ABSTRACT
Introduction
Mycophenolate mofetil (MMF), initially approved to prevent rejection in solid organ allograft, is now being increasingly used for other conditions. Over the last decade, MMF has emerged as a useful therapy for a variety of immune-mediated diseases.
Areas Covered
There has been a growing interest in the clinical use of MMF in the treatment of ILDs due to its versatile anti-inflammatory, immunomodulatory, anti-fibrotic and anti-proliferative properties. In this focussed review, we summarize the available literature using the Pubmed, Science Direct and EMBASE databases published until June 2021 on the efficacy and tolerability of MMF in various ILDs.
Expert Opinion
Other than idiopathic pulmonary fibrosis (IPF) and its broader category of progressive fibrosing ILD, there have been no drugs approved by relevant regulatory agencies for the treatment of the multiple other forms of ILD. Though results are limited, immunosuppressants such as MMF have shown promise as an effective and well-tolerated steroid-sparing agent, providing hope that the limited treatment armamentarium for ILDs can be expanded.
1. Introduction
Interstitial lung diseases (ILDs) aka diffuse parenchymal lung diseases encompass more than 200 disorders that are generally characterized by inflammation and/fibrosis in the lung parenchyma. Though the exact prevalence and incidence of ILDs remains unknown, there has been an increase in the recognized number of ILD cases across the globe. As per the Global Disease Burden Study report, ILDs were ranked at the 40th position among all diseases with respect to global years of life lost in 2013, representing an increase of 86% compared with 1990. Hence, for the first time, ILDs have been included in the top 50 causes of years of life lost [Citation1].
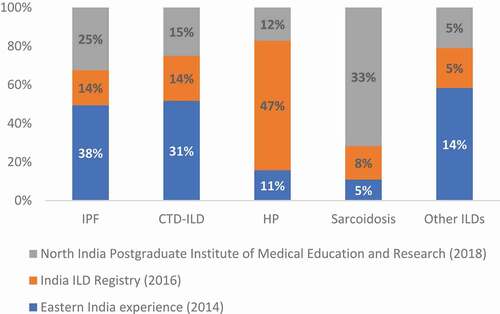
Today, ILDs have become a routine part of the daily clinical practice of a variety of specialists, viz. respiratory medicine, internal medicine, thoracic radiology, pathology, surgery, and geriatrics [Citation2]. Idiopathic pulmonary fibrosis (IPF), a prototype of an ILD, represents the most aggressive form of ILD. However, the India ILD Registry recently reported that chronic hypersensitivity pneumonitis (cHP) was the most prevalent in its cohort, accounting for 47% of all ILDs, followed by connective tissue disease-related ILD (CTD-ILD) (13.9%) and IPF (13.7%) [Citation3]. Similarly, additional prospective studies reported the common prevalence of ILDs other than IPF () [Citation4,Citation5].
Figure 1. Spectrum of ILDs in India from prospective studies
In several countries, two drugs have been approved for the treatment of IPF – nintedanib [Citation6] and pirfenidone [Citation7]. Based on the recent results of the INBUILD® trial, nintedanib – an intracellular tyrosine kinase inhibitor – has now been approved for the treatment of the broad category of chronic fibrosing ILDs with a progressive phenotype, marking a major therapeutic breakthrough for a group of fibrotic lung diseases where the treatment options are often of uncertain utility or not available [Citation8].
Patients with non-IPF ILDs have been historically treated with systemic oral corticosteroids (CS); however, their efficacy has rarely been tested prospectively, and the long-term use of CS is associated with numerous systemic side effects. Other studies suggest that patients with clinically significant and progressive ILD resistant to CS may slowly progress to respiratory failure [Citation9]. Therefore, there is a clear need for an immunosuppressive therapy other than CS that is well tolerated, to slow disease progression in a variety of ILDs.
2. Mycophenolate mofetil (MMF): a versatile immunosuppressant
MMF, a pro-drug of mycophenolic acid (MPA), is a noncompetitive, selective reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH) in stimulated lymphocytes. MPA blocks the de novo synthesis of guanosine nucleotides by which it exerts its immunosuppressive effect [Citation10]. MPA is reported to act even at the late stage of lymphocyte proliferation and is 5-fold more potent in inhibiting the isoform associated with stimulated cells than the isoform in resting cells [Citation11–13]. Evidence supports that, at clinically relevant concentrations, MPA and MMF inhibit proliferation of human peripheral blood lymphocytes induced by a variety of T-cell, T-dependent B-cells or B-cell mitogens and antigens [Citation11,Citation14]. MMF has an established role as a maintenance immunosuppressive regimen in improving cardiac and renal transplantation outcomes with acceptable efficacy and tolerability profile [Citation15,Citation16].
Beyond its anti-inflammatory activity, MMF exhibits important anti-proliferative and anti-fibrotic properties, including decreased expression of transforming growth factor-beta [Citation17,Citation18], making it a potentially attractive agent for the treatment of ILDs such as cHP, sarcoidosis and CTD-ILD (e.g. scleroderma, rheumatoid arthritis).
This article aims to review the currently available literature and published clinical studies for the use of MMF in patients with ILDs and summarizes the key considerations around its use in clinical practice. Literature review was performed by using the PubMed, Science Direct, and EMBASE databases using the terms ‘mycophenolate mofetil’ and ‘interstitial lung disease’ or ILD’. The search was further supplemented using the terms ‘chronic hypersensitivity pneumonitis’and ‘connective tissue disease-ILD or CTD-ILD’ and ‘scleroderma ILD’ and ‘idiopathic pulmonary fibrosis or ‘IPF’. Reviews on topics of interest were also included, as well as relevant evidence known to the authors. The search was restricted to clinical studies and English language publications. All studies published until June 2021 were included. Since the aim was not a statistical interpretation of the studies, the selected articles were not assessed for risk of bias in individual studies or the risk of bias across studies.
3. Evidence on the efficacy and tolerability of MMF in ILDs
The effectiveness of MMF has been assessed in retrospective reviews, small prospective case series and a few prospective randomized trials in various types of ILD. This section presents the summary of findings from a number of clinical studies ().
Table 1. Summary of Evidence from Various Clinical Studies with MMF in ILDs
3.1. Chronic hypersensitivity pneumonitis
In a retrospective analysis, the effects of MMF and azathioprine on lung function in patients (n = 70) with cHP were evaluated. All patients were either surgical lung biopsy-proven or had been exposed to an identified causative antigen. Prior to the onset of either MMF (n = 51; dose: 1–3 g/day) or azathioprine (n = 19; dose: 100–150 mg/day) therapy, 84% of patients were on CS (mean dose: 12.3 mg/day). Treatment for 1 year with either MMF or azathioprine failed to improve forced vital capacity (FVC) (0.5%, p = 0.46), but was associated with an improvement in diffusing lung capacity (DLco) (4.2%, p < 0.001). Similarly, in the sub-group of patients treated with MMF, a non-significant increase in FVC (1.3%) and a significant increase in DLco (3.9%), respectively, were noted, while in the patients treated with azathioprine, a statistically significant improvement in DLco (5.1%) and a non-statistical decline in FVC (–1.1%) were found. At the end of 6 months, the use of both MMF and azathioprine were associated with a reduction in the CS dose (from 12.35 mg to 3.78 mg) in over 40% of patients. Interestingly, there was no difference in the lung function decline between patients on CS and without CS before initiation of MMF or azathioprine, suggesting uncertainty about the efficacy of CS in the management of cHP. While the efficacy profile of both MMF and azathioprine were similar, the safety profile differed, with higher incidence of adverse events (AEs) and discontinuation of azathioprine compared with MMF [Citation19].
In a separate retrospective analysis, 22 cHP patients were treated with MMF at a daily dose of 1–2 g/day, while 8 patients were treated with azathioprine at a daily dose of 25–150 mg/day for 12 months. Compared with the study above [Citation20], patients in this study cohort had more severe disease as indicated by lower carbon monoxide diffusing capacity (TLco) (39% versus 50%). Despite treatment with CS, patients in this cohort reported a gradual increase in breathlessness, a decline in lung function, and clinically significant CS-related side effects. Treatment for 1 year with MMF or azathioprine allowed the overall CS dose to be reduced by 50%, viz. from 16.2 ± 9.7 mg to 8.2 ± 4.2 mg (p = 0.002). Treatment with MMF or azathioprine was associated with an improvement in TLco from – 0.55 + 0.96 mmol/kPa/min to +0.31 + 0.58 mmol/kPa/min followed by non-significant improvement in FVC from – 111 + 295 to +2.3 + 319 mL [Citation20].
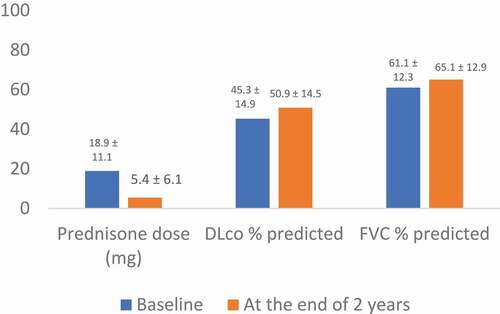
In another study, MMF was found to be a promising steroid-sparing agent. In 38 patients diagnosed with cHP, treatment with MMF over a 2-year period was associated with a reduction in the CS dose and stabilization of both DLco % predicted and FVC% predicted () [Citation21].
Figure 2. Prednisone Dose and Pulmonary Function Test Following Initiation with MMF
A large retrospective, observational study of patients with cHP determined that there was no difference in FVC decline or survival when comparing those (n = 93 of 131) receiving immunosuppressive therapy with either CS (median daily dose 40 mg), azathioprine (median daily dose: 125 mg) or MMF (median daily dose: 2 g) with those not treated with immunosuppressive therapy (n = 38). On an average, patients treated with azathioprine and MMF also received prednisone at a dose of 40 mg and 20 mg, respectively. The patients receiving immunosuppressive therapy had more severely impaired baseline FVC (60% versus 73% predicted) and DLco (47% versus 69% predicted); were more likely to be using supplemental oxygen (71% versus 24%, p < 0.001); and had a significantly higher radiographic pulmonary fibrosis score (126 versus 118, p = 0.01) compared with those not treated with immunosuppressive treatment. The study concluded that in recipients of immunosuppressive therapy, early transition to MMF or azathioprine may provide an appropriate therapeutic approach for patients with cHP. Treatment-emergent AEs were low in the MMF (66% less versus prednisone; p = 0.002) group compared with other therapies [Citation22].
While the evidence on MMF use in cHP is limited, the available findings suggest that MMF may be able to help stabilize the otherwise expected decline in lung function with a better safety profile compared with CS. Multiple studies suggest that the CS dose can be reduced significantly with the addition of MMF [Citation19–21].
3.2. Connective tissue disease- related-ILD
3.2.1. Scleroderma ILD
Over the past few years, there have been several publications on the effectiveness and tolerability of MMF in patients with ILD. However, the enthusiasm for its use increased following the Scleroderma Lung Study II (SLS II).
In SLS II, 126 patients included in the primary analysis were randomized to oral MMF for 2 years or cyclophosphamide for 1 year followed by 1 year of placebo. At the end of 2 years, improvements in the adjusted percent predicted FVC (2.19% in MMF and 2.88% in cyclophosphamide), skin thickness and dyspnea scores were observed in both treatment arms [Citation23]. However, the authors also reported a significant reduction in cough frequency (by 41%) among patients who received MMF over a period of 2 years. Though cyclophosphamide and MMF resulted in a lesser decrease in the percent predicted DLCO/alveolar volume ratio during treatment, MMF was shown to be superior in terms of safety profile and regarded as a safer, less toxic alternative to cyclophosphamide, which is associated with gonadal toxicity and cancer risk. In addition, those treated with MMF demonstrated stabilization of lung function for up to 36 months. This is in contrast with SLS I where the 12-month course of cyclophosphamide failed to achieve sustained improvement in the FVC at 36 months [Citation24].
In a retrospective study from India, the authors reported a statistically significant increase in the mean percentage of FVC in both the cyclophosphamide group (10.84 + 13.81%) and in the MMF group (6.07% + 11.92%) compared with baseline. There was no significant difference between the two groups. Notably, along with a numerical increase in FVC in both the MMF and intravenous cyclophosphamide groups, stabilization of the disease occurred in a majority of the patients, with no exacerbations, hospital admissions or fatalities reported in either group [Citation25].
A prospective single-center study in 5 patients with systemic sclerosis (SSc)-associated ILD (SSc-ILD) found that early treatment with MMF was as effective as cyclophosphamide [Citation26]. An additional retrospective study identified 13 patients with SSc-ILD who were treated with MMF and showed significant improvement in the FVC at 1 year after treatment [Citation27].
In the SENSCIS study, the largest Phase III double-blind, placebo-controlled, randomized trial in SSc-ILD to date, treatment with nintedanib over 52 weeks resulted in reduced decline in annual adjusted mean FVC compared with placebo (−52.4 mL/year versus 93.3 mL/year; p = 0.04). About half of the patients (139/228 [48.3%]) were also receiving mycophenolate (mofetil or sodium). Interestingly, in a pre-specified subgroup- analysis, the annual rate of decline in FVC in the group that received both mycophenolate and nintedanib was lower (26.3 mL/year) than in those who received only nintedanib (55.4 mL/year) [Citation28].
Additionally, a retrospective study evaluated the safety, tolerability and efficacy of MMF in a variety of defined CTD patients (predominant diagnosis of SSc [n = 9/25]). The key reason for initiating MMF was intolerance of a previous immunosuppressive agent and ILD progression. MMF was found to be associated with a clinically significant increase in FVC%, DLco% and TLco% by 2.3%, 2.6% and 4.0%, respectively, and a reduction in the daily CS dose. There were no serious side effects reported with MMF 2 g/day in divided doses [Citation29].
3.2.2. Other CTD-ILDs
In a longitudinal data analysis, researchers evaluated changes in pulmonary physiology, safety and tolerability with MMF in a larger group of patients with a diverse spectrum of CTD-ILDs (n = 125) followed over 3 years. Treatment with MMF was associated with a significant increase in FVC% at weeks 52, 104 and 156 (4.9% + 1.9%, p = 0.01; 6.1% + 1.8%; p = 0.0008; and 7.3% + 2.6%; p = 0.004) and DLco% at weeks 52 and 104 (6.3% + 2.8%; p = 0.02; 7.1% + 2.8%; p = 0.01) across the spectrum of CTD-ILDs. A subgroup analysis showed that MMF was associated with an improvement in FVC% and DLco% in the subgroup with non-usual interstitial pneumonia (UIP)-pattern injury and associated with stability in FVC% and DLco% in the subgroup with UIP pattern injury [Citation30].
In a retrospective study of 33 Indian patients with CTD-ILD, MMF was associated with ILD stability in a majority of the patients over a median of 24 months [Citation31]. Further evidence revealed that the use of MMF was associated with improvement or stability in pulmonary physiology in patients with CTD-ILD regardless of the underlying ILD subtype [Citation32]. Additionally, in a small series of 10 patients with autoimmune-related ILD treated with MMF, persistent symptomatic relief was observed in all patients within 2–6 weeks of treatment followed by a marked increase in the activity levels. Stabilization and/or improvement were noticed in repeat high-resolution computed tomography (HRCT) scans [Citation33]. It has been shown that azathioprine in combination with prednisone and N-acetylcysteine increases the risk of hospitalization and death in patients with IPF [Citation34]; however, the effect of this regimen in CTD-ILD is unknown. In a study conducted in patients with fibrotic CTD-ILD, including those with CTD-associated UIP, both azathioprine and MMF were found to be associated with stability in pulmonary function, but the medication discontinuation due to side effects and non-respiratory related discontinuation rate was higher in azathioprine-treated (13% and 27%) versus MMF-treated (5%) patients, respectively [Citation35].
Improvement in FVC % and DLco % have been reported in patients with primary Sjögren’s syndrome associated ILD and interstitial pneumonia with autoimmune features treated with MMF [Citation36,Citation37].
3.2.3. Polymyositis/dermatomyositis
In a case series of 4 patients diagnosed with dermatomyositis-associated ILD (2 with early-treated amyopathic dermatomyositis, 1 with classic dermatomyositis, and 1 with early treated hypomyopathic dermatomyositis) received MMF at a dose of 3 g/day. Complete normalization of both FVC% and DLco% and dyspnea was observed within 1 year of treatment, along with a reduction in dose of steroids from 60 mg to 4 mg in 2 patients and 15 mg to 4 mg in 1 patient. In 1 patient who received 5 months of MMF treatment, DLco% increased from 44% to 77% but there was no change in dyspnea [Citation38].
In another retrospective study conducted in 46 patients with steroid-resistant ILD associated with polymyositis/dermatomyositis, treatment with immunosuppressants such as cyclophosphamide (n = 24), azathioprine (n = 13) and MMF (n = 9) was associated with stabilization of pulmonary function and reduction in the CS dose. There were no significant differences in outcome among the immunosuppressants studied. A more favorable outcome was reported in patients treated with MMF who were anti-Jo-1 positive. At the end of 12 months, MMRC grade >2 was seen in 40% of patients compared with 92% of patients before treatment. The overall median change in FVC was +5.0%, corresponding to +0.02 L, while DLco increased by 2.93%, corresponding to 1 mm/mL/Hg, at the end of 6 months. The changes were steady at the end of 12 months. Further, 39 patients (85%) out of 46 were alive after a median follow-up of 5.1 years since the initial evaluation [Citation39].
In a case-based review of 11 patients diagnosed with hypomyopathic dermatomyositis/amyopathic dermatomyositis, MMF was found to be effective and safe in patients who were refractory to other immunosuppressants and improvements in clinical, radiological and/pulmonary variables were observed [Citation40].
In a retrospective observational study conducted at Johns Hopkins University School of Medicine, Baltimore, 110 patients with myositis related-ILD were evaluated to assess the effect of MMF (n = 44) and azathioprine (n = 66) on lung function and CS dose. Improvement in FVC% (MMF, 3.3%; azathioprine, 3.6%) and reduction in the CS dose was observed with both the therapies at the end of 24 months. However, there was a significantly higher rate of AEs with azathioprine compared with MMF (33.3% versus 13.6%; p = 0.04). Seventeen percent of patients on azathioprine discontinued the therapy compared with 7.5% of patients on MMF, with transaminitis being the common AE in the azathioprine group (15.2%; p = 0.04) [Citation41]. Furthermore, in another retrospective study conducted in patients with resistant inflammatory myopathy taking prednisolone, but refractory to conventional immunosuppressive therapy, the combination of MMF with calcineurin inhibitors (n = 7) showed a significant reduction in creatine kinase levels at the final visit (p = 0.04) versus MMF alone (n = 12) suggesting a potential role for this combination [Citation42].
MMF may be able to play a significant role in the treatment of polymyositis- and/or dermatomyositis-associated ILD. Compared with cyclosporine, tacrolimus and cyclophosphamide, the AE profile of MMF seems to be better [Citation43]. Further, MMF has been found to be an effective steroid-sparing agent for recalcitrant skin and muscle manifestations of dermatomyositis [Citation44].
3.3. Chronic sarcoidosis and/or refractory sarcoidosis
Though most patients with sarcoidosis do not require treatment, medical therapy is primarily indicated when clinically significant organ involvement is noted, particularly with evidence of disease progression, leading to significant impairment of the quality of life.
In a retrospective study of 8 patients with biopsy-proven sarcoidosis, the efficacy and safety of 2 g/day of MMF was evaluated for at least a year. All the patients had chronic and/or refractory sarcoidosis with the involvement of both pulmonary and extra-pulmonary disease as assessed by the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) Sarcoidosis Organ Assessment Tool. Patients also presented with various comorbid conditions such as diabetes mellitus, osteopenia, Cushing’s syndrome, cataract, myopathy, arterial hypertension, and significant body weight. The median duration of therapy was 48 months. During follow-up, a significant reduction in the daily dose of steroids from 15.0 mg to 2.5 mg (p = 0.016) and improvement in forced expiratory volume in 1 second (FEV1) and FVC (p = 0.010 and p = 0.021, respectively) was observed. All patients recorded symptomatic as well as radiological improvements, along with significant improvement for the dermal disease. Resolution of renal and cardiac disease was also noted [Citation45].
In another small study involving 8 patients with biopsy-proven sarcoidosis, 63 months of median treatment with MMF therapy at a daily dose of 1–2 g resulted in a significant reduction in steroid dose from 10 mg to 2.5 mg, with no serious AEs reported. While stability was achieved in 1 patient, 7 (87.5%) patients reported improvement in radiological signs, clinical symptoms as well as pulmonary function [Citation46]. Additional findings from a study evaluating the efficacy and safety of MMF in 10 patients with chronic pulmonary sarcoidosis demonstrated radiological improvement in 5/10 (50%) patients, with partial resolution of the reticulonodular pattern observed in 3 patients with stage II sarcoidosis and 1 patient with stage IV sarcoidosis. Prior to MMF therapy, all these patients were treated with CS therapy and experienced relapses whenever CS doses were stopped. Furthermore, the use of MMF was associated with an improvement in the FVC in those intolerable to prior therapies [Citation47]. Similarly, in another study conducted in 37 patients with either refractory sarcoidosis (71.4%) or those with intolerance to other immunosuppressive therapies (81.3%), pre- and post-MMF therapy showed no statistically significant changes in the absolute FVC and/or DLco. However, in patients who were started on MMF due to intolerance to previous immunosuppressive therapy, a trend toward improvement in DLco was observed at 12 months. The study investigators concluded that MMF can be an alternative treatment in patients intolerant to previous immunosuppressive therapies [Citation48].
3.4. Idiopathic pulmonary fibrosis
In a retrospective study conducted in 41 patients diagnosed with IPF, 11 were treated with MMF, 20 with a triple-drug combination (prednisone, azathioprine and/or N-acetylcysteine) while 10 did not receive any therapy. Ninety percent of patients across the treatment arms had definite UIP based on HRCT. Patients who received MMF had worse baseline FVC (mean 57.8 + 19.9%) compared with the triple-drug therapy arm (mean: 63.8 + 13.8%) and the no-treatment arm (mean: 68.3 + 20.4%).
At the end of 12 months, patients who received MMF showed lesser decline in FVC compared with the other two arms () and 87.5% of patients had a stable FVC. Although it is not possible to make direct comparisons between MMF and anti-fibrotics, the study investigators reported that the trend in FVC decline with MMF was similar to that observed with anti-fibrotics in the CAPACITY, ASCEND and INPLUSIS trials.
Table 2. Trends in FVC Decline in IPF patients at 1-Year Follow-up
Unlike the PANTHER trial, where a triple-drug therapy of prednisone, N-acetylcysteine plus azathioprine was associated with an increased rate of hospitalization and deaths, treatment with MMF in this cohort resulted in a trend toward improved survival (40.3 months) compared with the triple-drug combination (25.5 months) and no-treatment arms (29.3 months) [Citation49].
4. Safety
4.1. General profile
MMF has been generally safe and well tolerated, with low discontinuation rates (5–10%) as reported in clinical studies in patients with ILDs [Citation41]. Gastrointestinal and bone marrow suppression are the most observed AEs and are mostly dose-dependent and typically occur early in the course of treatment and decrease in frequency with continued use. The most common gastrointestinal side effects of MMF include nausea, vomiting, dyspepsia and abdominal pain. To reduce adverse GI events, mycophenolate sodium (MPS)- an enteric-coated formulation of MPA has been designed to overcome these challenges. MPS was found to achieve a longer peak plasma concentration (2 hours) compared to MMF (0.8 hours) allowing more sustained absorption of the MPA released to the small intestine [Citation50]. Although studies suggest that shifting MMF to MPS in renal transplant patients showed a trend favoring MPS for GI adverse event severity scores, further research is needed to confirm the efficacy and tolerability of MPS [Citation51]. Furthermore, as the tolerability problems associated with MPA, azathioprine, cyclophosphamide are substantial, guidelines recommend monitoring of patients with diffuse interstitial and inflammatory ILD (including CTD-ILD) when prescribed these immunosuppressive therapies [Citation52].
Additionally, rare hematological side effects including leukopenia, anemia, and thrombocytopenia have also been identified that are found to be rare and uncommon. Reduction in the daily dose or divided dosing can help improve some of these AEs. Co-administration of MMF with antiviral compounds such as aciclovir has been shown to increase plasma area under the concentration-time curve (AUC) of aciclovir. In combination with ganciclovir, MMF is anticipated to inhibit renal tubular secretion of ganciclovir resulting in increased blood levels of ganciclovir and prompting the risk of nephrotoxicity, neutropenia, and leukopenia) [Citation53]. In addition, patients receiving immunosuppressants, including MMF, are at increased risk of developing bacterial, fungal, protozoal and new or reactivated viral infections, including opportunistic infections, lymphomas and other malignancies, particularly of the skin. It is recommended to avoid MMF in pregnancy due to the risk of miscarriage and congenital anomalies. Complete blood counts weekly for the first month, every two weeks for the second and third months, and monthly for the remainder of the first year is recommended [Citation54].
4.2. Compared with other immunosuppressants
Though cyclophosphamide is considered as a modestly effective treatment for SSc-ILD based on SLS I [Citation55], its long-term use was associated with serious toxicities (bone marrow suppression, gonadal failure, teratogenicity and hemorrhagic cystitis). Studies that have compared MMF with cyclophosphamide have found MMF to be superior with respect to safety. In SLS II, a greater proportion of patients receiving cyclophosphamide developed leucopaenia (41% versus 6%) and thrombocytopaenia (6% versus 0) compared with MMF (). The premature withdrawal was also lower with MMF compared with cyclophosphamide. MMF therapy is also a safer alternative in patients for whom preserving reproductive ability is required as there is a high propensity of premature ovarian failure with cyclophosphamide [Citation23].
Table 3. Comparative AEs with Immunosuppressive Therapies
Several studies report higher discontinuation rates with azathioprine compared with mycophenolate in patients with cHP (10.5% versus 5.8%), SSc -ILD (27% versus 18%), and fibrotic ILDs (18% versus 56%) [Citation19,Citation24,Citation56]. There have been also reports of development of skin cancer with azathioprine. In a few studies, about 29% of patients receiving cyclophosphamide and 12% of patients receiving azathioprine were switched to MMF due to cyclophosphamide-associated toxicity and azathioprine-related progression of ILD. Moreover, available evidence reported greater number of treatment-emergent AEs with CS and azathioprine compared with MMF in cHP patients [Citation22]. Unlike azathioprine, MMF does not require monthly monitoring for liver toxicity and its activity is independent of thiopurine S-methyltransferase (TMPT) enzymes. TMPT deficiency is known to increase the risks of liver injury with azathioprine.
5. MMF: general dosing recommendations
The optimal daily dose range of MMF is 1.5–3 g in two divided doses. In patients with end-stage renal disease, dose reduction is recommended. It should be taken either 30 minutes before a meal or 2 hours after the meal. Use of antacids and mineral supplements should be separated by at least 2 hours from time of intake of MMF. The optimal duration of MMF remains unknown; however, it can be continued until the patient experiences stability (minimum of 12–24 months).
6. Conclusion
MMF is a potent immunosuppressant with anti-proliferative and anti-fibrotic properties. Its use in patients with ILDs has been associated with stabilization of the FVC and DLco and a significant reduction in the use of systemic CS. Compared with other immunosuppressants, MMF exhibits rapid onset of action with similar efficacy, but a generally better safety profile and lacking hepatic, renal or pulmonary toxicity. Treatment-emergent AEs such as gastrointestinal events and leucopaenia were generally mild to moderate in severity. Thus, though the evidence supporting the efficacy data is limited, MMF appears to be a safe and well-tolerated steroid-sparing agent in patients with ILDs.
7. Expert opinion
ILDs are often complex with more than 200 entities that often present with similar symptoms clinically but have different disease courses and prognosis. However, decades of research have been marked by changes in the understanding of ILDs to a large extent. The approvals of pirfenidone and nintedanib changed the paradigm of IPF treatment. Nonetheless, many challenges and unmet needs remain in the management of other progressive ILDs. A range of immunosuppressants are available for the treatment of ILDs, including cyclosporine, tacrolimus and cyclophosphamide. These drugs are reported to be associated with a variety of serious side effects that often limit and reduce tolerability. Therefore, there is a clear need for a therapy that is well tolerated and with fewer side effects. MMF, which is primarily used to suppress acute and chronic allograft rejection in post-transplant patients, has emerged as a wise choice of ILD therapy. It is an anti-proliferative, immunosuppressive agent with a relatively favorable safety profile. Observational data and clinical experience support the use of MMF in non-IPF ILDs, especially cHP and CTD-ILD. Leucopaenia, bone marrow suppression and gastrointestinal symptoms (such as nausea, diarrhea and abdominal cramping) are frequent and mild to moderate in severity, but may improve with divided dosing (e.g. three to four times a day) or a decrease in the total daily dose.
In SSc-ILD, evidence of the modest and temporary effectiveness of cyclophosphamide coupled with substantial drug toxicities over time highlights the need for safer alternatives. MMF, with its anti-inflammatory, anti-fibrotic and immunomodulatory properties, appears better tolerated than cyclophosphamide, based on the lower incidence of leucopaenia and thrombocytopaenia, and is associated with improved or stable pulmonary function. A number of studies have provided encouraging results for the use of MMF as the preferred choice of therapy in the treatment of SSc-ILD. Based on the findings from the above studies, MMF seems to be an effective steroid-sparing agent in those with refractory sarcoidosis and chronic progressive sarcoidosis with both pulmonary and extra-pulmonary involvements.
In summary, MMF is associated with lower CS doses, stabilization or improvement in the FVC% and/or DLCO % and has a low rate of discontinuation. In particular, the drug has been found to be safe and well tolerated in the long term. These early studies demonstrate that MMF with its safety, efficacy and tolerability represents a significant choice as a potential therapeutic regimen in the treatment of ILDs.
Going forward, the management of the large number and clinically varied disorders grouped together under the banner of inflammatory ILD will become increasingly personalized. For those patients who may benefit from an immunosuppressive strategy, MMF expands our limited treatment armamentarium, as the results from a substantial number of studies suggest that treatment with MMF is generally well-tolerated and safe, and can result in persistent control of inflammatory disease activity. Further prospective, randomized controlled trials in larger cohorts of patients comparing MMF to other treatment approaches will help stratify outcomes within these varied clinical disorders and their distinct subgroups.
Article highlights
Interstitial lung diseases (ILDs) are a large and diverse group of lung disorders recognised as posing a significant global burden.
Oral corticosteroids are frequently the preferred initial therapy for treatment of many ILDs; however, their use can be associated with serious side effects.
Mycophenolate Mofetil (MMF) is an immunosuppressive agent that may help to stabilise the decline in lung function seen in many of these disorders with a better safety profile than corticosteroids.
When compared with the use of cyclophosphamide and azathioprine in common conditions, the safety profile of MMF has been found to be generally superior.
In patients who are refractory to other immunosuppressants, MMF may be effective.
MMF represents a potential therapeutic option in the treatment of a variety of ILDs, with seemingly similar efficacy as other therapeutic options but a generally better safety profile.
Declaration of Interests
K. Brown serves on the Scientific Advisory Board of the Open-Source Imaging Consortium (OSIC), and has received grants from NHLBI, and personal fees from Biogen, Galecto, Third Pole, Galapagos, Boehringer Ingelheim, Theravance, Pliant, Blade Therapeutics, Huitai Biomedicine, Lilly, Dispersol, DevPro Biopharma, Sanofi, Bristol Myers Squibb, and Humanetics, all independent of the submitted work. S. Rajan serves on Scientific Advisory Board of Cipla Limited, Boehringer Ingelheim, Himalaya Drug. M. Mehta, M. Lopez, J. Gogtay and R. Hegde are employees of Cipla Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Kreuter M, Herth FJF, Wacker M, et al. Exploring clinical and epidemiological characteristics of interstitial lung diseases: rationale, aims, and design of a nationwide prospective registry—The EXCITING-ILD registry. Biomed Res Int. 2015;2015:123876.
- Richeldi L. Idiopathic pulmonary fibrosis: moving forward. BMC Med. 2015;13(1):231.
- Singh S, Collins BF, Sharma BB, et al. Interstitial lung disease in India. Results of a prospective registry. Am J Respir Crit Care Med. 2017;195(6):801–813.
- Kundu S, Mitra S, Ganguly J, et al. Spectrum of diffuse parenchymal lung diseases with special reference to idiopathic pulmonary fibrosis and connective tissue disease: an eastern India experience. Lung India. 2014;31(4):354–360.
- Dhooria S, Agarwal R, Sehgal IS, et al. Spectrum of interstitial lung diseases at a tertiary center in a developing country: a study of 803 subjects. Plos One. 2018;13(2):e0191938.
- Boehringer Ingelheim Pharmaceuticals, Inc. (OFEV) nintedanib capsules for oral use. U.S. Food and Drug Administration [cited 2020 Jun 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/205832s012lbl.pdf
- European Medicines Agency. Esbriet: EPAR - Product information [updated 2020 Jan 30; cited 2020 Jun 25]. Available from: https://www.ema.europa.eu/en/documents/product-information/esbriet-epar-product-information_en.pdf
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727.
- Ueda T, Sakagami T, Kikuchi T, et al. Mycophenolate mofetil as a therapeutic agent for interstitial lung diseases in systemic sclerosis. Respir Investig. 2018;56(1):14–20.
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2):85–118.
- Lipsky JJ. Mycophenolate mofetil. Lancet. 1996;348(9038):1357–1359.
- Fulton B, Markham A. Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs. 1996;51(2):278–298.
- Roos N, Poulalhon N, Farge D, et al. In vitro evidence for a direct antifibrotic role of the immunosuppressive drug mycophenolate mofetil. J Pharmacol Exp Ther. 2007;321(2):583–589.
- Qiu Y, Fairbanks LD, Rückemann K, et al. Mycophenolic acid-induced GTP depletion also affects ATP and pyrimidine synthesis in mitogen-stimulated primary human T -lymphocytes. Transplantation. 2000;69(5):890–897.
- Taylor DO, Ensley RD, Olsen SL, et al. Mycophenolate mofetil (RS-61443): preclinical, clinical, and three-year experience in heart transplantation. J Heart Lung Transplant. 1994;13(4):571–582.
- Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal transplant mycophenolate mofetil study group. Transplantation. 1995;60(3):225–232.
- Guo H, Leung JC, Chan LY, et al. Modulation of intra-pulmonary TGF-beta expression by mycophenolate mofetil in lupus prone MRL/lpr mice. Lupus. 2005;14(8):583–592.
- Morath C, Schwenger V, Beimler J, et al. Antifibrotic actions of mycophenolic acid. Clin Transplant. 2006;20(Suppl 17):25–29.
- Morisset J, Johannson KA, Vittinghoff E, et al. Use of mycophenolate mofetil or azathioprine for the management of chronic hypersensitivity pneumonitis. Chest. 2017;151(3):619–625.
- Fiddler CA, Simler N, Thillai M, et al. Use of mycophenolate mofetil and azathioprine for the treatment of chronic hypersensitivity pneumonitis—A single-centre experience. Clin Respir J. 2019;13(12):791–794.
- MacDonald D, Bhargava M, Tomic R, et al. Use of mycophenolate mofetil as a steroid-sparing agent in chronic hypersensitivity pneumonitis. Proceedings of the American Thoracic Society (ATS) International Conference; 2017 May 19-24; Washington, DC: American Thoracic Society; A1575.
- Adegunsoye A, Oldham JM, Fernández Pérez ER, et al. Outcomes of immunosuppressive therapy in chronic hypersensitivity pneumonitis. ERJ Open Res. 2017;3(3):00016–2017.
- Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719.
- Owen C, Ngian GS, Elford K, et al. Mycophenolate mofetil is an effective and safe option for the management of systemic sclerosis-associated interstitial lung disease: results from the Australian Scleroderma Cohort Study. Clin Exp Rheumatol. 2016;34(Suppl 100(5)):170–176.
- Shenoy PD, Bavaliya M, Sashidharan S, et al. Cyclophosphamide versus mycophenolate mofetil in scleroderma interstitial lung disease (SSc-ILD) as induction therapy: a single-centre, retrospective analysis. Arthritis Res Ther. 2016;18(1):123.
- Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology (Oxford). 2006;45(8):1005–1008.
- Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008;133(2):455–460.
- Highland KB, Distler O, Kuwana M, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med. 2021;9(1):96–106.
- Swigris JJ, Olson AL, Fischer A, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130(1):30–36.
- Fischer A, Brown KK, Du Bois RM, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40(5):640–646.
- Santhanam S, Rahulan V. AB0803 A real life experience on the efficacy and safety of mycophenolate mofetil in connective tissue disorder associated interstitial lung disease – a retrospective study. Ann Rheum Dis. 2018;77:1533.
- Omair MA, Alhamad EH. Mycophenolate mofetil is an effective therapy for connective tissue disease-associated interstitial lung disease. Int J Clin Rheumatol. 2017;12(3):67.
- Saketkoo LA, Espinoza LR. Experience of mycophenolate mofetil in 10 patients with autoimmune-related interstitial lung disease demonstrates promising effects. Am J Med Sci. 2009;337(5):329–335.
- Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, King TE Jr, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366(21):1968–1977.
- Oldham JM, Lee C, Valenzi E, et al. Azathioprine response in patients with fibrotic connective tissue disease-associated interstitial lung disease. Respir Med. 2016;121:117–122.
- Amlani B, Elsayed G, Barvalia U, et al. Treatment of primary sjögren’s syndrome-related interstitial lung disease: a retrospective cohort study. Sarcoidosis Vasc Diffuse Lung Dis. 2020;37(2):136–147.
- Sara S, Mukadam Z, Meyer KC, et al. Mycophenolate therapy in interstitial pneumonia with autoimmune features: a cohort study. Ther Clin Risk Manag. 2018;14(1):2171–2181.
- Morganroth PA, Kreider ME, Werth VP. Mycophenolate mofetil for interstitial lung disease in dermatomyositis. Arthritis Care Res. 2010;62(10):1496–1501.
- Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107(6):890–896.
- Koyama RVL, Braga TKK, da Silva Dias GA, et al. Hypomyopathic dermatomyositis associated with interstitial lung disease and good response to mycophenolate mofetil: case-based review. Clin Rheumatol. 2017;36(8):1919–1926.
- Huapaya JA, Silhan L, Pinal-Fernandez I, et al. Long-term treatment with azathioprine and mycophenolate mofetil for myositis-related interstitial lung disease. Chest. 2019;156(5):896–906.
- Hanaoka H, Iida H, Kiyokawa T, et al. Mycophenolate mofetil treatment with or without a calcineurin inhibitor in resistant inflammatory myopathy. Clin Rheumatol. 2019;38(2):585–590.
- Kameda H, Takeuchi T. Recent advances in the treatment of interstitial lung disease in patients with polymyositis/dermatomyositis. Endocr Metab Immune Disord Drug Targets. 2006;6(4):409–415.
- Edge JC, Outland JD, Dempsey JR, et al. Mycophenolate Mofetil as an effective corticosteroid-sparing therapy for recalcitrant dermatomyositis. Arch Dermatol. 2006;142(1):65–69.
- Papiris S, Stagaki E, Papadaki G, et al. Mycophenolate mofetil as an alternative treatment in sarcoidosis. Pulm Pharmacol Ther. 2019;58:101840.
- Stagaki E, Manali ED, Papadaki G, et al. Mycophenalate Mofetil as a corticosteroid-sparing agent in patients with sarcoidosis. Eur Respir J. 2018;52(suppl 62): PA4103.
- Brill AK, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration. 2013;86(5):376–383.
- Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med. 2014;108(11):1663–1669.
- Nambiar AM, Anzueto AR, Peters JI. Effectiveness and safety of mycophenolate mofetil in idiopathic pulmonary fibrosis. Plos One. 2017;12(4):e0176312.
- Bjarnason I. Enteric coating of mycophenolate sodium: a rational approach to limit topical gastrointestinal lesions and extend the therapeutic index of mycophenolate. Transplant Proc. 2001;33(7–8):3238–3240.
- Sollinger H. Enteric-coated mycophenolate sodium: therapeutic equivalence to mycophenolate mofetil in de novo renal transplant patients. Transplant Proc. 2004;36(2Suppl):517S–520S.
- Baughman RP, Meyer KC, Nathanson I, et al. Monitoring of nonsteroidal immunosuppressive drugs in patients with lung disease and lung transplant recipients: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;142(5):e1S–e111S.
- Wolfe EJ, Mathur V, Tomlanovich S, et al. Pharmacokinetics of mycophenolate mofetil and intravenous ganciclovir alone and in combination in renal transplant recipients. Pharmacotherapy. 1997;17(3):591–598.
- Gene.com. [Internet]. South San Francisco (CA): Genentech US Drug Safety; [Cited 2021 Jun 5]. Available from: https://www.gene.com/download/pdf/cellcept_prescribing.pdf
- Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–2666.
- Wong AW, Donohoe K, Marcoux. V, et al. Tolerability of mycophenolate and azathioprine in patients with fibrotic interstitial lung disease: a prospective cohort study using real-world data. Proceedings of the American Thoracic Society (ATS) International conference; 2021 May 14-19; virtual conference: American Thoracic Society; A1914
