ABSTRACT
Introduction
Asthma symptoms can be relieved through a maintenance treatment combining long-acting β2-agonist and inhaled corticosteroids (LABA/ICS). However, for patients with inadequately controlled asthma, the LABA/ICS combination might not be sufficient, and clinical guidelines recommend the administration of inhaled long-acting muscarinic antagonists (LAMA) as an add-on therapy to better control asthma and improve lung function. For nearly two decades, the only LAMA to be approved on the market has been tiotropium.
Areas covered
We reviewed recent clinical studies evaluating the safety and efficacy of LABA/LAMA/ICS fixed dose combinations by searching the PubMed database. Molecular mechanisms and clinical data support the use of a once-daily, single-inhaler fixed dose combination of the LABA/LAMA/ICS indacaterol/glycopyrronium/mometasone (IND/GLY/MF), the first therapy combining three agents in a fixed dose approved in Europe for the treatment of uncontrolled asthma.
Expert opinion
IND/GLY/MF was superior to both IND/MF and salmeterol/fluticasone, a well-established LABA/ICS combination improving the lung function in uncontrolled asthma. Moreover, IND/GLY/MF, delivered through the Breezhaler inhaler in a single inhalation, is the first inhaled therapy prescribed alongside a digital companion, a sensor and the Propeller app, allowing for improved treatment adherence, reduced rescue inhaler usage and hospitalizations, increased patient satisfaction and asthma control.
1. Introduction
1.1. Asthma: pathogenesis and treatment
Asthma is a disease involving chronic airway inflammation, reversible airway obstruction, bronchial hyperreactivity, and airways remodeling [Citation1]. Asthma is characterized by a complex relationship between inflammation, which represents the central component, airway hyper-responsiveness, and remodeling. Moreover, asthma can be triggered by different stimuli and can have an early or late-onset. The clinical characteristics of individuals led to the definition of asthma phenotypes and endotypes that allowed clinicians to titrate personalized therapy targeting specific pathways [Citation1]. Several phenotypic groups are defined by treatable or untreatable traits, measurable parameters, and risk predictors and are currently used for the stratification of asthma patients [Citation2]. However, the definition of a phenotype does not always correlate to or give insights into the underlying pathogenic mechanisms, which are associated with the disease endotypes. We can distinguish three different endotypes in relation to the biological and inflammatory mechanisms involved: type 2 high, type 2 low, and mixed [Citation3].
The main goal of asthma management is the reduction of individual risk factors, prevention of flares and symptoms control, assuring optimal lung functionality and management of comorbidities. The first-line therapy in all patients with persistent asthma is the treatment with low dose inhaled corticosteroids (ICS) [Citation1]. In addition, short-acting beta agonists (SABAs) or low dose ICS-formoterol can be used as a reliever inhaler. If symptoms are difficult to control and/or lung function is declining, the combined administration of ICS and long-acting β2 agonists (LABA) or an increased dose of ICS is required. Moreover, the recently introduced combination of LABA/ICS and long-acting muscarinic antagonists (LAMA) can be also considered for the treatment. The addition of oral corticosteroids could be considered in selected uncontrolled patients [Citation1] affected by severe asthma (SA) [Citation4,Citation5]. Multiple biologics targeting the Th2 pathway should be considered as add-on treatment after analyzing SA treatable or untreatable traits, measurable parameters, and risk predictors [Citation3].
All these interventions have shown a consistent effect on decreasing asthma exacerbations [Citation6,Citation7].
By screening the PubMed database, we reviewed the most recent clinical studies evaluating efficacy and safety of LABA/LAMA/ICS fixed dose combinations in asthma patients not adequately controlled.
1.2. Role of the triple therapy in the management of asthma
Although major improvements have been made in the treatment of patients with uncontrolled asthma, with an overall reduction in admissions to hospital – as documented by the GINA report – and asthma-related deaths, a considerable proportion of patients is still subjected to symptoms of uncontrolled asthma and experience exacerbations [Citation8]. Failure in proper control of asthma means a higher economic burden and, above all, a loss of quality-adjusted life-years for a number of patients [Citation9]. Up to 40% of the patients remain uncontrolled despite therapy with ICS alone or combined with a LABA [Citation10]. Therefore, the need to achieve disease control has led to consider anticholinergic drugs as an option for asthma treatment.
Tiotropium soft mist inhaler was the first LAMA approved for the maintenance of asthma, and it can be used as add-on therapy in patients requiring medium-to-high dose LABA/ICS (GINA step 4–5) from 6 years of age [Citation1]. Tiotropium Respimat modestly improves lung function and partially reduces the overall risk of severe exacerbations, and in clinical trials, it prolonged the time to first severe exacerbation (282 days vs. 226 days) when compared to placebo [Citation11,Citation12]. The benefit of tiotropium as an add-on therapy to a moderate-to-high dose of ICS and to LABA/ICS maintenance treatment was showed by two interesting large studies that assessed its efficacy in terms of lung function, risk of developing exacerbation and disease worsening, and its safety [Citation13,Citation14]. Recently, five large clinical trials on the use of fixed combination LABA/LAMA/ICS in asthma were published and added substantial knowledge on the use of the once daily therapy in asthma. The TRIMARAN study and TRIGGER trial showed that patients who received a twice daily combination of formoterol/glycopyrronium/beclomethasone had improvements in lung function (forced expiratory volume in 1 second [FEV1]) and, to some extent, a decrease in the number of exacerbations vs beclomethasone/formoterol combination [Citation15]. The authors found a statistically significant reduction of 15% in moderate and severe exacerbations in TRIMARAN, and a 12% decrease in TRIGGER study, which, however, was not significant. The CAPTAIN study concluded that in patients with uncontrolled moderate or severe asthma on fluticasone furoate plus vilanterol, the addition of umeclidinium improved the lung function without a significant reduction in moderate and/or severe exacerbations [Citation16]. Two additional and recently published trials, the IRIDIUM and the ARGON studies, have demonstrated significant efficacy of the IND/GLY/MF combination in improving the lung function and other clinically relevant outcomes such as exacerbation reduction [Citation17,Citation18]. The IRIDIUM and ARGON studies will be described in detail below.
The simultaneous use of the three classes of medications in a wide patient population with uncontrolled asthma represents a step forward in the treatment of severe asthma. That LABA/LAMA/ICS combinations can assure a better management of the disease for the patients when compared to other treatments, avoiding the side-effect profile of oral corticosteroids and resulting cost-effective with comparison to biologicals. Moreover, the possibility of simplifying the inhaler regimen, through the use of a fixed dose combination of the three components, could have advantages in terms of patients’ adherence and long-term compliance to treatment and reduced risks of missing doses.
2. Role of glucocorticoids in asthma
Asthma is treated with drugs blocking the underlying inflammatory mechanisms, the ones that cause the onset of clinical signs and symptoms, namely, corticosteroids [Citation19]. ICS are the cornerstone treatment for asthma, considered a precision medicine approach as they generally act locally and have a low systemic bioavailability [Citation20].
ICS can inhibit inflammation pathways by regulating gene transcription (either inactivating transcription factors with pro-inflammatory activity, like NF-kB, or by activating anti-inflammatory molecules such as IL-10 and adhesion molecules) or by a fast response that is not related to gene expression, i.e. acting directly on the cell membrane influencing blood flow and the function of cell membrane proteins [Citation21].
2.1. Mometasone furoate (MF)
Various ICS are available for asthma treatment with different chemical, pharmacological, and metabolic characteristics [Citation22]. Among them, mometasone furoate (MF), a derivative of hydrocortisone, is a topical glucocorticoid with an advantageous risk-benefit profile. It has a low oral bioavailability (around 1%) and is delivered as a dry powder, it has a long half-life and is suitable for daily dosing [Citation23]. A daily dose of 100 μg of inhaled MF is considered as a low/medium ICS dose showing strong efficacy and tolerability [Citation24]. MF is also widely used for treating several skin conditions such as eczema, psoriasis, and rashes with no significant effects on the hypothalamic–pituitary–adrenal axis, having low percutaneous absorption compared to other topical corticosteroids [Citation25].
The combination of the two inhaled molecules, a corticosteroid and a LABA, is more effective than the same dosage of MF alone in asthma symptoms control and improving the asthma related quality of life [Citation25]. The same superiority on the lung function and other outcomes was shown in the PALLADIUM study comparing high-dose indacaterol and mometasone furoate (IND/MF 150 μg/320 μg) or medium-dose IND/MF (150 μg/160 μg) administered once daily via Breezhaler, with high-dose MF (800 μg [400 μg twice daily]) or medium-dose MF (400 μg once daily) given via Twisthaler, or high-dose salmeterol xinafoate/fluticasone propionate (SAL/FLU). One possible limitation of this study is the administration of MF with two different delivery devices, an extra variable which can interfere with the comparison of the results. These results, described in detail below, correlate with the guidelines for asthma management that support the administration of LABA/ICS as maintenance therapy in moderate and severe asthma [Citation24].
3. Role of β2-agonists in asthma
3.1. Indacaterol (IND)
LABA are indicated in association with ICS as the preferred maintenance treatment in asthma, leading to reduction in exacerbation risk and higher lung function [Citation1]. Current recommendations also state that LABA are safe when used in combination with ICS, whereas they should not be used without ICS due to the increased risk of serious adverse outcomes.
A decade ago, the term ultra-LABA was coined to describe once-daily β2-agonists and differentiate them from the known twice-daily agonists, salmeterol and formoterol. In this regard, once-daily IND was shown to provide sustained 24-h bronchodilation, a fast activity and good profiles for tolerability and safety [Citation26]. This was the first study that demonstrated the ability of a bronchodilator to induce both a fast and a prolonged duration of action. From a patient perspective, this is what really matters, since asthmatics ask for a prompt and consistent relief of symptoms.
Studies involving IND both in vitro and in vivo have confirmed the quick onset of action and 24 h bronchodilating effect. In particular, the quick onset of action was comparable to the one of salbutamol and formoterol, and superior to salmeterol, whereas the duration of action of IND was higher than the one of salmeterol and formoterol [Citation27]. The safety and tolerability of IND was assessed in asthma patients [Citation28]. Over a period of 28 days, different dosages of the ultra-LABA were administered once daily and compared with placebo. IND, reported to be approved and marketed in more than 100 countries worldwide, was well tolerated, and no adverse cardiac or metabolic effects were observed. Moreover, the drug was shown to provide effective 24-h bronchodilation. In combination with MF, IND has shown improvements in lung function, symptom control and use of rescue medication, and reduction in the annual rate of exacerbations in asthmatics [Citation29,Citation30].
Interestingly, IND can be administered either in a maleate or in an acetate form in asthma patients, and both of the formulations resulted in effective improvement in lung function, with a safe profile, as demonstrated by a phase II study [Citation31]. The IND acetate can be used in combination with GLY and MF in a single inhaler, increasing the acceptability of a combined once daily therapy and the potential of the patient’s adherence to the treatment [Citation31].
The PLATINUM program is the phase III clinical plan which supports the development of IND/MF and IND/GLY/MF. These investigations open the way to the use of an ultra-LABA in the treatment of asthma.
4. Role of the muscarinic receptor antagonists in asthma
Anticholinergic bronchodilators antagonize the parasympathetic system by acting on the acetylcholine receptors expressed on airway smooth muscles and lung parasympathetic nerves. There are two groups of acetylcholine receptors: nicotinic- and muscarinic- and the muscarinic subtypes M1, M2 and M3 are primarily involved in the regulation of bronchoconstriction. All muscarinic receptor subtypes are widely expressed in different tissues (smooth muscles, brain, heart and the sinoatrial node, gastrointestinal tract, pupils, blood vessels and the parasympathetic nervous system). Muscarinic M2 receptors in the heart regulate heart beating by reducing the activation of the sinus node, while the M3 subtypes are responsible of contraction of the muscles of gastrointestinal tract, or blood vessel vasodilation [Citation32,Citation33]. Specifically referring to airway tract activity, M1 receptors are widely distributed in all parasympathetic ganglia and they act by regulating cholinergic transmission. M2 receptors are found in the pre-junctional membranes of the neuromuscular junctions of airway smooth muscles and reduce acetylcholine transmission through a negative feedback. M3 receptors are mainly expressed in smooth muscle cells in the lungs, regulating muscle contraction, while within the submucosal glands of the lung, M3 receptors regulate mucus secretion. Thus, it is preferable that antimuscarinic bronchodilators present higher affinity for M1 and M3 receptors, and lower affinity for M2 receptors [Citation34].
Although the mechanisms underlying bronchial obstruction and hyper-reactivity found in asthma are multiple and complicated, the contraction of bronchial smooth muscle is the main cause of reversible airway obstruction in asthma. Therefore, cholinergic activity plays a decisive role in bronchoconstriction [Citation34]. Additionally, patients with asthma have an increased bronchial smooth muscle tone suggesting that this is a result of an increased basal activity of the pulmonary parasympathetic cholinergic nerves, a phenomenon described as “cholinergic tone’ [Citation35]. Moreover, the non-neuronal cholinergic system of airway inflammatory cells represents a regulatory pathway which deserves further attention: indeed, potential immunomodulatory activities may influence the inflammation of obstructive respiratory diseases [Citation36].
4.1. Glycopyrronium/ glycopyrrolate (GLY)
Among the available anticholinergics, glycopyrronium also known as glycopyrrolate (GLY) is a LAMA with a higher selectivity for M3 receptors, thus promoting long-lasting and selective effects. Glycopyrronium bromide acts by blocking acetylcholine-induced bronchoconstriction on airway smooth muscle cells, thus dilating the airways. Through radioligand binding studies, it was demonstrated a four times greater selectivity of GLY for human M3 receptors compared to human M2 receptors, as well as a rapid onset of action and a longer duration, probably attributable to prolonged concentrations of active substance in the lungs [Citation37]. It has also been shown that differences in the affinity rate M3/M2 are present among different LAMAs [Citation38].
Many studies have investigated the action of LAMAs on the degree of bronchoconstriction of the airways. For example, a Japanese study showed how SABAs with LAMAs synergistically improve the inhibition of muscarinic contraction by diminishing the sensitization to Ca2+ [Citation39]. This results in less bronchoconstriction and better control of the respiratory pathology. In addition, other studies evaluating aclidinium, formoterol fumarate, GLY, and indacaterol fumarate have shown greater benefits in relaxing airway smooth muscle in isolated human bronchi.
In patients with mild to moderate asthma, GLY resulted more effective than placebo against methacholine-induced bronchoconstriction (p < 0.002) [Citation40]. In the same study, each inhalation of GLY provided bronchodilation lasting for up to 30 hours. In a recent Indian study [Citation41], the efficacy of GLY was evaluated in 53 patients with uncontrolled asthma and treated with LABA/ICS. This was an 8-week observational study in which 40 patients were treated with formoterol/budesonide plus GLY and 13 patients received only a LABA/ICS (formoterol/budesonide) combination. All patients included in the study were symptomatic and were assessed for FEV1. At eight weeks, the group of patients treated with formoterol/budesonide plus GLY showed a significant improvement in FEV1 compared to both baseline and the group of patients treated with the same LABA/ICS combination. Both groups showed improvements in terms of FEV1 and asthma control test (ACT) compared to their own baselines at the start of the study.
Two additional studies have compared the effects of GLY versus (i) the muscarinic receptor antagonist tiotropium and (ii) IND on the dose-response curve to methacholine in patients with mild asthma [Citation42,Citation43]. In the first study, both GLY and tiotropium were administered for a week as bronchoprotective medium before the methacholine test. This study showed that tiotropium is able to guarantee a statistically significant broncho-protection against methacholine only after 24 and 72 hours, while GLY was shown to be more effective, exerting broncho-protection already 1 hour post-treatment. In the second study, GLY alone and the IND/GLY combination were able to protect against methacholine-induced bronchoconstriction already after 1 hour from the administration, and they also kept mediating broncho-protection at 24 and 48 hours [Citation43].
In a phase II dose ranging study, GLY was administered to adult patients with either an intermittent or mild-to-moderate persistent asthma at the doses of 1.9 μg, 3.6 μg, 7.2 μg, 14.4 μg, and 28.8 μg. Results highlighted how patients experienced a significant improvement in FEV1 from baseline without an appreciable difference between the two higher doses (14.4 μg and 28.8 μg) versus the reference drug salbutamol 50 μg [Citation44].
Finally, a phase II/III clinical study evaluated the safety and efficacy of two doses (25 μg and 50 μg) of GLY as an add-on bronchodilator in asthmatic adult patients already treated with LABA/ICS. The patients were evaluated for an overall treatment time of 7 days and showed an improvement in lung function with both doses of GLY. The assessment of FEV1 after one week of treatment was the primary endpoint, and the change was in the order of 89 ml significantly different from placebo. Furthermore, after one week of treatment, the change in FEV1-peak at 4 hours post GLY administration was significantly different from placebo (173 mL with 25 μg GLY and 164 mL with 50 μg GLY, respectively) [Citation45].
In conclusion, the data emerging from the literature suggest that not only GLY is an effective molecule, but it is also safe in the control of respiratory pathology, establishing an important bronchodilator effect in addition to LABA/ICS therapy in asthma.
5. Anti-inflammatory and bronchodilating effects of the once daily inhaled therapy
5.1. Combined actions of indacaterol (IND), glycopyrronium (GLY), and mometasone furoate (MF)
In asthmatic patients not adequately controlled by LABA/ICS coformulations, the addition of a LAMA can further decrease asthma exacerbation rate and improve lung function [Citation11]. Within such a therapeutic context, recent phase III trials have provided very promising results about the clinical and functional effects of the fixed-dose LABA/LAMA/ICS association including IND/GLY/MF [Citation17,Citation18]. The pharmacological rationale underlying the combination of these three drugs is closely linked to the possibility of reciprocally potentiating their different mechanisms of action.
In particular, MF is characterized by a relatively strong in vitro potency, a powerful anti-inflammatory activity, and a high binding affinity to human glucocorticoid receptor (GR) [Citation46,Citation47]. Similar to all glucocorticoids (GC), MF is a liposoluble molecule that easily crosses the plasma membrane of cellular targets and interacts within the cytoplasm with GR, whose expression as GRα isoform largely prevails over the GRβ dysfunctional variant [Citation48,Citation49]. Following MF interaction with GR, the active ligand-receptor complex rapidly translocates into the nucleus, binding to genomic DNA at the level of regulatory nucleotide sequences named glucocorticoid response elements (GRE) (). As a consequence, GC-inducible genes are stimulated to enhance their transcriptional activity [Citation48], thus leading to an increased production of proteins with anti-inflammatory activity such as mitogen-activated protein kinase phosphatase-1 (MKP-1) and glucocorticoid-induced leucine zipper (GILZ) (). MKP-1 dephosphorylates and inactivates the pro-inflammatory mitogen-activated protein kinase (MAPK) family member p38 [Citation50,Citation51], whereas GILZ inhibits the pro-inflammatory functions of both activator protein-1 (AP-1) and nuclear factor-kB (NF-kB) [Citation52].
Figure 1. Molecular mechanisms underlying the therapeutic effects of mometasone furoate in asthma. Mometasone furoate is a liposoluble molecule which crosses the cell membrane and binds to the cytoplasmic glucocorticoid receptor (GR). Following mometasone binding, the activated GR migrates to the nucleus, where interacts with specific regulatory DNA sequences known as glucocorticoid response elements (GRE). GRE are located inside target genes encoding anti-inflammatory proteins such as GILZ (glucocorticoid-induced leucine zipper) and MKP-1 (mitogen-activated protein kinase phosphatase-1). The latter dephosphorylates and inactivates the pro-inflammatory p38 MAPK enzyme. Furthermore, activated GR can also bind via protein–protein interactions to the transcription factor nuclear factor-kB (NF-kB), thereby inactivating its proinflammatory functions by preventing DNA-binding.
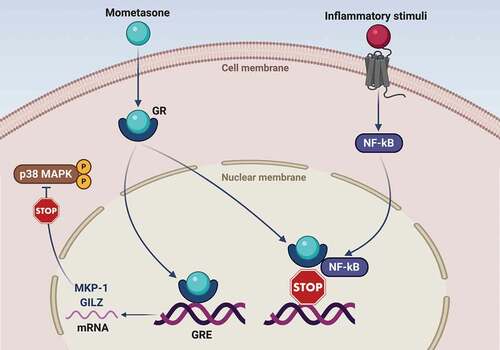
GRE are also located inside the gene encoding for the β2-adrenergic receptor (β2-AR) [Citation53,Citation54]. Indeed, by binding to these GRE, GR activated by ICS can stimulate β2-AR gene transcription and protein expression [Citation55]. ICS may also increase β2-AR number through inhibition of β2-AR mRNA degradation [Citation55]. Taken together, such GR-dependent mechanisms preserve a high β2-AR cellular density, thereby preventing the risk of β2-AR down-regulation and desensitization, possibly occurring as a consequence of patient chronic exposure to LABA [Citation56]. In addition to enhancing β2-AR synthesis, corticosteroids also promote β2-AR coupling to the post-receptor signaling machinery (see below) [Citation57,Citation58].
Besides targeting genomic DNA, activated GR can also directly bind to NF-kB and AP-1 via protein–protein interactions (), thus effectively neutralizing the powerful biological activities of such pro-inflammatory transcription factors. Hence, within the airways of asthmatic patients, this further mechanism contributes to GR-mediated inhibition of NF-kB/AP-1-dependent expression of cytokines and chemokines implicated in asthma pathobiology [Citation59].
Like all β2-AR agonists, IND induces bronchodilation by relaxing airway smooth muscle independently of the wide variety of agents eliciting bronchoconstriction, thereby acting as a functional antagonist of airway smooth muscle contraction. Hence, IND activates β2-AR coupled to the stimulatory G protein (Gs), which in turn stimulates adenylyl cyclase (AC) that is responsible for the subsequent increase in cytosolic levels of 3ʹ,5ʹ-cyclic adenosine monophosphate (cAMP), the second messenger ()
Figure 2. Molecular interactions involving the mechanisms of action of indacaterol, glycopyrronium and mometasone furoate. Indacaterol binds to the β2-adrenergic receptor (β2-AR), thus sequentially activating the stimulatory Gs protein and adenylyl cyclase (AC), which is responsible for cAMP synthesis and the subsequent activation of cAMP-dependent protein kinase A (PKA). This signaling pathway not only leads to bronchodilatation, but also promotes GR nuclear translocation, thus potentiating the pharmacologic effects of corticosteroids. The latter in turn increase β2-AR mRNA levels via GRE-dependent stimulation of β2-AR gene transcription. IND-induced bronchodilatation is synergistically potentiated by GLY through a competitive antagonism of M2 and M3 muscarinic receptors. With respect to the blockade of the M2 receptor coupled to AC inhibition operated by the inhibitory G protein (Gi), GLY occupies for a much longer time the M3 receptor, whose stimulation is the main mechanism underlying acetylcholine-induced bronchoconstriction, mediated by M3-coupling to Gq protein and phospholipase C (PLC). The latter enzyme catalyzes the generation of the intracellular second messenger inositol trisphosphate (IP3) that elicits airway smooth muscle contraction via mobilization of cytosolic calcium ions.
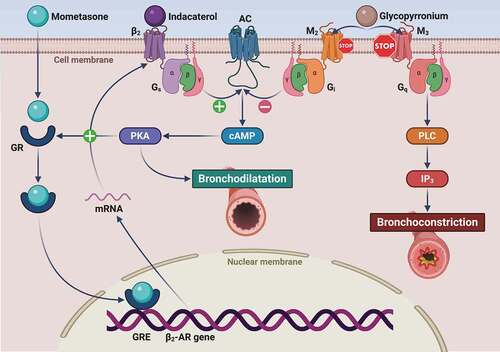
In addition to allowing airway smooth muscle relaxation, LABA-induced stimulation of β2-AR also promotes GR nuclear translocation via activation of the post-receptor signal transduction machinery, thus potentiating the anti-inflammatory effects of corticosteroids [Citation63]. The latter in turn facilitate β2-AR coupling with Gs through reduction of G-protein-coupled receptor kinase-2 (GRK2) expression [Citation58], which is responsible for β2-AR phosphorylation and the consequent receptor-Gs uncoupling [Citation56].
Similar to all anticholinergic bronchodilators, GLY acts through a competitive antagonism of airway muscarinic receptors [Citation61]. In particular, GLY exerts a prompt and persistent bronchodilating action via a long-lasting blockade of the M3 subtype of muscarinic receptors, which is mainly accountable for acetylcholine-dependent airway smooth muscle (ASM) contraction () [Citation38]. Hence, M3 competitive antagonism operated by GLY and IND-mediated functional antagonism of ASM contraction effectively cooperate to implement a reciprocal potentiation of these two different mechanisms, thereby resulting in maximization and optimization of bronchodilation [Citation61]. Moreover, IND can potentiate GLY action by inactivating the Gq protein associated with muscarinic M3 receptors within the signaling pathway leading to acetylcholine-induced bronchoconstriction. Indeed, LABA increase the expression of the regulator of G-protein signaling 2 (RGS2) that specifically inhibits Gq activation [Citation64]. This effect of β2-AR agonists is further enhanced by corticosteroids [Citation64]. Thus, through these complex mechanisms, IND and MF could amplify, at a post-receptor downstream level, GLY-induced bronchodilation resulting from upstream M3 receptor blockade. As a consequence of the latter mechanism, GLY may in turn contribute to protect asthmatic airways from β2-AR desensitization. In fact, GLY-dependent interruption of M3-Gq signaling cascade inhibits Gq coupling with phospholipase C, responsible for the generation of the intracellular second messenger diacylglycerol [Citation65]. Diacylglycerol activates protein kinase C, which not only sensitizes ASM contractile apparatus to calcium ions, but also phosphorylates both β2-AR and Gs, thus preventing their interaction and contributing to β2-AR desensitization [Citation66]. Overall, the above mechanisms explain the synergistic cooperation of GLY and IND with regard to their relaxant effects, exerted on ASM and demonstrated in isolated human bronchi, pre-contracted by acetylcholine [Citation67].
Therefore, by combining LAMA/LABA and the reciprocal potentiation of LABA/ICS mechanisms of action, IND/GLY/MF once daily therapy may provide a valuable option for inhaled treatment of asthmatic patients not adequately controlled by LABA/ICS associations.
6. PLATINUM clinical development program: clinical efficacy and safety of the once daily therapy with IND/GLY/MF
The PLATINUM clinical program includes phase III clinical studies evaluating safety and efficacy of the inhaled fixed dose combinations IND/GLY/MF and IND/MF in patients with inadequately controlled asthma [Citation68]. The MF doses administered in the studies have been determined by bridging the doses previously approved to the new formulation taking into account pharmacokinetic and pharmacodynamic evaluation. Upon this bridging, MF 80 μg and 160 once daily (medium and high-dose) in IND/GLY/MF combination resulted similar to MF 160 μg and 320 μg once daily (medium and high dose) in the IND/MF formulation, respectively [Citation68]. The main features of the clinical development program are summarized in .
Table 1. Summary of the phase III studies within PLATINUM program
The clinical program, which involved over 7500 patients, consisted of four clinical trials: two studies (QUARTZ and PALLADIUM) evaluating the combination IND/MF and two studies (IRIDIUM and ARGON) for the IND/GLY/MF combination. All aforementioned studies have been completed and published.
Overall, the administration of multiple inhaled doses of IND, GLY and MF, alone or combined, resulted to be safe and tolerated by the patients.
In addition, no relevant pharmacokinetic interaction between IND, GLY, and MF was reported when compounds were given in combination ‒IND/GLY/MF‒ [Citation69].
6.1. QUARTZ and PALLADIUM studies
The QUARTZ study is a 12-weeks multicenter, randomized, double-blind, double-dummy and parallel-group phase III study aiming at assessing the efficacy and safety of once daily IND/MF low-dose (150/80 μg) administered via Breezhaler versus a fixed once daily MF dose (200 μg) administered via Twisthaler in adult and adolescent (age range: 12–75 years) patients with asthma not adequately controlled [Citation70].
The primary outcome of the study was to show superiority of low-dose IND/MF to MF in terms of trough FEV1 after 12 weeks of treatment.
At 12 weeks, low-dose IND/MF significantly improved trough FEV1 and the Asthma Control Questionnaire (ACQ-7) versus MF, as well as all other secondary endpoints, thus supporting the use of the once daily low-dose of IND/MF for adult and adolescent patients with uncontrolled asthma.
The PALLADIUM study is a 52-week, double-blind, triple-dummy, parallel-group, phase III study, having the aim of verifying the efficacy and safety of high-dose IND/MF (150/320 μg) or IND/MF medium-dose (150/160 μg), administered once daily via Breezhaler versus MF monotherapy high-dose (800 μg total, in a twice daily administration of 400 μg) or MF medium-dose (400 μg) single daily dose via Twisthaler, in patients (≥12 ‒ < 75 years of age) with inadequately controlled asthma [Citation71].
The primary endpoint was improvement in trough FEV1 from baseline at 26 weeks, while the secondary outcome was the assessment of patients’ quality of life by ACQ-7.
Both medium- and high-dose IND/MF were superior in improving trough FEV1 over corresponding MF doses at 26 weeks. Moreover, the once-daily administration of high-dose IND/MF was able to elicit similar improvements to twice-daily high-dose SAL/FLU in trough FEV1 at 26 weeks, and significant improvements at week 52. The same improvements were observed on peak expiratory flow (PEF) and percentage of rescue medication free days.
The PALLADIUM study demonstrates that once-daily fixed-dose of IND/MF improves lung function over MF monotherapy over the course of 26 weeks and that the IND/MF combination is non-inferior to the twice-daily combination of SAL/FLU, showing also significant benefits in patients’ quality of life, thus providing a novel option in the management of asthma.
6.2. IRIDIUM and ARGON studies
The IRIDIUM study is a 52-week, multicenter, randomized, double-blind study in which the enrolled patients were diagnosed for asthma for at least 1 year before screening [Citation18]. In spite of a therapy with medium- or high-dose LABA/ICS, the individuals presented both symptomatic asthma and at least one exacerbation episode occurred in the previous year. Patients were randomized (1:1:1:1:1) to receive IND/GLY/MF medium-dose (either 15, 50, or 80 μg; n = 620 patients), IND/GLY/MF high-dose (150, 50,160 μg; n = 619), IND/MF medium-dose (150;160 μg; n = 617), high-dose (150;320 μg; n = 618) once daily via Breezhaler, or high-dose SAL/FLU (50;500 μg; n = 618) twice daily via Diskus.
The primary outcome was the change from baseline in trough FEV1 after 26 weeks of the once-daily treatment with either dose of IND/GLY/MF, compared to the respective dose of IND/MF. Trough FEV1 is the parameter of choice as recommended by the EMA guidelines, while the 26-week time point was chosen based on the pharmacokinetics and pharmacodynamics of the drugs, which reach a steady state by 4 weeks.
At week 26, trough FEV1 resulted in improvement with both medium-dose IND/GLY/MF and high-dose IND/GLY/MF, which were superior versus corresponding doses of IND/MF (medium-dose IND/GLY/MF: treatment difference [Δ] 76 mL [95% CI 41–111]; p < 0.001; high-dose IND/GLY/MF: Δ 65 mL [31–99]; p < 0.001).
The IRIDIUM study has been the first clinical study employing IND/GLY/MF to evaluate the once daily dosing of the IND/GLY/MF combination and to demonstrate that the addition of the GLY ‒a LAMA‒ to a medium- or high-dose IND/MF combination significantly ameliorates trough FEV1 over 26 weeks.
Moreover, from as early as 2 weeks and throughout the entire 52 weeks of treatment, both medium and high IND/GLY/MF doses proved to be superior in improving lung function in patients with inadequately controlled asthma as compared to high-dose SAL/FLU (medium dose IND/GLY/MF: treatment difference Δ 99 mL [64–133]; p < 0.001; high dose IND/GLY/MF: treatment difference Δ 119 mL [72–154]; p < 0.001), a benchmark LABA/ICS combination. Despite an overall non-significant reduction of exacerbation rate of IND/GLY/MF vs MF/IND, the IND/GLY/MF once daily therapy was correlated to a 36% reduction in moderate-to-severe exacerbations and a 42% reduction in severe exacerbations compared to high-dose SAL/FLU. Importantly, because IND/GLY/MF therapy is combined in a single inhaler, its once-daily dosing offers a strategy that can potentially enhance adherence to treatment, thus improving disease control.
The number of adverse events resulted similar among the groups.
The ARGON study is a 24-week, multicenter, randomized, parallel-group, open-label, active-controlled, non-inferiority phase IIIb study in patients with uncontrolled asthma [Citation17]. Criteria for patient inclusion were presence of asthma as diagnosed as early as six months prior to screening, being symptomatic despite treatment with medium- or high-doses of LABA/ICS and having a history of one or more asthma exacerbations. Patients were randomized (1:1:1) to IND/GLY/MF medium-dose (n = 474 patients) or IND/GLY/MF high-dose (n = 476) or concurrent administration of twice-daily SAL/FLU high-dose (50/500 μg) + tiotropium (5 μg, once-daily; n = 475) for 24 weeks.
The primary outcome was to evaluate the non-inferiority of both medium- and high-dose IND/GLY/MF administered via Breezhaler, compared to SAL/FLU twice daily via Diskus + tiotropium via Respimat after 24 weeks of treatment, by the Asthma Quality of Life Questionnaire (AQLQ).
At 24 weeks, the non-inferiority of either IND/GLY/MF medium- or high-dose to SAL/FLU high-dose + tiotropium was demonstrated by AQLQ score (least square mean treatment difference [Δ]: −0.038 and 0.073 form medium- and high-dose, respectively; both p < 0.001). With the high-dose, IND/GLY/MF ameliorated ACQ-7 (Δ: −0.124; p = 0.004), trough FEV1 (Δ: 96 mL; p < 0.001), PEF ([Δ: 9.56 L/min; p = 0.005] for the morning, [Δ: 9.15 L/min; p = 0.006] for the evening) and St. George’s Respiratory Questionnaire SGRQ (Δ: −2.00; p = 0.04) compared to SAL/FLU high dose + tiotropium. Specifically, improvements in trough FEV1 were detectable as early as 8 weeks from the beginning of the treatment and were maintained throughout the entire duration of the study. Comparable results were found for medium-dose of IND/GLY/MF and high dose of SAL/FLU + tiotropium, with the benefit of employing a lower steroid dose.
Of note, the TRIGGER phase III trial reported no difference in pre-dose FEV1 between formoterol/beclomethasone/GLY (FF/BDP/GLY, high-dose, twice daily) versus FF/BDP (high-dose, twice daily) +tiotropium (−45 mL; p = 0.13; at week 26).
In the ARGON study, exacerbation rates (mild, moderate and severe) were comparable between both IND/GLY/MF doses and twice daily SAL/FLU high-dose + tiotropium once daily.
Recently, two metanalyses have been published evaluating single inhaler triple therapy and its comparison to dual inhaler therapy [Citation72,Citation73]. It appears clear from current evidence that some patients may benefit differently from once daily triple therapy or biologic therapy, according to their phenotype, though the specific patient characteristics on which basing the choice is yet unknown [Citation72] Similarly, the choice of high ICS treatment is still reasonable and further studies are needed to understand whether high-dose single inhaler triple therapy might be an alternative choice to avoid maintenance therapy with systemic corticosteroids [Citation72].
7. Breezhaler and propeller sensor
IND/GLY/MF is delivered through the Breezhaler inhaler, a single dose DPI that allows confirmation of the inhaled dose via three feedback mechanisms (auditory, taste, and visual). The Breezhaler inhaler is approved for the use of long-term maintenance treatments for COPD and asthma. Unlike during randomized clinical trials, where patients are thoroughly instructed on the correct use of inhalers, in clinical practice errors made during inhalation are frequent, and some studies suggest a link between these errors and symptom control. The study by Molimard and colleagues in COPD patients found a correlation between critical errors and severe COPD exacerbations, highlighting the fact that device-handling errors could have an impact on clinical outcomes [Citation74]. In the same study, the use of the Breezhaler inhaler was related to a lower percentage of both device-dependent errors and critical errors compared to other inhalers evaluated [Citation74]. A subsequent analysis, The Real-life Experience and Accuracy of inhaLer use (REAL) survey, assessed the patients’ level of training in the correct use of the inhaler, factors affecting adherence to treatment and ease of use of the commercially available inhalers (Breezhaler, Ellipta, Genuair or Respimat) [Citation34]. The REAL survey showed a greater adherence to therapy and a greater likelihood of administering the correct dose of a given drug through Breezhaler compared to other inhalers, also avoiding the risk of under or overdosing.
The above-mentioned studies concern the use of Breezhaler in patients with COPD, therefore, further studies addressing the correct use of Breezhaler in asthma are needed to expand our knowledge of real clinical practice.
7.1. Propeller sensor for Breezhaler and Propeller app
Adherence to therapy in respiratory diseases is very low, and it is a recurrent problem in asthma therapy [Citation75]. It has been estimated that between 30–70% of asthma patients do not adhere to their treatment [Citation76,Citation77], and that as much as 50% of patients are unable to correctly use their inhaler, despite having received training [Citation78].
Poor adherence, especially in severe asthma cases can contribute to substantial worsening of the disease. Asthma patients are often asked to comply with treatments that require medications to be taken at different dosages, under different times, with different kinds of inhalers, all factors that could contribute to poor adherence. Adherence to any given therapy has been shown to improve when the dosing frequency is reduced (e.g. once daily) and when reinforcing visits are increased [Citation79]. In this sense, the management of uncontrolled asthma through the use of the single-inhaler fixed dose combination of IND/GLY/MF could prove beneficial in enhancing patients’ adherence, as it reduces the administration frequency to once daily. Moreover, the IND/GLY/MF therapy is the first inhaled therapy that can be prescribed alongside a digital companion (Propeller Health, USA). Propeller is a digital health platform for patients with asthma and COPD. The patient easily attaches the sensor to the Breezhaler device, and the sensor communicates with the smartphone app via Bluetooth. Through the use of the app the patient can obtain inhalation confirmation, reminders at appropriate time (when medication is needed), and access to objective data (e.g. reports of medication use and trends) to better support therapeutic decisions. This is optional and IND/GLY/MF therapy can be prescribed to patients also without the sensor, if not needed or useful.
Here below, we summarize some clinical evidence regarding the use of this innovative digital system in asthma patients.
Improved Medication Adherence: in a six-month randomized study including 125 adults with asthma, patients using a Propeller’s asthma platform had higher adherence than those in the control group [Citation80]. Propeller consistently provides higher levels of medication adherence [Citation81], which may lead to a reduced risk of asthma exacerbations [Citation82].
Reduced Need of Rescue Inhaler Use: The Propeller platform is also accompanied by reductions in rescue inhaler use as demonstrated by several studies [Citation83,Citation84]. A pragmatic controlled study aiming to measure real-world effectiveness of the Propeller platform demonstrated that the system significantly decreased SABA use, increased SABA-free days, and improved ACT scores [Citation84]. By improving medication adherence and reducing rescue inhaler use, the sensor and app associated with IND/GLY/MF Breezhaler may provide patients with enhanced asthma control, longer time without symptoms and a better quality of life.
Reduced Healthcare Utilization: The Propeller use allows better adherence and therefore better asthma control. This can be translated into reduced patients’ hospitalizations and emergency department (ED) visits, meaning decreased healthcare costs. During a study lasting 12 months, there was a general reduction in both ED visits and ED visits and hospitalizations (reduced by 53% and 57%, respectively) with a digital health intervention consisting in the administration of SABA with the Propeller [Citation85]. There is therefore good indication to speculate a plausible similar result with the use of the monitored IND/GLY/MF inhaler.
Patient Satisfaction: Participants of a randomized controlled study (a total of 89 subjects, adults and children with asthma) completed a satisfaction survey at the end of the trial. The results of the survey showed 79% of the patients being ‘very satisfied’ with the use of the device, and 20% being ‘somewhat satisfied’ [Citation86]. Moreover, 93% were satisfied with the reports generated, 90% reported that the information collected by the sensor was useful to knowing more about their asthma condition, and 72% expressed their interest in continuing to use the sensor and platform even after the end of the study [Citation86].
This digital solution represents an innovation in the asthma treatment and has the potential to improve treatment adherence and efficiency by introducing a simple and effective tool able to support the patients in the self-management of their own condition. Moreover, monitoring asthma therapy adherence is fundamental to healthcare professionals in order to share decisions with the patient and to tailor their treatments. Thus, the introduction of a supporting digital system would translate into better monitoring progress of the disease in asthma patients by healthcare professionals and in a general optimization of the resources.
As confirmation, recently EMA gave a significant award to IND/GLY/MF Breezhaler for the ‘Outstanding contributions to public health’ as the only product approved in 2020 that represent significant progress in the asthma therapeutic area as ‘the first asthma once daily combination therapy that includes an optional electronic sensor to collect data on the use of the inhaler by the patient’ [Citation87].
8. Conclusions
The management of asthma aims to efficiently control clinical symptoms, reduce individual risk factors and comorbidities, and assure patients an improved quality of life.
The introduction of IND/GLY/MF once daily therapy represents a step forward in the treatment of severe asthma. The evaluation of the results of phase III clinical trials supports both the clinical efficacy on lung function, exacerbation reduction, and the safety of the once daily therapy.
The IND/GLY/MF once daily therapy administered with Breezhaler and Propeller sensor is the first asthma treatment that is supported by a digital companion, allowing patients’ self-management of their condition, thus resulting in better adherence and improved monitoring of the disease, meeting the satisfaction of both the patients and the healthcare professionals.
9. Expert opinion
The introduction in clinical practice of a single fixed dose inhaled combination including the three principles LABA/LAMA/ICS represents a valuable advance in the management of asthma in a wide percentage of moderate-to-severe patients. In this regard, the potential benefits of the IND/GLY/MF inhaled formulation include both improvement of lung function and reduction of the asthma exacerbation rate. Therefore, it is likely that these therapeutic effects can be achieved by patients with not adequately controlled asthma. Indeed, the different mechanisms of action of GLY and IND guarantee a maximal bronchodilation resulting from the competitive antagonism of muscarinic receptors, induced by GLY, optimally combined with the bronchodilation implemented by IND. In addition, IND and MF reciprocally integrate their respective pharmacological actions. Indeed, IND improves the anti-inflammatory effects of MF, which in turn potentiates the bronchodilation induced by IND.
The practical advantages arising from the adoption of a single daily fixed dose of IND/GLY/MF, demonstrated by recent trials included within the PLATINUM study program, are as follows:
An effective therapy that can simultaneously target lung functions and exacerbation rate will help to better treat the patients, reducing hospitalizations, and emergency treatments.
Providing a better control of asthma would also allow a decreased health economic burden to the health care system and society, relieving the pressure on hospitals particularly crucial in the current health emergency situation.
The IND/GLY/MF combination can play a competitive role vs alternative step-up option, considering both the side-effect profile challenge of systemic oral corticosteroids and the cost/availability/management challenges that come with the use of biological treatments.
An effective and safe treatment administered once daily for asthma management could be a valuable option for a cutting-edge management of moderate to severe asthma, allowing to increase adherence to recommended doses of prescribed treatments.
Improvement of symptoms’ control means the increased quality of life for a large percentage of patients (up to 40% that remain uncontrolled despite usual therapy with ICS alone or combined with a LABA).
The administration of a unique dose by means of a handy and dose confirming inhaler can help to increase patient independence and ability to manage his/her own treatment.
Increased patient engagement and self-management would allow an increment in adherence to therapy while minimizing the risk of missing doses, particularly when the use of IND/GLY/MF will be supported by the optional digital sensor
On the basis of these considerations, it can be inferred that once daily fixed drug combination could represent an improvement in key areas of asthmatic patient treatment. One additional positive aspect is the presence of a dedicated app that can increase patients’ engagement to asthma management and assist in the control of the dosage administration. This technological support can provide a valuable option in modern medicine.
Therefore, inhaled triple LABA/LAMA/ICS combinations and particularly IND/GLY/MF provide a real possibility, for moderate-to-severe asthmatic patients, to achieve the main goals of asthma treatment. Among the positive outcomes of this triple therapeutic approach, prevention of asthma exacerbations, as well as better improvements in lung function and overall quality of life, can be considered the most important results of LABA/LAMA/ICS treatment.
Article highlights
Despite the available treatments, there is still a proportion of patients who suffer from uncontrolled asthma.
The introduction of the therapies combining three agents (a corticosteroid, a LABA, and a LAMA) represents a step forward in the treatment of moderate-to-severe asthma
The therapy combining IND/GLY/MF in a single daily fixed dose is clinically effective and safe, allowing the improvement of lung function and reduction of asthma exacerbation rate.
The positive effect is guaranteed by the different mechanisms of action of the principles on bronchodilation and on inflammation.
The IND/GLY/MF therapy is the first treatment that can be administered with a digital companion.
The IND/GLY/MF once daily fixed dose increases adherence, minimizing the risk of missing doses, meeting both patients’ and clinicians’ satisfaction.
Declaration of interests
P. Morini, A. Rizzi, and O. Bonavita declare to be Novartis employees. S. Andaloro is a former Novartis employee, employed during the drafting of the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer of this manuscript has disclosed that they have worked as a consultant, contract investigator and lecturer for Novartis. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
Medical writing support was provided by Irene Sebastianutto and Barbara Bartolini on behalf of Health Publishing & Services Srl, which was funded by Novartis Farma SpA.
Additional information
Funding
References
- Global Initiative for Asthma GINA. 2020 [cited 2021 Oct 10]. Available from: https://ginasthma.org/
- Agache I, Akdis C, Jutel M, et al. Untangling asthma phenotypes and endotypes. Allergy. 2012 Jul;67(7):835–846.
- Chung KF, Adcock IM. Precision medicine for the discovery of treatable mechanisms in severe asthma. Allergy. 2019 Sep;74(9):1649–1659.
- Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015 Apr;135(4):896–902.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014 Feb;43(2):343–373.
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014 Sep 25;371(13):1189–1197.
- Schumann C, Kropf C, Wibmer T, et al. Omalizumab in patients with severe asthma: the XCLUSIVE study. Clin Respir J. 2012 Oct;6(4):215–227.
- Stanford RH, Gilsenan AW, Ziemiecki R, et al. Predictors of uncontrolled asthma in adult and pediatric patients: analysis of the Asthma Control Characteristics and Prevalence Survey Studies (ACCESS). J Asthma. 2010 Apr;47(3):257–262.
- Yaghoubi M, Adibi A, Safari A, et al. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med. 2019 Nov 1;200(9):1102–1112.
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004 Oct 15;170(8):836–844.
- Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012 Sep 27;367(13):1198–1207.
- Kew KM, Dahri K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database Syst Rev. 2016 Jan;21(1):CD011721.
- Kerstjens HA, Casale TB, Bleecker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015 May;3(5):367–376.
- Paggiaro P, Halpin DM, Buhl R, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016 Jan-Feb;4(1):104–13 e2.
- Virchow JC, Kuna P, Paggiaro P, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet. 2019 Nov9;394(10210):1737–1749.
- Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2020 Sep 9; 9(1):69-84.
- Gessner C, Kornmann O, Maspero J, et al. Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: a randomised, Phase IIIb, non-inferiority study (ARGON). Respir Med. 2020;170:106021.
- Kerstjens HAM, Maspero J, Chapman KR, et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020 Oct;8(10):1000–1012.
- Canonica GW. Treating asthma as an inflammatory disease. Chest. 2006 Jul;130(1Suppl):21S–28S.
- Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol. 2019 Jul;144(1):1–12.
- Horvath G, Wanner A. Inhaled corticosteroids: effects on the airway vasculature in bronchial asthma. Eur Respir J. 2006 Jan;27(1):172–187.
- Hubner M, Hochhaus G, Derendorf H. Comparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroids. Immunol Allergy Clin North Am. 2005 Aug;25(3):469–488.
- Cowie RL, Giembycz MA, Leigh R. Mometasone furoate: an inhaled glucocorticoid for the management of asthma in adults and children. Expert Opin Pharmacother. 2009 Aug;10(12):2009–2014.
- Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J. 2019 Jun;53(6):1901046.
- Spada F, Barnes TM, Greive KA. Comparative safety and efficacy of topical mometasone furoate with other topical corticosteroids. Australas J Dermatol. 2018 Aug;59(3):e168–e174.
- Beeh KM, Derom E, Kanniess F, et al. Indacaterol, a novel inhaled beta2-agonist, provides sustained 24-h bronchodilation in asthma. Eur Respir J. 2007 May;29(5):871–878.
- Sturton RG, Trifilieff A, Nicholson AG, et al. Pharmacological characterization of indacaterol, a novel once daily inhaled 2 adrenoceptor agonist, on small airways in human and rat precision-cut lung slices. J Pharmacol Exp Ther. 2008 Jan;324(1):270–275.
- Chuchalin AG, Tsoi AN, Richter K, et al. Safety and tolerability of indacaterol in asthma: a randomized, placebo-controlled 28-day study. Respir Med. 2007 Oct;101(10):2065–2075.
- Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015 Feb 3;5(2):e006131.
- Murphy L, Rennard S, Donohue J, et al. Turning a molecule into a medicine: the development of indacaterol as a novel once-daily bronchodilator treatment for patients with COPD. Drugs. 2014 Sep;74(14):1635–1657.
- Miller D, Vaidya S, Jauernig J, et al. Lung function, pharmacokinetics, and tolerability of inhaled indacaterol maleate and acetate in asthma patients. Respir Res. 2020 Sep 23;21(1):248.
- Kudlak M, Tadi P Physiology, muscarinic receptor. StatPearls. Treasure Island (FL); 2021.
- Moss R, Sachse FB, Moreno-Galindo EG, et al. Modeling effects of voltage dependent properties of the cardiac muscarinic receptor on human sinus node function. PLoS Comput Biol. 2018 Oct;14(10):e1006438.
- Price D, Fromer L, Kaplan A, et al. Is there a rationale and role for long-acting anticholinergic bronchodilators in asthma? NPJ Prim Care Respir Med. 2014 Jul 17;24(1):14023.
- Gosens R, Gross N. The mode of action of anticholinergics in asthma. Eur Respir J. 2018 Oct;52(4):1701247.
- Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol Ther. 2007 Aug;115(2):208–222.
- Haddad EB, Patel H, Keeling JE, et al. Pharmacological characterization of the muscarinic receptor antagonist, glycopyrrolate, in human and Guinea-pig airways. Br J Pharmacol. 1999 May;127(2):413–420.
- Sykes DA, Dowling MR, Leighton-Davies J, et al. The Influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther. 2012 Nov;343(2):520–528.
- Fukunaga K, Kume H, Oguma T, et al. Involvement of Ca(2+) signaling in the synergistic effects between muscarinic receptor antagonists and beta(2)-adrenoceptor agonists in airway smooth muscle. Int J Mol Sci. 2016 Sep 21;17(9):1590.
- Hansel TT, Neighbour H, Erin EM, et al. Glycopyrrolate causes prolonged bronchoprotection and bronchodilatation in patients with asthma. Chest. 2005 Oct;128(4):1974–1979.
- Talwar D, Bendre S. Health-related effects of home nebulization with glycopyrronium on difficult-to-treat asthma: post-hoc analyses of an observational study. Interact J Med Res. 2020 Apr 29;9(2):e17863.
- Blais CM, Davis BE, Cockcroft DW. Duration of bronchoprotection of the long-acting muscarinic antagonists tiotropium & glycopyrronium against methacholine-induced bronchoconstriction in mild asthmatics. Respir Med. 2016 Sep;118:96–101.
- Blais CM, Davis BE, Cockcroft DW. The effect of glycopyrronium and indacaterol, as monotherapy and in combination, on the methacholine dose-response curve of mild asthmatics: a randomized three-way crossover study. Respir Res. 2017 Aug 2;18(1):146.
- Matera MG, Belardo C, Rinaldi M, et al. Emerging muscarinic receptor antagonists for the treatment of asthma. Expert Opin Emerg Drugs. 2020 Jun;25(2):123–130.
- Kerwin E, Wachtel A, Sher L, et al. Efficacy, safety, and dose response of glycopyrronium administered by metered dose inhaler using co-suspension delivery technology in subjects with intermittent or mild-to-moderate persistent asthma: a randomized controlled trial. Respir Med. 2018 Jun;139:39–47.
- Sharpe M, Jarvis B. Inhaled mometasone furoate: a review of its use in adults and adolescents with persistent asthma. Drugs. 2001;61(9):1325–1350.
- Valotis A, Neukam K, Elert O, et al. Human receptor kinetics, tissue binding affinity, and stability of mometasone furoate. J Pharm Sci. 2004 May;93(5):1337–1350.
- Pelaia G, Vatrella A, Cuda G, et al. Molecular mechanisms of corticosteroid actions in chronic inflammatory airway diseases. Life Sci. 2003 Feb 21;72(14):1549–1561.
- Barnes PJ. Corticosteroids: the drugs to beat. Eur J Pharmacol. 2006 Mar 8;533(1–3):2–14.
- Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? J Endocrinol. 2003 Jul;178(1):5–12.
- Pelaia C, Vatrella A, Crimi C, et al. Clinical relevance of understanding mitogen-activated protein kinases involved in asthma. Expert Rev Respir Med. 2020 May;14(5):501–510.
- Pelaia G, Vatrella A, Busceti MT, et al. Molecular and cellular mechanisms underlying the therapeutic effects of budesonide in asthma. Pulm Pharmacol Ther. 2016 Oct;40:15–21.
- Mak JC, Nishikawa M, Barnes PJ. Glucocorticosteroids increase beta 2-adrenergic receptor transcription in human lung. Am J Physiol. 1995 Jan;268(1 Pt 1):L41–6.
- Scott MG, Swan C, Wheatley AP, et al. Identification of novel polymorphisms within the promoter region of the human beta2 adrenergic receptor gene. Br J Pharmacol. 1999 Feb;126(4):841–844.
- Profita M, Gagliardo R, Di Giorgi R, et al. Biochemical interaction between effects of beclomethasone dipropionate and salbutamol or formoterol in sputum cells from mild to moderate asthmatics. Allergy. 2005 Mar;60(3):323–329.
- Pelaia G, Muzzio CC, Vatrella A, et al. Pharmacological basis and scientific rationale underlying the targeted use of inhaled corticosteroid/long-acting beta2-adrenergic agonist combinations in chronic obstructive pulmonary disease treatment. Expert Opin Pharmacother. 2015;16(13):2009–2021.
- Barnes PJ. Biochemical basis of asthma therapy. J Biol Chem. 2011 Sep 23;286(38):32899–32905.
- Mak JC, Hisada T, Salmon M, et al. Glucocorticoids reverse IL-1beta-induced impairment of beta-adrenoceptor-mediated relaxation and up-regulation of G-protein-coupled receptor kinases. Br J Pharmacol. 2002 Feb;135(4):987–996.
- Vatrella A, Maglio A, Pelaia C, et al. Pharmacotherapeutic strategies for critical asthma syndrome: a look at the state of the art. Expert Opin Pharmacother. 2020 Aug;21(12):1505–1515.
- Cazzola M, Proietto A, Matera MG. Indacaterol for chronic obstructive pulmonary disease (COPD). Drugs Today (Barc). 2010 Mar;46(3):139–150.
- Pelaia G, Maselli R, Matera MG. Treatment of chronic obstructive pulmonary disease by dual bronchodilation with coformulation of indacaterol/glycopyrronium. Pharmacology. 2014;94(5–6):249–258.
- Lombardi D, Cuenoud B, Kramer SD. Lipid membrane interactions of indacaterol and salmeterol: do they influence their pharmacological properties? Eur J Pharm Sci. 2009 Dec 8;38(5):533–547.
- Eickelberg O, Roth M, Lorx R, et al. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999 Jan 8;274(2):1005–1010.
- Holden NS, Bell MJ, Rider CF, et al. beta2-Adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19713–19718.
- Hall IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J. 2000 Jun;15(6):1120–1127.
- Pelaia G, Marsico SA. Regulation of beta 2-adrenergic receptors and the implications for bronchial asthma: an update. Monaldi Arch Chest Dis. 1994 Apr;49(2):125–130.
- Cazzola M, Calzetta L, Segreti A, et al. Translational Study Searching for Synergy between Glycopyrronium and Indacaterol. COPD. 2015 Apr;12(2):175–181.
- Buhl R, Nikolaev I, Tillmann HC, et al. Dose bridging data for mometasone furoate in once-daily fixed-dose inhaled combinations of mometasone furoate/indacaterol and mometasone furoate/ indacaterol/glycopyrronium in patients with asthma. Pulm Pharmacol Ther. 2021 Jul 28;70:102068.
- Vaidya S, Jauernig J, Ethell B, et al. Pharmacokinetics of indacaterol, glycopyrronium and mometasone furoate following once-daily inhalation as a combination in healthy subjects. Pulm Pharmacol Ther. 2020 Oct;64:101964.
- Kornmann O, Mucsi J, Kolosa N, et al. Efficacy and safety of inhaled once-daily low-dose indacaterol acetate/mometasone furoate in patients with inadequately controlled asthma: phase III randomised QUARTZ study findings. Respir Med. 2020 Jan;161:105809.
- van Zyl-smit RN, Krull M, Gessner C, et al. Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): a randomised, double-blind, triple-dummy, controlled phase 3 study. Lancet Respir Med. 2020 Oct;8(10):987–999.
- Agusti A, Fabbri L, Lahousse L, et al. Single inhaler triple therapy (SITT) in asthma: Systematic review and practice implications. Allergy. 2021 Sep 3.
- Kim LHY, Saleh C, Whalen-Browne A, et al. Triple vs Dual Inhaler Therapy and Asthma Outcomes in Moderate to Severe Asthma: A Systematic Review and Meta-analysis. JAMA. 2021 Jun 22;325(24):2466–2479.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017 Feb;49(2).
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001 Aug;23(8):1296–310.
- Lindsay JT, Heaney LG. Nonadherence in difficult asthma - facts, myths, and a time to act. Patient Prefer Adherence. 2013;7:329–36.
- Elliott RA. Poor adherence to anti-inflammatory medication in asthma. Dis Manag Health Out. 2006;14(4):223–233.
- Azzi E, Srour P, Armour C, et al. Practice makes perfect: self-reported adherence a positive marker of inhaler technique maintenance. NPJ Prim Care Respir Med. 2017 Apr 24;27(1):29.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005 Aug 4;353(5):487–97.
- Van Sickle D, Barrett MA, Humblet O, et al. Randomized, controlled study of the impact of a mobile health tool on asthma SABA use, control and adherence. European Respiratory Journal 2016;48:PA1018.
- Mosnaim GS, Stempel DA, Gonzalez C, et al. The Impact of Patient Self-Monitoring Via Electronic Medication Monitor and Mobile App Plus Remote Clinician Feedback on Adherence to Inhaled Corticosteroids: A Randomized Controlled Trial. J Allergy Clin Immunol Pract. 2021 Apr;9(4):1586–1594.
- Delea TE, Stanford RH, Hagiwara M, et al. Association between adherence with fixed dose combination fluticasone propionate/salmeterol on asthma outcomes and costs*. Curr Med Res Opin. 2008 Dec;24(12):3435–42.
- Barrett M, Combs V, Su JG, et al. AIR Louisville: Addressing Asthma With Technology, Crowdsourcing, Cross-Sector Collaboration, And Policy. Health Aff (Millwood). 2018 Apr;37(4):525–534.
- Merchant RK, Inamdar R, Quade RC. Effectiveness of Population Health Management Using the Propeller Health Asthma Platform: A Randomized Clinical Trial. J Allergy Clin Immunol Pract. 2016 May-Jun;4(3):455–63.
- Merchant RK, Szefler SJ, Bender BG, et al. Impact of a digital health intervention on asthma resource utilization. World Allergy Organ J. 2018;11(1):28.
- Merchant R, Inamdar R, Henderson K, et al. Digital health intervention for asthma: patient-reported value and usability. JMIR Mhealth Uhealth. 2018 Jun 4;6(6):e133.
- Eurpean Medicines Agency E. Human medicines: highlights of 2020; 2020 [cited 2021 Oct 10]. Available from: https://www.ema.europa.eu/en/news/human-medicines-highlights-2020
