ABSTRACT
Objectives
Metabolic-associated fatty liver disease (MAFLD) has clinical relevance in patients with acute-on-chronic liver failure (ACLF). We investigated the association between MAFLD and prognosis in patients with ACLF.
Methods
We included patients with ACLF with available clinical data who visited our hospital for nearly 9 years. We compared the prognosis of patients in the different subgroups of ACLF and predicted the incidence of adverse outcomes. Moreover, a new model based on MAFLD was established.
Results
Among 339 participants, 75 had MAFLD. The prognosis of patients with ACLF was significantly correlated with MAFLD. Patients with ACLF with concomitant MAFLD tended to have a lower cumulative survival rate (p = 0.026) and a higher incidence of hepatorenal syndrome (9.33% versus 3.40%, p = 0.033) than those without MAFLD. We developed an TIM2 model and the area under the ROC curve of the new model for 30-day and 60-day mortality (0.759 and 0.748) was higher than other predictive methods.
Conclusion
The presence of MAFLD in patients with HBV-related ACLF was associated with an increased risk of in-hospital mortality. Moreover, The TIM2 model is a high-performance prognostic score for HBV-related ACLF.
1. Introduction
Hepatitis B virus (HBV) infections pose a threat to public health worldwide. It is a leading cause of mortality globally, with an estimated prevalence of 5–6% in China [Citation1]. HBV-induced liver injury is a major cause of liver failure, which is associated with high short-term mortality rates [Citation2]. Acute-on-chronic liver failure (ACLF), a common subtype of liver failure, was first reported by Ohnishi et al. [Citation3]. According to the Asia-Pacific ACLF consensus, ACLF is characterized by acute decompensation of chronic liver disease or cirrhosis and complications within 4 weeks of ascites and/or encephalopathy [Citation4]. Asian diagnostic criteria mainly focus on the clinical manifestations of liver failure for an early diagnosis [Citation5]. Timely treatment is critical for reversibility and good prognosis in the early phase of ACLF and for improving the survival rate of patients with liver failure [Citation6].
In recent years, the incidence of nonalcoholic fatty liver disease (NAFLD) has increased at an alarming pace owing to the obesity epidemic and improvements in living standards in China. Accumulating evidence suggests that NAFLD increases the risk of advanced fibrosis and all-cause mortality in patients with HBV [Citation7–9]. In Asia, emerging evidence highlights the importance of concomitant NAFLD in the development of hepatocellular carcinoma (HCC) in patients with HBV [Citation10,Citation11]. Therefore, concurrent NAFLD is associated with adverse liver-related outcomes in patients with chronic hepatitis B (CHB).
NAFLD is currently the main driver of the increased incidence of chronic liver disease and global public health burden [Citation12]. Diagnosis of NAFLD requires the exclusion of patients with other chronic liver diseases; hence, a need exists for ‘definitive’ diagnostic criteria [Citation13]. A panel of international experts have proposed a new definition for diagnosing metabolic-associated fatty liver disease (MAFLD) that is simple and comprehensive [Citation14]. More importantly, the new definition can effectively identify metabolically unhealthy normal-weight patients with MAFLD, referred to as ‘lean MAFLD,’ who exhibited both hepatic steatosis and metabolic disorders [Citation15]. MAFLD is more practical than NAFLD for identifying patients with fatty liver with a high risk of disease progression in the real world [Citation16]. Notably, MAFLD is associated with a higher risk of HCC or advanced fibrosis in patients with CHB [Citation17,Citation18]. A paucity of data exists regarding the clinical impact of MAFLD on the outcomes of patients with ACLF. Therefore, we aimed to evaluate the correlation between MAFLD and poor clinical prognosis in patients with HBV with ACLF. Additionally, MAFLD was used as a prognostic indicator in this study.
2. Methods
2.1. Eligible participants
In this retrospective single-center study, we identified and included patients with ACLF who were hospitalized at the Department of Hepatology Research Institute of the First Affiliated Hospital, Fujian Medical University between 2013 and 2022. ACLF was diagnosed according to the latest guidelines (the Asian Pacific association for the study of the liver definition, 2019) [Citation4]. Patient complications were also assessed using these guidelines. The criteria for diagnosing MAFLD are based on a radiological or ultrasound diagnosis of fatty liver and the presence of any one of the following three conditions: overweight/obesity, diabetes mellitus (DM), or evidence of metabolic dysregulation [Citation14]. All patients with ACLF were excluded if they had the following conditions at baseline or follow-up: (1) de novo tumors; (2) other concomitant liver diseases (including autoimmune liver disease, alcoholic liver disease, human immunodeficiency virus, and hepatitis C virus coinfection); and (3) liver transplantation, pregnancy, or other fatal diseases. The requirement for informed consent was waived owing to the retrospective nature of the study, which was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University, China.
2.2. Data acquisition
Data on patient demographics (i.e. sex, age, and body mass index [BMI]) and clinical information (history or presence of DM or hypertension) were collected from the electronic medical record system by two well-trained investigators. Baseline biochemical and hematological data were also retrieved, including prothrombin time; international normalized ratio; total bilirubin (TBIL), alanine aminotransferase, albumin, fasting blood glucose (FBG), C-reactive protein, triglyceride, and high-density lipoprotein levels; and platelet count. The Model for End-Stage Liver Disease (MELD), Child-Turcotte-Pugh (CTP), and MELD-Na scores were calculated using the latest published criteria [Citation19–21]. Data on clinical laboratory variables were collected within 48 h of admission.
2.3. Endpoint outcomes
The primary endpoint of the study was death. We also observed occurrences of other adverse clinical events, including spontaneous peritonitis (SBP), ascites, hepatic encephalopathy (HE), hepatorenal syndrome (HRS), gastrointestinal hemorrhage, and other infections. Follow-ups started on the date of the first hospital admission and ended on the date of death or 60 days. Clinical outcome data were obtained using an electronic medical record system.
2.4. Statistical analyses
Statistical analyses were performed using SPSS (Version 23.0) and R (version 3.5.1). Continuous variables are expressed as mean ± standard deviation and were further evaluated using Student’s t-test (for normally distributed variables) and the Mann – Whitney U-test (for non-normally distributed variables). Categorical variables are described using frequencies and proportions, and the chi-square test was used to investigate differences between groups.
A Cox proportional hazards regression analysis was used to estimate hazard ratios (HRs) for the association between MAFLD and all-cause mortality. Variables with p < 0.05 from univariable analyses were included in a multivariable Cox model. A prognostic model was built on the basis of the results of the Cox regression analyses and presented as a nomogram. Decision curve analysis (DCA) and Calibration curve analysis were used to evaluate the clinical utility and accuracy of the model. Kaplan – Meier survival curves were generated, and a log-rank test was performed to compare differences in overall survival between the two groups. The area under the receiver operating characteristic curve (AUC) was measured and compared to evaluate the predictive ability of the different models. All tests were two-tailed, and a p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Characteristics and demographics of the study population
A study flowchart is shown in . Our study cohort included 339 patients with ACLF, of whom 83.19% were men, with a mean age of 49.98 ± 13.49 years. The patient characteristics stratified by the presence or absence of MAFLD are shown in . The MAFLD group included more patients with DM (32.0% versus 13.64%, p < 0.000) and hypertension (26.67% versus 15.15%, p = 0.021) than did the non-MAFLD group. The patients in the MAFLD group had higher BMI (23.14 ± 3.61 versus 21.64 ± 3.31, p < 0.000) and FBG (5.23 ± 3.75 versus 4.89 ± 8.37, p = 0.007) levels than did patients in the non-MAFLD group. The CTP, MELD, and MELD-Na scores were not statistically different between the MAFLD and non-MAFLD groups. The mortality rate in the MAFLD group was significantly higher than that in the non-MAFLD group (56.00% versus 31.44%, p = 0.034).
Figure 1. The flowchart of study design and patient enrollment. HBV: hepatitis B virus; ACLF: acute-on-chronic liver failure; MAFLD: metabolic associated fatty liver disease.
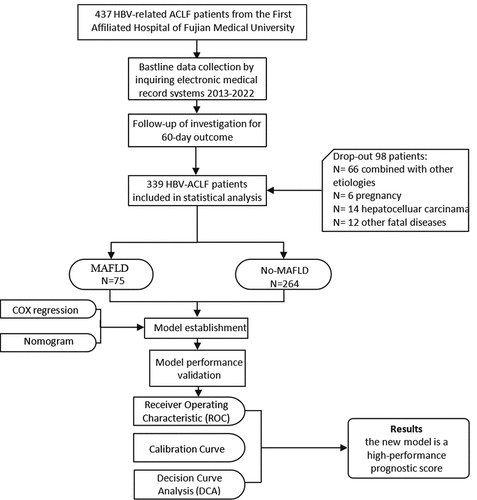
Table 1. Demographic and characteristics of the study cohort.
3.2. Incidence of complications in the MAFLD and non-MAFLD groups
Although ascites was the most frequent complication in patients with ACLF (), most cases were mild. The MAFLD group had a higher incidence of HRS complications than did the non-MAFLD group (p = 0.033). However, there were no significant differences in the other clinical complications, including ascites, HE, and GIB, between the two groups. The common sites of infection among the participants with ACLF were the SBP (47/339, 13.86%) and lungs (95/339, 28.02%); other sites were the urine, blood, cholecyst, and intestines. No significant difference was observed in the occurrence of infectious events between the two groups.
Table 2. The prevalence of complications between MAFLD and non- MAFLD patients.
3.3. Development of a new prognostic model and nomogram
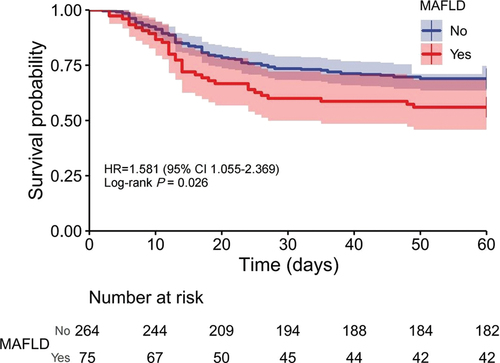
shows that the cumulative survival rate of patients was significantly distinguished between the MAFLD and non-MAFLD groups (p = 0.0031). With an increase in the follow-up time, concomitant MAFLD was associated with a higher mortality risk in patients with ACLF.
Figure 2. Cumulative survival time was stratified by presence or absence of MAFLD in HBV-related ACLF. MAFLD: metabolic associated fatty liver disease.
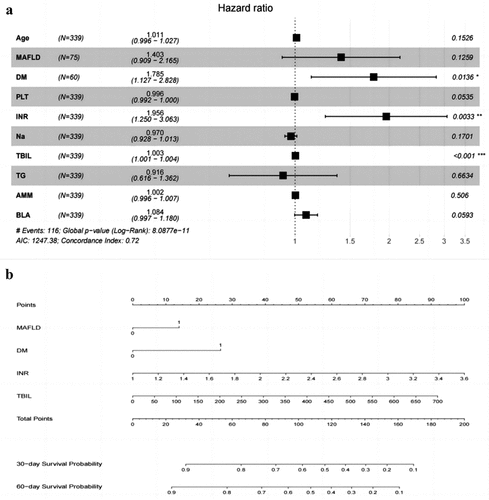
Univariable and multivariable HRs for the key characteristics of predictive death are shown in . The risk factors that were statistically associated with death in univariable analyses were included as covariates in the multivariate regression analysis. The following independent risk factors based on multivariate Cox regression analysis could be used for a new prognostic model: DM, INR,and TBIL. Based on the result of our study, MAFLD was significantly correlated with the death of liver failure and therefore was included in the model. Ultimately, the new prognostic model for ACLF was constructed as follows: TIM2 = 0.339 × MAFLD (yes, 1; no, 0) + 0.579 × DM (yes, 1; no, 0) + 0.003 × TBIL (mmol/L)+ 0.671 ×INR.
Table 3. Hrs for death of the patient with HBV-related ACLF.
Furthermore, the nomogram and forest plot were constructed as shown in . As shown in the , the total points accumulated by the relative variables predicted individual survival probabilities at 30 or 60 days. The forest plot illustrates the correlation between each risk factor and ACLF prognosis ().
Figure 3. Forest plot and nomogram for patients with HBV-related ACLF. Forest plot of prognostic risk factors for patients with HBV-related ACLF based on multivariate cox regression analysis (a). The nomogram predicted the probability of 30- and 60-day survival rate (b). HBV: hepatitis B virus; ACLF: acute-on-chronic liver failure; MAFLD: MAFLD: metabolic associated fatty liver disease; DM: diabetes mellitus; PLT: platelet count; INR: international normalized ratio; na: Natriumion; TBIL: total bilirubin; TG: Triglyceride; AMM: Ammonia; BLA: blood lactic acid; ACLF: acute-on-chronic liver failure.
3.4. Discriminative performance and clinical usefulness of the new model in prognostic prediction
The AUC of TIM2 for 30-day and 60-day mortality was 0.759 and 0.748 (), which was significantly higher than those of the CTP, MELD, and MELD-Na scores (AUC = 0.680 and 0.682, 0.682 and 0.673, 0.692 and 0.681, respectively; all p < 0.001; ). The calibration curve of the TIM2 had good consistency for the 30-day and 60-day prognosis of patients with HBV-related ACLF; meanwhile, the DCA showed that use of the TIM2 for prognostic prediction provided more benefit than that of other scores ().
Figure 4. Prediction of 30-day (a) and 60-day (b) mortality for patients with HBV-related ACLF according to the TIM2 and other scores. CTP: child-Turcotte-Pugh; MELD: Model for end-stage liver disease; MELD-Na: Model for end-stage liver disease with serum sodium; AUC: area under the receiver operating characteristic curve.
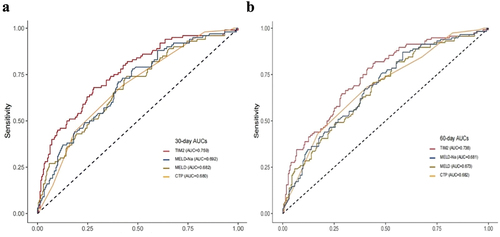
Figure 5. Calibration curves analysis and DCA of the TIM2. The calibration curve and DCA for the 30-day (a,b) and 60-day (c,d) prognosis of patients. CTP: child-Turcotte-Pugh; MELD: Model for end-stage liver disease; MELD-Na: Model for end-stage liver disease with serum sodium; DCA: decision curve analysis.
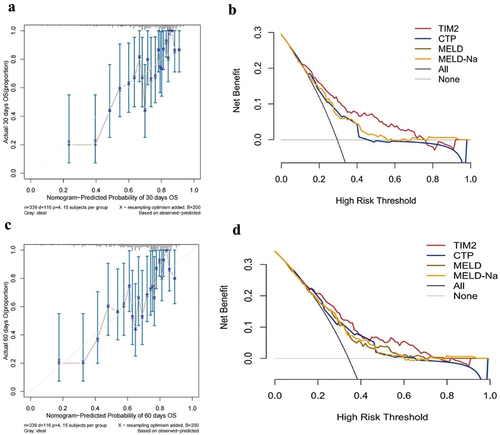
Table 4. Comparison of the predictive prognosis of different models for patients with HBV-related ACLF.
4. Discussion
In recent years, the role of abnormal lipid metabolism in patient with ACLF has attracted enough attention, and lipid metabolism disorders may play a important role in the development of ACLF [Citation22]. To the best of our knowledge, this is the first study to demonstrate that MAFLD is independently associated with adverse outcomes in patients with HBV-ralated ACLF. The main finding of our study was that, compared with the non-MAFLD group, the concomitant MAFLD group was associated with an increased risk of mortality and HRS.
ACLF is a complex pathological process associated with adverse outcomes [Citation23]. Effective prognostic predictors would help identify more patients at risk of ACLF to guide clinical management and decrease mortality [Citation24,Citation25]. The results of our study revealed that concomitant MAFLD was a prognostic factor for patients with ACLF, and the incidence of mortality was higher in the MAFLD group than in non-MAFLD group. DM, TBIL, and INR were independent prognostic risk factors for ACLF in the multivariable analysis, and an effective prognostic nomogram for patients with HBV-related ACLF was established. The new nomogram showed good performance for prediction of short-term survival rate in patients with ACLF concomitant MAFLD. Previous studies have shown that nomograms can accurately predict individual survival rate and demonstrate superior net benefits in patients with ACLF [Citation26,Citation27].
The global prevalence of NAFLD is estimated to be 24%, and it is the most common cause of liver disease worldwide [Citation28]. However, given the increasing prevalence of NAFLD, positive diagnostic criteria are required [Citation14]. With improvements in living standards in China, the coexistence of HBV infection and NAFLD has been commonly observed. Identifying the risk of fatty liver disease in patients with HBV would be difficult using the previous exclusionary diagnostic criteria. Compared with NAFLD criteria, the MAFLD criteria do not require the exclusion of other chronic liver diseases; this new definition can specifically identify high-risk patients for early interventions [Citation29]. A recent study reported that the presence of MAFLD in patients was associated with an increased risk of liver-related clinical events and death [Citation30]. With the ‘positive’ MAFLD diagnostic criteria, our study found that concomitant MAFLD increased the risk of death in patients with HBV-related ACLF, and we elucidated the advantages of this new definition in clinical practice. A recent study showed patients with CHB + MAFLD had a greater proportion of advanced fibrosis/cirrhosis (22.6% vs. 11.8%, p = 0.043) compared to patients with CHB + NAFLD outside the MAFLD criteria [Citation31]. Therefore, switching the nomenclature to MAFLD may help clinicians in identifying high-risk patients with concomitant hepatic steatosis and viral hepatitis.
The liver is a major target for the action of insulin and plays an important role in glucose homeostasis by regulating glycogen synthesis and decomposition. We developed an TIM2 model based on MAFLD that involves DM, TBIL and INR. TBIL and INR as well-known prognostic indicators of liver disease, have been widely used in various prognostic model for patients with ACLF. Meanwhile, DM increases the risk of developing liver disease and leads to fibrosis progression in patients with NAFLD [Citation32,Citation33]. Several surveys have indicated that the survival rate is significantly lower in patients with cirrhosis and DM than in those without DM [Citation34,Citation35]. Our research confirms that DM may be an effective risk indicator for predicting the progression of chronic liver disease. DM and fatty liver are metabolic risk factors that are strongly related to the pathogenesis of metabolic syndrome. The advantage of the current definition of MAFLD is that it accounts for factors that are major predictors of metabolic dysfunction such as DM and obesity.
In terms of liver failure complications, patients with MAFLD had a higher incidence of HRS than did patients without MAFLD (p = 0.033), whereas other clinical complications, especially the common infections, were not statistically different. A recent meta-analysis reported a significantly higher prevalence of chronic kidney disease (CKD) complications in individuals with MAFLD than in those without MAFLD [Citation36]. Another study showed that MAFLD is strongly and independently associated with CKD, and that MAFLD identified patients with CKD better than did NAFLD [Citation37]. These findings suggest that MAFLD is associated with a high incidence of renal damage, which is consistent with the findings of our study. The pathophysiologic mechanisms linking MAFLD and kidney damage remain to be poorly understood. Experimental and clinical evidence indicate that NAFLD or MAFLD exacerbates insulin resistance, predisposes patients to atherogenic dyslipidemia, and releases various proinflammatory factors, prothrombotic factors, and profibrogenic molecules that promote renal injury [Citation38–40]. MAFLD is associated with an increased risk of renal injury, suggesting that close monitoring of renal function is necessary in patients with ACLF with MAFLD. In addition to CKD, MAFLD was a vital risk factor for a variety of diseases, such as cardiovascular disease, colorectal adenoma, liver cirrhosis, liver cancer and other cancers [Citation41,Citation42], thus the utility of the MAFLD definition has been proved across a wide spectrum of hepatic and extrahepatic outcomes.
Our study had few limitations. First, the study was retrospective in nature, and the number of patients with concomitant MAFLD was relatively small. Therefore, the magnitude of the association between MAFLD and the prognosis of patients with ACLF requires further evaluation. Second, the study was an Asian population – based cohort, and all the participants were from China. Whether our results can be generalized to patients with ACLF defined according to Western criteria remains unknown. Owing to the drawbacks of this retrospective study, further prospective multicenter studies are needed to confirm our conclusions.
5. Conclusions
In conclusion, our study showed that MAFLD is associated with adverse outcomes in patients with HBV-related ACLF. In patients with ACLF, concomitant MAFLD increased the risk of mortality and HRS. Our findings provide compelling evidence for the clinical usefulness of the novel MAFLD criteria in patients with HBV-related ACLF and highlight the importance of positive diagnostic criteria.
Abbreviations
ACLF: Acute-on-chronic liver failure; ALB: Albumin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; AUGIH: Acute upper gastrointestinal hemorrhage; AMMON: Ammonia; AFP: Alpha fetal protein; AUC: Area under the curve; BMI: Body mass index; BLA: Blood lactic acid; BUN: Blood urea nitrogen; CTP: Child-Turcotte-Pugh; CI: Confidence interval; CKD: Chronic kidney disease; CRP: C-reactive protein; DM: Diabetes mellitus; DNA: Deoxyribonucleic acid; FBG: Fasting blood-glucose; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; HDL: High density lipoprotein; HE: Hepatic encephalopathy; HRS: Hepatorenal syndrome; HRs: Hazard ratios; INR: International normalized ratio; LDL: Low-density lipoprotein; MELD: Model for End-Stage Liver Disease; MELD-Na: Model for End-Stage Liver Disease with serum sodium; MAFLD: Metabolic associated fatty liver disease; NAFLD: Non-alcoholic fatty liver disease; Na+: Natriumion; PLT: Platelet count; PT: Prothrombin time; SBP: Spontaneous peritonitis;Scr: Serum creatinine; TBIL: Total bilirubin; TCHO: Total cholesterol; TG: Triglyceride; WBC: White blood cell.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors had access to relevant data and approved the final version. Study concept and design (R Lai, Y Zhu, L Yao); acquisition of data (L Yao, Shan Lin, B Liu, Z Liang); statistical analysis and interpretation of data (J Zhou, T Chen, J Jiang); drafting of the manuscript (R Lai, Qi Zheng); critical revision of the manuscript for important intellectual content (R Lai, Y Zhu).
Informed consent statement
Informed consent was waived because of the retrospective nature of the study.
Data sharing statement
All data generated during the project will be made freely available by the corresponding author when reasonably required. There are no security, licensing, or ethical issues related to these data.
Acknowledgments
All patients who participated in this study are appreciated.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/17474124.2024.2314810).
Additional information
Funding
References
- Liu J, Liang W, Jing W, et al. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97(3):230–238. doi: 10.2471/BLT.18.219469
- Mezzano G, Juanola A, Cardenas A, et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut. 2022;71(1):148–155. doi: 10.1136/gutjnl-2020-322161
- Ohnishi H, Sugihara J, Moriwaki H, et al. [Acute-on-chronic liver failure]. Ryoikibetsu shokogun shirizu. 1995;7:217–219.
- Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(4):353–390. doi: 10.1007/s12072-019-09946-3
- Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatology. 2011;54(5):1864–1872. doi: 10.1002/hep.24622
- Xue R, Duan Z, Liu H, et al. A novel dynamic model for predicting outcome in patients with hepatitis B virus related acute-on-chronic liver failure. Oncotarget. 2017;8(65):108970–108980. doi: 10.18632/oncotarget.22447
- Wong SW, Chan WK, Mohamed R. Fatty liver is associated with advanced fibrosis but does not predict adverse outcomes in patients with chronic hepatitis B. J Viral Hepat. 2020;27(12):1297–1305. doi: 10.1111/jvh.13361
- Choi HSJ, Brouwer WP, Zanjir WMR, et al. Nonalcoholic Steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–548. doi: 10.1002/hep.30857
- Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, et al. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int. 2017;37(4):542–551. doi: 10.1111/liv.13271
- Yip TC, Lee HW, Chan WK, et al. Asian perspective on NAFLD-associated HCC. J Hepatol. 2022;76(3):726–734. doi: 10.1016/j.jhep.2021.09.024
- Lee YB, Ha Y, Chon YE, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25(1):52–64. doi: 10.3350/cmh.2018.0040
- Paik JM, Kabbara K, Eberly KE, et al. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology. 2022;75(5):1204–1217. doi: 10.1002/hep.32228
- EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004
- Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039
- Eslam M, El-Serag HB, Francque S, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19(10):638–651. doi: 10.1038/s41575-022-00635-5
- Lin S, Huang J, Wang M, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089. doi: 10.1111/liv.14548
- Wang QX, Xue J, Shi MJ, et al. Association between metabolic dysfunction-associated fatty liver disease and the risk of cirrhosis in patients with chronic hepatitis B-A retrospective cohort study. Diabetes Metab Syndr Obes. 2022;15:2311–2322. doi: 10.2147/DMSO.S369824
- Yun B, Ahn SH, Oh J, et al. Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: a nationwide study. Hepatol Res. 2022;52(12):975–984. doi: 10.1111/hepr.13830
- Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–1047. doi: 10.1016/j.jhep.2014.06.012
- Perdigoto DN, Figueiredo P, Tomé L. The role of the CLIF-C of and the 2016 MELD in prognosis of cirrhosis with and without acute-on-chronic liver failure. Ann Hepatol. 2019;18(1):48–57. doi: 10.5604/01.3001.0012.7862
- Song DS, Kim TY, Kim DJ, et al. Validation of prognostic scores to predict short-term mortality in patients with acute-on-chronic liver failure. J Gastroenterol Hepatol. 2018;33(4):900–909. doi: 10.1111/jgh.13991
- Li J, Liang X, Jiang J, et al. PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF. Gut. 2022;71(1):163–175. doi: 10.1136/gutjnl-2020-323395
- Hernaez R, Sola E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut. 2017;66(3):541–553. doi: 10.1136/gutjnl-2016-312670
- Gustot T, Fernandez J, Garcia E, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62(1):243–252. doi: 10.1002/hep.27849
- Li J, Liang X, You S, et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75(5):1104–1115. doi: 10.1016/j.jhep.2021.05.026
- Lin S, Chen J, Wang M, et al. Prognostic nomogram for acute-on-chronic hepatitis B liver failure. Oncotarget. 2017;8:109772–109782. doi: 10.18632/oncotarget.21012
- Gao F, Li X, Wan G, et al. Development and external validation of a prognostic nomogram for acute decompensation of chronic hepatitis B cirrhosis. BMC Gastroenterol. 2018;18(1):179. doi: 10.1186/s12876-018-0911-y
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109
- Nguyen VH, Le MH, Cheung RC, et al. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19(10):2172–2181.e6. doi: 10.1016/j.cgh.2021.05.029
- Van Kleef LA, Choi HSJ, Brouwer WP, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3(5):100350. doi: 10.1016/j.jhepr.2021.100350
- Mak LY, Yuen MF, Seto WK. Letter regarding “A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement”. J Hepatol. 2020;73(6):1573–1574. doi: 10.1016/j.jhep.2020.07.008
- Hamed AE, Elsahar M, Elwan NM, et al. Managing diabetes and liver disease association. Arab J Gastroenterol. 2018;19(4):166–179. doi: 10.1016/j.ajg.2018.08.003
- Pelusi S, Petta S, Rosso C, et al. Renin-Angiotensin system inhibitors, type 2 diabetes and fibrosis progression: an observational study in patients with nonalcoholic fatty liver disease. PLoS One. 2016;11(9):e0163069. doi: 10.1371/journal.pone.0163069
- Elkrief L, Chouinard P, Bendersky N, et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60(3):823–831. doi: 10.1002/hep.27228
- Elkrief L, Rautou PE, Sarin S, et al. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36(7):936–948. doi: 10.1111/liv.13115
- Quek J, Ng CH, Tang ASP, et al. Metabolic associated fatty liver disease increases the risk of systemic complications and mortality. A meta-analysis and systematic review of 12 620 736 individuals. Endocr Pract. 2022;28(7):667–672. doi: 10.1016/j.eprac.2022.03.016
- Sun DQ, Jin Y, Wang TY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433. doi: 10.1016/j.metabol.2020.154433
- Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol. 2017;13(5):297–310. doi: 10.1038/nrneph.2017.16
- Mantovani A, Lombardi R, Cattazzo F, et al. MAFLD and CKD: an updated narrative review. Int J Mol Sci. 2022;23(13):7007. doi: 10.3390/ijms23137007
- Wang TY, Wang RF, Bu ZY, et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. 2022;18(4):259–268. doi: 10.1038/s41581-021-00519-y
- Alharthi J, Gastaldelli A, Cua IH, et al. Metabolic dysfunction-associated fatty liver disease: a year in review. Curr Opin Gastroenterol. 2022;38(3):251–260. doi: 10.1097/MOG.0000000000000823
- Kawaguchi T, Tsutsumi T, Nakano D, et al. MAFLD enhances clinical practice for liver disease in the Asia-Pacific region. Clin Mol Hepatol. 2022;28(2):150–163. doi: 10.3350/cmh.2021.0310