ABSTRACT
Introduction
Half of all children will experience an episode of wheezing by their sixth birthday and acute episodes of wheezing in preschool children account for the majority of all childhood hospital admissions for wheeze. Recurrent preschool wheezing associates with early loss of lung function and a life-long impact on lung health.
Areas covered
We reviewed the literature on PubMed from August 2010–2020 focussing on factors associated with wheeze inception and persistence, paying specific attention to mechanistic studies that have investigated the impact of early life exposures in shaping immune responses in children with underlying susceptibility to wheezing. In particular, the role of early allergen sensitization, respiratory infections, and the impact of the environment on shaping the airway microbiome and resulting immune responses are discussed.
Expert opinion
There is an abundance of associative data showing the role of in utero and postnatal factors influencing wheeze onset and persistence. However, mechanistic and stratified, biomarker-based interventional studies that confirm these associations are now needed if we are to impact the significant healthcare burden resulting from preschool wheezing disorders.
1. Introduction
Wheezing in the preschool years (before age 6) is common, occurring in a third of children by their third birthday and half of children by 6 years [Citation1]. The prevalence of parent reported wheeze in 2-year-old children was 2–17% in the European EuroPrevall birth cohort study [Citation2]. Iceland had the highest prevalence, followed by the UK. Wheezing in early childhood is associated with significant healthcare resource utilization, accounting for approximately 75% of all childhood hospital admissions for acute wheeze [Citation3] and associated with significant impact on family quality of life [Citation4]. In the USA, preschool wheezers have double the rate of emergency department attendances and five times the rate of hospital admissions, compared with school-age asthmatics [Citation5]. Birth cohort studies, animal models, and clinical trials have identified early lower respiratory tract illness (LRTI), daycare attendance, and antenatal and postnatal smoke exposure among the risk factors for the development of preschool wheeze [Citation6–11]. However, due to the heterogeneity (both clinical and mechanistic) of preschool wheeze and difficulty obtaining objective measurements of airway inflammation and function in young children, there are significant gaps in our current understanding of the factors driving preschool wheeze, limiting our ability to identify novel therapies. This is reflected in the persistently high and unchanged disease burden resulting from preschool wheezing disorders over the last two decades. The aim of this review is to consolidate current evidence about the underlying mechanisms contributing to the onset and persistence of wheezing in preschool children aged between 1 and 5 years, outline gaps in knowledge and highlight directions for future research. We reviewed the literature on PubMed from August 2010–2020 focussing on factors associated with preschool wheeze inception and mechanisms mediating persistence. Only full manuscripts published in English language were included.
2. Recurrent wheezing episodes
2.1. Current knowledge of the pathophysiology of recurrent preschool wheeze
Compared to school age (6–16 years) asthma, in which allergen sensitization and exacerbated type 2 immune responses to inhaled environmental exposures are key (), relatively little is known about the immunopathology of preschool wheeze. However, several clinical features of preschool wheezers suggest the underlying pathological mechanisms are likely to be distinct from school-age atopic asthma. Firstly, over half of children with recurrent preschool wheeze have clinical symptom resolution by school age and do not develop allergic asthma [Citation12], though they may have residual lung function deficits. Secondly, many do not benefit significantly from therapies, such as corticosteroids, that are usually effective in allergic asthma. Specifically in viral preschool wheeze exacerbations, use of systemic corticosteroids has been shown to confer no benefit in terms of symptom scores, risk of hospital admission, need for further systemic corticosteroid courses or unscheduled visits for wheeze in the following month [Citation13–17]. And while a post-hoc superiority analysis reported a small reduction in length of hospital stay for oral corticosteroids (OCS) compared to placebo of 2.8 hours [Citation18], four studies have shown no difference in this parameter [Citation16,Citation19–21]. Similarly, leukotriene receptor antagonists given as maintenance or intermittent treatment have not been shown to confer benefit in terms of number of wheeze episodes progressing requiring oral steroids in two meta-analyses [Citation22,Citation23]. Although two studies of intermittent montelukast administration in episodic viral wheezers showed reductions in unscheduled medical visits due to wheeze, the effects were small [Citation24,Citation25]. Thirdly, children with transient wheezing that resolves during the preschool years are typically not atopic suggesting allergy is not the main driver of disease in these patients [Citation26]. Finally, growing evidence shows the pathogens associated with acute episodes of wheezing in preschool children may be distinct from those in school-age asthma [Citation27], and the underlying airway inflammatory profile also differs [Citation28–30]. Acute wheeze episodes in preschool children were as frequently associated with hypopharyngeal bacterial infection as nasopharyngeal viral infection [Citation31]. Eighty-five percent had positive hypopharyngeal culture for Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis compared with 65% having positive detection of a respiratory virus, while 55% had both bacterial and viral pathogens detected [Citation32]. For this reason, ‘infection associated wheeze’ has been suggested as a more appropriate term than ‘viral wheeze.’
Figure 1. Pathology of preschool wheeze and school-age allergic asthma
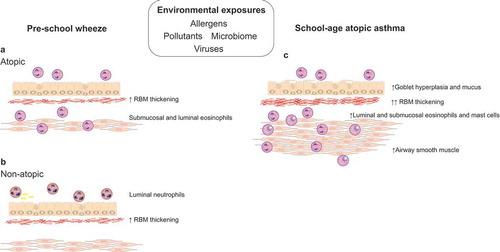
2.2. The role of eosinophils in preschool wheeze
Severe recurrent wheezers undergoing bronchoscopy and endobronchial biopsy, have evidence of increased numbers of tissue eosinophils irrespective of atopic status [Citation33,Citation34] (). Eosinophils have been shown to be present in increased numbers in the submucosa in established severe recurrent wheeze by 3 years of age [Citation34], but eosinophilic inflammation has not been found in the airways prior to wheeze onset, even in infants with atopy and reversible airflow obstruction [Citation35]. An increase in urinary eosinophil activation markers has been reported during acute wheeze episodes [Citation36], but it is unclear whether these reflect airway eosinophilic inflammation. In a mouse model of house dust mite induced allergic airway disease, depletion of eosinophils did not alter the development of airway inflammation, remodeling or hyperresponsiveness, suggesting eosinophils are not driving wheeze inception [Citation37]. In support of this, trials of inhaled corticosteroids, which reduce eosinophil recruitment and activation, given to preschool wheezers with the aim to prevent asthma development have been unsuccessful [Citation38,Citation39]. Although many persistent wheezers benefit from reduced exacerbations and improved symptoms while taking inhaled corticosteroids [Citation40], they have no disease modifying effect on lung function decline or airway hyper-responsiveness [Citation39]. Importantly, evidence for lower airway eosinophilia suggests the presence of a corticosteroid responsive phenotype. An unbiased analysis of airway luminal inflammation in bronchoalveolar lavage from preschool children with severe wheezing has shown a cluster with mean age 57 months who had aeroallergen sensitization and were responsive to inhaled corticosteroids [Citation41]. This suggests preschool wheezers with aeroallergen sensitization may also have lower airway eosinophilia. Although this relationship has not been shown directly, the Individualized Therapy for Asthma in Toddlers (INFANT) study which was a multi-center, double blinded RCT of daily inhaled corticosteroids vs daily LTRA vs intermittent ICS in (n = 300) children aged 12–59 months old, has shown the greatest reduction in asthma symptoms in children receiving daily ICS who also had aeroallergen sensitization and/or blood eosinophils >300 cells/µl [Citation42]. These data demonstrated two objective biomarkers, aeroallergen sensitization and blood eosinophils, can be used to identify response to ICS in recurrent preschool wheeze. However, in order to confirm this relationship, a prospective study with children randomized/stratified to treatment based on aeroallergen sensitization and/or blood eosinophils is needed.
2.3. Neutrophils in recurrent preschool wheeze
Neutrophilic inflammation in bronchoalveolar lavage fluid has been identified in children with recurrent preschool wheeze (). An unbiased analysis of bronchoalveolar lavage inflammation from severe wheezers identified a cluster mean age 40 months with neutrophilic, steroid refractory wheeze [Citation41]. Interestingly, severe wheezers with neutrophilia may also have associated lower airway positive bacterial culture [Citation43] or a Moraxella-dominant airway microbiome profile [Citation44]. These findings are apparent when the child is well and in the absence of acute symptoms of infection. In an observational case series, prolonged (2–16 weeks) treatment with an appropriate antibiotic resulted in reduced wheeze episodes in 92% of children who had lower airway neutrophilia and positive bacterial culture up to 6 months later [Citation43]. This suggests chronic infection may be causally associated with persistent wheezing and lower airway neutrophilia in a subgroup of preschool wheezers. However, there have been no prospective trials that have investigated the role of antibiotics in preventing attacks of preschool wheeze. It is not known whether neutrophilic inflammation is beneficial in preschool wheezing and a response to lower airway bacterial infection, or potentially detrimental, and a lack of data on lower airway inflammation prior to wheeze onset means the potential contribution of neutrophils at wheeze inception is unknown. Importantly, although aeroallergen sensitization is a marker of a steroid responsive phenotype, the majority (approximately 75%) of preschool wheezers are non-atopic and do not have aeroallergen sensitization [Citation44]. There is therefore a need to investigate whether use of targeted antibiotics for airway bacterial infection and neutrophilia in non-atopic wheezers may offer a management strategy to prevent attacks.
2.4. The airway epithelium and structural cells in preschool wheeze
The airway epithelium has a vital role as a continuous highly regulated physical barrier to penetration by inhaled microbes and allergens, which is maintained by intercellular epithelial junctions [Citation45]. It has been proposed that abnormal epithelial barrier integrity predisposes to aeroallergen sensitization and ongoing inflammation by increased contact of subepithelial tissues with allergens and microbes [Citation46]. Persistent preschool wheezers and school-age asthmatics are reported to have a defective epithelial barrier, with increased epithelial shedding in bronchial biopsies [Citation47] and bronchoalveolar lavage fluid [Citation30], reduced tight junction integrity [Citation48], and delayed wound repair in cultured primary bronchial epithelial cells compared with controls [Citation49]. The central role of the airway epithelium in orchestrating immune responses involved in asthma pathogenesis is being recognized, and there is evidence that airway epithelial cell innate immune function is impaired in children with persistent wheeze compared with healthy children. In vitro nasal epithelial cells from children aged 0–15 years with wheezing or asthma produce less IL-8, IL-6, MCP-1 and G-CSF [Citation50], while bronchial epithelial cells produce more IL-6, prostaglandin E2, and epidermal growth factor at baseline [Citation51]. Impaired epithelial antiviral responses, with reduced interferon-β production and increased viral replication in response to human rhinovirus (HRV) [Citation52] and respiratory syncytial virus (RSV) [Citation53] infection has also been shown in children with wheeze/asthma in vitro. Similar responses in airway epithelial cells sampled from neonates in the first 48 hours of life prior to wheeze onset [Citation54] suggest a potentially important role of altered airway epithelial cell function in the development of wheezing.
One of the characteristic pathological features of established asthma, apparent in school-age children, is airway remodeling. This includes the development of increased thickness of the subepithelial reticular basement membrane, increased airway smooth muscle, and angiogenesis [Citation47,Citation55], which likely develop in parallel with airway inflammation [Citation56]. Importantly, the presence of remodeling does not relate to the type of airway inflammation or to allergic sensitization [Citation33,Citation57]. Reticular basement membrane thickening was not seen in wheezing infants with reversible airflow obstruction at 12 months [Citation35], but reported in two cohorts of severe persistent preschool wheezers by 3–5 years of age [Citation33,Citation34]. Increased bronchial airway smooth muscle (ASM) has also been reported in severe preschool wheezers by 2.2 years of age, and contrary to RBM thickness and eosinophilic inflammation may be a predictor of school-age asthma [Citation58]. Airway remodeling is closely linked with lung function in older children with asthma [Citation59] and is therefore likely to be associated with early lung function changes in preschool wheezers. However, studies are needed to confirm this association. Although early airway remodeling has been described in severe, recurrent preschool wheeze, little is known about the mechanisms mediating the development of these changes. The inability to achieve disease modification by targeting airway inflammation in preschool wheeze suggests therapies targeting structural airway changes are critical to allow a change in the natural history of the disease. Studies investigating mechanisms mediating early life airway remodeling are therefore a critical unmet need if we are to succeed in achieving secondary asthma prevention.
3. Factors associated with wheeze inception
3.1. Genetic/host
A twin study of >25,000 twin pairs in Sweden estimated heritability accounted for up to 82% of childhood asthma risk [Citation60]. But the rapid rise in childhood wheeze and asthma prevalence over the last century emphasizes the additional importance of environment exposures, including cigarette smoke, pollution, aeroallergen and endotoxin exposure, in modifying this risk [Citation61]. Genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNPs) associated with early asthma development in children, including the 17q12–21 region, which increases the risk of persistent wheeze and asthma before 5 years of age [Citation62,Citation63]. In one study, the asthma risk associated with 17q12–21 locus was dependent on tobacco smoke exposure [Citation64], while, in another, it only conferred risk in those with a history of early life human rhinovirus (HRV) illness and was associated with ORMDL3 and GSDMB gene expression [Citation65]. The importance of gene by environment interactions was demonstrated since the genetic susceptibility risk resulted in increased rhinovirus associated wheezing illnesses in the first year, but the same gene locus conferred protection if children were brought up on a cattle farm [Citation66]. Other loci identified include genes for IL-33 and its receptor IL1RL1, known to be involved in airway remodeling, and CDHR3, which mediates binding and replication of human rhinovirus C (HRV-C) in the epithelium, and associates with severe exacerbations of childhood asthma [Citation67,Citation68]. Little can be done at present to alter genetic susceptibility for wheeze development, but manipulation of environmental exposures to alter immune responses is increasingly being investigated as a means for primary asthma prevention [Citation69].
3.2. Prematurity/chronic lung disease
The prevalence of premature birth (<37 weeks gestation) is ~10% worldwide [Citation70] and increasing [Citation71], while improvements in perinatal care mean survival rates for very (<32 weeks gestation) and extremely (<28 weeks gestation) preterm infants are also increasing [Citation71]. Consequently, long-term respiratory morbidity as a result of prematurity is also rising [Citation72]. Even in children born late preterm (33–34 weeks gestation), lung function measured by forced expiratory volume in 1 second (FEV1), forced expiratory flow at 25–75% of forced vital capacity (FEF25–75) and FEV1/FVC ratio at 8–9 years of age was comparable to that in children born at 25–32 weeks, and significantly lower than in children born at term [Citation73]. Preterm birth was associated with an increased risk of wheezing disorders, particularly in those born very preterm, which persisted into late adult life [Citation74]. The mechanisms underlying the relationship between preterm birth and wheeze are difficult to discern, but include common triggers of preterm birth including maternal smoking, restricted growth, and inflammation. Premature birth also has direct negative impacts on lung development, airway reactivity, and immune responses, which further increase susceptibility to postnatal lung injury through sepsis, mechanical ventilation, and hyperoxia [Citation74]. The most likely factor resulting in wheezing in children born prematurely is the presence of smaller airways, since reduced lung function is apparent in preterm wheezers despite similar rates of early allergen sensitization as in term children. Thus, airway eosinophilia may not be present and indiscriminate prescription of inhaled corticosteroids should be questioned, as bronchodilators alone may be the most appropriate option to achieve symptom control.
3.3. Maternal and infant nutrition
Maternal nutrition during pregnancy has been linked to risk of wheezing in their offspring (), most notably for low intake of vitamin D and long-chain polyunsaturated fatty acids (LC-PUFAs). Observational studies are inconsistent with the role of vitamin D in wheeze inception; some have suggested vitamin D deficiency is associated with increased risk of wheeze and asthma development, whilst few studies suggest excess vitamin D supplementation might contribute to increased allergic diseases and asthma. However, a meta-analysis of two large RCTs comparing high-dose vitamin D supplementation to standard care in pregnancy for the primary prevention of asthma in their offspring showed a 25% reduction in risk of recurrent wheeze at 3 years of age [Citation75]. Using a higher dose and starting earlier in pregnancy was associated with a greater effect. Vitamin D has known roles in immune regulation and fetal lung maturation [Citation75], suggesting plausible mechanisms for protection against wheeze inception. The offspring of mothers supplemented with high-dose vitamin D in pregnancy had significantly upregulated airway immune profiles at 1 month of age, supporting immune regulation as a potential mechanism [Citation76]. A meta-analysis including six RCTs investigating pre- and/or postnatal LC-PUFA supplementation for the primary prevention of allergies in early childhood found no significant reduction in asthma [Citation77]. However, a more recent RCT of LC-PUFA supplementation in pregnancy found a 30% reduction in persistent wheezing in their offspring by 5 years of age, with the effect being most pronounced in mothers that had low dietary intake of PUFAs prior to supplementation and those with a FADS gene variant associated with low serum PUFA levels [Citation78]. Maternal LC-PUFA supplementation in pregnancy has been shown to balance Th1/Th2 and modulate IFNγ and IL-13 in infants [Citation79], suggesting a potential mechanism for reducing wheeze risk. Maternal obesity is a growing form of malnutrition and is associated with wheeze in infancy in several studies including a pooled analysis of 14 birth cohorts (85 509 mother-child pairs). Here, the risk was not mediated by low birth weight, prematurity, mode of delivery, or breastfeeding [Citation80]. The mechanisms underlying this association are not clear but may in part be related to obesity and/or weight gain in the infant.
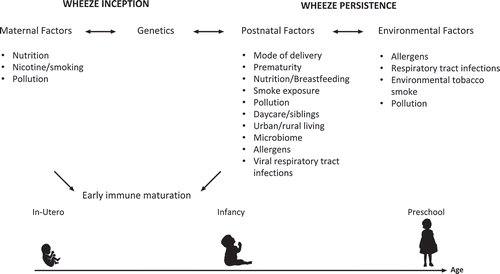
Figure 2. Factors associated with wheeze inception and persistence
3.4. Mode of delivery
Cesarean section delivery is associated with an increased risk of childhood asthma in most studies [Citation81]. It is unclear to what extent this is related to the delivery itself or the associated use of intrapartum antibiotics and the resulting effect on the newborn microbiome [Citation82], or to factors associated with the indication for Cesarean delivery. The Canadian CHILD (Canadian Healthy Infant Longitudinal Development) national birth cohort study found the use of intrapartum antibiotics led to alterations in the infant gut microbiota at 3 months of age, which persisted to 12 months of age in those delivered by emergency cesarean section, suggesting cesarean delivery itself alters the microbiota [Citation83]. In contrast, another study found that the risk of asthma medication was increased with emergency but not elective cesarean section delivery, suggesting altered microbiota is not as important as the indication for emergency delivery [Citation84].
3.5. Breastfeeding
Breastfeeding has benefits in terms of early life immunity and a reduction in respiratory tract infections [Citation85], and a meta-analysis has shown a strong protective association with wheezing in the first 2 years of life, but this diminished with time [Citation86]. This suggests breastfeeding is protective against transient early wheezing through a reduction in respiratory tract infections known to trigger wheeze attacks. Confounding by associated lifestyle factors, such as socioeconomic status, may explain the apparent association with persistent wheeze and asthma seen in some studies.
3.6. Environmental exposures and pathophysiology of preschool wheeze
3.6.1. Tobacco and nicotine exposure
There is a large body of evidence showing tobacco smoke exposure antenatally in utero, even more so than postnatally, is associated with reduced lung function at birth [Citation6,Citation7] that persists into later life [Citation87–90], airway hyperresponsiveness [Citation91], and an increased risk of sudden infant death syndrome [Citation92], severe bronchiolitis [Citation93], wheezing [Citation8,Citation91,Citation94] and asthma [Citation8,Citation88]. In a study of 5,762 school-aged children in California, in utero exposure to maternal smoking without postnatal environmental tobacco smoke (ETS) exposure was associated with persistent wheezing and physician-diagnosed asthma f[Citation8]. Postnatal ETS exposure was associated with wheezing, particularly in children exposed to two or more smokers even in the absence of inutero exposure, but was not associated with physician-diagnosed asthma. Several other large epidemiological studies, including the ALSPAC (Avon study of parents and children) [Citation10], BAMSE (Barn Allergi Milieu Stockholm Epidemiologi) [Citation95] and Tucson Children’s Respiratory Study [Citation94] have reported similar significant effects of maternal smoking on preschool wheezing. Passive exposure to ETS postnatally has also been associated with frequency and severity of respiratory tract infections [Citation96] and wheezing [Citation97] in systematic reviews. Together these studies suggest in utero exposure is causally associated with persistent wheeze, but whilst postnatal ETS exposure triggers wheezing attacks, particularly in combination with other insults such as viral infection in non-atopic children, it is not driving asthma development in school-age children [Citation8,Citation98].
Many studies have investigated the mechanisms underlying the relationship between cigarette smoke exposure and preschool wheezing. Whilst traditional cigarettes contain many toxic components detrimental to lung health, recent research in animal models has also shown that nicotine alone has detrimental effects on lung growth and development. Specifically, prenatal nicotine exposure is associated with fetal lung airway remodeling, with increased collagen type 1 and 3 deposition and airway wall diameter [Citation99], and narrowing and lengthening of airways [Citation100]. These changes manifest as decreased forced expiratory flow in offspring, and increased airway hyperresponsiveness to methacholine challenge into adulthood, even in the absence of allergic sensitization [Citation100]. Cigarette smoke exposure prenatally has been associated with loss of alveolar tethering points, leading to a reduction in the forces opposing airway narrowing and airway hyperresponsiveness [Citation92,Citation101], as well as emphysematous changes with a reduction in number but increase in the size of alveoli [Citation102]. Nicotine has also been shown to promote a more contractile airway smooth muscle cell phenotype [Citation103], reduce elastin synthesis [Citation104], and increase mucus production from bronchial epithelial cells [Citation105]. The association between smoking in pregnancy and intrauterine growth restriction and prematurity is widely reported, which in turn has negative consequences for future lung function and risk of preschool wheezing [Citation106,Citation107].
As well as causing structural airway remodeling, there is evidence that prenatal nicotine exposure predisposes to wheezing and asthma through effects on the developing immune system. Nicotine exposure has been shown to promote a more inflammatory environment in the neonatal lung, with increased expression of interleukin (IL)-13 and transforming growth factor-β1 (TGFβ1), and increased alternative activation of resident airway macrophages which consequently have reduced phagocytic ability [Citation108]. Maternal smoking has also been associated with altered cord blood mononuclear cell immune responses, including increased proliferation in response to stimulation with house dust mite allergen [Citation109] and reduced cytokine release in response to toll-like receptor ligands [Citation110], and lower numbers of cord blood T regulatory cells [Citation111]. These effects may result in altered innate immune responses to pathogens and allergens in the postnatal period and contribute to wheezing. Whilst nicotine replacement strategies such as e-cigarettes, chewing gum or patches are often considered safer than traditional cigarettes, their use in pregnancy is likely to have significant impacts on offspring lung health [Citation112]. But even if the mother smoked during pregnancy, parents stopping smoking after birth would still confer substantial benefits in terms of lung health [Citation113].
3.6.2. Pollution
Ambient pollution has been consistently linked with negative effects on respiratory health, particularly in children who are undergoing rapid lung growth and maturation, and have the greatest exposure to inhaled pollution through higher activity levels and time spent outdoors [Citation114]. Specifically, environmental air pollution has been shown to affect lung growth, with long-term exposure to small particulate matter and nitrogen dioxide associated with significantly lower growth in FVC and FEV1 during school-age in cohort studies [Citation115–117]. Pollution also acts synergistically with respiratory tract infections to increase the frequency and severity of exacerbations of pre-school wheeze [Citation118]. However, the data is conflicting on whether pollution exposure is causally associated with development of wheeze. A meta-analysis of five large European birth cohort studies found no significant association between asthma prevalence and pollution exposure [Citation119]. In contrast, a meta-analysis of traffic-related air pollution (TRAP) included 41 studies and found significant associations between asthma beyond 6 years and prior levels of exposure to black carbon, nitrogen dioxide, and particulate matter [Citation120]. A recent case–control registry study of all Danish children born between 1997 and 2014 also showed a significant association between increased 12-month average particulate matter and nitrogen dioxide exposure and risk of persistent wheezing in children <6 years, but not in children >6 years [Citation121], suggesting timing of exposure is important. Another study showed high TRAP exposure at birth was associated with transient and persistent wheeze, but only high average TRAP exposure from birth through to age 7 years was associated with school-age asthma, suggesting cumulative dose is important. Exposure to biomass fuels in indoor environments is also a significant source of pollution in developing countries, and while the effects on lung health are not as thoroughly researched, exposure in the home in early infancy has been associated with preschool wheeze prevalence [Citation122] and significant reductions in peak flow growth [Citation123]. In utero exposure to cigarettes and maternal exposure to high ambient pollution during pregnancy has been associated with reduced lung function in offspring at 4.5 years [Citation124] and development of early childhood asthma [Citation125,Citation126].
Studies investigating gene–environment interactions suggest air pollution is significantly associated with wheeze and asthma development in children with specific risk alleles, including genes involved in innate immune responses (TLR2 and TLR4) [Citation127], airway inflammation (TNF) and management of oxidative stress (GSTP1) [Citation128], and loci previously associated with chronic obstructive pulmonary disease (COPD) in adults (ADCY2 and DLG2) [Citation129]. Other potential mechanisms underlying the association between air pollution and persistent wheeze include oxidative stress-induced tissue damage, airway remodeling, IgE-mediated and non-allergic inflammation, and increased susceptibility to respiratory infections [Citation130]. Overall, exposure to inhaled oxidants such as cigarette smoking or particulate matter increases the risk of early wheezing and involves multiple mechanisms, which require investigation to enable adequate and effective treatments, rather than the blanket use of inhaled corticosteroids [Citation131].
3.6.3. Allergens
Multiple early aeroallergen sensitization is associated with persistent wheezing and progression to asthma [Citation132–134]. In the Multicentre Allergy Study (MAS), children were followed from birth to 13 years, and sensitization to perennial aeroallergens (house dust mite, cat and dog) by age 3 years was associated with persistent wheeze and reduced lung function at school age, particularly in those with the highest levels of allergen exposure [Citation135]. In the Urban Environment and Childhood Asthma (URECA) longitudinal birth cohort study, cumulative exposure over the first 3 years of life to cockroach, mouse, and house dust mite allergens in the home was associated with sensitization to those allergens at age 3 and sensitization to any food or any aeroallergen was associated with recurrent wheeze at 3 years [Citation136]. In contrast, exposure to the same allergens only in the first year had little or no association with sensitization at age 3 years and was negatively associated with recurrent wheeze, highlighting the importance of timing of exposure [Citation136]. This is supported by animal studies that have shown age at first allergen exposure determines the type of immune response and lung disease generated. In a neonatal mouse model of allergic airway disease, exposure to inhaled house dust mite from day 3 of life was associated with significantly increased eosinophilia, airway hyperresponsiveness, and type 2 immune responses compared to adult mice [Citation133]. The role of early allergic sensitization in wheeze development appears to be linked to viral respiratory tract infections and microbiome composition (discussed in more detail later). Despite multiple sources of evidence showing early allergen sensitization is associated with risk of persistent wheezing, avoidance of any individual allergen has not been successful in preventing wheeze or asthma development.
3.6.4. Viral respiratory tract infections
Viral respiratory tract infections, particularly caused by RSV and HRV, are known to be important triggers of wheezing episodes in infancy, and many studies have shown an association with subsequent development of persistent wheezing in the preschool years. But it remains unclear whether viral infections play a causative role in the development of persistent wheeze and asthma, or whether more frequent and/or severe viral infections result from underlying immune or structural airway pathology that predisposes to asthma. Several studies have shown that the immune system is altered prior to the onset of wheezing. In nasal epithelial cells sampled at birth, release of the cytokines IL-6, IL-8, GM-CSF, and ICAM-1 after treatment with pro-inflammatory TNFα/IL-1β was reduced in children that subsequently developed wheezing, compared to those that did not wheeze [Citation54]. Similarly, in healthy infants in the Tucson study, sampled at 9 months of age, low antiviral interferon gamma (IFN-y) production by stimulated peripheral blood mononuclear cells (PBMCs) was associated with subsequent onset of chronic wheezing [Citation137]. These studies suggest that dysregulation of innate immune responses and premorbid lung function are important mechanisms explaining the apparent association between severe early life viral infections and persistent wheezing.
3.6.5 Respiratory syncytial virus (RSV)
RSV respiratory infection affects ~70% children in the first year of life, with peak hospital admissions for RSV bronchiolitis occurring at 2 months of age [Citation138]. Several studies including two large birth cohort studies have shown an association with subsequent wheeze and asthma: the Tucson Children’s Respiratory study [Citation9] and the ALSPAC study [Citation139].
Whether RSV LRTI causes persistent preschool wheezing, or is simply a marker of predisposition to wheezing, remains controversial. Evidence for a causal relationship comes from a retrospective birth cohort of almost 100,000 infants, which showed that asthma risk was highest among those born 4 months prior to the peak in winter respiratory viruses each year [Citation140]. However, in a study of 8280 twin pairs, hospitalization for RSV infection was associated with subsequent asthma risk, but genetic determinants for both completely overlapped, suggesting rather than severe RSV infection causing asthma it was an indicator of genetic predisposition to asthma [Citation141]. Gene association studies have shown several polymorphisms in innate immune response genes are associated with both severity of RSV infection and risk of persistent wheezing, including in IL-4 R, IL-13, TNF, IL1R1, IL-10 and TLR-4 genes [Citation61]. A recent meta-analysis of 35 studies estimated the effect of RSV infections on subsequent wheezing illnesses as OR 4.17 (95% CI 2.36–7.37), but after adjustment for some genetic and environmental influences the effect size was significantly lower (aOR 2.45, 95% CI 1.23–4.88), suggesting a significant amount of the association was non-causal [Citation142]. If RSV infections played a causal role in wheeze development, we would expect effective RSV immunoprophylaxis to reduce subsequent risk of wheezing. Several immunoprophylaxis studies have shown a significant reduction in parent-reported wheezing in the preschool years [Citation143–145], but no difference in physician diagnosed asthma at 6 years [Citation143,Citation144].
Potential mechanisms whereby RSV infection influences wheeze development are suggested by animal studies investigating the immune response to RSV infection. In a neonatal mouse model of allergic airway disease, RSV infection compromised the suppressive function of pulmonary T regulatory cells, and resulted in a Th2-type inflammatory response in the lung on re-infection with RSV or subsequent allergen exposure [Citation11]. In another neonatal mouse model, initial infection with RSV in the immediate newborn period resulted in Th2 inflammation and eosinophilia during subsequent re-infection in adulthood, but initial RSV infection beyond the neonatal period was associated with increased IFNγ production (Th1 response) and less severe disease during later re-infection [Citation146]. These results suggest that the relationship between RSV infection and later persistent wheeze risk is likely to be complex and depend on timing of infection, immune maturation, and lung developmental stage.
3.6.6 Human rhinovirus (HRV)
Recently with the use of molecular diagnostic techniques, HRV infection has been shown to be the most common cause of LRTI and wheezing after 6 months of age [Citation147] as well as the most common trigger of acute preschool wheeze episodes [Citation148]. HRV infection is strongly associated with risk of persistent wheeze and asthma [Citation149,Citation150]. In the COAST study, children at high risk of developing allergies and/or asthma based on parental atopy were monitored with nasal lavage for asymptomatic and symptomatic viral infections between 2 and 12 months of age [Citation149]. HRV infection with wheeze in the first year of life was most strongly associated with wheezing at 3 years of age (OR 10, 95% CI 4.7–23), while RSV infection had a much weaker effect (OR 3, 95% CI 1.6–5.8) [Citation149]. When followed to 6 years of age, those with HRV associated wheeze in the first year had increased risk of asthma, but those with RSV wheeze did not [Citation134]. Interestingly, early aeroallergen sensitization in the first year of life led to an increased risk of wheezing with HRV but not RSV [Citation151]. Conversely, HRV infection was not associated with subsequent allergic sensitization, supporting a causal role of allergic sensitization in modifying the risk of wheeze development with HRV. In a similar high-risk cohort, HRV wheezing illness in the first year of life was associated with increased asthma risk at 5 years only in those with aeroallergen sensitization at 2 years of age [Citation152]. Gene studies suggest host genotype has a significant interaction with HRV infection leading to increased risk of wheeze and asthma development. Variants in the 17q21 locus, which includes ORMDL3 and GSDM genes, have been identified in GWAS as risks for childhood onset asthma in those that experience wheezing with HRV infection [Citation65,Citation153]. Cadherin-related family member 3 (CDHR3) serves as a binding site for HRV-C on epithelial cells, facilitating viral entry and replication, and a polymorphism associated with enhanced cell surface expression is related to increased risk of severe wheezing illnesses [Citation68]. The strength of association and interaction with early aeroallergen sensitization and heritable risk suggests HRV infection plays a causal role in wheeze development in some children, but mechanistic studies to confirm this are needed.
Potential mechanisms by which HRV infection leads to wheeze development, particularly in those with a background of early aeroallergen sensitization, include allergic inflammation impairing antiviral responses. This may be through bias toward Th2 rather than effective anti-viral Th1 T cell responses [Citation154] and synergistic interactions between allergic and anti-viral inflammatory pathways [Citation155]. Impaired epithelial cell barrier integrity and goblet cell hyperplasia associated with allergic airway inflammation may allow more extensive viral spread and damage to the airways [Citation151]. This is supported by in vitro studies showing reduced barrier integrity in airway epithelial cells cultured from asthmatic children compared to non-asthmatic controls at baseline, which becomes even more significantly impaired by HRV infection [Citation48]. Airway epithelial cells from asthmatic children also have reduced IFN-β production and reduced apoptosis, associated with increased HRV proliferation and viral shedding in vitro [Citation52]. But it is not known if these same responses are found in atopic infants prior to persistent wheeze development.
3.6.7 Bacterial pathogens and the microbiome
Over recent years, there has been growing interest in the role of airway bacterial colonization and the airway microbiome in wheeze and asthma pathogenesis. A significant proportion of children with established preschool wheeze have chronic lower airway infection or colonization with Moraxella catarrhalis, Haemophilus influenzae, or Streptococcus pneumoniae, even between exacerbations during periods of relative disease stability [Citation43,Citation44,Citation156]. Administration of a prolonged course of appropriate antibiotics after positive BAL bacterial culture in one observational study was associated with reduced frequency of dyspnea and hospitalizations due to wheeze for at least 6 months [Citation43], supporting the role of bacterial infection in driving disease persistence, and the role for antibiotics in preventing attacks. However, several RCTs have evaluated the effectiveness of azithromycin treatment initiated during acute wheeze episodes, and while one showed a reduction in need for rescue oral corticosteroids [Citation157], and another showed a reduction in duration of respiratory symptoms from 7.7 to 3.4 days [Citation158], none showed a difference in time to next wheeze episode or progression to more severe symptoms [Citation159]. It is possible that any benefit of azithromycin was immunomodulatory rather than antibacterial [Citation160]. The role for targeted antibiotics in preventing attacks of pre-school wheeze needs to be further investigated, their role in treating acute attacks does not appear beneficial.
To investigate the potential role of the airway microbiome in wheeze inception, studies have examined the upper airway in early life prior to onset of wheeze symptoms. Most infants at 2 months are colonized with Staphylococcus or Corynebacterium, and then become more stably colonized with Alloiococcus or Moraxella by 12 months [Citation161]. Viral respiratory tract infections have been associated with microbiome instability with shifts to a Moraxella, Streptococcus, or Haemophilus dominant state [Citation161]. Indeed, prevalence of Moraxella was seen to increase 1–2 weeks prior to LRTI symptoms and respiratory virus detection [Citation162]. Antibiotic use, exposure to pets, and exposure to other children (daycare or siblings at home) have also been associated with changes in the airway microbiome [Citation161,Citation163]. In the prospective cohort Childhood Asthma Study (CAS), asymptomatic colonization with Streptococcus by 2 months was associated with earlier respiratory tract infections and chronic wheeze at 5 years [Citation161]. In the same cohort, asymptomatic colonization up to 2 years of age with Moraxella, Streptococcus, or Haemophilus was associated with persistent wheeze at 5 years old in those with early allergic sensitization (≤2 years), but only transient wheezing that resolved by 4 years in non-atopic children [Citation162]. In the COPSAC cohort, hypopharyngeal colonization in the first month of life with the same respiratory pathogens M.catarrhalis, H. influenzae, or S. pneumoniae was also associated with increased risk of recurrent wheezing and asthma diagnosis at 5 years [Citation164]. But it is not known whether these pathogens play a causative role in wheeze pathogenesis or their presence is the result of an intrinsic immune defect that also predisposes to wheezing and asthma.
There is growing evidence of a bidirectional relationship between the airway microbiome and respiratory viruses, which may be causally associated with wheeze development. Bacterial promotion of viral infection is suggested by outgrowth of Moraxella 1–2 weeks prior to viral infection [Citation162], which may increase the risk of viral infection or symptoms associated with it through damage to the airway epithelium or immune dysregulation. Bacteria may also enhance viral receptor expression on host cells, as has been shown for H.influenzae and HRV, or increase viral replication and release from host cells [Citation165]. Viral infection may promote bacterial colonization and outgrowth through impairment of mucociliary function, reduced expression of antimicrobial peptides, impaired neutrophil, and macrophage recruitment, and enhanced bacterial adherence to airway epithelial cells [Citation165].
Diverse environmental microbial exposures have also been associated with reduced risk of wheezing. In the URECA high-risk urban cohort study, higher bacterial diversity and increased Firmicutes and Bacteroidetes content in house dust samples, in combination with higher perennial allergen exposure, in the first year of life was associated with reduced atopy and recurrent wheezing at age 3 years [Citation136]. Diverse microbial exposure in children growing up on traditional cattle farms has also been linked to reduced persistent wheezing and asthma risk [Citation166,Citation167]. Two cross-sectional studies have looked at children living in rural farming communities in Central Europe, the German PARSIFAL (Prevention of Allergy-Risk Factors for Sensitization in Children Related to Farming and Anthroposophic Lifestyle) study [Citation168], and Bavarian GABRIELA (Multidisciplinary Study to Identify the Genetic and Environmental Causes of Asthma in the European Community Advanced) study [Citation169]. In both studies, the diversity of microbial exposure in dust samples from children’s bedrooms was higher and inversely associated with risk of asthma compared to non-farming communities [Citation170]. Comparison of two farming communities in America with similar European genetic backgrounds and lifestyles but different farming practices has allowed a clearer understanding of the mechanisms involved [Citation171]. The Amish practice traditional cattle farming, while the Hutterites have adopted industrialized cattle farming techniques, which are associated with a fourfold higher prevalence of asthma and allergic sensitization in the Hutterite compared to the Amish [Citation172]. Analysis of house dust samples showed endotoxin levels (lipopolysaccharide from the cell walls of gram-negative bacteria) were 6.8 times higher in Amish compared to Hutterite homes, and microbial composition of house dust differed, most notably with increased Bartonellaceae in Amish compared to Hutterite homes [Citation172]. The mechanisms underlying the reduced risk of asthma and allergies in Amish children appear to be related to these different microbial exposures, which trigger innate immune activation and consequent dampening of downstream adaptive responses. Amish children had increased neutrophil and decreased eosinophil proportions in peripheral blood, and the neutrophils expressed fewer CXCR4 chemokine receptors and CD11b and CD11c adhesion molecules, which suggest recent recruitment from the bone marrow. In Amish children, peripheral monocytes had a more suppressive phenotype, and gene expression profiles of peripheral leukocytes differed significantly in tumor necrosis factor (TNF) and interferon regulatory factor 7 (IRF7) pathways involved in innate immune responses to microbes. In an adult mouse model of ovalbumin induced allergic airway disease, Amish but not Hutterite house dust extracts given intranasally significantly reduced airway hyperreactivity and eosinophilia in response to ovalbumin in wild-type mice, but not in mice with deficient innate immune signaling, suggesting innate immune activation is essential for the protective effect of microbial exposure [Citation173]. In a neonatal mouse model, inhaled Acinetobacter lwoffii, a bacterial isolate found in high concentrations in cattle farm dust, prevented airway hyperresponsiveness driven by house dust mite induced allergic airway disease and reduced IL-13 secreting CD4 + T cells [Citation174], supporting a causal link between microbial exposure in early life and protection from wheeze and asthma development. Treatment with oral bacterial lysates containing inactivated respiratory pathogens has been shown to significantly reduce the frequency of respiratory tract infections and associated wheezing episodes in preschool children [Citation175]. It is not yet clear whether this treatment can prevent persistent preschool wheezing and childhood asthma, but randomized controlled trials including the ORBEX study (ClinicalTrials.gov Identifier: NCT02148796) are underway.
Cross-talk between the lung and gut microbiome and immune systems, referred to as the gut-lung axis, has been implicated in wheeze and asthma development. A study in a mouse model of allergic airway disease showed reducing gut microbial load and diversity with vancomycin in the first 3 weeks of life increased eosinophilic airway inflammation and airway hyperresponsiveness, and reduced T regulatory cells in the gut but not lung, after allergen exposure [Citation176]. Bacterial metabolites such as short-chain fatty acids (SCFAs) produced by the gut microbiome promote epithelial barrier integrity and immune homeostasis in the gut, regulate T reg and dendritic cell function, alter lung epithelial gene expression, and protect against HDM-induced allergic airway disease in mice [Citation177,Citation178].
3.6.8 Early immune maturation
Various risk factors discussed above including tobacco smoke and pollution exposure, allergen exposure, and pathogens exert more significant effects on wheeze development when experienced in early life. This is likely due to a combination of lung and immune system immaturity and rapid development in early life which renders the infant more susceptible to environmental factors. Due to difficulties collecting airway samples in early life, mechanistic studies in this age group are lacking. Recently, systems biology studies utilizing small quantities of peripheral blood from infants and age-appropriate animal models have improved our understanding of the potential mechanisms linking early immune maturation and development of wheezing. Profiling 58 immune cell populations and 267 proteins in infants from only 100 µl of peripheral blood between birth and 3 months showed rapid development of B lymphocytes, dendritic and natural killer cells, which reached adult-like phenotypes by 3 months, and changes in T cell phenotypes driven by microbial interactions [Citation179]. In a neonatal mouse model of HDM-induced allergic airway disease, airway hyperresponsiveness, eosinophilic inflammation, and airway remodeling occurred in parallel, as in preschool children with severe recurrent wheeze [Citation56]. This model has shown that response to allergen exposure in early life is significantly different from that in adult mice, with greater eosinophilic airway inflammation, and AHR driven by IL-13 secreting CD4 + T cells, with the likely mechanism being a lower bacterial load and less diverse airway microbiome in neonatal mice resulting in more T regulatory cells and thus exaggerated type 2 immune responses [Citation174]. Similarly, initial infection with RSV in the immediate newborn period concomitant with neonatal allergen exposure resulted in Th2 inflammation and eosinophilia, contrasting with an effective Th1 response with initial infection in adulthood [Citation146]. Altering the airway microbiome with vancomycin in sensitized neonatal mice increased eosinophilic airway inflammation and airway hyperresponsiveness after allergen exposure, but had no effect on asthma induction in adult mice [Citation176]. These studies confirm the critical time window during which environmental exposures impact wheeze development and support the concept of a ‘window of opportunity’ in early life during which modification of these risk factors may reduce the risk of developing preschool wheeze.
4. Conclusion
Whilst there is some overlap, factors that initiate preschool wheezing are different from the factors maintaining the disease (). In utero exposure to cigarette smoke, and early postnatal exposures altering the microbiome and immune system development seem particularly important in disease onset, while the most prominent risk factors for persistent and recurrent symptoms are frequent and severe lower respiratory tract infections and early sensitization to multiple aeroallergens (). The genetic and environmental risk factors for persistent wheeze individually have relatively weak effects, and it is becoming apparent that the effect of environmental exposures differs between individuals with different genetic backgrounds. Studies looking at specific environmental exposures may miss the relationship with persistent wheeze development where that environmental factor only imposes increased risk of wheezing in individuals with certain genetic polymorphisms, emphasizing the need for large comprehensive studies that can control for multiple environmental factors and characterize individual genetic backgrounds.
Table 1. Key factors associated with the development of preschool wheezing
5. Expert opinion
Current medical management to prevent attacks of preschool wheeze and improve daily symptoms is focussed on parental reported symptoms and limiting environmental triggers. Little emphasis is given to the assessment of objective markers that might determine a response to treatment, and we do not define objective phenotypes. Recent clinical studies have highlighted aeroallergen sensitization and raised blood eosinophil count as predictors of a good clinical response to treatment with daily ICS [Citation42]. It is important to ensure some tests that show evidence of a phenotype that is likely respond to ICS are undertaken, and that we do not continue to rely on parental reports of symptom pattern alone to decide whether or not to prescribe ICS. However, the utility and efficacy of these biomarkers, either alone or in combination, in identifying children who respond best to inhaled steroids do need to be confirmed in prospective studies. The biggest limitation of the current approach is that only approximately one-quarter of preschool wheezers have allergen sensitization and similarly only a proportion have elevated blood eosinophils. A big gap in our current management of preschool wheezing is optimal therapies to prevent attacks of wheeze in children who do not have aeroallergen sensitization or elevated blood eosinophils (). The presence of airway bacterial and viral infections, in association with neutrophilia, in between episodes in non-atopic children, suggests targeted antibiotic therapy may be an option for these patients. But, to date, we have no evidence of efficacy or mechanism of action of this proposed approach. Mechanistic research to further understand the underlying etiology and pathophysiology of preschool wheezing disorders is limited by difficulties obtaining lower airway samples and measuring lung function in young children. However, we believe this challenge can be overcome by the utility of newer technologies that allow investigation of mechanisms with very small sample volumes.
Table 2. Mechanisms and management of persistent wheeze phenotype and acute infection induced wheeze episodes
An increasing understanding of underlying mechanisms contributing to the inception of preschool wheeze is another important unmet need because no intervention to date, including the use of inhaled corticosteroids, has been shown to have a modifiable effect on progression of preschool wheeze to asthma [Citation38]. A better understanding of individual endotypes and the identification of clinical biomarkers linking endotypes with phenotypes is the only way to enable personalized targeted medicine to minimize current treatment burden, but also to have an impact on the long-term adverse outcomes of preschool wheezing in adulthood.
Article highlights
Factors associated with preschool wheeze inception include in utero exposures, genetic susceptibility, prematurity and immediate postnatal exposures, such as early allergen sensitisation and respiratory infections.
Persistence of preschool wheeze symptoms is influenced by environmental factors including allergen exposure, respiratory infections and pollution. Protection may be achieved by favourable microbial exposures, such as farming environments.
Airway epithelial cell innate immune function is impaired in children with persistent wheeze compared with healthy children. This may predispose to aeroallergen sensitisation and ongoing inflammation by increased contact of subepithelial tissues with allergens and microbes.
Aero-allergen sensitisation and blood eosinophilia are markers of a steroid responsive pre-school wheeze phenotype. However, approximately 75% of pre-school wheezers are non-atopic, although some of these may have elevated blood eosinophils, a significant proportion of non-atopic children may not respond to steroids.
Acute wheeze episodes in preschool children are associated with hypopharyngeal bacterial infection as often as nasopharyngeal viral infection. Targeted antibiotics for airway bacterial infection and neutrophilia in non-atopic wheezers may offer a management strategy to prevent wheeze attacks in preschool age children.
The inability to achieve disease modification by targeting airway inflammation in preschool wheeze suggests that therapies targeting structural airway changes are critical to allow a change in the natural history of the disease. Therefore, studies investigating mechanisms mediating early-life airway remodelling are essential.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The group health medical associates. N Engl J Med. 1995;332(3):133–138.
- Selby A, Munro A, Grimshaw KE, et al. Prevalence estimates and risk factors for early childhood wheeze across Europe: the EuroPrevall birth cohort. Thorax. 2018;73(11):1049–1061.
- Davies G, Paton JY, Beaton SJ, et al., Children admitted with acute wheeze/asthma during November 1998–2005: a national UK audit. Arch Dis Child. 93(11): 952–958. 2008.
- Stevens CA, Turner D, Kuehni CE, et al. The economic impact of preschool asthma and wheeze. Eur Respir J. 2003;21(6):1000–1006.
- Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat.2012;3(35):1–58.
- Stick SM, Burton PR, Gurrin L, et al. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348(9034):1060–1064.
- Hanrahan JP, Tager IB, Segal MR, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145(5):1129–1135.
- Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163(2):429–436.
- Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–545.
- Henderson AJ, Sherriff A, Northstone K, et al. Pre- and postnatal parental smoking and wheeze in infancy: cross cultural differences. Avon Study of Parents and Children (ALSPAC) study team, European Longitudinal Study of Pregnancy and Childhood (ELSPAC) co-ordinating centre. Eur Respir J. 2001;18(2):323–329.
- Krishnamoorthy N, Khare A, Oriss TB, et al., Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 18(10): 1525–1530. 2012.
- Grad R, Morgan WJ. Long-term outcomes of early-onset wheeze and asthma. J Allergy Clin Immunol. 2012;130(2):299–307.
- Beigelman A, Durrani S, Guilbert TW. Should a preschool child with acute episodic wheeze be treated with Oral Corticosteroids? A Pro/Con Debate. J Allergy Clin Immunol Pract. 2016;4(1):27–35.
- Beigelman A, Ts K, Mauger D, et al. Do oral corticosteroids reduce the severity of acute lower respiratory tract illnesses in preschool children with recurrent wheezing? J Allergy Clin Immunol. 2013;131(6):1518–1525.
- Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003 Nov;362(9394):1433–1438.
- Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009 Jan;360(4):329–338.
- Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51(8):868–876.
- Foster SJ, Cooper MN, Oosterhof S, et al. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):97–106.
- Jartti T, Lehtinen P, Vanto T, et al. Efficacy of prednisolone in children hospitalized for recurrent wheezing. Pediatr Allergy Immunol. 2007;18(4):326–334.
- Gleeson JG, Loftus BG, Price JF. Placebo controlled trial of systemic corticosteroids in acute childhood asthma. Acta Paediatr Scand. 1990;79(11):1052–1058.
- Fox GF, Marsh MJ, Milner AD. Treatment of recurrent acute wheezing episodes in infancy with oral salbutamol and prednisolone. Eur J Pediatr. 1996;155(6):512–516.
- Brodlie M, Gupta A, Rodriguez-Martinez CE, et al. Leukotriene receptor antagonists as maintenance or intermittent treatment in pre-school children with episodic viral wheeze. Paediatr Respir Rev. 2016;17:57–59.
- Hussein HR, Gupta A, Broughton S, et al. A meta-analysis of montelukast for recurrent wheeze in preschool children. Eur J Pediatr. 2017;176(7):963–969.
- Nwokoro C, Pandya H, Turner S, et al. Intermittent montelukast in children aged 10 months to 5 years with wheeze (WAIT trial): a multicentre, randomised, placebo-controlled trial. Lancet Respir Med. 2014;2(10):796–803.
- Robertson CF, Price D, Henry R, et al. Short-course montelukast for intermittent asthma in children: a randomized controlled trial. Am J Respir Crit Care Med. 2007;175(4):323–329.
- Collins SA, Pike KC, Inskip HM, et al. Validation of novel wheeze phenotypes using longitudinal airway function and atopic sensitization data in the first 6 years of life: evidence from the Southampton Women’s survey. Pediatr Pulmonol. 2013;48(7):683–692.
- Miller EK, Avila PC, Khan YW, et al. Wheezing exacerbations in early childhood: evaluation, treatment, and recent advances relevant to the genesis of asthma. J Allergy Clin Immunol. 2014;2(5):537–543.
- Krawiec ME, Westcott JY, Chu HW, et al. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001;163(6):1338–1343.
- Hauk PJ, Krawiec M, Murphy J, et al. Neutrophilic airway inflammation and association with bacterial lipopolysaccharide in children with asthma and wheezing. Pediatr Pulmonol. 2008;43(9):916–923.
- Marguet C, Jouen-Boedes F, Dean TP, et al. Bronchoalveolar cell profiles in children with asthma, infantile wheeze, chronic cough, or cystic fibrosis. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1533–1540.
- Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341(oct04 1):c4978.
- Carlsson CJ, Vissing NH, Sevelsted A, et al., Duration of wheezy episodes in early childhood is independent of the microbial trigger. J Allergy Clin Immunol. 136(5): 1208–14.e145. 2015.
- Turato G, Barbato A, Baraldo S, et al. Nonatopic children with multitrigger wheezing have airway pathology comparable to atopic asthma. Am J Respir Crit Care Med. 2008;178(5):476–482.
- Saglani S, Payne DN, Zhu J, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176(9):858–864.
- Saglani S, Malmström K, Pelkonen AS, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171(7):722–727.
- Oommen A, McNally T, Grigg J. Eosinophil activation and preschool viral wheeze. Thorax. 2003;58(10):876–879.
- Fattouh R, Al-Garawi A, Fattouh M, et al. Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease. Am J Respir Crit Care Med. 2011;183(2):179–188.
- Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–1997.
- Murray CS, Woodcock A, Langley SJ, et al. Secondary prevention of asthma by the use of inhaled fluticasone propionate in Wheezy INfants (IFWIN): double-blind, randomised, controlled study. Lancet. 2006;368(9537):754–762.
- Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics. 2009;123(3):e519–25.
- Guiddir T, Saint-Pierre P, Purenne-Denis E, et al., Neutrophilic Steroid-Refractory Recurrent Wheeze and Eosinophilic Steroid-Refractory Asthma in Children. J Allergy Clin Immunol. 5(5): 1351–1361.e2. 2017.
- Fitzpatrick AM, Jackson DJ, Mauger DT, et al., Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 138(6): 1608–18.e12. 2016.
- Schwerk N, Brinkmann F, Soudah B, et al. Wheeze in preschool age is associated with pulmonary bacterial infection and resolves after antibiotic therapy. PLoS One. 2011;6(11):e27913.
- Robinson PFM, Pattaroni C, Cook J, et al., Lower airway microbiota associates with inflammatory phenotype in severe preschool wheeze. J Allergy Clin Immunol. 143(4): 1607–1610.e3. 2019.
- Hallstrand TS, Hackett TL, Altemeier WA, et al. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. 2014;151(1):1–15.
- Holtzman MJ, Byers DE, Alexander-Brett J, et al. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol. 2014;14(10):686–698.
- Barbato A, Turato G, Baraldo S, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174(9):975–981.
- Looi K, Buckley AG, Rigby PJ, et al. Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin Exp Allergy. 2018;48(5):513–524.
- Kicic A, Hallstrand TS, Sutanto EN, et al. Decreased fibronectin production significantly contributes to dysregulated repair of asthmatic epithelium. Am J Respir Crit Care Med. 2010;181(9):889–898.
- McDougall CM, Helms PJ, Walsh GM. Airway epithelial cytokine responses in childhood wheeze are independent of atopic status. Respir Med. 2015;109(6):689–700.
- Kicic A, Sutanto EN, Stevens PT, et al. Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am J Respir Crit Care Med. 2006;174(10):1110–1118.
- Kicic A, Stevens PT, Sutanto EN, et al. Impaired airway epithelial cell responses from children with asthma to rhinoviral infection. Clin Exp Allergy. 2016;46(11):1441–1455. 11.
- Spann KM, Baturcam E, Schagen J, et al. Viral and host factors determine innate immune responses in airway epithelial cells from children with wheeze and atopy. Thorax. 2014;69(10):918–925.
- Turner S, Miller D, Walsh GM, et al. Pro-inflammatory mediator responses from neonatal airway epithelial cells and early childhood wheeze. Pediatr Pulmonol. 2018;53(1):10–16.
- Bossley CJ, Fleming L, Gupta A, et al. Pediatric severe asthma is characterized by eosinophilia and remodeling without TH2 cytokines. J Allergy Clin Immunol. 2012;129(4):974–982.e13.
- Saglani S, Mathie SA, Gregory LG, et al. Pathophysiological features of asthma develop in parallel in house dust mite-exposed neonatal mice. Am J Respir Cell Mol Biol. 2009;41(3):281–289.
- Baraldo S, Turato G, Bazzan E, et al. Noneosinophilic asthma in children: relation with airway remodelling. Eur Respir J. 2011;38(3):575.
- O’Reilly R, Ullmann N, Irving S, et al. Increased airway smooth muscle in preschool wheezers who have asthma at school age. J Allergy Clin Immunol. 2013;131(4):1024–1032.
- Regamey N, Ochs M, Hilliard TN, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2008;177(8):837–843.
- Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242(1):10–30.
- Larkin EK, Hartert TV. Genes associated with RSV lower respiratory tract infection and asthma: the application of genetic epidemiological methods to understand causality. Future Virol. 2015;10(7):883–897.
- Halapi E, Gudbjartsson DF, Jonsdottir GM, et al. A sequence variant on 17q21 is associated with age at onset and severity of asthma. Eur J Hum Genet. 2010;18(8):902–908.
- Sarnowski C, Sugier PE, Granell R, et al. Identification of a new locus at 16q12 associated with time to asthma onset. J Allergy Clin Immunol. 2016;138(4):1071–1080. 10.
- Bouzigon E, Corda E, Aschard H, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med. 2008;359(19):1985–1994.
- Calışkan M, Bochkov YA, Kreiner-Møller E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368(15):1398–1407.
- Loss GJ, Depner M, Hose AJ, et al., The early development of wheeze. Environmental determinants and genetic susceptibility at 17q21. Am J Respir Crit Care Med. 193(8): 889–897. 2016.
- Bønnelykke K, Sleiman P, Nielsen K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46(1):51–55.
- Bochkov YA, Watters K, Ashraf S, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112(17):5485–5490.
- Von Mutius E, Smits HH. Primary prevention of asthma: from risk and protective factors to targeted strategies for prevention. Lancet. 2020;396(10254):854–866. 09.
- Crump C. Preterm birth and mortality in adulthood: a systematic review. J Perinatol. 2020;40(6):833–843.
- Gibbons JTD, Wilson AC, Simpson SJ. predicting lung health trajectories for survivors of preterm birth. Front Pediatr. 2020;8:318.
- Crump C, Sundquist K, Sundquist J. Adult outcomes of preterm birth. Prev Med. 2016;91:400–401. [ 10].
- Kotecha SJ, Watkins WJ, Paranjothy S, et al. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax. 2012;67(1):54–61.
- Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11(1):e1001596.
- Wolsk HM, Chawes BL, Litonjua AA, et al. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: a combined analysis of two randomized controlled trials. PLoS One. 2017;12(10):e0186657.
- Chawes BL, Bønnelykke K, Stokholm J, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353–361.
- Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;(7):CD010085.
- Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and Asthma in Offspring. N Engl J Med. 2016;375(26):2530–2539. 12.
- Lee HS, Barraza-Villarreal A, Hernandez-Vargas H, et al. Modulation of DNA methylation states and infant immune system by dietary supplementation with ω-3 PUFA during pregnancy in an intervention study. Am J Clin Nutr. 2013;98(2):480–487.
- Zugna D, Galassi C, Annesi-Maesano I, et al. Maternal complications in pregnancy and wheezing in early childhood: a pooled analysis of 14 birth cohorts. Int J Epidemiol. 2015;44(1):199–208.
- Huang L, Chen Q, Zhao Y, et al. Is elective cesarean section associated with a higher risk of asthma? A meta-analysis. J Asthma. 2015;52(1):16–25.
- Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975.
- Azad MB, Konya T, Persaud RR, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983–993.
- Almqvist C, Cnattingius S, Lichtenstein P, et al. The impact of birth mode of delivery on childhood asthma and allergic diseases–a sibling study. Clin Exp Allergy. 2012;42(9):1369–1376.
- Oddy WH, Sly PD, De Klerk NH, et al. Breast feeding and respiratory morbidity in infancy: a birth cohort study. Arch Dis Child. 2003;88(3):224–228.
- Dogaru CM, Nyffenegger D, Pescatore AM, et al. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014;179(10):1153–1167.
- Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med. 1995;152(3):977–983.
- Hollams EM, De Klerk NH, Holt PG, et al. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med. 2014;189(4):401–407.
- Gilliland FD, Berhane K, McConnell R, et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55(4):271–276.
- Gilliland FD, Berhane K, Li YF, et al. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167(6):917–924.
- Young S, Arnott J, O’Keeffe PT, et al. The association between early life lung function and wheezing during the first 2 yrs of life. Eur Respir J. 2000;15(1):151–157.
- Elliot J, Carroll N, Bosco M, et al. Increased airway responsiveness and decreased alveolar attachment points following in utero smoke exposure in the guinea pig. Am J Respir Crit Care Med. 2001;163(1):140–144.
- Stevenson MD, Mansbach JM, Mowad E, et al. Prenatal versus postnatal tobacco smoke exposure and intensive care use in children hospitalized with Bronchiolitis. Acad Pediatr. 2016;16(5):446–452.
- Stein RT, Holberg CJ, Sherrill D, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson children’s respiratory study. Am J Epidemiol. 1999;149(11):1030–1037.
- Lannerö E, Wickman M, Pershagen G, et al. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res. 2006;7(1):3.
- Strachan DP, Cook DG. Health effects of passive smoking. 1. Parental smoking and lower respiratory illness in infancy and early childhood. Thorax. 1997;52(10):905–914.
- Cook DG, Strachan DP. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax. 1997;52(12):1081–1094.
- Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53(3):204–212.
- Sekhon HS, Keller JA, Proskocil BJ, et al. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol. 2002;26(1):31–41.
- Wongtrakool C, Wang N, Hyde DM, et al. Prenatal nicotine exposure alters lung function and airway geometry through α7 nicotinic receptors. Am J Respir Cell Mol Biol. 2012;46(5):695–702.
- Elliot JG, Carroll NG, James AL, et al. Airway alveolar attachment points and exposure to cigarette smoke in utero. Am J Respir Crit Care Med. 2003;167(1):45–49.
- Collins MH, Moessinger AC, Kleinerman J, et al. Fetal lung hypoplasia associated with maternal smoking: a morphometric analysis. Pediatr Res. 1985;19(4):408–412.
- Wongtrakool C, Grooms K, Bijli KM, et al. Nicotine stimulates nerve growth factor in lung fibroblasts through an NFκB-dependent mechanism. PLoS One. 2014;9(10):e109602.
- Maritz GS, Woolward K. Effect of maternal nicotine exposure on neonatal lung elastic tissue and possible consequences. S Afr Med J. 1992;81(10):517–519.
- Fu XW, Wood K, Spindel ER. Prenatal nicotine exposure increases GABA signaling and mucin expression in airway epithelium. Am J Respir Cell Mol Biol. 2011;44(2):222–229.
- Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306(6881):817–820.
- Urs R, Kotecha S, Hall GL, et al. Persistent and progressive long-term lung disease in survivors of preterm birth. Paediatr Respir Rev. 2018;28:87–94.
- Wongtrakool C, Grooms K, Xd P, et al. In utero nicotine exposure promotes M2 activation in neonatal mouse alveolar macrophages. Pediatr Res. 2012;72(2):147–153.
- Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32(1):43–50.
- Noakes PS, Hale J, Thomas R, et al. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J. 2006;28(4):721–729.
- Hinz D, Bauer M, Röder S, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67(3):380–389.
- Spindel ER, McEvoy CT. The role of nicotine in the effects of maternal smoking during pregnancy on lung development and childhood respiratory disease. Implications for dangers of E-cigarettes. Am J Respir Crit Care Med. 2016;193(5):486–494.
- Cook DG, Strachan DP. Health effects of passive smoking-10: summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357–366.
- Li S, Williams G, Jalaludin B, et al. Panel studies of air pollution on children’s lung function and respiratory symptoms: a literature review. J Asthma. 2012;49(9):895–910.
- Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico city. Am J Respir Crit Care Med. 2007;176(4):377–384.
- Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–1067.
- Rice MB, Rifas-Shiman SL, Litonjua AA, et al. Lifetime exposure to ambient pollution and lung function in children. Am J Respir Crit Care Med. 2016;193(8):881–888.
- Andersen ZJ, Loft S, Ketzel M, et al. Ambient air pollution triggers wheezing symptoms in infants. Thorax. 2008;63(8):710–716.
- Mölter A, Simpson A, Berdel D, et al. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J. 2015;45(3):610–624.
- Khreis H, Kelly C, Tate J, et al. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1–31.
- Holst GJ, Pedersen CB, Thygesen M, et al. Air pollution and family related determinants of asthma onset and persistent wheezing in children: nationwide case-control study. BMJ. 2020;370:m2791.
- Norbäck D, Lu C, Zhang Y, et al. Sources of indoor particulate matter (PM) and outdoor air pollution in China in relation to asthma, wheeze, rhinitis and eczema among pre-school children: synergistic effects between antibiotics use and PM(10) and second hand smoke. Environ Int. 2019;125:252–260.
- Heinzerling AP, Guarnieri MJ, Mann JK, et al. Lung function in woodsmoke-exposed guatemalan children following a chimney stove intervention. Thorax. 2016;71(5):421–428.
- Morales E, Garcia-Esteban R, De La Cruz OA, et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax. 2015;70(1):64–73.
- Hsu HH, Chiu YH, Coull BA, et al. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med. 2015;192(9):1052–1059.
- Deng Q, Lu C, Li Y, et al. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. 10 2016;150: 119–127.
- Kerkhof M, Postma DS, Brunekreef B, et al. Toll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthma. Thorax. 2010;65(8):690–697.
- MacIntyre EA, Brauer M, Melén E, et al. GSTP1 and TNF Gene variants and associations between air pollution and incident childhood asthma: the traffic, asthma and genetics (TAG) study. Environ Health Perspect. 2014;122(4):418–424.
- Gref A, Merid SK, Gruzieva O, et al. Genome-Wide interaction analysis of air pollution exposure and childhood Asthma with functional follow-up. Am J Respir Crit Care Med. 2017;195(10):1373–1383.
- Esposito S, Tenconi R, Lelii M, et al. Possible molecular mechanisms linking air pollution and asthma in children. BMC Pulm Med. 2014;14(1):31.
- Wang B, Chen H, Chan YL, et al. Why do intrauterine exposure to air pollution and cigarette smoke increase the risk of Asthma? Front Cell Dev Biol. 2020;8:38.
- Sly PD, Boner AL, Björksten B, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372(9643):1100–1106.
- Lloyd CM, Saglani S. Development of allergic immunity in early life. Immunol Rev. 2017;278(1):101–115.
- Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672.
- Illi S, Von Mutius E, Lau S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763–770.
- Lynch SV, Wood RA, Boushey H, et al., Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 134(3): 593–601.e12. 2014.
- Stern DA, Guerra S, Halonen M, et al. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120(4):835–841.
- Singh AM, Moore PE, Gern JE, et al. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175(2):108–119.
- Henderson J, Hilliard TN, Sherriff A, et al. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16(5):386–392.
- Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178(11):1123–1129.
- Thomsen SF, Van Der Sluis S, Stensballe LG, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179(12):1091–1097.
- Brunwasser SM, Snyder BM, Driscoll AJ, et al. Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: a systematic review and meta-analysis. Lancet Respir Med. 2020;8(8):795–806.
- Scheltema NM, Nibbelke EE, Pouw J, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med. 2018;6(4):257–264.
- Mochizuki H, Kusuda S, Okada K, et al. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. Six-year follow-up study. Am J Respir Crit Care Med. 2017;196(1):29–38.
- Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151(1):34–42. 42.e1.
- Culley FJ, Pollott J, Openshaw PJM. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196(10):1381–1386.
- Korppi M, Kotaniemi-Syrjänen A, Waris M, et al. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23(11):995–999.
- Jartti T, Gern JE. Rhinovirus-associated wheeze during infancy and asthma development. Curr Respir Med Rev. 2011;7(3):160–166.
- Lemanske Jr. RF, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577.
- Van Der Gugten AC, Van Der Zalm MM, Uiterwaal CS, et al. Human rhinovirus and wheezing: short and long-term associations in children. Pediatr Infect Dis J. 2013;32(8):827–833.
- Jackson DJ, Evans MD, Gangnon RE, et al., Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 185(3): 281–285. 2012.
- Kusel MM, De Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105–1110.
- Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–473.
- Papadopoulos NG, Stanciu LA, Papi A, et al. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57(4):328–332.
- Holt PG, Strickland DH, Hales BJ, et al. Defective respiratory tract immune surveillance in asthma: a primary causal factor in disease onset and progression. Chest. 2014;145(2):370–378.
- De Schutter I, Dreesman A, Soetens O, et al. In young children, persistent wheezing is associated with bronchial bacterial infection: a retrospective analysis. BMC. Pediatr. 2012;12(1):83.
- Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034–2044.
- Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(1):19–26.
- Mandhane PJ, P PZDS, Yn A, et al. Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: a placebo-controlled randomized trial. PLoS One. 2017;12(8):e0182411.
- Bush A. Azithromycin is the answer in paediatric respiratory medicine, but what was the question? Paediatr Respir Rev. 2020;34:67–74.
- Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–715.
- Teo SM, Tang HHF, Mok D, et al., Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and Allergy in childhood respiratory disease. Cell Host Microbe. 24(3): 341–352. 2018.
- Bosch AATM, Waa DSP, Van Houten MA, et al. Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. A prospective cohort study. Am J Respir Crit Care Med. 2017;196(12):1582–1590.
- Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–1495.
- Brealey JC, Sly PD, Young PR, et al. Viral bacterial co-infection of the respiratory tract during early childhood. FEMS Microbiol Lett. 2015;362(10):10.
- Genuneit J. Exposure to farming environments in childhood and asthma and wheeze in rural populations: a systematic review with meta-analysis. Pediatr Allergy Immunol. 2012;23(6):509–518.
- Von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10(12):861–868.
- Alfvén T, Braun-Fahrländer C, Brunekreef B, et al. Allergic diseases and atopic sensitization in children related to farming and anthroposophic lifestyle–the PARSIFAL study. Allergy. 2006;61(4):414–421.
- Genuneit J, Büchele G, Waser M, et al. The GABRIEL advanced surveys: study design, participation and evaluation of bias. Paediatr Perinat Epidemiol. 2011;25(5):436–447.
- Ege MJ, Mayer M, Normand A-C, et al. Exposure to environmental microorganisms and childhood Asthma. N Engl J Med. 2011;364(8):701–709.
- Ober C, Sperling AI, Von Mutius E, et al. Immune development and environment: lessons from Amish and Hutterite children. Curr Opin Immunol. 2017;48:51–60.
- Stein MM, Hrusch CL, Gozdz J, et al., Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 375(5): 411–421. 2016.
- Eder W, Klimecki W, Yu L, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113(3):482–488.
- Saglani S, Gregory LG, Manghera AK, et al. Inception of early-life allergen-induced airway hyperresponsiveness is reliant on IL-13. Sci Immunol. 2018;3(27):27.
- Rossi GA, Pohunek P, Feleszko W, et al. Viral infections and wheezing-asthma inception in childhood: is there a role for immunomodulation by oral bacterial lysates? Clin Transl Allergy. 2020;10(1):17.
- Russell SL, Gold MJ, Hartmann M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13(5):440–447.
- Thorburn AN, McKenzie CI, Shen S, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6(1):7320.
- McKenzie C, Tan J, Macia L, et al. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev. 2017;278(1):277–295.
- Olin A, Henckel E, Chen Y, et al. Stereotypic immune system development in newborn Children. Cell. 2018;174(5):1277–1292.
