1. Introduction
Community-acquired pneumonia (CAP) is a worldwide prevalent infection and the most frequent cause of mortality due to infectious reasons [Citation1]. Sepsis is present in one out of three hospitalized CAP patients already at diagnosis, and in some cases it appears within the first 48 h since onset of symptoms [Citation2]. Sepsis, as defined in the New Consensus Sepsis (Sepsis-3), is considered a life-threatening organ dysfunction caused by a dysregulated host response against infection. In fact, it has been reported that, in CAP, the number of organ dysfunctions is the main mortality driver [Citation3].
An adequate host response against infection requires an innate and adaptive immune response to locally get rid of microorganisms, avoiding its spread with deleterious systemic consequences. Neutrophils are rapidly recruited to lung after the innate response recognizes, through toll-like receptors (TLR), some patterns from microorganisms. TLRs are essential although they also contribute to tissue injury amplifying inflammation. Thus, that response needs to be well regulated because, as it has been found in previous studies, an excessive systemic inflammatory response may be detrimental for the host and increase treatment failure and mortality. In fact, initial elevated levels of interleukin (IL)-6, IL-8, and IL-10 depicted an inflammatory scenario that has been related to poor outcome [Citation4]. Nevertheless, heterogeneous cytokine patterns have been reported across patients [Citation5], related to the initial severity, the presence of comorbidities, and the causal microorganism [Citation6]. All those studies bring to light how intricate the host response is.
2. Body
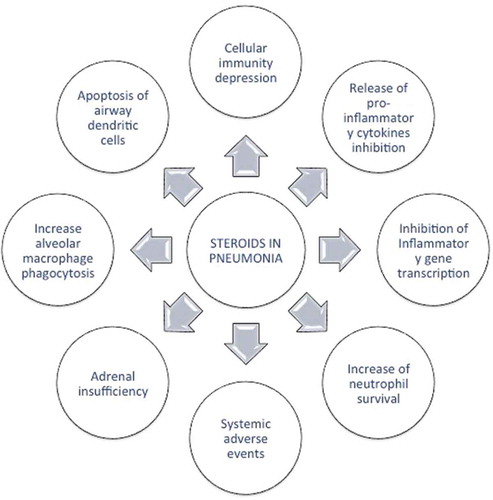
The rationale for using corticosteroids as an adjunctive CAP therapy is to diminish the excessive inflammation in order to avoid organ injury [Citation7]. Glucocorticosteroids have anti-inflammatory activity as they reproduce the effect of endogenous cortisol, resulting in a reduction of inflammatory cytokines and chemokines; they modify the carbohydrate metabolism and also depress the immune system and regulate the stress response. Corticosteroids may also play a beneficial role in the presence of adrenal insufficiency or inadequate adrenal function due to sepsis. On the other hand, corticosteroids have also adverse effects such as hyperglycemia, gastrointestinal bleeding, superinfection by cellular immunosuppression, delirium, and others. The main effects of corticosteroids in pneumonia are depicted in .
Confalonieri et al. [Citation8] were the first authors to show an increase in survival in severe CAP treated with corticosteroids. In their study, intravenous hydrocortisone was prescribed (200 mg as an initial bolus followed by 10 mg/h) during 7 days in CAP patients admitted to the intensive care unit, showing a significant reduction in mortality and multiorgan dysfunction syndrome, with improvement of oxygenation and reduction of C-reactive protein (CRP) levels. This study created a huge debate because in the arm treated with hydrocortisone there was no death, and the main criticism was a possible flaw in the selection of patients in each arm. Besides these considerations, it was also pointed out that there was a potential role for corticosteroids on the adrenal insufficiency justifying part of the results. Some other studies revealed interesting findings, such as a reduction in systemic cytokine inflammatory systemic in CAP [Citation9] and in pneumonia animal models [Citation10]. Some posterior studies along with the impressive results of Confalonieri et al. were incorporated in meta-analyses reaching the conclusion that, in severe CAP, corticosteroids decreased mortality [Citation11,Citation12]. The potential weakness at that period was to elucidate how to define severe CAP and how to measure an excessive inflammatory response. Other important aspects are the type of corticosteroid used, the dosages and its duration.
Two randomized studies have shed light on this topic. Torres et al. [Citation13] found that in patients with higher severity, measured by severe criteria of ATS/IDSA and higher initial levels of CRP (>15 mg/dl), there was a reduction on treatment failure without differences in mortality when using low dosages of corticosteroids. The corticosteroid dosages were 0.5 mg/kg/12 h methylprednisone or equivalent during the 5 days. In this study, the presence of H1N1 influenza A pneumonia was excluded. Blum et al. [Citation14] also randomized 785 CAP patients to receive 50 mg of prednisone daily for 7 days versus placebo with the end point of clinical stability, but not mortality. The median time for clinical stability was one day shorter related to a faster resolution of inflammation with greater decrease in CRP levels and fever in the treatment arms. In contrast, a significant increment on hyperglycemia was reported.
One decisive aspect that should be taken into account concerning the effect of corticosteroids in CAP is linked to the causal microorganisms, and this aspect is crucial because at CAP diagnosis the etiology is unknown in more than 50% of patients. In CAP caused by Pneumocystis jirovecii, a benefit has been established, although this finding is based on the results of small randomized studies. On the contrary, in viral CAP, the effect of corticosteroids seems to be more harmful than beneficial, as reported in H1N1 infection, where they were associated to higher pneumonia mortality [Citation15]. Delaney et al. [Citation16] performed an observational multicenter study in patients with severe A (H1N1 pdm09) and found a negative impact on mortality related to corticosteroids. These results were not confirmed after adjusting for confounders. Rodrigo et al. [Citation15], in a meta-analysis performed with ten studies (9 during pandemic A influenza), also reported an increase in mortality (OR, 2.12; 95% CI, 1.36–3.29) associated with corticosteroid therapy. Noteworthy, the potential interaction between corticosteroids and macrolides has been taken into account for its immunomodulatory effect. However, its association in CAP has not shown a beneficial or negative effect on treatment failure or mortality [Citation17,Citation18].
Three recent meta-analyses have been published with slightly different conclusions. Stern et al. [Citation19] found that corticosteroid therapy reduces mortality in patients with severe CAP and also estimated that the number needed to treat to prevent one death was 18 (95% CI: 12–49). They included 13 randomized clinical trials with a total of 1549 adults recruited in studies with different outcomes – mortality, treatment failure, clinical stability, or length of hospitalization– and different dosages of corticosteroids. The second one, published by Wan et al. [Citation20], included patients from 9 randomized control trials with 1667 patients and 6 cohort studies with 4095. They showed a beneficial effect to reduce ARDS progression, length of stay, time to clinical stability, and the duration of IV antibiotic, but not mortality rates. Noteworthy, one limitation of meta-analyses is the difference in selection of patients, definitions, dosages, and duration of corticosteroids and evaluated outcomes. There is more agreement with regard to the lack of beneficial effect in non-severe CAP and the increase in adverse events, specifically hyperglycemia requiring insulin.
The latest recent published meta-analysis did not verify the benefit of corticosteroids on mortality. Briel et al. [Citation21] used a slightly different methodology – individual patient data meta-analysis – that offers the advantage of standardized definitions and adjustments for variation on individual prognosis at baseline. With this approach, only 6 randomized trials were included, and patients in both arms were 748 vs. 758 representing 88.4% of the eligible population. In this meta-analysis, the main outcome, benefit on 30-day mortality, was not demonstrated (OR 0.75, 95% CI 0.48–1.21) although a trend for a beneficial effect is found in those initially admitted to the intensive care unit (0.34, 95% CI 0.10–1.12). The positive findings in favor of corticosteroid treatment were the reduction in length of stay (one day), mainly in those with higher severity (Pneumonia Severity Index), and in the time to clinical stability (from 4 to 3 days). However, an increase in CAP-related readmission was reported for the first time (OR 1.85, 95% CI 1.03–3.32), and no significant difference was found regarding mortality although there was a trend for a reduction. The different conclusion in the main outcome – 30-day mortality – was explained because they eliminated three studies without individual data and with potential risk of bias and, thus, their findings are not identical in mortality. Siemineuk et al. [Citation12] also included the requirement of mechanical ventilation and development of secondary outcomes.
One of the limitations of current publications and meta-analyses evaluating the role of corticosteroids in severe CAP, explaining their divergent findings, might be also related to this one-dimensional approach relying only on inflammation. The current studies of corticosteroids are measuring mainly their impact on the inflammatory host response without considering the other important component, the adaptive immunity, and they rely on a rather simplistic perspective. Immediately after the innate immune response takes place, the host has to mount an adequate adaptive response. In infection with sepsis, some characteristics of immunosuppression such as depletion of lymphocytes CD4+ and T cells and B cells have been reported. There is an increasing body of evidence demonstrating that the evaluation of the immune response during CAP could provide valuable information for prognosis prediction. An initial reduction of lymphocyte count was associated with higher mortality adjusted for other confounders [Citation22]. In severe CAP and sepsis, a reduction of CD4+ or NK cell counts has been reported as well as a reduction of IgG2. It is plausible to suggest that in those cases a different approach to restore the immune response is probably needed [Citation23]. Current clinical trials evaluating inflammation and other clinical severity scores in CAP are probably missing this neglected perspective.
The current knowledge about the potential role of corticosteroids on CAP is pointing out to a trend to decreased mortality (except in viral pneumonia) without statistical significance, and a significant reduction in the number of days to reach clinical stability in those with higher initial severity (or inflammation). As an increase in adverse effects has also been proved, the indication needs to be highly individualized. The debate about corticosteroids is not finished yet and, to clarify this important topic, more information about the complete host scenario – innate and adaptive host immune response – would be helpful. Although the ideal candidate is not totally known, we are better targeting the adequate patient. The current evidence is pointing out in favor for corticosteroids in ARDS and in a CAP endotype patient characterized by a high systemic inflammatory response with higher severity. The indication of corticosteroids in CAP needs to be highly individualized to get their beneficial effect.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. Internet. 2012;67:71–79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20729232
- Montull B, Menéndez R, Torres A, et al. Predictors of severe sepsis among patients hospitalized for community-acquired pneumonia. PLoS One. 2016Jan 4; 11(1):e0145929.
- Menéndez R, Montull B, Reyes S, et al. Pneumonia presenting with organ dysfunctions: causative microorganisms, host factors and outcome. J Infect. 2016;73:419–426. Internet. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0163445316302043
- Menéndez R, Torres A, Zalacaín R, et al. Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. Internet. 2004;59:960–965. cited 2018 Jun 27. Available from: http://thorax.bmj.com/cgi/doi/10.1136/thx.2003.017756
- Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. Internet.
- Torres A, Menéndez R. Enterobacteriaceae and pseudomonas aeruginosa in community-acquired pneumonia: the reality after a decade of uncertainty? Eur Respir J. Internet. 2010;35:473–474. cited 2018 Jun 27. Available from: http://erj.ersjournals.com/cgi/doi/10.1183/09031936.00198109
- Prina E, Ceccato A, Torres A. New aspects in the management of pneumonia. Crit Care. Internet. 2016;20:267. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27716262
- Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. Internet. 2005;171:242–248. Available from: http://www.atsjournals.org/doi/abs/10.1164/rccm.200406-808OC
- Fernández-Serrano S, Dorca J, Garcia-Vidal C, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care. Internet. 2011;15:R96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21406101
- Sibila O, Luna CM, Agustí C, et al. Effects of glucocorticoids in ventilated piglets with severe pneumonia. Eur Respir J. Internet. 2008;32. Available from: http://erj.ersjournals.com/content/32/4/1037.long
- Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23112872
- Siemieniuk RAC, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia. Ann Intern Med. 2015;163:519. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26258555
- Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. Internet. 2015;313:677–686. Available from.
- Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511–1518.
- Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam JS, et al. Effect of corticosteroid therapy on influenza-related mortality: a systematic review and meta-analysis. J Infect Dis. 2015;212:183–194. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25406333
- Delaney JW, Pinto R, Long J, et al. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care. 2016;20:75. Internet. Available from: http://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1230-8
- Wirz SA, Blum CA, Schuetz P, et al. Pathogen-and antibiotic-specific effects of prednisone in community-acquired pneumonia. Eur Respir J. Internet. 2016;48:984–986. cited 2016 Oct 26. Available from: http://ow.ly/xeoY302nZPx
- Ceccato A, Cilloniz C, Ranzani OT, et al. Treatment with macrolides and glucocorticosteroids in severe community-acquired pneumonia: a post-hoc exploratory analysis of a randomized controlled trial. PLoS One. 2017;12:e0178022. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28617807
- Stern A, Skalsky K, Avni T, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017;12:CD007720. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29236286
- Wan Y-D, Sun T-W, Liu Z-Q, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209–219.
- Briel M, Spoorenberg SMC, Snijders D, et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: systematic review and individual patient data metaanalysis. Clin Infect Dis. 2018;66:346–354. Internet. Available from: https://academic.oup.com/cid/article/66/3/346/4110206
- Bermejo-Martin JF, Cilloniz C, Mendez R, et al. Lymphopenic community acquired pneumonia (L-CAP), an immunological phenotype associated with higher risk of mortality. EBioMedicine. 2017;24:231-236.
- Bermejo-Martin J, Almansa R, Martin-Fernandez M, et al. Immunological profiling to assess disease severity and prognosis in community-acquired pneumonia. Lancet Respir Med. 2017;5(12):e35–e36.