ABSTRACT
Introduction: Sarcoidosis is a multisystemic inflammatory disorder with a great variety of symptoms, including fatigue, dyspnea, pain, reduced exercise tolerance and muscle strength. Physical training has the potential to improve exercise capacity and muscle strength, and reduce fatigue. The aim of this review and survey was to present information about the role of physical training in sarcoidosis and offer practical guidelines.
Areas covered: A systematic literature review guided an international consensus effort among sarcoidosis experts to establish practice-basic recommendations for the implementation of exercise as treatment for patients with various manifestations of sarcoidosis. International sarcoidosis experts suggested considering physical training in symptomatic patients with sarcoidosis.
Expert commentary: There is promising evidence of a positive effect of physical training. Recommendations were based on available data and expert consensus. However, the heterogeneity of these patients will require modification and program adjustment of the standard rehabilitation format for e.g. COPD or interstitial lung diseases. An optimal training program (types of exercise, intensities, frequency, duration) still needs to be defined to optimize training adjustments, especially reduction of fatigue. Further randomized controlled trials are needed to consolidate these findings and optimize the comprehensive care of sarcoidosis patients.
1. Introduction
Physical training or pulmonary rehabilitation (PR) is an important element of the comprehensive care of people with pulmonary diseases and other chronic diseases, including musculoskeletal disorders, neurological diseases, and psychiatric conditions [Citation1–Citation6]. Of note, exercise also has a role as treatment in diseases such as those of the locomotive apparatus or respiratory system that do not primarily manifest as organ-specific disorders, but also are accompanied by many other clinical manifestations such as fatigue and other disabling nonspecific symptoms [Citation3,Citation6]. In selected cases, exercise therapy might be just as effective as medical treatment, and in special situations it might be more effective or add to its effect [Citation6].
Sarcoidosis, a multisystem inflammatory disorder, has many faces and phenotypes. It may occur at all ages and presents with lung involvement in the majority of cases [Citation7–Citation11]. Extrapulmonary manifestations of this disease involve the heart, joints, kidney, liver, eyes, nervous system, and skin. A growing body of evidence has demonstrated the impact of not only organ-specific symptoms but also nonspecific problems, including lack of energy, fatigue, pain, anxiety, depression, and cognitive symptoms, on patients’ lives, inducing significant worsening of (health-related) quality of life (HR QoL) [Citation10,Citation12–Citation14]. Multifactorial influences include systemic inflammation, decreased pulmonary function, sleeping disorders, small fiber neuropathy (SFN), sarcoid myopathy, hypoxia or glucocorticoid use, and deconditioning [Citation15–Citation18]. This can lead to physical inactivity, loss of fitness and muscle strength, and thus increased fatigue [Citation19,Citation20]. Considerable knowledge has accumulated concerning the significance of exercise as the first-line treatment of several chronic diseases [Citation6]. To date, no formal consensus exists regarding the role of exercise programs for sarcoidosis.
To collect information about the benefits of physical training in sarcoidosis, a comprehensive literature review was performed, which was then used to guide an international consensus effort among sarcoidosis experts to establish practical recommendations – based on evidence, experience, and common sense – for the use of physical training in the management of patients with manifestations of sarcoidosis.
2. Material and methods
This study consisted of four phases.
2.1. Phase I
A computerized comprehensive search of the literature from January 1971 until December 2015 was performed. Results were identified in PubMed, MEDLINE, and CINAHL. Combinations of the following Medical Subject Headings (MeSH) and free text words were used: sarcoidosis, interstitial lung disease, physical training, training, physical therapy, exercise, exercise training, exercise capacity, outcome, evaluation.
The following criteria were used to identify relevant studies:
patients: sarcoidosis, interstitial lung disease;
intervention: physical training/exercise training/physical therapy/PR; and
language: English.
We augmented our search by reviewing the reference lists of retrieved articles, including review articles. The initial selection was done by two authors (LAS and BS). Consensus regarding ‘title and abstracts’ was reached by two authors (MD and BS). Data was extracted by BS and checked by MD, see . A descriptive summary of studies included presents the study design, participant and treatment characteristics, as well as objective and patient-reported outcomes (see also ).
Table 1. Data-extraction from studies on physical training in sarcoidosis patients.
The study quality was assessed using appropriate instruments, viz. the STROBE Statement [Citation25] for observational studies and PEDro scale [Citation26,Citation27] for randomized trials.
2.2. Phase II
The results and conclusions from the literature review in Phase I provided key concepts regarding PR and physical training in interstitial lung disease (ILD) and in sarcoidosis. These concepts reflect the scientific efforts and experienced opinion of the expert community.
Thus, Phase II involved content analysis of the literature review (LAS, BS), deconstructing the text of each paper into individual topics and reassembling them into a nonredundant and categorized item list. Each item from Phase II was directly translated into a representative survey question, which together formed the questionnaire used in Phase III of the study.
2.3. Phase III
Phase III comprised an evaluation of physical training in sarcoidosis by international sarcoidosis experts. The active data collection occurred during 6 weeks in August and September 2015; 165 international sarcoidosis experts were invited by email to complete a web-based survey on physical training in sarcoidosis. The experts selected were members of the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) or the American Association of Sarcoidosis and Other Granulomatous Disorders (AASOG), or identified by authorship in peer-reviewed journals in related studies. Additionally, the respondents were subdivided by region: Europe, United States and Canada, and rest of the world (including Russia, Asia, Australia).
2.4. Survey
The survey consisted of two parts. All participants were asked to complete Part I of the survey, which collected sarcoidosis-specific demographic data including specialty, degree of clinical experience, and perceptions and regional availability of physical training.
Part II of the survey included only participants self-defined as being familiar with physical training in sarcoidosis. Items were divided into two major areas: (1) potential sarcoidosis manifestations (e.g. pulmonary, cardiac) and symptoms (e.g. fatigue, dyspnea) as indications for physical training; and (2) potential domains and tools to monitor the impact of physical training (e.g. fatigue or dyspnea scales, lung function).
The survey items were rated on a 10-point Likert scale from 1 (not at all) to 10 (absolutely), anchored in either useful or appropriate depending on the item content. This included the degree of appropriateness of physical function testing (e.g. exercise capacity, muscle function, activities of daily living) as part of the standard assessment of any patient with sarcoidosis. Additionally, open-ended questions were posed, such as those querying relative contraindications of physical training in sarcoidosis. Finally, participants ranked the top three domains for monitoring physical training in sarcoidosis.
2.5. Phase IV
In Phase IV, results obtained during Phases I and III were assembled to prepare 10 recommendations (MD, LAS, BS). Finally, the recommendations were submitted to a panel of 15 leading international sarcoidosis experts familiar with exercise in sarcoidosis who each saw over 100 new sarcoidosis patients a year. The experts indicated their level of agreement on a 10-point Likert scale from 1 (no agreement) to 10 (full agreement) [Citation28]. The experts were also asked to comment on each recommendation. The specific comments on the recommendations were gathered and grouped (by BS and MD), and recommendations were refined on the basis of these comments. Recommendations with an agreement level less than 75% were excluded from the final selection.
2.6. Statistical analysis
Standard proportional analyses were performed on aggregate responses. Chi-square analyses were used to assess regional differences. Descriptive statistics were used for the recommendations of Phase IV. All statistical analyses were performed using SPSS statistical software (version 22.0 for Windows, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Phase I
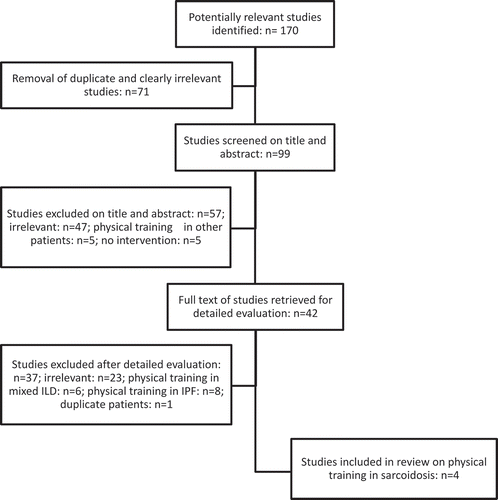
A systematic literature review () yielded 42 studies on PR or physical training in ILD. Twenty-three were irrelevant after detailed evaluation, eight studied patients suffering from idiopathic pulmonary fibrosis (IPF), six patients with mixed ILD etiology, and one study analyzed patients also included in one of the other included studies. Therefore, finally, only four studies were included. Average observational study quality, assessed by the STROBE checklist, was 25 out of 34 points (range 20–30). The quality of the randomized trial, assessed by the PEDro scale, was 9/11, see also .
Two of the four included studies were retrospective cohort studies [Citation21,Citation23], one was a prospective cohort study [Citation22], and one was a randomized controlled trial [Citation24]. Specific information regarding patient characteristics for the sarcoidosis subpopulation in the study by Huppmann et al. could not be determined from the paper or from personal correspondence with the authors [Citation21]. Information regarding the outcomes for the sarcoidosis subpopulation was presented by the authors in personal correspondence.
The age of the patients was similar across all other studies (average 48 years), and the percentage of women in the studies was also similar (ranging from 42% to 66%). However, the study by Marcellis et al. had a smaller proportion of women (22%) [Citation22].
Chest radiographic stages showed some variance across the study populations. All of the sarcoidosis patients in the study by Karadalli et al. had chest X-ray stage I or II [Citation24]. In the other two studies, most patients had stage II or III (50–66%) [Citation22,Citation23].
The majority of patients were outpatients who performed their specific training regimen two to three times a week [Citation22,Citation23,Citation29]. Patients in the study by Huppmann et al. trained four to five times a week on an inpatient basis [Citation21]. The interventions consisted of endurance training, peripheral muscle training [Citation22,Citation23,Citation29] and inspiratory muscle training [Citation24], exercise training, breathing training, and education [Citation21].
Each study suggested benefits in the areas of exercise capacity, fatigue, and QoL [Citation21–Citation24].
In all of the studies, the exercise capacity (6-minute walking distance, 6MWD) improved, ranging from 34 to 70 m improvement. In the study of Marcellis et al. and Strookappe et al., fatigue decreased significantly, −2.7 points (CI −4.4 to 1.1) and −4.2 (CI −5.4 to −2.7) points on the Fatigue Assessment Scale (FAS), respectively [Citation22,Citation23]. Huppmann et al. and Marcellis et al. found improvement of health status [Citation21,Citation22]. Marcellis et al. also showed improvement of quadriceps femoris muscle strength (+10.7 kg; CI 5.5 to 15.9) [Citation22]. In the RCT of Karadalli et al., patients who performed the inspiratory muscle training program improved their inspiratory strength significantly compared with the controls (PImax +45.9 cmH2O, CI 39.3 to 52.8, p < 0.001) [Citation24].
More information is provided in .
3.2. Phase II
Content analysis of the literature review resulted in 28 draft recommendations, 10 of which were related to specified symptoms or organ manifestations that justified indications for physical training, 15 to clinical end points in physical training, and three to general assessment in sarcoidosis. These were further deconstructed and translated into discrete survey items with 28 questions and 15 suggestions for rating and priority ranking.
3.3. Phase III
In Phase III of the study, 165 of the world’s leading sarcoidosis experts of varying specialties were consulted with a web-based survey, of whom 108 (65%) participated. The great majority of the participants were pulmonologists (82%). Other specialists and health-care workers included: rheumatologists (7%), internists (4%), cardiologists (3%), neurologists (2%), and other specialists (all <1%, including immunologists, physical therapists, and oncologists). More than half (55%) had more than 15 years’ experience with sarcoidosis (70% >10 years). Of the overall patient populations seen by the participants, 61% had severe pulmonary sarcoidosis and 49% had severe extrapulmonary sarcoidosis. Participant demographics are shown in .
Table 2. Demographics of respondents to the survey [Citation30–Citation32].
The majority of participants rated physical training as valuable in sarcoidosis (81%, n = 87). Only 3% (n = 4) considered it not valuable and 16% (n = 17) expressed being uncertain about this. These latter respondents also indicated being unfamiliar with physical training in sarcoidosis. The majority of respondents (69%, n = 75) reported that their patients generally had access to physical training. However, insurance coverage of and access to physical training for sarcoidosis were available only in some regions (see ). Most respondents (62%, n = 67) would refer patients ‘regularly, often or always’ if physical training was available for sarcoidosis, with pulmonary involvement and fatigue being the most prominent indications for referral (see ).
Table 3. Questions on physical training in sarcoidosis.
Respondents familiar with physical training in sarcoidosis (n = 60) also completed part II of the survey. In their assessment of physical training, several domains were rated as important (median Likert 1–10, range for all respondents): QoL (9; range 2–10), exercise capacity (9; range 2–10), activity level (9; range 3–10), fatigue (8.5; range 0–10), health status (8; range 2–10), muscle strength (8; range 2–10), dyspnea (8; range 0–10), and mental health (8; range 2–10). The highest levels of agreement on indications for physical training were pulmonary involvement, fatigue, and muscular and extrapulmonary involvement (see ). Sarcoidosis experts from Europe reported a higher likelihood of physical training referral for extrapulmonary sarcoidosis than respondents from other regions.
Table 4. Indications for physical training in sarcoidosis.
Half of the respondents considered physical training a safe intervention in sarcoidosis without need for restrictions. Almost 50% of respondents (29/60) indicated situations of potential harm from physical training, e.g. for patients with cardiac involvement (e.g. untreated arrhythmias (n = 17, 28%)) or sarcoidosis-associated pulmonary hypertension.
3.4. Phase IV
In Phase IV, results obtained during Phases I and III were assembled to prepare 10 recommendations (MD, LAS, BS) (see Section 2.5). The recommendations were reviewed by 15 leading international sarcoidosis experts familiar with exercise in sarcoidosis. Recommendations with an agreement level less than 75% were excluded from the final selection. This review process led to the exclusion of two recommendations, resulting in the final eight recommendations.
The eight key remaining recommendations are presented in with their levels of agreement. The mean level of agreement for the total set of these initial recommendations among the 15 leading sarcoidologists was 7.8 ± 0.8.
Table 5. International experts’ recommendations for the use of physical training in sarcoidosis.
4. Discussion
Physical activity brings health benefits [Citation33,Citation34]. However, the best way to implement this awareness into the care of sarcoidosis patients to reduce physical inactivity and fatigue has to be explored. A comprehensive literature review was performed regarding the role of physical training in sarcoidosis patients, to guide an international consensus effort among sarcoidosis experts to establish practical recommendations for the use of physical training in the management of various manifestations of sarcoidosis. Although relatively few studies have been done so far, there is encouraging evidence of a positive effect of physical training on the devastating symptoms of sarcoidosis. Despite the paucity of studies, available data and scientific rationale induced a multinational committee of sarcoidosis experts to recommend that sarcoidosis patients might benefit from supervised tailored physical training with serial assessment of muscle strength and exercise capacity. Physical training was recognized as a strategy to reduce fatigue and dyspnea, as well as to improve QoL.
4.1. Heterogeneity of sarcoidosis
Due to the heterogeneity of the disease and the diversity in severity, sarcoidosis patients may present with a variety of organ-related symptoms and functional impairments. Moreover, they are often affected by rather nonspecific disabling symptoms. In addition to the impact of inflammation and treatment on muscles on the one hand, there is a well-described relationship of reduced physical activity/deconditioning, fatigue, and exercise intolerance with peripheral muscle integrity, comparable to that in other chronic diseases, and not only respiratory disorders, on the other hand [Citation3,Citation6,Citation23]. This underlines that the treatment should be individualized and tailored to the personal needs and cover all clinically relevant symptoms (see also ). Due to its complexity, sarcoidosis requires a multidisciplinary approach [Citation10,Citation35].
4.1.1. Fatigue and dyspnea
Fatigue is the most frequently described and disabling symptom in sarcoidosis and can be nonspecific and difficult to characterize for both patients and clinicians [Citation20,Citation36]. Sarcoidosis-associated fatigue and exercise capacity have important associations with QoL, especially in the domain of physical health [Citation17,Citation37–Citation40]. Even so, the fatigue associated with systemic inflammation is multidimensional and can be subclassified as general, mental, physical, or motivational fatigue. Inflammation-related fatigue exerts cytokine/chemokine influences on the hypothalamus, muscle (including respiratory muscles), nerve, and bone, leading to mental exhaustion, sleep disorders, loss of muscle and bone mass and autonomic dysfunction, as well as the exhausting psychological burden of pain in addition to that of living with a chronic illness. In patients with sarcoidosis, sarcoidosis-associated pulmonary hypertension and sleep apnea are important disease aspects that are potential causes of fatigue, which need to be excluded [Citation20,Citation41–Citation43]. Fatigue can also be a consequence of the treatment itself, such as corticosteroid therapy, which also affects the hypothalamic axis as well as other endocrine functions and muscle health [Citation7]. Like fatigue, dyspnea is a significant symptom and multifactorial phenomenon in sarcoidosis [Citation41,Citation44–Citation49]. Dyspnea appears to be related to fatigue, low levels of energy, and chest pain [Citation22,Citation46,Citation49,Citation50]. However, the degree of dyspnea in sarcoidosis does not correlate with lung function tests [Citation51].
In line with the studies done so far, practical recommendations () show that fatigue was considered by the sarcoidosis experts to be a key element in the management of sarcoidosis patients. This makes assessment of fatigue an important metric in addition to objective clinical and laboratory data. Accordingly, it was recommended by 15 leading sarcoidologists that measurement of patient-reported fatigue in combination with assessment of physical activity and functional performance may offer useful clinical information in the evaluation of fatigue in patients with sarcoidosis. Moreover, it was recognized that – as the etiology of fatigue is elusive and may be multifactorial – the diagnosis of sarcoidosis-associated fatigue requires extensive evaluation to identify and treat potentially reversible causes, including non-disease-related causes such as hypothyroidism () [Citation7,Citation20].
4.1.2. Muscle strength and deconditioning
Patients with sarcoidosis may experience respiratory as well as limb muscle dysfunction, and the ensuing deconditioning, inactivity, and exercise tolerance [Citation3,Citation5,Citation11,Citation19,Citation23,Citation52]. Wirnsberger et al. found reduced respiratory muscle strength and endurance time in a small population of sarcoidosis patients with normal lung function [Citation53]. More recently, it was demonstrated that not only fatigue but also exercise intolerance and muscle weakness were frequently reported, with substantial reduction of maximal inspiratory pressure (PImax) [Citation11]. Interestingly, maximal inspiratory and expiratory mouth pressures in sarcoidosis patients demonstrated a more consistent gradual decline with increasing dyspnea and diminishing activity levels than lung volumes and gas transfer [Citation54].
However, the assessment of muscle strength is variable in clinical practice. The Biodex System 3 Pro Dynamometer (Biodex Medical Systems, Shirley, New York, USA), which is the gold standard in muscle strength testing, was used to assess muscle function in sarcoidosis. However, this system is quite expensive, not portable, and has limited availability in clinical practice, which limits its practical usability. The microFET (Biometrics, Almere, The Netherlands), used in the study of Marcellis et al. and Strookappe et al., is a handheld dynamometer and could offer a reliable alternative to measure peripheral muscle strength [Citation22,Citation23,Citation55].
Although asymptomatic muscle involvement in sarcoidosis has been reported in up to 80% of cases, symptomatic involvement is thought to be less frequent [Citation7,Citation56–Citation59]. Symptomatic muscle involvement may include palpable nodules, acute myositis, and chronic myopathy with or without functional impairment [Citation60]. According to the results reported in the literature, respiratory as well as limb muscle dysfunction are also important in considering when to start a physical training program. These latter considerations were appreciated by the experts as recommendation (). Whether impaired respiratory muscle function impacts on morbidity and mortality, in sarcoidosis as in other ILDs, needs to be further investigated [Citation61].
4.1.3. Small fiber neuropathy
SFN has been recognized as a serious phenomenon in sarcoidosis [Citation62]. Symptoms affecting the autonomic nervous system generally take the form of pain, constipation, incontinence, and in some cases erectile dysfunction and orthostatic hypotension. Patients also experience insomnia and depression at an advanced stage of the disease, with some patients experiencing memory problems and a lack of concentration and initiative [Citation63]. There is a positive association between SFN-associated symptoms and fatigue [Citation17,Citation64]. Moreover, SFN may at least partly explain muscle dysfunction and exercise limitations. Since symptoms of SFN are disabling for patients, they can also significantly reduce their health status [Citation64–Citation66]. To date, SFN itself is often difficult to treat [Citation10,Citation67].
4.1.4. Side effects of medical treatment
Medical treatment for sarcoidosis is often associated with burdensome side effects, with glucocorticoids being known to cause myopathy [Citation68], fatigue, psychological burden, and sleeping problems [Citation42,Citation47,Citation69]. In a study among 25 patients with sarcoidosis, only in the patients who received oral corticosteroid treatment (n = 11) was the quadriceps peak torque inversely related to the mean daily dose of corticosteroids received in the 6 months before testing [Citation18]. Thus, steroid myopathy may be a clinically relevant entity in sarcoidosis, especially with intensified corticosteroid treatment. However, two studies found that the medication did not contribute much and does not impact on the health status more than the symptoms of sarcoidosis [Citation14,Citation49]. Sarcoidosis-related muscle effects at the tissue, cellular, and molecular levels require further investigation.
4.1.5. Quality of life
The majority of patients with sarcoidosis have impaired QoL and health status due to the burden of the disease, leading to limitations in activities of daily living, social isolation, and depression [Citation10,Citation13–Citation15]. In line with others, Salligan demonstrated in a small study that sarcoidosis patients were more fatigued, more depressed, more dyspneic, and less physically active, and had lower physical performance than their age- and race-matched controls [Citation70].
Sarcoidosis-related complaints, including fatigue, may become chronic and affect patients’ QoL even after all other signs of disease activity have disappeared; this appears to be unique to sarcoidosis-related fatigue as compared to other inflammatory conditions [Citation36,Citation71]. Physicians generally assess disease severity and progression in sarcoidosis on the basis of so-called ‘objective measures’ such as pulmonary function tests, chest radiographs, and serologic tests. However, these parameters correlate poorly with the patients’ subjective sense of well-being. Marcellis et al. demonstrated that fatigue and QoL were closely correlated over a 2-year follow-up period, suggesting that reduced muscle strength and exercise intolerance underlie fatigue in sarcoidosis [Citation45]. Inspiratory muscle endurance and quadriceps strength each correlated strongly with SF-36 (medical outcomes study 36-item short form health survey) scores, especially the physical subscales [Citation72]. In recent years, patient-related outcome measures (PROMs) have gained increasing recognition in terms of their value in clinical trials to quantify patient-perceived health status, which is now a standard outcome measure [Citation13]. Moreover, patient involvement can influence the priorities of clinical care. In the management of sarcoidosis, therapeutic approaches should include strategies to restore QoL, with special emphasis on energy and fatigue [Citation9,Citation38]. In terms of the impact on patients’ lives, lack of energy, physical impairment, and fatigue are the most important QoL domains affecting them. Therefore, in line with the results of earlier studies, the experts agreed on recommending a physical training program in sarcoidosis patients suffering from substantial symptoms, complementary to the medical treatment ().
4.2. Physical training in sarcoidosis
The literature review revealed that the evidence for the role of physical training is limited but promising () [Citation21–Citation24]. The only three currently available observational non-randomized studies and one randomized controlled trial evaluating physical training in sarcoidosis found significant and clinically relevant benefits [Citation21–Citation24]. Two studies reported that a physical training program improved exercise capacity and muscle strength and reduced fatigue in sarcoidosis, and recommended that physical training be included as a first-line therapy in sarcoidosis [Citation22,Citation23]. Both Marcellis et al. and Strookappe et al. found a significantly greater decrease of fatigue in physical training groups compared with the patients who did not complete a physical training program. These findings show consistent observational relationships between fatigue and reduced 6MWD, respiratory muscle weakness, and reduced peripheral muscle strength, as well as significant tandem improvement in sarcoidosis-associated fatigue, psychological health, and physical functioning after a period of physical training [Citation22,Citation23]. Huppmann et al. described that an inpatient PR program had a positive impact on the functional status and HR QoL of patients with ILD, including sarcoidosis patients (n = 50; 12%) [Citation21]. In the original article, data on the sarcoidosis patients was not presented separately [Citation21]. Specifically, Karadalli et al. demonstrated that inspiratory muscle training improves functional capacity, maximal exercise capacity, and respiratory muscle strength, while reducing severe perceived fatigue and dyspnea in the early stages of sarcoidosis, and could be safely added to rehabilitation programs [Citation24]. Early referral to physical training should be considered, as less severe physiological limitation may provide greater opportunity to successfully undertake training [Citation52]. But several studies found that patients with very low functional exercise capacity and severe symptoms should be offered the opportunity to undertake a training program and may experience clinically important benefits [Citation1,Citation73].
In one of the studies excluded of final analysis (due to duplicate patients with an included study) [Citation29], patients (n = 12) with severe respiratory involvement (stage IV fibrotic sarcoidosis) were analyzed after a 12-week training program. Exercise capacity and muscle strength were improved in half of the patients. An increased 6MWD of >10% was found in 50% of the patients, and 58% of the patients improved their hand grip strength by >10%. There was also a trend regarding improvement of the forced vital capacity (FVC) % of predicted (Δ = 9.7 ± 11.4; p = 0.075).
4.3. Survey
Eight recommendations for the practical use of physical training in sarcoidosis were developed by integrating evidence from both our systematic literature review and the experiential opinions of sarcoidosis experts worldwide (). In the consensus process, the knowledge and experience of 108 international sarcoidosis experts were harnessed to bridge the gaps in the available evidence. The agreement by this large expert group provides a valuable platform for the implementation and adjustment of these physical training recommendations for sarcoidosis. Such strength of consensus is anticipated to increase the awareness and availability of physical training as a safe and cost-effective strategy in the management of sarcoidosis. The present study was also a first attempt to increase the awareness that sarcoidosis patients might benefit from physical training, like many other patients suffering from any kind of chronic disease [Citation6]. Further studies are urgently needed.
4.4. Effect of physical training
Physical activity increases aerobic capacity and muscle strength, and thus physical well-being.
Rehabilitation has many benefits for patients with sarcoidosis, including social participation, psychological well-being, maintaining levels of activity, learning to use breathing exercises, and ways to adapt exercises to the home environment [Citation5,Citation22,Citation29,Citation74]. In the broader context of medical management, physical therapy or rehabilitation can help to avoid a negative vicious circle of deconditioning and improve coping with the disease [Citation18,Citation22].
4.5. Optimizing physical therapy
The duration, frequency, and intensity of exercise programs are critical to achieve physical benefits [Citation12,Citation23,Citation52]. Although physical training interventions described for ILD show great similarities with interventions used in other chronic lung diseases, e.g. COPD [Citation2,Citation75], lessons learned from other disorders, like neurological and rheumatologic disorders, should be taken into account as well [Citation6]. Studies carried out so far involving subjects with sarcoidosis have shown that physical activity can reduce their symptoms. The positive effect of physical training in sarcoidosis is believed to be multifactorial. The physical training program in sarcoidosis must be individualized and should focus on the patients’ needs and symptoms. Generally speaking, ‘high-frequency, low-impact’ exercise can be recommended. Further prospective studies are warranted to fine-tune the training parameters, duration, frequency, and ways to achieve an optimal and long-lasting effect.
4.6. Safety and other considerations
Emerging evidence suggests that in a disease with severe functional impairment, exercise training may serve as a safe, feasible, and beneficial adjunct therapy [Citation3,Citation5,Citation23,Citation52]. The suggested indications for initiating physical training in sarcoidosis are broad, but due to the heterogeneity of manifestations and symptomatology, the management of sarcoidosis patients is complex and indications as well as relative contraindications should be taken carefully into account. Moreover, the impact of disease severity on the response to exercise training in sarcoidosis is still unclear. Multifactorial sarcoidosis-related pulmonary hypertension is a serious concern in severe sarcoidosis; however, current international guidelines by the American Thoracic Society and the European Respiratory Society support exercise training within the context of PR for pulmonary arterial hypertension (PAH) [Citation76]. The recommended forms of exercise include light or moderate aerobic and light resistive training in patients with stable disease [Citation3,Citation19,Citation52]. The recommendations endorsed by the experts indicate that supervision in these cases is beneficial ().
Future studies need to include larger and more homogeneous samples in terms of sarcoidosis phenotypes, disease severity and duration, age, nutritional status, comorbidities, and treatments. This may help reveal possible variations in fatigue, muscle function, and exercise capacity while drawing attention to the most severely affected phenotypes.
5. Conclusion
Sarcoidosis has many faces and many phenotypes as well as a wide spectrum of symptoms. This justifies the fact that the treatment strategies should be tailored to the specific needs of the individual sarcoidosis patient, including use of training modalities. Emerging evidence suggests that sarcoidosis involves adverse alterations of respiratory and peripheral skeletal muscle morphology and function. These alterations are clinically relevant and appear to be associated with functional limitations, dyspnea, and fatigue. Ultimately, muscle dysfunction is a useful indication for therapeutic intervention, as it seems partially reversible by exercise training. Observational studies have shown that sarcoidosis patients benefit from physical training by improving their exercise capacity as well as reducing sarcoidosis-associated fatigue and dyspnea. An exercise-based rehabilitation program should be offered to all sarcoidosis patients suffering from fatigue, dyspnea, and/or exercise intolerance. Expected outcomes are improvements in muscle strength and endurance, reduction in fatigue, and ultimately improvement in QoL. A thorough patient assessment should be performed at the beginning and end of rehabilitation to evaluate program outcomes, including assessment of fatigue, muscle strength, and exercise capacity. Addressing these issues in the management of sarcoidosis patients enables clinicians to tailor their therapies. Even more importantly, it helps the patients in their struggle with this devastating disease and to gain more understanding. The present study developed practical recommendations for the use of physical training, based on available data and expert consensus. This review provides further justification to prioritize the promotion of regular physical activity as part of a comprehensive management strategy of symptomatic sarcoidosis patients to reduce physical inactivity and fatigue. However, further randomized controlled trials are needed to consolidate these findings into specific recommendations for including physical training and exercise rehabilitation in the comprehensive care of patients with sarcoidosis.
6. Expert commentary
The indications for physical training and rehabilitation in sarcoidosis are broad, but still have to be defined. So far, no studies have evaluated this extensively. The unique clinical picture and underlying pathophysiology of sarcoidosis may require sarcoidosis-specific exercise prescription. Furthermore, organ-specific manifestations, such as joint pain and stiffness, may require modification of the standard PR program [Citation2], including reduction of weight-bearing exercise [Citation52].
The heterogeneity of patients with sarcoidosis, representing different phenotypes who may or may not have lung parenchymal involvement, pain, fatigue, and/or muscle impairment, may require modification and program adjustment of the standard physical training format. The intensity of the training should be personalized, tailored to the individual – which might also include adjustments for daily fluctuations in energy levels – to avoid aggravating the impairments, which would result in high dropout rates [Citation2]. Besides, as with other chronic cardiopulmonary diseases, exercise limitation in sarcoidosis is most likely to be multifactorial, meaning that exercise capacity is not limited by any single component of the disease process, but rather by their collective quantitative interaction(s). This reinforces the need for clinicians to tailor all components of the rehabilitation program to the specific needs of people with sarcoidosis. Despite the limited studies, the initial results are promising, providing sufficient justification for further investigation with multicenter randomized trials. Challenges for future research include patient selection, along with the specific components of physical training to optimize the benefits. The consensus results presented here are a first attempt to produce recommendations for the use of physical training for various manifestations of sarcoidosis. In view of the paucity of data, an optimal training program (types of exercises, intensities, frequency, and duration) still needs to be defined to optimize training adjustments, especially regarding the reduction of fatigue. Again, characterization of disease phenotypes may provide the guidance that is necessary for a structured tailored physical training and lifestyle intervention program, with an emphasis on determinants of modifiable lifestyle habits [Citation77]. Moreover, psychological aspects and coping with the disease should be covered as well. Prospective studies should be designed to answer lingering questions about the value of exercise training for patients with sarcoidosis, including finding the optimal type and dosage of exercise, the benefits that can be expected from maintenance programs, and how long these benefits will last. The accumulated knowledge about the importance of physical training in symptomatic sarcoidosis patients is promising enough for it to be implemented.
7. Five-year view
Sarcoidosis patients generally benefit from additional non-pharmacologic treatments, not only physical training but also nutritional supplements and counseling [Citation56,Citation78]. Therefore, patients should be aware of their opportunities for managing their own condition, including ways to engage different services when required, and lifestyle, for example, the importance of regular exercise as well as physical training programs. Patients’ knowledge about the importance of exercise for their health (in addition to drug therapy) should be improved [Citation10].
A growing body of evidence has demonstrated the exercise-limiting effects of sarcoidosis, suggesting that, in general, patients with sarcoidosis may indeed benefit from an exercise program. However, evidenced-based guidelines have to be established. A thorough patient assessment at entry into rehabilitation will assist in tailoring the exercise program to their individual needs. Health education, using self-administered modules and continued supervised home practice of physical training for chronic symptomatic sarcoidosis-associated fatigue, will add significant and sustained benefits to conventional therapy while reducing costs [Citation79].
The phenomenon of muscle dysfunction in sarcoidosis demands a wider appreciation and deeper understanding. The pathogenesis, molecular basis, and extent of muscle dysfunction should be further explored. Larger, robustly designed studies can help establish whether both respiratory and limb muscles are affected. Whether the demonstrated muscle defects represent the consequences of systemic abnormalities stemming from the primary pathobiology and multisystemic character of sarcoidosis, or constitute manifestations of a primary myopathic process, remains to be explored. The role of inflammation, oxidative stress, physical inactivity and the possible effect of sarcoidosis-specific therapy are likely to be better characterized. Finally, studies exploring sarcoidosis-specific treatment influences on aspects of skeletal muscle function, morphology, and enzyme activities should provide the required insights. Ultimately, all aspects of muscle alterations in sarcoidosis should be considered and interpreted within the context of disease heterogeneity, duration, and severity, with disease phenotypes identified and physical training targeted appropriately to these differing needs.
Studies in IPF and Parkinson’s disease have reported increased awareness of the benefits of home-based physical training, supervised by physical therapists online and/or by phone calls, in terms of reduction of burden of disease (muscle strength, exercise capacity, fatigue, mental status, and QoL), and the same benefits may be achieved in patients with sarcoidosis [Citation6,Citation79,Citation80]. These findings could guide a feasibility study outlining ‘best practice’ in other chronic disorders, in which home-based supervised physical training programs are expected to improve QoL and reduce the burden of disease. The results can be used to stimulate broader initiatives to promote supervised physical training in sarcoidosis as well as other ILDs, and help develop national and international guidelines. Just as in many other chronic diseases, it is now time for the health-care systems to create the necessary infrastructure to ensure that supervised exercise can be prescribed as treatment. Moreover, it is important that society in general supports a physically active lifestyle. People do not exercise when you just tell them to; people start to exercise when the context compels them to do so. In order to enhance the physical activity level of a population, accessibility is important. There is a need for political statements and regulations about ‘health consequences’. Politicians should also consider health aspects, including how infrastructure and architecture may influence the population’s physical activity levels.
Key issues
Sarcoidosis is a systemic heterogenic disease affecting the lungs in most cases. The evolution (progression, improvement, or stability) and impact of sarcoidosis are variable.
Sarcoidosis patients often present with non-specific symptoms, such as reduced exercise capacity, peripheral and respiratory muscle strength impairment, and dyspnea. The hallmark and most frustrating symptom of sarcoidosis is fatigue.
International sarcoidosis experts suggest considering physical training in patients suffering from sarcoidosis-associated fatigue. Important indications for initiating physical training by fatigued sarcoidosis patients were considered to be the presence of pulmonary, muscular, as well as other extra-pulmonary involvement.
The heterogeneity of patients with sarcoidosis will require modification and program adjustments to the standard rehabilitation format for e.g. COPD, ILD or other chronic diseases.
In view of the paucity of data, an optimal training program (types of exercises, intensities, frequency, duration) still needs to be defined in order to optimize training specifications, especially with the aim reducing fatigue.
A thorough characterization of the sarcoidosis phenotypes in terms of manifestations and limitations is necessary to find determinants of physical activities that are modifiable by changing lifestyle habits and to develop structured tailored exercise training and lifestyle interventions.
Randomized controlled trials are needed to consolidate the limited data into specific recommendations for physical training in patients with sarcoidosis.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgments
The authors greatly appreciate the efforts of the sarcoidosis experts who participated in this study by completing the questionnaires.
Additional information
Funding
References
- Holland AE, Hill CJ, Glaspole I, et al. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med. 2012;106:429–435.
- Holland AE, Wadell K, Spruit MA. How to adapt the pulmonary rehabilitation programme to patients with chronic respiratory disease other than COPD. Eur Respir Rev. 2013;22:577–586.
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–64.
- Swigris JJ, Brown KK, Make BJ, et al. Pulmonary rehabilitation in idiopathic pulmonary fibrosis: a call for continued investigation. Respir Med. 2008;102:1675–1680.
- Swigris JJ, Fairclough DL, Morrison M, et al. Benefits of pulmonary rehabilitation in idiopathic pulmonary fibrosis. Respir Care. 2011;56:783–789.
- Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72.
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155–1167.
- Judson MA, Mack M, Beaumont JL, et al. Validation and important differences for the sarcoidosis assessment tool. A new patient-reported outcome measure. Am J Respir Crit Care Med. 2015;191:786–795.
- Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63–78.
- Drent M, Strookappe B, Hoitsma E, et al. Consequences of sarcoidosis. Clin Chest Med. 2015;36:727–737.
- Marcellis RG, Lenssen AF, Elfferich MD, et al. Exercise capacity, muscle strength and fatigue in sarcoidosis. Eur Respir J. 2011;38:628–634.
- De Vries J, Drent M. Quality of life and health status in sarcoidosis: a review of the literature. Clin Chest Med. 2008;29:525–532.
- Judson MA. Quality of life assessment in Sarcoidosis. Clin Chest Med. 2015;36:739–750.
- Patel AS, Siegert RJ, Creamer D, et al. The development and validation of the King’s sarcoidosis questionnaire for the assessment of health status. Thorax. 2013;68:57–65.
- De Vries J, Wirnsberger RM. Fatigue, quality of life and health status in sarcoidosis. Eur Respir Mon. 2005;32:92–104.
- De Vries J, Drent M. Relationship between perceived stress and sarcoidosis in a Dutch patient population. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:57–63.
- Drent M, Marcellis R, Lenssen A, et al. Association between physical functions and quality of life in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:117–128.
- Spruit MA, Thomeer MJ, Gosselink R, et al. Skeletal muscle weakness in patients with sarcoidosis and its relationship with exercise intolerance and reduced health status. Thorax. 2005;60:32–38.
- Panagiotou M, Polychronopoulos V, Strange C. Respiratory and lower limb muscle function in interstitial lung disease. Chron Respir Dis. 2016;13:162–172.
- Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J. 2012;40:255–263.
- Huppmann P, Sczepanski B, Boensch M, et al. Effects of inpatient pulmonary rehabilitation in patients with interstitial lung disease. Eur Respir J. 2013;42:444–453.
- Marcellis RG, Veeke MAF, Mesters I, et al. Does physical training reduce fatigue in sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:53–62.
- Strookappe B, Swigris J, De Vries J, et al. Benefits of physical training in Sarcoidosis. Lung. 2015;193:701–708.
- Karadalli MN, Bosnak-Guclu M, Camcioglu B, et al. Effects of inspiratory muscle training in subjects with sarcoidosis: a randomized controlled clinical trial. Respir Care. 2015;61:483–494.
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7:e1000251.
- Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–1241.
- Roddy E, Zhang W, Doherty M, et al. Evidence-based clinical guidelines: a new system to better determine true strength of recommendation. J Eval Clin Pract. 2006;12:347–352.
- Strookappe B, Elfferich M, Swigris J, et al. Benefits of physical training in patients with idiopathic or end-stage sarcoidosis-related pulmonary fibrosis: a pilot study. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:43–52.
- Baughman RP, Drent M, Culver DA, et al. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:90–98.
- Judson MA, Baughman RP, Costabel U, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. 2008;31:1189–1196.
- Judson MA, Costabel U, Drent M, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27.
- Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229.
- Ding D, Lawson KD, Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016; [Epub ahead of print]. DOI:10.1016/S0140-6736(16)30383-X
- Drent M. Sarcoidosis: benefits of a multidisciplinary approach. Eur J Intern Med. 2003;14:217–220.
- Korenromp IH, Heijnen CJ, Vogels OJ, et al. Characterization of chronic fatigue in patients with sarcoidosis in clinical remission. Chest. 2011;140:441–447.
- Michielsen HJ, Peros-Golubicic T, Drent M, et al. Relationship between symptoms and quality of life in a sarcoidosis population. Respiration. 2007;74:401–405.
- De Vries J, Rothkrantz-Kos S, van Dieijen-Visser MP, et al. The relationship between fatigue and clinical parameters in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:127–136.
- De Boer S, Kolbe J, Wilsher ML. The relationships among dyspnoea, health-related quality of life and psychological factors in sarcoidosis. Respirology. 2014;19:1019–1024.
- Wirnsberger RM, De Vries J, Jansen TL, et al. Impairment of quality of life: rheumatoid arthritis versus sarcoidosis. Neth J Med. 1999;54:86–95.
- Bosse-Henck A, Wirtz H, Hinz A. Subjective sleep quality in sarcoidosis. Sleep Med. 2015;16:570–576.
- Verbraecken J, Hoitsma E, van der Grinten CP, et al. Sleep disturbances associated with periodic leg movements in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:137–146.
- Baughman RP, Engel PJ, Nathan S. Pulmonary hypertension in sarcoidosis. Clin Chest Med. 2015;36:703–714.
- Rp B, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132:207–213.
- Marcellis RG, Lenssen AF, de Vries J, et al. Reduced muscle strength, exercise intolerance and disabling symptoms in sarcoidosis. Curr Opin Pulm Med. 2013;19:524–530.
- Jastrzebski D, Ziora D, Lubecki M, et al. Fatigue in sarcoidosis and exercise tolerance, dyspnea, and quality of life. Adv Exp Med Biol. 2015;833:31–36.
- Hinz A, Brähler E, Möde R, et al. Anxiety and depression in sarcoidosis: the influence of age, gender, affected organs, concomitant diseases and dyspnea. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:139–146.
- Kabitz HJ, Lang F, Walterspacher S, et al. Impact of impaired inspiratory muscle strength on dyspnea and walking capacity in sarcoidosis. Chest. 2006;130:1496–1502.
- Van Manen MJ, Wapenaar M, Strookappe B, et al. Validation of the King’s Sarcoidosis Questionnaire (KSQ) in a Dutch sarcoidosis population. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:75–82.
- Wirnsberger RM, de Vries J, Breteler MH, et al. Evaluation of quality of life in sarcoidosis patients. Respir Med. 1998;92:750–756.
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889.
- Holland AE, Dowman LM, Hill CJ. Principles of rehabilitation and reactivation: interstitial lung disease, sarcoidosis and rheumatoid disease with respiratory involvement. Respiration. 2015;89:89–99.
- Wirnsberger RM, Drent M, Hekelaar N, et al. Relationship between respiratory muscle function and quality of life in sarcoidosis. Eur Respir J. 1997;10:1450–1455.
- Baydur A, Alsalek M, Louie SG, et al. Respiratory muscle strength, lung function, and dyspnea in patients with sarcoidosis. Chest. 2001;120:102–108.
- Bohannon RW. Test-retest reliability of hand-held dynamometry during a single session of strength assessment. Phys Ther. 1986;66:206–209.
- Cremers JP, Drent M, Elfferich MD, et al. Body composition profiling in a Dutch sarcoidosis population. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:289–299.
- Cremers JP, Van Kroonenburgh MJ, Mostard RL, et al. Extent of disease activity assessed by 18F-FDG PET/CT in a Dutch sarcoidosis population. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:37–45.
- Silverstein A, Siltzbach LE. Muscle involvement in sarcoidosis. Asymptomatic, myositis, and myopathy. Arch Neurol. 1969;21:235–241.
- Wirnsberger RM, de Vries J, Wouters EF, et al. Clinical presentation of sarcoidosis in The Netherlands an epidemiological study. Neth J Med. 1998;53:53–60.
- Costabel U. Skeletal muscle weakness, fatigue and sarcoidosis. Thorax. 2005;60:1–2.
- Walterspacher S, Schlager D, Walker DJ, et al. Respiratory muscle function in interstitial lung disease. Eur Respir J. 2013;42:211–219.
- Hoitsma E, Marziniak M, Faber CG, et al. Small fibre neuropathy in sarcoidosis. Lancet. 2002;359:2085–2086.
- Elfferich MD, Nelemans PJ, Ponds RW, et al. Everyday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-alpha treatment. Respiration. 2010;80:212–219.
- Hoitsma E, De Vries J, Drent M. The small fiber neuropathy screening list: construction and cross-validation in sarcoidosis. Respir Med. 2011;105:95–100.
- Judson MA. Small fiber neuropathy in sarcoidosis: something beneath the surface. Respir Med. 2011;105:1–2.
- Hoitsma E, Drent M, Sharma OP. A pragmatic approach to diagnosing and treating neurosarcoidosis in the 21st century. Curr Opin Pulm Med. 2010;16:472–479.
- Tavee J, Culver D. Sarcoidosis and small-fiber neuropathy. Curr Pain Headache Rep. 2011;15:201–206.
- Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95–103.
- Elfferich MD, De Vries J, Drent M. Type D or ‘distressed’ personality in sarcoidosis and idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:65–71.
- Saligan LN. The relationship between physical activity, functional performance and fatigue in sarcoidosis. J Clin Nurs. 2014;23:2376–2378.
- Michielsen HJ, Drent M, Peros-Golubicic T, et al. Fatigue is associated with quality of life in sarcoidosis patients. Chest. 2006;130:989–994.
- Brancaleone P, Perez T, Robin S, et al. Clinical impact of inspiratory muscle impairment in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:219–227.
- Ryerson CJ, Cayou C, Topp F, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med. 2014;108:203–210.
- Spruit MA, Wouters EFM, Gosselink R. Rehabilitation programmes in sarcoidosis: a multidisciplinary approach. Eur Respir Mon. 2005;32:316–326.
- Holland AE, Hill CJ, Conron M, et al. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax. 2008;63:549–554.
- Panagiotou M, Peacock AJ, Johnson MK. Respiratory and limb muscle dysfunction in pulmonary arterial hypertension: a role for exercise training? Pulm Circ. 2015;5:424–434.
- Hebestreit H, Kriemler S, Radtke T. Exercise for all cystic fibrosis patients: is the evidence strengthening? Curr Opin Pulm Med. 2015;21:591–595.
- Boots AW, Drent M, de Boer VC, et al. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 2011;30:506–512.
- Rammaert B, Leroy S, Cavestri B, et al. Home-based pulmonary rehabilitation in idiopathic pulmonary fibrosis. Rev Mal Respir. 2011;28:e52–57.
- Lingner H, Grosshennig A, Flunkert K, et al. ProKaSaRe study protocol: a prospective multicenter study of pulmonary rehabilitation of patients with sarcoidosis. JMIR Res Protoc. 2015;4:e134.