ABSTRACT
Introduction
Bronchoscopic lung volume reduction treatment with one-way valves is an effective guideline treatment option for patients with severe emphysema. However, important challenges and adverse reactions may occur after treatment.
Areas covered
This review summarizes the complications after endobronchial and intrabronchial valve treatment that have been described by the currently published randomized controlled trials and other relevant papers regarding the complications and its management. In case there was no relevant literature regarding these subjects, recommendations are based on expert opinion. Complications include pneumothorax, post-obstruction pneumonia and hemoptysis. Also, the treatment may not be effective due to the presence of collateral ventilation or misplaced valves. Furthermore, an initial beneficial effect may vanish due to granulation tissue formation, valve dysfunction or valve migration. Careful follow-up after treatment with valves is important. Evaluation with a CT-scan and/or bronchoscopy is needed if there is no improvement after treatment, loss of benefit, or occurrence of important adverse events during follow-up.
Expert opinion
Treating severe emphysema patients with one-way valves requires continuous dedication and expertise, especially to achieve an optimal outcome and elegantly deal with the various complications after treatment.
1. Introduction
Patients with chronic obstructive pulmonary disease may suffer from severe emphysema and hyperinflation [Citation1]. In carefully selected patients, lung volume reduction surgery or bronchoscopic lung volume reduction can be a successful addition to the treatment of this very disabled patient group. Bronchoscopic lung volume reduction treatment with one-way endobronchial valves (Zephyr EBV (Pulmonx, Redwood City, CA, USA) or the Spiration® Valve System (SVS, Olympus, Redmond, WA, USA)) has developed into an important treatment strategy for carefully selected emphysema patients [Citation2–12]. The goal of bronchoscopic lung volume reduction treatment (BLVR) is to achieve a complete occlusion of the most diseased lobe with valves, which induces a lobar atelectasis, thereby causing reduction of hyperinflation. This results in clinically relevant improvements in quality of life, lung function, and exercise capacity, which has been proven by various randomized controlled trials () [Citation2–10].
Table 1. Efficacy outcomes and reported serious adverse events (SAE) for the seven published randomized controlled trials using valves for emphysema. * follow -up: 0–30 days and 30 days – 6 months; # follow -up: 0–45 days and 45 days – 12 months; $ follow -up: 0–6 months and 6–12 months. Abbreviations: SVS: Spiration Valve System; EBV: Endobronchial valves; FEV1: Forced Expiratory Volume in the first second; RV: Residual Volume; SGRQ: St George’s Respiratory Questionnaire; 6MWD: 6 minute walking distance
Valve treatment is a valuable enrichment in the therapy of this severely diseased end-stage patient group who have limited treatment options, and is included in the GOLD COPD treatment recommendations [Citation13]. However, patient selection, treatment, logistics and follow-up need to be very dedicated and require sufficient expertise to achieve full benefit of the valve treatment [Citation12]. Current recommendations regarding these subjects are described in the best practice recommendations by Slebos et al [Citation12]. After placement of valves, there are potential future challenges such as lack of benefit after treatment, or loss of benefit during follow -up. Furthermore, adverse events like pneumothorax and granulation tissue formation can occur. Although most adverse events are reported in the published papers, there is no extensive overview of these problems and its dedicated management.
After valve treatment, it is recommended to monitor the patient for clinical, radiological, and lung functional improvement (or deterioration). Generally, a patient should be reevaluated if there is no improvement after initial treatment, or if there is loss of the initially observed benefit during the follow-up. Furthermore, clinical signs like persistent cough, recurrent pneumonias, and hemoptysis require an additional evaluation with computed tomography (CT) scan and/or bronchoscopy.
In this review, we will discuss challenges and common adverse events effects following bronchoscopic valve treatment and share our experience in the management of these situations. We used all published randomized controlled trials regarding the treatment with endobronchial (Zephyr) and intrabronchial (SVS system) valves and screened these for complications. The PubMed database was searched using the terms ‘COPD’, ‘emphysema’, ‘hyperinflation’, and other synonyms, combined with ‘bronchoscopic lung volume reduction’ and synonyms, combined with ‘complications’ and all frequently described complications and synonyms (e.g. ‘pneumothorax’, ‘granulation tissue formation’). As the current available literature does not always provide definite evidence regarding these problems and its management, a part of this review is based on expert opinion.
2. Follow-up
Follow-up after treatment is important to monitor for treatment effect, loss of effect or complications. Most patients will be treated by their own pulmonologist and collaboration with the treating center and the primary pulmonologist is essential. Some complications can be managed by the patient’s pulmonologist; however, valve-specific problems will require more specific expertise.
In our center, our follow-up after valve treatment is arranged as follows: At 1 week, a brief consultation by telephone. After approximately 6–8 weeks, the patient will visit the outpatient clinic with a low dose chest CT-scan, pulmonary function tests and health status update. If patient experiences clinical benefit, the follow-up is after 6 months and yearly after the procedure up to 5 years. All this data is captured in our registry (‘BREATH-NL’; NCT0281568). If there is no clinical benefit or no volume reduction, earlier follow-up is indicated. Patients are instructed to contact their pulmonologist or us in case of clinical deterioration or emergencies. In this case, earlier visit to the outpatient clinic, with CT-scan and/or lung function is indicated.
3. Lack of efficacy after initial valve treatment
After valve treatment, the first follow-up at approximately is recommended at 6–8 weeks to assess the initial outcome, as the treatment effect is not immediate in all patients. If there is no clinical benefit, it is important to review the patient carefully. The two most common causes of lack of effect are the presence of collateral ventilation and valve misplacement or migration. Follow-up may consist of a (low dose) chest CT, pulmonary function test (spirometry and body plethysmography) and health status of the patient.
3.1. Presence of collateral ventilation
An important predictor of effect is the absence of collateral ventilation between the target lobe and the ipsilateral lobe(s). If a patient does not have clinical effect after treatment, it is important to review the possibility of collateral ventilation. The presence or absence of collateral ventilation can be assessed with a Chartis (Pulmonx, Redwood City, CA, USA) measurement during bronchoscopy [Citation14]. If collateral ventilation is present, valve therapy will not be effective as air leakage from the ipsilateral lobe will prevent substantial volume reduction of the treated lobe [Citation7].
An alternative method to predict the presence of potential collateral ventilation is the fissure completeness score (FCS) as determined by quantitative CT-analysis. Using quantitative CT analysis by the StratX platform (PulmonX, CA, USA), the FCS has shown good correlations with functionally assessed air leakage assessed with Chartis [Citation15,Citation16].
In patients with (nearly) complete fissures between the target lobe and ipsilateral lobe(s) (>95%), there is frequently no collateral ventilation. However, a part of the patients may still have collateral ventilation and thus no lung volume reduction effect [Citation16]. Recent evidence shows that there is also a difference between the right and left fissures. In patients with a left major fissure completeness score of 95–100%, approximately 10% of the patients will still have collateral ventilation, whereas for the right major fissure, with fissure completeness score of 95–100%, approximately 25% of the patients will still have collateral ventilation [Citation15]. Therefore, if Chartis measurement was not performed, the presence of collateral ventilation may well be the cause of the lack of effect, particularly in patients with lower FSC scores.
However, even Chartis measurements may fail to predict absence of collateral ventilation and thus successful valve treatment. Such a failure may be due to a challenging measurement. For example, if the Chartis catheter is obstructed by mucus plugs or due to contact with the mucosa of the airways, this can incorrectly be interpreted as absence of collateral ventilation. Another example is if the lower lobes exhibit a low-flow or no-flow pattern, which may also be falsely interpreted as absence of collateral ventilation [Citation17]. If this is the case, collateral ventilation over the major fissure can be measured in the left upper lobe for the left major fissure, or the right upper lobe with temporary occlusion of the middle lobe with a Watanabe spigot or balloon for the right major fissure [Citation12,Citation18]. However, a small defect in the right major fissure at the middle lobe level can be hard to detect with Chartis and provide false-negative results.
If there is no radiological lung volume reduction effect in patients, the fissures on CT scan and the results of the Chartis measurement should be critically reviewed. A bronchoscopy may prove collateral ventilation by demonstrating a clear picture of continuous opening and closing (‘venting’) of the valves. A Chartis measurement may yet be performed or repeated, but if collateral ventilation is proven in a patient without effect, the valves have to be removed, as there will not be effect in the future, and they may cause complications as granulation tissue formation, hemoptysis, and bacterial colonization [Citation19,Citation20]. In this case, other therapies can be considered, for example, lung volume reduction surgery or coils. However, before removing valves, proper placement of the valves needs to be confirmed.
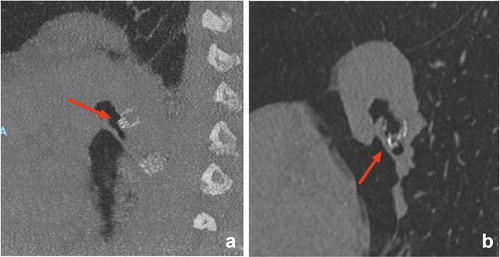
3.2. Valve misplacement
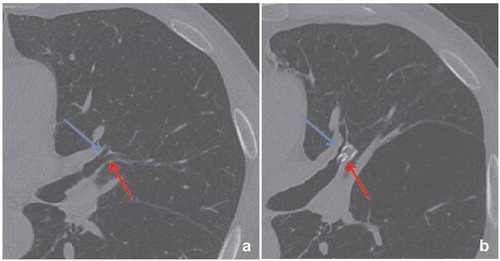
Complete lobar occlusion of the treatment target is obligatory to achieve successful lung volume reduction. Due to collateral ventilation between lobar segments, a complete lobar occlusion is only guaranteed if every valve is placed optimally. If there is no significant lung volume reduction visible on the CT scan, it is recommended to carefully evaluate on CT-scan the position of every valve and search for the presence of untreated airways (). In case of an untreated airway or valve dislocation, it is recommended to perform a revision bronchoscopy. However, if the CT-scan does not give a clue for the lack of effect, a revision bronchoscopy might still be considered to assess for small missed subsegments or a slightly misplaced valve. Furthermore, it is important that the sizing of the valves has been done correctly. There are several available sizes of both the SVS and the EBV, adequate placement of the valves depends on the size and anatomy of the airways. Undersized valves will lead to leakage and incomplete lobar occlusion, oversizing might lead to pressure necrosis of the local airway and granulation tissue formation. During the procedure, airway edema may arise due to suctioning or airway manipulation (e.g. Chartis measurement). If this is the case, it may adversely affect the valve sizing decisions [Citation12].
3.3. Adhesions
Extensive adhesions between the target lobe and the parietal pleura may prevent the lobe from collapsing. From the NETT trial publications, we know that 18% of 552 evaluable patients had ‘marked’ adhesions [Citation21]. The presence of adhesions is also the reason that patients with prior surgery on the same side as the target lobe (e.g. pleurodesis or pleurectomy) are most often excluded for endobronchial valve treatment [Citation22]. Adhesions may be visible on CT-scan, but currently it is not known whether this correlates to treatment success or the ability to achieve an atelectasis [Citation23]. If there is no effect after treatment with valves, there is no presence of collateral ventilation and revision bronchoscopy did not show evidence of valve dysfunction, adhesions may be the cause of the treatment failure. In this case, removal of valves can be considered.
Removal of valves is bronchoscopically best performed via a rigid or flexible intubation to prevent damage to the vocal cords. However, this can also be done very cautiously without endobronchial tube. Removal will be easier by using rat-tooth graspers or alligator jaw grasping forceps and slowly apply pulling pressure. Some local airway bleeding may occur. It has been shown that endobronchial valves can be removed safely [Citation24].
3.4. Temporary shunting
After a successful lung volume reduction treatment with atelectasis of the treated lobe, shunting and subsequent hypoxemia may develop directly after treatment due to decreased ventilation in combination with intact perfusion. In most cases, the hypoxemia is temporary and self-resolving because the alveolar hypoxemia causes pulmonary vasoconstriction [Citation25]. However, sometimes hypoxemia may persist because the patient uses medication that inhibits the desired vasoconstriction, such as calcium channel blockers [Citation26]. If this is the case, discontinuation of this medication may be considered. If the hypoxemia due to shunting does not resolve and the patient has no clinical benefit after treatment, or needs excessive amounts of oxygen, it may be necessary to remove the valves.
3.5. Central airway ‘folding’
Due to the desired valve-induced atelectasis, there will be a change in the position of the remaining lobe(s) and airways. Very rarely this may lead to bronchial folding, airway narrowing and even torsion of the non-treated lobe. These problems have been described after surgical lobectomy, and mainly after treatment of the upper lobes [Citation27,Citation28].
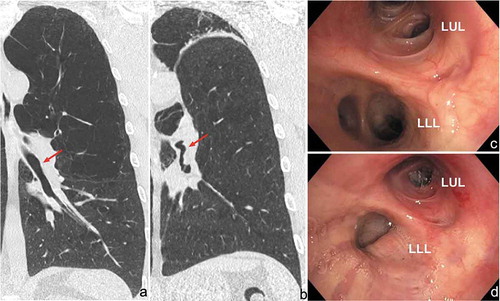
The occurrence of bronchial angulation after valve treatment has been reported in less than 5% of treated patients [Citation29–31]. The clinical presentation may be relatively mild, but due to the narrowing or folding of airways there may be complaints of dyspnea caused by a ventilation/perfusion mismatch and mucus retention with persistent cough (). Especially in a patient with a perfect treatment and subsequent radiological response, but worsening of clinical parameters and symptoms, this problem should be acknowledged. A CT-scan and bronchoscopy can be performed to confirm this diagnosis. Depending on the magnitude of symptoms a patient exhibits, airway folding can be accepted in combination with sputum expectoration techniques. However, if there is no clinical effect after treatment, the valves can be removed, to regain the original position of the airways [Citation30].
4. Loss of benefit after valve treatment
After initially successful valve treatment, patients may complain about a deterioration in their clinical situation over time. This loss of benefit can be temporarily due to intercurrent problems such as a COPD exacerbation or pneumonia. However, when this decline persists despite being recovered from these events, it is important to evaluate whether there are other underlying causes. Both a (low dose) CT scan and bronchoscopy can be performed in these situations.
In case the desired complete atelectasis is still visible on the CT scan, the decline of the patients’ condition may possibly be explained as a result of either ongoing progression of COPD, or due to compensatory expansion of the adjacent lobe [Citation32].
Significant lobar volume reduction after treatment will lead to volume redistribution to the ipsilateral lobe(s). The expansion of the ipsilateral lobe involves the relatively less diseased tissues [Citation33,Citation34]. Nevertheless, there is still a significant improvement of the expiratory low attenuation area and air trapping with an improvement of lung mechanisms [Citation33,Citation34]. However, in some cases there may be an over-hyperinflation of the non-treated ipsilateral lobe, leading to less (and sometimes zero) effect on lung function even with a complete atelectasis. This compensatory overexpansion of residual lung lobes has been described after surgery, but for lung volume reduction in patients with severe emphysema this is currently mainly observational and there are currently no studies to further characterize the patients at risk for compensatory overexpansion [Citation35,Citation36].
Other causes of decline may be concurrent local issues like a foreign body aspiration, or new underlying diseases such a pulmonary embolism, pulmonary hypertension or cardiac pathology.
If the obtained lung volume reduction after treatment has disappeared on the follow-up scan, the decline is probably caused by local airway wall reaction to the valves, leading to malfunctioning of the valves and re-expansion of the treated lobe. In this case, it is recommended to perform a ‘revision bronchoscopy’ after thorough evaluation of the valve position on CT scan. Detection of valve-related issues on CT scan (and even during bronchoscopy) can however be very challenging and frequently needs a very precise comparison with previous valve positions on CT-scan (). There are several valve-related causes for the loss of lung volume reduction, which will be described in more detail below.
Figure 3. Images of thorax CT scan showing valve migration. In this case, the left lower lobe was treated with endobronchial valves and 6 weeks after treatment a complete atelectasis of the target lobe was achieved with a significant clinical benefit. After six months, there was loss of clinical benefit and no more lung volume reduction. (a): EBV well positioned in LB6. (b): Complete re-expansion of the treated lobe, the valve in LB6 is dislocated and now in a different position compared to the follow-up scan at six weeks
4.1. Valve malfunction
One-way valve function can be compromised due to abundant mucus impaction and bacterial or fungal colonization (). In this situation, the valve will be in ‘open’ position continuously, and during inhalation air will flow into the target lobe, resulting in re-expansion. This cannot be detected by CT-scan easily. The affected valve(s) can be removed bronchoscopically and new valve(s) can be replaced during the same session. Furthermore, antibiotics can be prescribed based on culture samples obtained in the same session.
4.2. Incomplete occlusion of airway
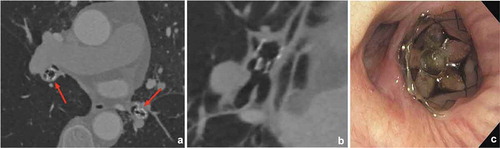
Dislocation of a valve may lead to an incomplete occlusion of the airway. Subsequently, air can leak alongside the valve, resulting in re-expansion of the target lobe. Sometimes, this incomplete occlusion of the airways only takes place during inspiration, when the airways tend to distend. This may be difficult to detect during bronchoscopy, with minor variations in airway movements. Dislocation can be caused by the formation of granulation tissue, significant local bronchomalacia, bronchitis and other local factors. Also, hyperdynamic airways, or excessive dynamic airway collapse (even of segmental bronchi)—often present in emphysema—may promote migration of the valves over time due to excessive variations in airway diameter.
During revision bronchoscopy it is recommended to evaluate the position of each valve. Due to the presence of excessive granulation tissue it can be very challenging to detect which valve is responsible for the incomplete occlusion of a (sub)segment (). Partial patent airway lumen alongside the valve is not always directly visible. Investigation of possible leakage can be performed by carefully flushing saline at the valve location and check for air bubbles appearing outside the valve mechanism itself, proving the presence of air leakage. Another way to demonstrate incomplete occlusion, is by flushing saline or air into the treated segment. If the valve is undersized, this will easily pass the valve. Affected valves can be removed and replaced during the same procedure. Depending on the local situation the size of the valves may be adjusted or they can be replaced more distally [Citation12,Citation37]. A more proximal valve positioning using a larger sized valve is not recommended in these cases.
4.3. Valve migration
Valve migration may occur spontaneously and in particular when sizing or placement of the valves has been done incorrectly. Valve migration has been described in the lower lobes more often, probably due to more collapse of the bronchi compared to the upper lobes [Citation38]. Therefore, although it might be tempting to place a single valve in the lower lobes, it is recommended to place more valves distally in the individual segments [Citation12].
The extent of the valve migration is most often limited to minor changes in the original position; however, sometimes the valves may migrate to the ipsilateral lobe(s), the contralateral lung or be expectorated. Clinical signs that are suggestive of valve migration are sudden loss of beneficial effect, increased coughing and sudden chest discomfort [Citation12,Citation29]. Also, the history may reveal a whistling sound on inspiration [Citation39]. It is important that patients with acute loss of initial benefit and increased dyspnea are evaluated carefully and in short-term. A chest X-ray should be used to exclude a pneumothorax, valve migration can sometimes also be observed. Using a CT scan in these situations, valve position, migration, and target lobe volume reduction can be examined much more precisely. Seldomly, a migrated valve can occlude another, non-treated segment or lobe, potentially leading to (obstruction-) pneumonia, or even a pneumothorax. Furthermore, if the valve is migrated into a reversed position, hyperinflation of the obstructed lung part may follow due to a reversed one-way valve mechanism, potentially leading to a pneumothorax (). If there is (suspicion of) dislocation or migration of valves, a bronchoscopy can be performed to evaluate and replace the affected valves [Citation12].
Figure 6. Migration of EBV to the contralateral lung. Endobronchial valves were placed into the left lower lobe resulting in a complete atelectasis and clinical benefit. One year after the treatment, the patient presented with complaints of progressive dyspnea. (a): The CT-scan showed migration of a valve from the left lower lobe to the right lower lobe, the arrows indicate the position of the valves on both sides. (b): The valve in the entrance of the non-target right lower lobe in reversed position. (c): Endoscopic image of the valve position in reversed position, which was removed and replaced by a new valve in the original left lower lobe position
5. Complications directly related to valve treatment
5.1. Granulation tissue
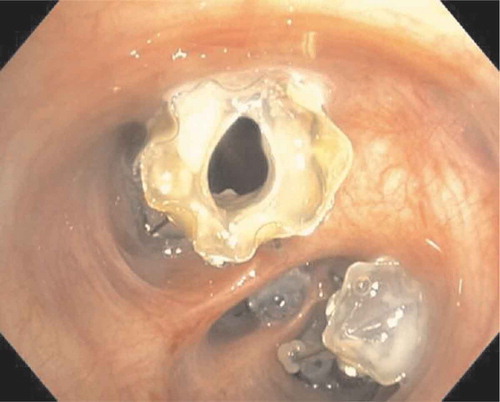
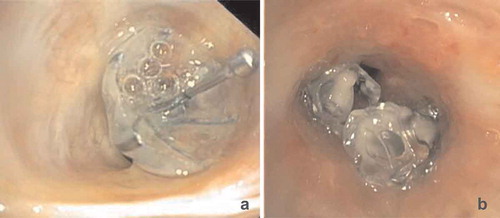
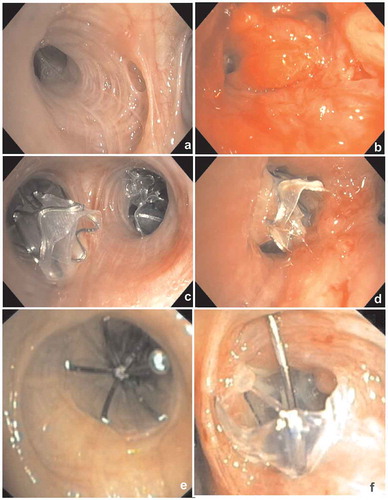
The formation of granulation tissue is a medium to longer term complication of the treatment with valves (both EBV and SVS). It is hypothesized that the cause of the granulation tissue formation is the contact of the foreign body with the airway mucosa, where both shape, pressure and repetitive motion will cause an inflammatory response, with granulation tissue formation as result () [Citation30]. Furthermore, contact of the valve with the adjacent or opposite bronchial wall during movement or coughing may also induce granulation tissue. Granulation tissue formation is described in approximately 40–50% of patients that need valve removal or revision bronchoscopy [Citation30].
Figure 7. Endoscopic images showing granulation tissue in the segmental airways. (a): RB3 (anterior segment of the right upper lobe) before treatment. B: Same segment (RB3), after removal of the valve, which shows an extensive granulation reaction behind the valve. (c): RB3 and RB2 (posterior segment of the right upper lobe) directly after placement of EBVs. (d): Same position as in C with granulation tissue. E: Endobronchial view of an SVS directly after placement. (f): Endobronchial view of the SVS being displaced with granulation tissue formation
The occurrence of airway granulation may also be associated with an increased bacterial load [Citation20,Citation40]. In a single-center study, it has been reported that there is an increased bacterial colonization after valve implantation. Treatment with valves, acting as a foreign body, can promote fungal colonization and impede mucociliary clearance, subsequently leading to retention of secretions, stimulating the bacterial colonization [Citation40]. It is currently not known whether maintenance antibiotics, prednisolone, or high-dose inhaled corticosteroids prevent or reduce the formation of granulation tissue in patients with valves.
Due to the granulation tissue, a valve might (slightly) dislocate, leading to loss of the lung volume reduction effect. In case of loss of benefit due to the formation of granulation tissue, it is advisable to bronchoscopically remove this valve. In case of mild to moderate granulation tissue formation, valves can be replaced during the same procedure. If possible, a valve can be replaced more distally to prevent the formation of granulation tissue at the same site. More proximal replacement may be possible, but in our opinion this should be avoided as granulation tissue may be more likely to develop due to more movement of the valves in the larger airways.
In case of severe granulation tissue formation, we recommend to remove the valves and wait for approximately 10–12 weeks before revision and re-treatment, to promote the recovery of the airways. In case of severe granulation tissue formation, we recommend to remove the valves and wait for approximately 10–12 weeks before revision and re-treatment, to promote the recovery of the airways. Earlier treatment is possible, but with the risk of granulation tissue still being present, thus causing the treatment to be more difficult to perform.
In addition, it is our practice to prescribe a course of corticosteroids (30 mg prednisolone) a few days before valve removal (to facilitate ease of removal) and approximately 1 week after (to facilitate airway healing) in case of severe granulation tissue formation. Macrolide antibiotics (azithromycin) or ‘culture-based’ antibiotics can be prescribed after the treatment.
In general, there is no need for other interventional treatment with laser, cryoablation, or coagulation, as the underlying cause has already been removed.
If there is a re-occurrence of granulation tissue formation or if the granulation reaction was very severe, it is an option not to replace but to remove all valves. As an alternative, a VATS (video-assisted thoracic surgery) lobectomy can be considered, especially if the treatment was initially very beneficial for the patient (and confirmed by radiological and pulmonary function improvement), and the patient is still fit for this intervention [Citation41].
5.2. Pneumothorax
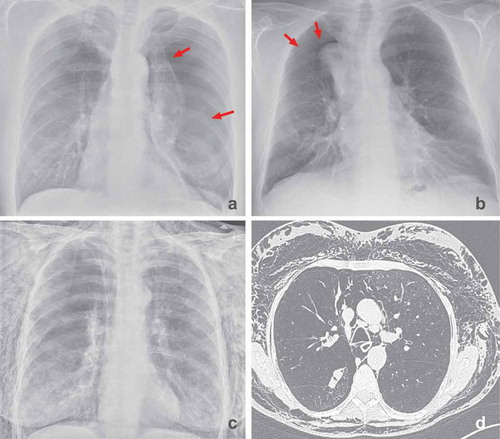
A pneumothorax is a common complication after valve treatment and occurs in 4.2–26.6% of the treated patients [Citation2,Citation3,Citation5–10]. After treatment with valves, the volume of the emphysematous target lobe is reduced. Part of this volume reduction is compensated by the expansion of the untreated ipsilateral lobe [Citation34,Citation42]. Due to the volume reduction, the negative pleural pressure may drop significantly and promote bullae or bleb rupture in the expanded ipsilateral lung tissue. Furthermore, the shifts of the lung may lead to ruptures of bullae or blebs due to preexisting pleural adhesions () [Citation42]. Symptomatic pneumothoraces may constitute life-threatening conditions in severe emphysema patients, requiring immediate drainage with a chest tube.
Figure 8. (a): Pneumothorax 1 day after treatment with endobronchial valves in the left upper lobe (indicated by arrows). There is a mediastinal shift to the right, indicating volume expansion due to the pneumothorax. This patient was treated with a chest tube only. (b): Pneumothorax ex vacuo (indicated by arrows) 1 day after treatment with endobronchial valves in the right upper lobe, the pneumothorax resolved spontaneously. Note: there is still volume loss on the site of the pneumothorax. (c and d): Extensive subcutaneous emphysema and pneumomediastinum due to a large air leak after treatment of the left lower lobe. This patient was successfully treated with surgical bullectomy to treat the air leak
Another potential manifestation is the ‘pneumothorax ex vacuo’, which means there is air in the pleural space, but no active air leak. There are two hypotheses as to how this develops. It is possible that there is a trauma of the treated lobe in which a part of the volume expands to the pleural cavity, but the valves prevent an active air leak. Most often, the size of the pneumothorax is small [Citation42]. Another hypothesis is that due to the increase in negative intrapleural pressure after the acute lobar collapse, air from the surrounding tissue and blood is drawn into the pleural space. In this case, the pleura remains intact and there is no bronchopleural fistula [Citation42,Citation43]. In case of a pneumothorax ex vacuo, drainage is normally not necessary and a ‘wait-and-see’ policy can be successful in these patients as the pneumothorax will slowly resolve ().
In case of a small pneumothorax without dyspnea or pain, prolonged observation is recommended. However, if the symptoms deteriorate or the size of the pneumothorax is increasing, a chest tube should be placed. Normally, a 14 French chest tube should suffice, but in case of a non-re-expanding pneumothorax or the development of subcutaneous emphysema, a large-bore chest tube should be considered [Citation44].
In most situations, chest tube drainage is sufficient to treat the pneumothorax [Citation7,Citation10]. In case the lung does not expand and/or there is a persistent or significant air leak, removal of valves can be considered after a few days. The removal of one or more valves will lead to re-expansion of the treated lobe and improve the contact of the pleural surfaces to promote healing of the pneumothorax. If the air leak stops and pneumothorax resolves after valve removal, the chest tube can be removed. Because valve removal can be performed rather easily, general anesthesia is not necessary in most cases. An extra reason to avoid general anesthesia is that positive pressure ventilation of emphysema patients with a pneumothorax is considered less safe. Not all valves have to be removed to re-expand the treated lobe and to recover from a pneumothorax. It is sufficient to remove only those valves that are most easy to reach. Valves can be replaced after approximately 6–8 weeks after removal of the chest tube, as it has been shown that these patients can experience significant functional improvements [Citation42,Citation45].
In case of a persistent air leak despite the removal of valves, other options should be considered, comparable to the standard care of a pneumothorax. However, in the majority of the patients this is not necessary. This includes mechanical or chemical pleurodesis or use of a Heimlich valve. Furthermore, one-way valves can be used to treat persistent air leaks. In this case, valve insertion in the targeted airways leading to the region of the air leakage may lead to a resolution or reduction of the air leak [Citation42,Citation46]. Furthermore, video-assisted thoracoscopic surgery (VATS) with bullectomy can be performed to treat the air leak [Citation42]. However, the choice of and timing of therapy are dependent on both clinical parameters, patient preference, and the availability of several options within the institution [Citation42].
5.3. Pneumonia and COPD exacerbation
After valve placement, approximately 0–8% of the patients develop a pneumonia in the first year [Citation2–10]. This is reported in both the treated lobe (0.9–3.6% of the patients [Citation2,Citation5,Citation7,Citation9]) as in the non-treated lobes (2.3%-7.1% of patients) [Citation2,Citation5,Citation9].
To potentially decrease the incidence of respiratory exacerbations, a course of prophylactic antibiotics and steroids are often prescribed around the bronchoscopy according to local guidelines. This intervention is mainly pragmatic and not evidence based.
In case of a bacterial pneumonia, a broad-spectrum oral antibiotic is advised. In case the pneumonia is located distal to the valves, removal of the valves should strongly be considered if the pneumonia does not clearly respond to treatment with (intravenous) broad-spectrum antibiotics (). Approximately 6 weeks after treatment, the valves can be replaced [Citation12]. A COPD exacerbation should be treated in accordance with standard care, with oral corticosteroids and inhaled bronchodilators. The LIBERATE study showed a strong trend toward a reduction of serious COPD exacerbations requiring hospitalization in patients treated with endobronchial valves in long-term follow -up [Citation3].
Figure 9. This patient was successfully treated with endobronchial valves in the left lower lobe. Two years later the patient presented with a postobstruction pneumonia. (a): The CT-scan showed an increased volume of the atelectasis of the left lower lobe. (b): After treatment with antibiotics and removal of two of the valves, there was clinical improvement and partial resolution of the consolidation. Due to the important initial clinical benefit of the valve treatment, this patient was treated by VATS lobectomy after full recovery of the pneumonia, rather than removal of the valves and experienced persistent clinical benefit
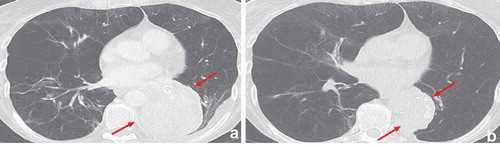
5.4. Hemoptysis
Directly after treatment, many patients may experience minor hemoptysis for a few days which is related to the procedure. This is self-limiting in most patients and in this case no further evaluation is required. However, during follow-up hemoptysis is reported in 1.5–5.6% of treated patients [Citation5,Citation6,Citation9,Citation47]. In most cases, the cause of hemoptysis is granulation tissue at the site of the valves or mucosal damage/ulceration due to valve movements (), and might be more prevalent in patients on systemic anticoagulation.
Figure 10. Airway ulceration due to contact of the valve with the opposite airway wall which led to hemoptysis. In this case valve removal was sufficient to treat the hemoptysis
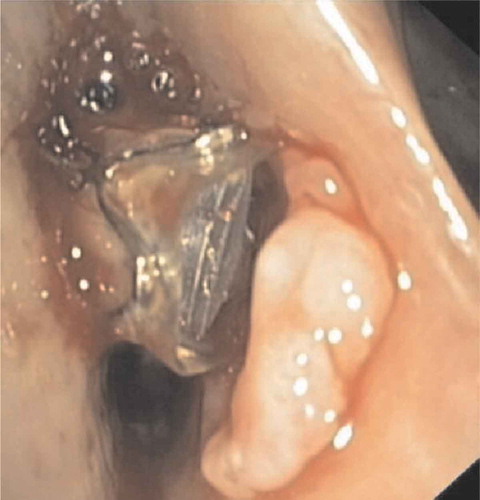
In patients with minor hemoptysis, a bronchoscopic evaluation is warranted to assess the possible causes and precise location [Citation12]. In case of minor mucosal damage or granulation tissue, a wait and see policy can be justified. A culture sample can guide treatment with antibiotics and a course of prednisolone is advised to treat granulation tissue formation. Small focused local lesions can be treated with coagulation therapy.
However, if the hemoptysis is more pronounced, or the granulation reaction is more severe, valve removal is indicated. In these cases, direct replacement is not recommended, to allow full recovery of the bronchus. Furthermore, future treatment at the same position is most often not rational. A more distal replacement can be considered in those situations.
Next to local hemoptysis guidelines, there are several treatment options in case of severe hemoptysis [Citation48]. Pharmacological treatment includes prednisolone and antibiotics, and tranexamic acid may be considered. If a patient uses anticoagulants, these should be discontinued or antagonized. Primarily, a bronchoscopy should be performed. Valves can be removed to treat the cause of the granulatory reaction. If there is a severe granulomatous reaction, this can be treated with coagulation therapy. It Is advised to have a bronchus blocker readily available during the procedure in case of extensive hemoptysis after valve removal.
Endovascular interventions may also be considered in case of severe hemoptysis. A CT-scan with intravenous contrast can show pathologically altered bronchial arteries and bronchial artery embolization may be successful if a bronchial artery is thought to be the cause of the hemoptysis.
If it is not possible to regain local control of the bleeding, a thoracic surgeon may be consulted for a lobectomy. If a patient with hemoptysis had a favorable outcome after the valve treatment, a lobectomy can be even more feasible for the patient’s outcome, with preservation of the lung volume reduction effects [Citation12].
5.5. Cough
Some patients may exhibit a persistent cough after treatment [Citation4,Citation7]. The cause of coughing is probably the local reaction of the airways to the valves and can be related to the formation of granulation tissue [Citation30]. However, even in patients without evidence of granulation tissue there may be coughing. Furthermore, it may be due to an incomplete atelectasis after valve insertion. Also, airway folding of the untreated lobe can lead to coughing and decreased sputum expectoration ().
A bronchoscopy can be performed to review local irritation or granulation tissue, perform local cleaning of secretions and take a culture to guide antibiotic treatment. A trial period of antibiotics and prednisolone may be prescribed, but this is not always effective. If, despite these efforts, the cough is persistent in time and invalidating, removal of valves may be considered. However, if there is lung volume reduction and clinical benefit regarding the exercise tolerance and dyspnea, it is very important to discuss the treatment options with the patient as removal of the valves will lead to a relapse of the hyperinflation and thus increase in symptoms, often back to baseline.
6. Conclusion
In conclusion, treatment with one-way valves is a successful treatment option in carefully selected patients with severe emphysema, with benefits on lung function, quality of life, and exercise tolerance. After treatment, both acute (e.g. pneumothorax) and late-onset (e.g. granulation tissue) manageable complications do occur. It is important to be aware of the possible complications, perform adequate follow-up and handle various complications. The most important tools to identify and manage complications are a dedicated review of the CT-scan and revision bronchoscopies.
7. Expert opinion
Patients with severe emphysema are severely disabled and have very limited treatment options. Endobronchial lung volume reduction is an additional treatment for this patient group, and this treatment option is becoming more and more important as worldwide awareness and acceptance in the pulmonary field increases driven by solid science, guidelines, and reimbursement. Treatment with one-way valves is currently the most effective treatment, but only suitable in a small percentage of the patients. Careful patient selection is important to treat only patients that will benefit from the valves. However, even if successful, these patients need dedicated follow-up to monitor treatment effect, and in case of deterioration or complications, a critical reevaluation should be performed to try to regain the effect or treat the complication.
Patients with COPD are a very heterogeneous group and bronchoscopic treatment options are not suitable for all. In the upcoming years, new treatment options will hopefully be developed or become more widely available. Coils, vapor, biologic lung volume reduction or airway bypass can be alternative options for patients with the presence of collateral ventilation [Citation41,Citation49–51]. Further, the presence of collateral ventilation could be blocked by completing the fissure surgically, or endobronchially by delivering a blocking substance at the incomplete part of the fissure [Citation52,Citation53]. An important issue is the biocompatibility of the devices in the lung. A frequent complication of all endobronchial devises (e.g. silicon and metal stents, EBV, and SVS valves and also the former examined airway bypass) is the formation of granulation tissue. If this can be prevented, this would increase the treatment success, treatment options, and decrease the number of complications and need for revision bronchoscopies. Furthermore, in patients with chronic bronchitis, hopefully there will be more successful endobronchial treatment options as targeted lung denervation or nitrogen therapy [Citation54,Citation55].
Article highlights
Bronchoscopic lung volume reduction is an important treatment option in carefully selected patients with severe emphysema with significant benefits in lung function, quality of life and exercise capacity.
After treatment with one-way valves, there are several possible complications and challenges. Careful follow-up after treatment is important to monitor for possible complications.
In case of complications, a CT-scan and/or bronchoscopy can be performed to identify and treat the problem.
If there is no initial effect after treatment with valves, this may be due to presence of collateral ventilation or displaced valves.
Loss of initial effect may be due to valve dislocation or granulation tissue formation. This can be treated bronchoscopically with valve removal and replacement.
Acute complications that should be anticipated after treatment are pneumothorax, pneumonia and COPD-exacerbations.
Declaration of interest
K Klooster has received travel reimbursement and speakers fee from PulmonX Inc., Redwood City, CA, USA. DJ Slebos is a physician advisor, consultant, and investigator for PulmonX Inc., Redwood City, CA, USA, and gave scientific presentations for Olympus Europe, Hamburg, Germany. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187–201.
- Criner GJ, Delage A, Voelker K, et al. improving lung function in severe heterogenous Emphysema with the Spiration Valve System (EMPROVE). A multicenter, open-label randomized controlled clinical trial. Am J Respir Care. 2019 Jul 31;200(11):1354–1362.
- Criner GJ, Sue R, Wright S, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE). Am J Respir Crit Care Med. 2018 Nov 1;198(9):1151–1164.
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015 Sep 12;386(9998):1066–1073.
- Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012 Jun;39(6):1334–1342.
- Kemp SV, Slebos DJ, Kirk A, et al. A multicenter randomized controlled trial of zephyr endobronchial valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med. 2017 Dec 15;196(12):1535–1543.
- Klooster K, Ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015 Dec 10;373(24):2325–2335.
- Li S, Wang G, Wang C, et al. The REACH trial: A randomized controlled trial assessing the safety and effectiveness of the Spiration(R) valve system in the treatment of severe emphysema. Respiration. 2019;97(5):416–427.
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010 Sep 23;363(13):1233–1244.
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT study. Am J Respir Crit Care Med. 2016 Nov 1;194(9):1073–1082.
- Shah PL, Slebos DJ. Bronchoscopic interventions for severe emphysema: where are we now? Respirology. 2020 May 3. DOI:10.1111/resp.13835
- Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration. 2017;93(2):138–150.
- Singh D, Agusti A, Anzueto A, et al. Global Strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019 May;53(5). DOi:10.1183/13993003.00164-2019
- Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur Respir J. 2013 Feb;41(2):302–308.
- Klooster K, Koster TD, Ruwwe-Glosenkamp C, et al. An integrative approach of the fissure completeness score and chartis assessment in endobronchial valve treatment for emphysema. Int J Chron Obstruct Pulmon Dis. 2020;15:1325–1334.
- Koster TD, van Rikxoort EM, Huebner RH, et al. Predicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic tools. Respiration. 2016;92(3):150–157.
- Gesierich W, Samitas K, Reichenberger F, et al. Collapse phenomenon during Chartis collateral ventilation assessment. Eur Respir J. 2016 Jun;47(6):1657–1667.
- Welling JBA, Koster TD, Hartman JE, et al. Temporary right middle lobe occlusion with a blocking device to enable collateral ventilation measurement of the right major fissure. Respiration. 2020;99(6):516–520.
- Nouraei SA, Petrou MA, Randhawa PS, et al. Bacterial colonization of airway stents: a promoter of granulation tissue formation following laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 2006 Oct;132(10):1086–1090.
- Sarmand N, Gompelmann D, Kontogianni K, et al. New bacterial growth in bronchial secretions after bronchoscopic valve implantation. Int J Chron Obstruct Pulmon Dis. 2018;13:565–570.
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg. 2006 Jul;82(1):197–206;discussion 206–7.
- Valipour A. Valve therapy in patients with emphysematous type of chronic obstructive pulmonary disease (COPD): from randomized trials to patient selection in clinical practice. J Thorac Dis. 2018 Aug;10(Suppl 23):S2780–S2796.
- van Geffen WH, Klooster K, Hartman JE, et al. Pleural adhesion assessment as a predictor for pneumothorax after endobronchial valve treatment. Respiration. 2017;94(2):224–231.
- Hubner RH, Ruwwe-Glosenkamp C, Saccomanno J, et al. Endoscopic lung volume reduction: can endobronchial valves be safely removed? Respiration. 2020;99(5):459–460.
- Sylvester JT, Shimoda LA, Aaronson PI, et al. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012 Jan;92(1):367–520.
- Mishra A, Reed RM, Eberlein M. Severe, rapidly reversible hypoxemia in the early period after bilateral lung transplantation. Ann Am Thorac Soc. 2016 Jun;13(6):979–985.
- Cable DG, Deschamps C, Allen MS, et al. Lobar torsion after pulmonary resection: presentation and outcome. J Thorac Cardiovasc Surg. 2001 Dec;122(6):1091–1093.
- Seok Y, Cho S, Lee JY, et al. The effect of postoperative change in bronchial angle on postoperative pulmonary function after upper lobectomy in lung cancer patients. Interact Cardiovasc Thorac Surg. 2014 Feb;18(2):183–188.
- Fiorelli A, D’Andrilli A, Bezzi M, et al. Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: results of a multicenter study. J Thorac Dis. 2018 Oct;10(Suppl 27):S3315–S3325.
- Klooster K, Hartman JE, Ten Hacken NH, et al. One-year follow-up after endobronchial valve treatment in patients with emphysema without collateral ventilation treated in the STELVIO Trial. Respiration. 2017;93(2):112–121.
- Caviedes IR, Labarca G, de Oliveira HG, et al. Non-answered questions in patients with endobronchial valve placement for lung volume reduction. Respiration. 2018;95(4):269–272.
- Gompelmann D, Heinhold T, Rotting M, et al. Long-term follow up after endoscopic valve therapy in patients with severe emphysema. Ther Adv Respir Dis. 2019 Jan-Dec;13:1753466619866101.
- Becker MD, Berkmen YM, Austin JH, et al. Lung volumes before and after lung volume reduction surgery: quantitative CT analysis. Am J Respir Crit Care Med. 1998 May;157(5 Pt 1):1593–1599.
- Brown MS, Kim HJ, Abtin FG, et al. Emphysema lung lobe volume reduction: effects on the ipsilateral and contralateral lobes. Eur Radiol. 2012 Jul;22(7):1547–1555.
- Ueda K, Tanaka T, Hayashi M, et al. Compensation of pulmonary function after upper lobectomy versus lower lobectomy. J Thorac Cardiovasc Surg. 2011 Oct;142(4):762–767.
- Ueda K, Tanaka T, Hayashi M, et al. Computed tomography-defined functional lung volume after segmentectomy versus lobectomy. Eur J Cardiothorac Surg. 2010 Jun;37(6):1433–1437.
- Klooster K, van Dijk M, Koster TD, et al. First in human experience of the performance of the new 5.5-LP size zephyr endobronchial valve. Respiration. 2020;99(1):50–55.
- Kontogianni K, Gompelmann D, Valipour A, et al. Efficacy and safety of the 9-mm Intrabronchial valve in patients with advanced emphysema. Respiration. 2020;99(4):333–343.
- Fiorelli A, Reginelli A, Santini M. Endobronchial valve migration: A “Whistle Blower”. J Bronchology Interv Pulmonol. 2016 Jul;23(3):e24–6.
- Gompelmann D, Gerovasili V, Kontogianni K, et al. Endoscopic valve removal >180 days since implantation in patients with severe emphysema. Respiration. 2018;96(4):348–354.
- Hornemann K, Gompelmann D, Herth FJF, et al. Lung volume reduction surgery (LVRS) after endoscopic lung volume reduction (ELVR) in severe emphysema - A case series. Eur Respir J. 2012 Sep 1; 40. DOI:10.1183/09031936.00213711
- Valipour A, Slebos DJ, de Oliveira HG, et al. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema–potential mechanisms, treatment algorithm, and case examples. Respiration. 2014;87(6):513–521.
- Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest. 1996 Oct;110(4):1102–1105.
- MacDuff A, Arnold A, Harvey J. Group BTSPDG. Management of spontaneous pneumothorax: British thoracic society pleural disease guideline 2010. Thorax. 2010 Aug;65(Suppl 2):ii18–31.
- Gompelmann D, Herth FJ, Slebos DJ, et al. Pneumothorax following endobronchial valve therapy and its impact on clinical outcomes in severe emphysema. Respiration. 2014;87(6):485–491.
- Travaline JM, McKenna RJ Jr., De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest. 2009 Aug;136(2):355–360.
- Franzen D, Straub G, Freitag L. Complications after bronchoscopic lung volume reduction. J Thorac Dis. 2018 Aug;10(Suppl 23):S2811–S2815.
- Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis. 2017 Sep;9(Suppl 10):S1069–S1086.
- Herth FJF, Slebos DJ, Shah PL, et al. Protocol of a randomized controlled study of the PneumRx Endobronchial Coil System versus Standard-of-Care Medical Management in the Treatment of Subjects with Severe Emphysema (ELEVATE). Respiration. 2019;98(6):512–520.
- Snell G, Herth FJ, Hopkins P, et al. Bronchoscopic thermal vapour ablation therapy in the management of heterogeneous emphysema. Eur Respir J. 2012 Jun;39(6):1326–1333.
- Shah PL, Slebos DJ, Cardoso PF, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011 Sep 10;378(9795):997–1005.
- Ing A, Sullivan C, Hersch N, et al. Reversal of Collateral Ventilation Using Endobronchial Polymer Sealant in a Patient With Emphysema Undergoing Endoscopic Lung Volume Reduction (ELVR) With Valves: A Case Report and Proof of Concept. J Bronchology Interv Pulmonol. 2020 Jan;27(1):e14–e16.
- Majid A, Kheir F, Alape D, et al. Combined thoracoscopic surgical stapling and endobronchial valve placement for lung volume reduction with incomplete lobar fissures: an experimental pilot animal study. J Bronchology Interv Pulmonol. 2020 Apr;27(2):128–134.
- Slebos DJ, Breen D, Coad J, et al. Safety and histological effect of liquid nitrogen metered spray cryotherapy in the lung. Am J Respir Crit Care Med. 2017 Nov 15;196(10):1351–1352.
- Slebos DJ, Shah PL, Herth FJF, et al. Safety and Adverse Events after Targeted Lung Denervation for Symptomatic Moderate to Severe Chronic Obstructive Pulmonary Disease (AIRFLOW). A multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019 Dec 15;200(12):1477–1486.