increased from 743 in 2010 to 1,875 in 208 (a 2.5-fold increase). The overall prevalence of HEN increased from 104.1 cases in 2010 to 270.3 per million children in 2018. The most common indications were neurological disorders (21.9%) and endocrine, nutritional, or metabolic diseases (21.9%) [Citation5]. These results were consistent with another national survey from Poland, which showed an increase in the prevalence of HEN in children from 11.34 cases per million in 2010 to 525 per million in 2011. The median age at the onset of HEN was six years, and almost two-thirds of the cases had neurological diseases [Citation31].
The significant increase in HEN use in children can be attributed to several reasons. Advances in nutritional technology have greatly improved the safety and efficacy of HEN devices, making them more acceptable and easier to use for caregivers and patients in the home [Citation33]. From an economic perspective, HEN is a potentially more cost-effective strategy than hospital care, as it shortens the length of hospital stay and minimizes malnutrition-related readmissions [Citation34]. Healthcare policies that emphasize patient-centered care and shorten hospital stays may have contributed to the increased acceptance of HEN [Citation6]. Additionally, HEN can be a preferred option for children and their families as it allows for nutritional support while being able to enjoy the comfort of their homes [Citation35]. The growing interest in mixed diets may also have contributed to the increased use of HEN [Citation36].
2.2. Impact of HEN on patients’ nutritional status and quality of life
The clinical outcomes of HEN have increasingly been studied in randomized trials and observational studies. Regarding nutritional status, several studies have shown a reduction in the risk of malnutrition and improvement in body composition, indicating improved protein status and overall nutritional status in patients receiving HEN treatment [Citation37,Citation38]. The HEN also demonstrated safety, feasibility, and acceptability among patients and their caregivers [Citation35]. The impact of HEN on quality of life (QoL) appears to be well documented in adult patients. Previous studies and observational studies showed that HEN was associated with a significant improvement in quality of life in patients with cancer after oesophagectomy [Citation39], patients on chemoradiotherapy [Citation40], and malnourished patients with bowel failure [Citation38].
In children, HEN has shown significant improvements in body weight, height, body mass index (BMI), and overall nutritional status in children with chronic conditions following a gastrostomy tube [Citation41]. HEN significantly improved the growth profile in children with neurological impairment and reduced gastric reflux and aspiration [Citation42]. In terms of the impact of HEN on quality of life, the ability to regulate a child’s nutritional needs at home provides a sense of normality and may help reduce stress and anxiety associated with hospital visits and stays. Being at home can also facilitate a child’s educational continuity and allow them to lead a normal life despite their illness. In addition, HEN enables the child to participate in regular daily activities and social events, thereby contributing to their psychological and emotional well-being [Citation35,Citation43]. Recently, Dipasquale et al. [Citation44] conducted a cross-sectional study in three Italian centers recruiting caregivers of neurologically impaired children with HEN. The results showed an acceptable to excellent health-related quality of life and underscored the positive impact of HEN on the quality of life of children and their caregivers.
3. Access and methods of administration of HEN in children
The decision on EN access devices and delivery methods depends on the patient’s age, disease status, digestive and absorptive capacity of the GI tract, expected duration of therapy, and risk of aspiration. In addition, technical experience or costs can play a role in decision-making [Citation3]. Below, we discuss the advantages and limitations of commonly used routes of administration and HEN delivery methods.
3.1. Access devices for HEN
Nasogastric (NG) tubes are a short-term enteral feeding option that directs food into the stomach. While NG tubes are the most effective and easiest route to insert for patients requiring short-term EN (<4 weeks), they carry a high risk of obstruction and aspiration [Citation45].
Gastrostomy tubes (G-tubes), on the other hand, are intended for long-term use and are inserted endoscopically through the abdominal wall directly into the patient’s stomach. They are suitable for patients who require long-term EN because they allow more flexibility in daily activity and pose a lower risk of aspiration [Citation46]. Percutaneous endoscopic gastrostomy (PEG) and sometimes surgically placed gastrostomies are the most commonly used access procedures for long-term EN in children. The body of evidence favors PEG over NG tubes in patients requiring long-term EN. Previous studies and systematic reviews showed that PEG or percutaneous endoscopic jejunostomy (PEJ), when indicated, was either as effective or even more effective than NG tubes and was associated with a reduced risk of tube dislocation [Citation47] and postoperative wound infection [Citation48], and intervention failure [Citation49]. It was also found that PEG was associated with a better quality of life than NG tubes [Citation50]. In children receiving HEN, PEG nutrition significantly improved anthropometric measures and nutritional status [Citation41,Citation51,Citation52]. In addition, PEG was associated with acceptable levels of satisfaction and improved quality of life in children and caregivers [Citation53]. Therefore, PEG is the preferred access tool for children requiring long-term (>4 weeks) HEN [Citation17].
3.2. Feeding methods for HEN and the benefits of bolus feeding
The methods of EN feeding are typically classified into continuous, intermittent, and bolus feeding (). ‘Continuous’ means the continuous delivery of a predefined amount of formula over a period of time, typically 24 hours, via a pump. Continuous feeding is often used in critical care settings or when the patient’s GI system cannot process large amounts of formula at once. While continuous feeding can be used for HEN in certain clinical settings, it is typically unsuitable for most children receiving HEN due to the lack of flexibility and normal daily activities, as well as the complexity of home administration [Citation4,Citation7,Citation8]. Cyclic feeding can be administered for less than 24 hours as a transition phase from continuous feeding to stimulate the patient’s appetite [Citation7].
Table 2. Types and characteristics of the feeding methods for EN in clinical practice [Citation7].
With intermittent feeding, small amounts of feed are cyclically released, typically every few hours (every 4–6 hours for almost 60 minutes each) during the day or night via an automatic portable pump or gravity [Citation7,Citation8]. With the advancement of pump technology, it is now possible to provide intermittent feedings overnight without interrupting daily activities or sleep, as no flow adjustments are required at night. Additionally, mobile portable feeding pumps with lighter weight and more user-friendly operating systems are now available, which have improved their acceptability among patients receiving HEN [Citation54]. However, intermittent pump feeding does not fully resemble normal feeding patterns and may present certain limitations for mobile, restless patients who desire short feeding times or require less frequent feeding sessions, a profile commonly seen in children. According to the ESPEN guideline on HEN, the social and daily activities of the patients should be taken into account [Citation17].
In turn, the use of bolus feeding in HEN has attracted growing interest in both adult and pediatric cohorts over the past few decades. Bolus feeding is a quick and easy-to-use enteral feeding method in which the infant’s formula is given nearly 3–6 times a day over a short period of time (typically 4–10 minutes). Recent studies have shown a growing trend toward bolus feeding in HEN. A multicenter cross-sectional survey from the UK (n = 1830) showed that 37% of adult patients received HEN via bolus tube feeding. The use of bolus tube feeding has been observed frequently in patients with neurological diseases (41–57%) and patients with head and neck cancer (45%). PEG was the most common route of bolus feeding (72%), followed by a low-profile G-tube (12%) [Citation4]. A national survey from Poland, collecting data from 4586 adult patients having HEN, found that bolus feeding was the most commonly used method (74.4%), with PEG being the main use [Citation55]. Another multicenter study collected data from 23 centers in seven European countries and found that 34.1% of patients having HEN used bolus feeding [Citation28].
Limited data exist regarding the prevalence of bolus feeding in children receiving HEN. In a recent report from Italy, Diamanti et al. [Citation42]. retrospectively analyzed the data of neurologically impaired children who received HEN between 2011 and 2019. The results showed that nearly one-third of the children with HEN receive bolus feeding. At the same time, other reports showed a higher prevalence approaching 92% [Citation44].
Several benefits have been associated with bolus feeding, which may account for its increasing use in HEN. From a biological perspective, bolus feeding mimics normal physiological eating patterns, potentially leading to greater satiety and more physiological release of gut hormones [Citation56]. It was found that bolus feeding resulted in better satiety effects and a more physiological release of circulating ghrelin than continuous feeding in healthy adults [Citation57,Citation58]. Furthermore, intermittent bolus feeding was associated with better accumulation of muscle protein and better GI development than continuous feeding [Citation59]. Although initial reports suggested that bolus feeding has lower food tolerance than continuous tube feeding, more recent evidence did not show significant differences in food tolerance and safety outcomes between bolus and continuous feeding [Citation13,Citation14,Citation60]. In a recent report by O’Connor et al. [Citation36], there was no significant difference in food tolerance between bolus and continuous feeding in patients with HEN-fed compound diets.
The physiological benefits of bolus feeding in patients with HEN are also related to no worse feeding outcomes compared to continuous feeding. When managed properly, bolus feeding can effectively meet nutritional needs, and previous studies have shown comparable outcomes in feeding intake between the two feeding methods [Citation36,Citation61,Citation62]. Bolus feeding can have several advantages from the patient and caregiver perspective and increase patient preference. Bolus feeding is inherently flexible and convenient, allowing feeding to be administered at times that suit the patient’s daily schedule. This flexibility can improve adherence to the diet, particularly in children, where traditional feeding schedules may not always be feasible. In addition, bolus feeding is a practical approach because formula amounts can be customized, and feeding methods can be easily transported outside the home [Citation63–65].
From a technical perspective, although enteral portable pumps are easy to use and user-friendly, syringe-assisted bolus feeding usually requires less technical expertise and less equipment than continuous or pump-assisted feeding. This simplicity can reduce the complexity of care and potentially reduce the burden on caregivers. Compared to continuous feeding, bolus feeding reduces the time required for feeding. This shorter feeding time can improve the patient’s freedom and quality of life, especially in-home care. The similarity of bolus feeding to normal eating habits can facilitate social integration, especially for children. It syncs meals with other family members, promotes a sense of normalcy, and potentially improves psychological well-being. Finally, bolus feeding may be safer for patients prone to restlessness or excessive exercise [Citation63–65].
The impact of these benefits on the use of bolus feeding was reviewed by Hubbard et al [Citation4]. In this survey, the top reasons for bolus feeding were complementation with an oral diet (39%), meal timing similarity (31%), ease of use (29%), and rapid method (23%). Another report also showed that patients and caregivers preferred bolus feeding due to its flexibility [Citation66]. The decision to start bolus feeding should be individualized based on the patient’s age, underlying disease, degree of dependency, dietary needs, and preferences [Citation17]. We recommend bolus feeding for mobile and active children who want short feeding times. Appropriate candidates for bolus feeding are restless patients and patients who are unable to remain in the same position for long periods. Candidates for bolus feeding may include patients who need to complement other feeding methods. On the other hand, bolus feeding can be difficult for the following patient groups: patients with NG tubes, patients who experience frequent nausea or vomiting, patients who require postpyloric feeding, and patients who may be uncomfortable with bolus amounts of food administered at one time.
3.3. Challenges in current bolus feeding techniques
Despite the known benefits, particularly for active children with HEN, bolus feeding has several limitations. If handled improperly, bolus feeding can pose a risk of malnutrition. The timing and amount of bolus delivery must be carefully adjusted to the patient’s individual nutritional needs. Patients may not be able to stick to their feeding schedule if they are uncomfortable with giving infant formula in public or if they require a high level of caregiver dependency [Citation63]. The risk of malnutrition may be increased in patients who receive a homemade mixed diet [Citation67]. In addition, there are technical and social challenges in the administration of bolus feeding, mainly due to the limitations of current bolus feeding delivery methods. Bolus feeding is most commonly done using a syringe or feeding pump [Citation4]. shows the limitations of these methods.
Table 3. Description and limitations of bolus feeding delivery methods in clinical practice.
One of the technical limitations of bolus feeding compared to continuous feeding methods is the need for frequent handling of feeding equipment and manipulation of the formula. This can increase the time caregivers spend preparing and administering feed and potentially increase the risk of feed contamination if proper hygiene protocols are not followed [Citation68]. Contaminated pediatric enteral formula increases the risk of diarrhea, infectious enterocolitis, septicemia, and pneumonia [Citation69]. In addition, bolus feeding is typically associated with longer pediatric enteral formula storage times, which may increase the risk of contamination and require appropriate storage conditions to prevent spoilage [Citation70].
From a social perspective, bolus feeding can lead to social limitations that impact the quality of life of children and their caregivers. Committing to multiple feeding sessions throughout the day can limit the ability to engage in normal activities of daily living, such as school, play, or recreational activities. At the same time, the social life of caregivers can be significantly affected by the need to be present when feeding is given. For caregivers, their personal, professional, and social responsibilities may be limited by the diet plan [Citation71,Citation72]. In addition, the practical challenges and time-consuming nature of bolus feeding can limit family meal options. Therefore, delivery methods involving longer handling time or a slow feeding rate (such as syringes) can be associated with significant social limitations and an impaired quality of life. According to Apezetxea et al. [Citation73], a bolus syringe was associated with a poorer quality of life than gravity and infusion pumps.
In turn, there is a need for innovative delivery methods in bolus feeding to address unmet needs in terms of reducing feeding burden and improving social activities and quality of life of patients.
4. New cap-based bolus feeding system SimpLinkTM (Nestlé Health Science, Switzerland): benefits and users’ experience
4.1. Benefits of the new cap-based feeding system
As discussed above, optimal delivery systems for home bolus feeding should include improved patient autonomy, reduced handling of formula and equipment (thereby reducing the risk of contamination), increased portability for greater patient mobility, simplified feeding preparation and delivery for Time savings and improved results provide overall adherence to diet plans. SimpLinkTM (Nestle Health Science, Switzerland) is a new cap-based bolus feeding system that connects the enteral formula directly to the feeding tube without the need for a syringe or other adapted equipment. The closure is connected to a collapsible, semi-rigid bottle that allows for tube feeding with reduced formula manipulation (). The SimpLinkTM technology can deliver up to 250 mL of the complete formula in a single dose to patients on tube feeding. In addition, the SimpLinkTM system is compatible with current nutritional devices via a secure ENFit connection to enable a secure connection and avoid the risk of spillage in restless or active patients [Citation74].
Figure 1. A graphical representation of SimpLink™ system. This new cap-based bolus feeding system is securely fixed onto Nestle Health Science’s SmartFlex™ collapsible semi-rigid bottles allowing secure and convenient administration of formula without the need for open administration through a syringe. SimpLink™ is compatible with ENFit feeding tubes, ensuring a tight connection.
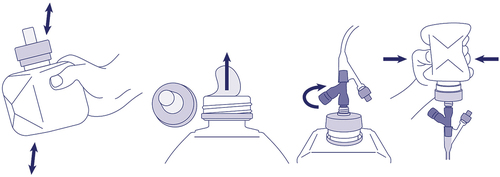
The SimpLinkTM cap offers several advantages over traditional bolus-feeding delivery methods. The SimpLinkTM system can potentially minimize the risk of contamination by eliminating the need to reuse equipment, empty feed into a syringe, and refill the syringe multiple times to deliver the required formula volume. In turn, this innovation significantly reduces the overall time required to prepare and administer feed while reducing the exposure of infant formula to potentially contaminating external environments. By eliminating a syringe, IV set, and/or pump, SimpLinkTM can improve patient autonomy and mobility. This also leads to a reduction in dependency on caregivers. From a practical point of view, the SimpLinkTM device helps reduce the stress of maintaining a regular feeding routine; This may reduce the number of missed feeds and improve food intake and adherence to diet plans. Previous reports indicated that a significant proportion of patients having HEN reported that duration of food administration, limited social events, and reduced mobility were the main reasons for restricted daily activities and impaired quality of life [Citation75].
Thus, SimpLinkTM can improve the QoL of the patients and their caregivers by improving their ability to participate in daily activities.
4.2. Users’ experience with SimpLink: results of the Delphi questionnaire
As part of the evaluation of SimplinkTM in clinical practice, we answered the online user experience questionnaire. The average monthly number of patients having EN per specialist was 41 patients who received an average of 127 ml per single bolus administration, to be reasonable. The polymer diet was the most commonly used formula in these patients (45%). Almost 15% of patients in clinical practice received bolus feeding via syringe. Satisfaction with bolus feeding by syringe was lower than with continuous and intermittent feeding.
Five authors rated the new cap-based bolus feeding system SimpLinkTM as very good, two as excellent, and the remaining one as good. Based on the individual items of the questionnaire, six authors rated SimpLinkTM as an excellent device in terms of hygiene. Other characteristics rated as excellent included safety, short preparation time, lack of leakage, and the convenience of this diet in an outdoor context. In addition, we used a semantics-based aggregation analysis to determine perspectives on the key benefits of SimpLinkTM. The analysis revealed that ease of use, security, and reduced preparation times were the key benefits of the new cap-based bolus feeding system. SimpLinkTM was chosen over the syringe in all aspects of the user experience, particularly ease of use, hygiene, and the amount of equipment needed.
There are no specific disadvantages of the new cap-based bolus feeding system. Nevertheless, we attach great importance to clearly explaining how the system works using user-friendly approaches such as videos. Compared to the peristaltic pump, SimpLinkTM also requires the assistance of a caregiver for administration. In addition, it is important that patients using SimpLinkTM must have a good tolerance to bolus feeding.
Based on the survey results, we believe that SimpLink’s stated beneficiaries should be characterized by several factors. This includes those over the age of 12 who require an active social life or those over the age of 5 who demonstrate a high level of EN tolerance and an active social life. Patients who are already balanced and bolus-accustomed, including both patients with neurological diseases and those dependent on EN nutrition for support, are also identified as potential candidates. Individuals with sufficient interaction skills to communicate dyspeptic symptoms should also be considered appropriate. Cerebral palsy patients equipped with PEG and fed via a bolus injection are also recommended as suitable candidates. Patients on bolus or mixed diets, particularly those eating meals away from home and stable patients with defined needs and set maximum tolerated infusion rates, may benefit from SimpLinkTM technology. On the other hand, patients fed by a portable peristaltic pump are not the best candidates for SimpLinkTM since caregivers are accustomed to the pump and continue to use it.
Regarding the impact of the SimpLinkTM device on quality of life, most authors rated the force required to deliver the formula as acceptable. Additionally, we believe that the need for fewer tools and ease of use on the go can significantly improve the quality of life for the ideal patient (a person who can tolerate a bolus) with this new system due to the positive impact on social activities with less risk of bacterial contamination.
5. Conclusion
In summary, the use of HEN in children has increased dramatically. Characterized by its practicality and flexibility, bolus feeding offers numerous potential benefits, including reduced feeding time, increased social integration, tailored nutrient delivery, and improved safety, particularly for restless or active patients. However, the success of bolus feeding depends on several patient-specific factors, and traditional bolus feeding methods (such as syringes and plungers) may limit the benefits of bolus feeding in some children. New technologies aimed at improving the efficiency and convenience of bolus feeding show promise and may improve the practice of HEN in children. The user experience of the novel cap-based bolus feeding system SimpLinkTM suggests that it offers significant advantages over traditional bolus feeding methods, such as administration with a syringe.
6. Expert opinion
Recent evidence shows a dramatic increase in HEN use in children, which positively impacts patients’ nutritional status and quality of life. Bolus feeding for HEN is increasing amongst children with functioning GI tract requiring nutritional support. As an alternative to using syringes, innovative techniques such as the SimpLinkTM system have been developed to improve flexibility and reduce feeding time. The management of HEN through this system can also improve the quality of life of the patient and his family or caregivers.
One of the most common side effects of bolus HEN by syringe is diarrhea. The SimpLinkTM system significantly reduces this risk. It significantly reduces the overall time required to prepare and administer feed while reducing infant formula exposure to potentially contaminating external environments. This also leads to a reduction in dependency on caregivers. In fact, we have children with medical complexity [Citation76,Citation77] who suffer from a congenital or acquired multisystem disorder, a severe neurological disease with marked functional impairment and technology dependence in activities of daily living. Many of them are entitled to palliative care and are completely dependent on the support of carers; most of them are on HEN. Easy-to-use and user-friendly nutritional care systems can be extremely useful for these caregivers, giving them more time for rehabilitation and social and family activities with other relatives and siblings.
In addition, many patients having HEN suffering from complex and chronic diseases (such as cancer, heart disease, congenital digestive and kidney diseases) are regularly included in an educational, social and work environment. Previous studies showed that a significant proportion of patients having HEN reported that feeding duration, limited social events and limited mobility were the main reasons for limited daily activities and impaired quality of life. Treating EN with simplified delivery methods offers significant advances in this clinical setting. Therefore, the method of delivering EN using SimplinkTM could become the method of choice for clinically stable patients who need to access EN in work and school contexts.
From a metabolic point of view, the SimplinkTM system allows for a better insulinemic and glycemic response comparable to that after an oral meal. Many GI symptoms, such as diarrhea and constipation, are better controlled in patients fed with SimplinkTM. Bolus feeding mimics a ‘normal’ eating pattern. The practice of bolus feeding has evolved over time in response to the preferences and needs of individual patients using enteral tube feeding, their social circumstances, and the experience of the healthcare professionals caring for them. The timing of bolus nutrition administration can be optimized to give patients some control over when they want to receive their nutrition and to allow for uninterrupted activity/rehabilitation sessions. For restless patients who move around in bed or are unable to maintain an upright position for long periods, meals may be given when the patient is in the correct position to allow a break between meals and gastric pH to be restored, which may help minimize gastric acid colonization. With regular feeding intervals, physicians should try to meet the patient’s fluid needs during bolus feedings and medication administration to reduce the frequency of bolus feedings and flushes required throughout the day. If there are long gaps between feedings, physicians should maintain hydration with water rinses before and after each feed. Further clinical studies with larger numbers of patients could be useful to confirm these results.
Article highlights
Recent data suggest a significant increase in the number of children receiving home enteral nutrition (HEN) therapy.
Bolus feeding mimics physiological eating habits, promoting patient mobility and independence. However, current home methods of bolus feeding have certain limitations, particularly in mobile or restless patients with excessive movement.
An ideal delivery method for bolus HEN feeding should consider activity, social interactions, and other aspects of the patient’s quality of life.
A new cap-based bolus feeding system (SimpLinkTM, Nestlé Health Science, Switzerland) can improve flexibility and reduce feeding time, positively impacting user experience and patients’ quality of life.
In a Delphi-based questionnaire, we highlighted the positive users’ experience with the new cap-based bolus feeding system regarding hygiene, safety, short preparation time, lack of leakage, and convenience in an outdoor context.
Declaration of interests
C Romano received consulting fees from Nestlè Health Science. P Lionetti received consulting fees from Nestlé Health Science, Takeda, Firma Menarini, Dr Falk, and Sandoz in the last 36 months. A Diamanti participated in Data Safety Monitoring Board and served in a Fiduciary role on the advisory boards of Nutricia, Takeda, and Baxter. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors acknowledge Content Ed Net Switzerland for their medical writing assistance.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Mehta NM, McAleer D, Hamilton S, et al. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN J Parenter Enteral Nutr. 2010;34(1):38–45.
- Mehta NM, Bechard LJ, Cahill N, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children—an international multicenter cohort study. Crit Care Med. 2012;40(7):2204–2211.
- Yi DY. Enteral nutrition in pediatric patients. Pediatr Gastroenterol Hepatol Nutr. 2018;21(1):12–19. doi: 10.5223/pghn.2018.21.1.12
- Hubbard GP, Andrews S, White S, et al. A survey of bolus tube feeding prevalence and practice in adult patients requiring home enteral tube feeding. Br J Nutr. 2019;122(11):1271–1278.
- Wyszomirska K, Wyszomirski A, Brzeziński M, et al. Home artificial nutrition in polish children: an analysis of 9-year national healthcare provider data. Nutrients. 2021;13(3):1007–1011.
- Martin K, Gardner G. Home enteral nutrition: updates, trends, and challenges. Nutr Clin Pract. 2017;32(6):712–721. doi: 10.1177/0884533617701401
- Ichimaru S. Methods of enteral nutrition administration in critically ill patients: continuous, Cyclic, intermittent, and bolus feeding. Nutr Clin Pract. 2018;33(6):790–795. doi: 10.1002/ncp.10105
- Fogg L. Home enteral feeding part 1: an overview. Br J Community Nurs. 2007;12(6):250–52. 246, 248. doi: 10.12968/bjcn.2007.12.6.23771
- Serpa LF, Kimura M, Faintuch J, et al. Effects of continuous versus bolus infusion of enteral nutrition in critical patients. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:9–14. doi: 10.1590/S0041-87812003000100003
- Krom H, de Winter JP, Kindermann A, et al. Development, prevention, and treatment of feeding tube dependency. Eur J Pediatr. 2017;176(6):683–688. doi: 10.1007/s00431-017-2908-x
- Littler H, Tume LN. Is bolus or continuous enteral feeding better in critically ill children: an evidence-based review. Nurs Crit Care. 2023;28(1):36–39. doi: 10.1111/nicc.12788
- Santos HO, Genario R, Tinsley GM, et al. A scoping review of intermittent fasting, chronobiology, and metabolism. Am J Clin Nutr. 2022;115(4):991–1004.
- Evans DC, Forbes R, Jones C, et al. Continuous versus bolus tube feeds: does the modality affect glycemic variability, tube feeding volume, caloric intake, or insulin utilization? Int J Crit Illn Inj Sci. 2016;6(1):9–15.
- Lee JSW, Auyeung TW. A comparison of two feeding methods in the alleviation of diarrhoea in older tube-fed patients: a randomized controlled trial. Age Ageing. 2003;32(4):388–393. doi: 10.1093/ageing/32.4.388
- Bowling TE, Cliff B, Wright JW, et al. The effects of bolus and continuous nasogastric feeding on gastro-oesophageal reflux and gastric emptying in healthy volunteers: a randomized three-way crossover pilot study. Clin Nutr. 2008;27(4):608–613.
- Cederholm T, Barazzoni R, Austin P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64.
- Bischoff SC, Austin P, Boeykens K, et al. ESPEN guideline on home enteral nutrition. Clin Nutr. 2020;39(1):5–22.
- Braegger C, Decsi T, Dias JA, et al. Practical approach to paediatric enteral nutrition: a comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;51(1):110–122.
- Heuschkel RB, Gottrand F, Devarajan K, et al. ESPGHAN position paper on management of percutaneous endoscopic gastrostomy in children and adolescents. J Pediatr Gastroenterol Nutr. 2015;60(1):131–141.
- Hays T, Kerner JA. Special considerations for managing food allergies. JPEN J Parenter Enteral Nutr. 2012;36(1 Suppl):56S–9S. doi: 10.1177/0148607111429127
- Daveluy W, Guimber D, Mention K, et al. Home enteral nutrition in children: an 11-year experience with 416 patients. Clin Nutr. 2005;24(1):48–54.
- Howard L, Ament M, Richard Fleming C, et al. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology. 1995;109(2):355–365.
- Mundi MS, Pattinson A, McMahon MT, et al. Prevalence of home parenteral and enteral nutrition in the United States. Nutr Clin Pract. 2017;32(6):799–805.
- British artificial nutrition survey (BANS) [Internet]. Accessed from: https://www.bapen.org.uk/screening-and-must/28-about-bapen/committees-and-groups/41-british-artificial-nutrition-survey-bans#:~:text=Overall%20Aim%20of%20BANS%3A&text=To%20monitor%20at%20national%20and,the%20use%2Flack%20of%20ANS
- Klek S, Pawlowska D, Dziwiszek G, et al. The Evolution of Home Enteral Nutrition (HEN) in Poland During Five Years After Implementation: a Multicentre Study. Nutrición Hospitalaria. 2015;32:196–201. doi: 10.1016/S0261-5614(15)30327-7
- Pironi L, Candusso M, Biondo A, et al. Prevalence of home artificial nutrition in Italy in 2005: a survey by the Italian Society for Parenteral and enteral nutrition (SINPE). Clin Nutr. 2007;26(1):123–132.
- Wanden-Berghe C, Luengo LM, Álvarez J, et al. Spanish home enteral nutrition registry of the year 2014 and 2015 from the NADYA-SENPE group. Nutrición Hospitalaria. 2017;34(1):15–18.
- Hebuterne X, Bozzetti F, Moreno Villares JM, et al. Home enteral nutrition in adults: a European multicentre survey. Clin Nutr. 2003;22(3):261–266.
- Diamanti A, Di Ciommo VM, Tentolini A, et al. Home enteral nutrition in children: a 14-year multicenter survey. Eur J Clin Nutr. 2013;67(1):53–57.
- Santarpia L, Pagano MC, Pasanisi F, et al. Home artificial nutrition: an update seven years after the regional regulation. Clin Nutr. 2014;33(5):872–878.
- Szlagatys-Sidorkiewicz A, Popińska K, Toporowska-Kowalska E, et al. Home enteral nutrition in children—2010 nationwide survey of the polish society for clinical nutrition of children. Eur J Pediatr. 2012;171(4):719–723.
- Daveluy W, Guimber D, Uhlen S, et al. Dramatic changes in home-based enteral nutrition practices in children during an 11-year period. J Pediatr Gastroenterol Nutr. 2006;43(2):240–244.
- Sevilla WMA, McElhanon B. Optimizing transition to home enteral nutrition for pediatric patients. Nutr Clin Pract. 2016;31(6):762–768. doi: 10.1177/0884533616673348
- Wong A, Goh G, Banks MD, et al. A systematic review of the cost and economic outcomes of home enteral nutrition. Clin Nutr. 2018;37(2):429–442.
- Boland K, Maher N, O’Hanlon C, et al. Home enteral nutrition recipients: patient perspectives on training, complications and satisfaction. Frontline Gastroenterol. 2017;8(1):79–84.
- O’Connor G, Hartfiel-Capriles Z, Saduera S. Intermittent bolus versus continuous feeding in children receiving an enteral formula with food derived ingredients: a national multicentre retrospective study. Clin Nutr ESPEN. 2023;54:175–179. doi: 10.1016/j.clnesp.2023.01.029
- Bowrey DJ, Baker M, Halliday V, et al. A randomized controlled trial of six weeks of home enteral nutrition versus standard care after oesophagectomy or total gastrectomy for cancer: report on a pilot and feasibility study. Trials. 2015;21(16):53.
- Gao X, Zhang Y, Zhang L, et al. Effect of home enteral nutrition on nutritional status, body composition and quality of life in patients with malnourished intestinal failure. Front Nutr. 2021;8:643907. doi: 10.3389/fnut.2021.643907
- Wu Z, Wu M, Wang Q, et al. Home enteral nutrition after minimally invasive esophagectomy can improve quality of life and reduce the risk of malnutrition. Asia Pac J Clin Nutr. 2018;27(1):129–136.
- Yu FJ, Shih HY, Wu CY, et al. Enteral nutrition and quality of life in patients undergoing chemoradiotherapy for esophageal carcinoma: a comparison of nasogastric tube, esophageal stent, and ostomy tube feeding. Gastrointest Endosc. 2018;88(1):21–31.e4.
- Martínez-Costa C, Calderón C, Gómez-López L, et al. Nutritional outcome in home gastrostomy-fed children with chronic diseases. Nutrients. 2019;11(5):11.
- Diamanti A, Capriati T, Mosca A, et al. Neurological impairment and malnutrition in children: the role of home enteral nutrition in real life. Front Nutr. 2023;10:10. doi: 10.3389/fnut.2023.1087603
- Backman E, Granlund M, Karlsson AK. Parental perspectives on family mealtimes related to gastrostomy tube feeding in children. Qual Health Res. 2021;31(9):1596–1608. doi: 101177/1049732321997133
- Dipasquale V, Ventimiglia M, Gramaglia SMC, et al. Health-related quality of life and home enteral nutrition in children with neurological impairment: report from a multicenter survey. Nutrients. 2019;11(12):11.
- Huerta G, Puri VK. Nasoenteric feeding tubes in critically ill patients (fluoroscopy versus blind). Nutrition. 2000;16(4):264–267. doi: 10.1016/S0899-9007(99)00307-X
- Alhaffaf FA, Alqahtani AS, Alrobyan AA, et al. Percutaneous endoscopic gastrostomy in children: a single center experience in Saudi Arabia. Saudi Med J. 2021;42(2):205–208.
- Wang J, Liu M, Liu C, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: A systematic review. J Radiat Res. 2014;55(3):559–567.
- Tabrizi R, Hosseinpour S, Taghizadeh F. Feeding in oral cancer patients after massive ablative surgery: percutaneous endoscopic gastrostomy or nasogastric tube. J Craniofac Surg. 2016;27(4):1010–1011. doi: 10.1097/SCS.0000000000002662
- Gomes Jr CA, Andriolo RB, Bennett C, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev. 2015;2017(1). doi: 10.1002/14651858.CD008096.pub4
- Corry J, Poon W, McPhee N, et al. Prospective study of percutaneous endoscopic gastrostomy tubes versus nasogastric tubes for enteral feeding in patients with head and neck cancer undergoing (chemo)radiation. Head Neck. 2009;31(7):867–876.
- Truby H, Cowlishaw P, O’dneil C, et al. The long term efficacy of gastrostomy feeding in children with Cystic Fibrosis on anthropometric markers of nutritonal status and pulmonary function. Open Respir Med J. 2009;3(1):112–115.
- Suh CR, Kim W, Eun BL, et al. Percutaneous endoscopic gastrostomy and nutritional interventions by the pediatric nutritional support team improve the nutritional status of neurologically impaired children. J Clin Med. 2020;9(10):1–13.
- Grzybowska-Chlebowczyk U, Wiecek S, Popińska K, et al. The evaluation of life quality of families of children after percutaneous endoscopic gastrostomy. Pediatr Pol. 2015;90(2):103–107.
- White H, King L. Enteral feeding pumps: efficacy, safety, and patient acceptability. Med Devices Evidence Res. 2014;291–298. doi: 10.2147/MDER.S50050
- Folwarski M, Bartoszewska L, Figuła K, et al. Home enteral nutrition in adults—nationwide multicenter survey. Nutrients. 2020;12(7):2087–2089.
- Chowdhury AH, Murray K, Hoad CL, et al. Effects of bolus and continuous nasogastric feeding on gastric emptying, small bowel water content, superior mesenteric artery blood flow, and plasma hormone concentrations in healthy adults: a randomized crossover study. Ann Surg. 2016;263(3):450–457.
- Stratton RJ, Elia M, Stubbs RJ. Short-term continuous enteral tube feeding schedules did not suppress appetite and food intake in healthy men in a placebo-controlled trial. J Nutr. 2003;133(8):2570–2576. doi: 10.1093/jn/133.8.2570
- Stratton RJ, Stubbs RJ, Elia M. Bolus tube feeding suppresses food intake and circulating ghrelin concentrations in healthy subjects in a short-term placebo-controlled trial. Am J Clin Nutr. 2008;88(1):77–83. doi: 10.1093/ajcn/88.1.77
- El-Kadi SW, Suryawan A, Gazzaneo MC, et al. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol - Endocrinol Metab. 2012;302(6):E674–E686.
- Mahoney LB, Liu E, Rosen R. Continuous feedings are not associated with lower Rates of Gastroesophageal reflux when compared with bolus feedings. J Pediatr Gastroenterol Nutr. 2019;69(6):678–681. doi: 10.1097/MPG.0000000000002464
- Bruch S, Paige T, Saez K, et al. Bolus versus continuous feeding regimens post gastrostomy tube placement in children. J Pediatr Surg. 2021;56(4):717–720.
- Theodoridis X, Chrysoula L, Evripidou K, et al. Continuous versus intermittent enteral feeding in critically ill children: a systematic review. Nutrients. 2023;15(2):288.
- Fletcher A. Bolus feeding: an overview. Internet. 2018.
- Bolus feeding in adults: A practical guide | Nutricia UK [Internet].
- Martin L, Blomberg J, Lagergren P. Patients’ perspectives of living with a percutaneous endoscopic gastrostomy (PEG). BMC Gastroenterol. 2012;12(1):12. doi: 10.1186/1471-230X-12-126
- Brotherton A, Abbott J, Aggett P. The impact of percutaneous endoscopic gastrostomy feeding upon daily life in adults. J Hum Nutr Diet. 2006;19(5):355–367. doi: 10.1111/j.1365-277X.2006.00712.x
- Vieira MMC, Santos VFN, Bottoni A, et al. Nutritional and microbiological quality of commercial and homemade blenderized whole food enteral diets for home-based enteral nutritional therapy in adults. Clin Nutr. 2018;37(1):177–181.
- Boullata JI, Carrera AL, Harvey L, et al. ASPEN safe practices for enteral nutrition therapy. J Parenter Enter Nutr. 2017;41(1):15–103.
- Anderton A. Bacterial contamination of enteral feeds and feeding systems. Clin Nutr. 1993;12:S16–S32. doi: 10.1016/S0261-5614(09)90005-X
- Ojo O, Adegboye ARA, Ojo OO, et al. The microbial quality and safety of Blenderised enteral nutrition formula: a systematic review. Int J Environ Res Public Heal. 2020;17(24):9563.
- Cuerda MC, Apezetxea A, Carrillo L, et al. Development and validation of a specific questionnaire to assess health-related quality of life in patients with home enteral nutrition. NutriQol® Development Patient Prefer Adherence. 2016;10:2289. doi: 10.2147/PPA.S110188
- Alsaeed D, Furniss D, Blandford A, et al. Carers’ experiences of home enteral feeding: a survey exploring medicines administration challenges and strategies. J Clin Pharm Ther. 2018;43(3):359–365.
- Apezetxea A, Carrillo L, Casanueva F, et al. The NutriQoL ® questionnaire for assessing health-related quality of life (HRQoL) in patients with home enteral nutrition (HEN): validation and fi rst results. Nutrición Hospitalaria. 2016;33(6):1260.
- Compleat® paediatric SimpLink® |. Nestlé Health Science. Internet
- Bjuresäter K, Larsson M, Athlin E. Patients’ experiences of home enteral tube feeding (HETF) – a qualitative study. J Res Nurs. 2015;20(7):552–565. doi: 10.1177/1744987114568655
- Cohen E, Kuo DZ, Agrawal R, et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- Kuo DZ, Houtrow AJ, Norwood KW, et al. Recognition and management of medical complexity. Pediatrics. 2016;138(6). doi: 10.1542/peds.2016-3021
