ABSTRACT
Background
During the pandemic, there have been disruptions to how patients seek care.
Research design and methods
To investigate monthly prescription claims for asthma and chronic obstructive pulmonary disease (COPD) medicines during the first UK wave, interrupted time series (ITS) analysis was used. A national cohort of community patients’ data were examined.
Results
Descriptive statistics show salbutamol, aminophylline, ipratropium, and theophylline remain below pre-pandemic levels.
Montelukast showed pre-pandemic monthly increase (Est. 67,151 doses, P = 0.05, 95% CI: 1011, 133,291), followed by a jump of 1.6 million doses at March , followed by monthly declines (Est. −112,098 doses, P = 0.216, 95% CI: -293,499, 69,303).
Before the pandemic, tiotropium, salbutamol, aminophylline, and ipratropium (P = 0.003) show monthly declines but theophylline and beclometasone showed increases. In March , salbutamol (P = 0.033) and ipratropium (P = 0.001) show a significant jump. After March , ipratropium continues with a downward trajectory (P = 0.001), with a generalized negative trend for all other agents. Salbutamol confidence bounds become negative after March 2020. Some brands were unavailable.
Conclusions
An ‘unmet’ medical gap is identified. While it is essential to understand the underlying reasons, urgent action needs to be taken to reassess patients and ensure continuity of care.
PLAIN LANGUAGE SUMMARIES (PLS)
Asthma and chronic obstructive pulmonary disease (COPD) are long-term lung conditions, affecting 6 million & 1.2 million people respectively and causing breathing difficulties. Sufferers are at a higher risk of chest infections including the coronavirus. Regular use of prescribed medication stabilizes these conditions and prevents them from getting worse. It is common to be prescribed a combination of five to eight oral and inhaled medications.
We investigated the impact of the pandemic on the dispensing of these specific medicines across England during the first wave. The English Prescribing Dataset was checked from January 2019 to February 2020 (14 months before the pandemic) and March to October 2020 (8 months after its onset).
We find that since March 2020, salbutamol, aminophylline, ipratropium, and theophylline have not returned to their pre-pandemic levels. However, for all agents, there is great variability. Further analysis suggests these trends are not reversing, suggesting that people have not been using their medication as anticipated for 8 months, which is concerning.
As a consequence of this work, we recommend that doctors specifically call these patients and discuss their health as a matter of urgency, we encourage patients to continue to take their medication. We advise policy changes to waive the NHS prescription levy for asthma and COPD medication and we seek more granular data for further harm quantification. There are several strengths and weaknesses to our analysis, and we need to conduct more studies to ask patients about their experiences.
1. Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are long-term chronic conditions that affect a large proportion of the United Kingdom (UK) population. Overall UK asthma prevalence was 6.5% in 2016 as compared to a decade before 7.2% in 2006. [Citation1], p.2006–2016]. The prevalence of asthma is estimated at 6.0 million sufferers annually, resulting in at least 6.3 million primary care consultations, 93 thousand hospital in-patient episodes, 1800 intensive-care unit episodes, and 36.8 thousand disability living allowance claims per year [Citation2]. Similarly, the overall UK prevalence of COPD is estimated at 1.7% [Citation3] with an estimated 1.2 million people diagnosed with COPD [Citation4]. A subset of these patients have a concurrent asthma and COPD diagnosis and the figures depend on which diagnosis is used as the reference point: 14.5% of patients with COPD also have asthma, and 14.8% of the people with asthma also have COPD. However, evidence suggests that an asthma diagnosis may be over-recorded in people with COPD [Citation5]. Conservatively, this puts the UK's estimate at 7 million people diagnosed with asthma and/or COPD. Misdiagnosis of COPD in primary care is also common, with particular diagnostic confusion between COPD and asthma, so assessing medicines used for both conditions (which often overlap by indication, but at different doses) together is important [Citation6].
Sufferers have been shown to be more at risk of contracting illness and chest infections, including the coronavirus [Citation7–9]. National [Citation10] and international [Citation11–13] guidelines for both these conditions recommend clinical management plans to keep acute attacks under control and prevent hospitalization by the routine and continued use of medications that patients have been stabilized on. During the pandemic, there have been disruptions to how patients have sought care, avoidance of care-settings [Citation14], with socioeconomic disparities deepening health disparities for patients with asthma during the COVID-19 pandemic [Citation15]. Patients with asthma and COPD are advised to closely adhere to their prescribed inhaler medication therapy [Citation16]. Early-stage pandemic research showed that adherence was high in global patient populations [Citation16–18].
It is quite common for patients to be prescribed a combination of five to eight oral and inhaled medications [Citation19]. Inhaled short acting beta2-agonists, e.g. salbutamol/albuterol, and corticosteroids e.g. beclometasone are the mainstay for asthma and COPD management [Citation20]. Assuming five prescription medications per person per month are dispensed for 7 million sufferers annually, a reasonable expectation of 35 million items per month (or up to 980 million doses per month) are made.
This study aims to assess the impact of the coronavirus pandemic on the dispensing of normal therapies used in the long-term, management and control of respiratory conditions such as asthma and COPD in the first wave.
2. Material and methods
2.1. Study design
This was a retrospective cohort study of all patients in England, within primary care who were prescribed asthma or COPD medicines. These were identified to be medicines with reimbursement volumes consistently above 200,000 doses in primary care settings in England (before the pandemic). The exposure was to the global pandemic. Prescription claims data in England before and after the pandemic’s onset were compared. Statistical variations were the outcome of interest.
2.2. Data source
The ‘English Prescribing Dataset’ (EPD) [Citation21] provided by the National Health Service (NHS) Business Services Authority (NHSBSA) provided anonymized prescription data in England covered by Open Government License (OGL) which is not linked to other datasets. All prescription data processed across primary care within the NHS is included in this study. The data includes processing periods (months, years) and aggregated total dosage-quantities issued against each clinical commissioning groups (CCGs). Tablets are counted at single doses, while inhalers are counted per unit, i.e. tablets of montelukast, aminophylline, and theophylline will be counted individually (per tablet), while inhalers like tiotropium, salbutamol, ipratropium, and beclometasone are counted per inhaler (200 metered doses). Data from January 2019 to October 2020 were examined with March 2020 as the interrupt point. January 2019 to February 2020 (14 months before the pandemic) and March to October 2020 (8 months after its onset). The EPD does not provide demographic or individual-level data. The UK’s Central Alerting System https://www.cas.mhra.gov.uk/SearchAlerts.aspx was interrogated for medicines shortages.
2.3. Changes in the population
In 2019, there were 712,680 live births in the UK (731,213 in 2018) and 604,707 deaths (616,014 in 2018) [Citation22], net growth (107,973). Provisional statistics put 608,016 deaths in England and Wales in 2020 [Citation23]. The number of births for the first three-quarters in England and Wales for 2020 were 464,437 (481,767 in 2019) [Citation24]. Extrapolating provisional estimates (Birth 580,546, Death 760,020 Net −179,474) gives a net decline of approximately 180-thousand. Hence, the 7 million suffers from asthma and COPD were assumed to be constant because of the small variations expected as per the above estimates and travel restrictions imposed as a consequence of the pandemic. While mortality may be higher for these respiratory patients, no data currently exists to quantify these claims [Citation8,Citation25].
2.4. Outcomes measures
The primary outcome was the total quantity of each medicine. These were total quantities per month of individual medicines, including branded and generics. To assess changes, a rolling continuous period from January 2019 to October 2020 was identified. Asthma/COPD medicines (montelukast, tiotropium bromide, salbutamol, aminophylline hydrate, ipratropium bromide, theophylline, and beclometasone dipropionate) account for approximately 90% of all medicines used. Formulations included inhalers, nebulizers, dry powder inhalers, oral preparations, and solid doses (see supplemental). Other medicines used in management were excluded, e.g., acetylcysteine, antihistamine, adrenaline. A 10% sampling validation was conducted against https://openprescribing.net/ . Findings are presented according to the RECORD statement [Citation26].
2.5. Statistical analysis
All prescription data were extracted within a specified time period and examined 387,288,884 rows of data (528.8GB) against inclusion criteria. Of these, 169,094 rows were identified and analyzed in this publication. An interrupted time series (ITS) design [Citation27–29] at 95% confidence level was used, which provides powerful evidence of causal effects because it controls for secular trends in study outcomes. A commonly used time-series modeling framework (autoregressive integrated moving average, or ARIMA) to analyze the monthly total-quantity of prescription data from the EPD was employed. ARIMA is a flexible modeling construct, allowing lagged (auto)correlations and seasonal differences to be modeled, while controlling for confounding. Such analysis measures whether a natural event like the pandemic causes abrupt changes in the level or the preexisting trends (slope) of study outcomes and is appropriate for examining the impact of natural events at a population level. A ‘step change’ or a ‘change in trend’ was assessed before and after March 2020. Autocorrelation and the influence of seasonality was assessed by including lag terms in sensitivity analysis (see Supplemental Sensitivity analysis). Ethical approval was not required for this database study. Patients or members of the public were not involved in the design, conduct, reporting, or dissemination of this research.
3. Results
A total of 22 months’ worth of data were analyzed (or 668,334,660 total items, monthly average 30,378,848 items), using March 2020 as the cut-point for the first lockdown in England, making the finding nationally representative. While all the data from the EPD was extracted, only 169,094 rows pertained to the medications in this analysis, i.e. montelukast, tiotropium bromide, salbutamol, aminophylline hydrate, ipratropium bromide, theophylline, and beclometasone dipropionate. Analyzed formulations were used for respiratory conditions rather than other conditions, such as topical applications, formulations used for the management of rhinorrhea (associated with allergic and non-allergic rhinitis). From the above assumption, it was reasonably expected that 35 million items (or 980 million doses) per month would be used. This estimate closely matches monthly use approximations, except for Apr-20, Jun-20, Aug-20, and Sept-20 (see 2a. Supplemental Total Quantities). Much seasonal variation is not expected from clinical experience, except in February where a dip is observed annually (for most medicines as a New Year’s slump). Seasonal variation assessment did not find any significant patterns over the study period nor in sensitivity analysis (see supplemental), other than those believed to be consequent to the pandemic, described below.
shows descriptive statistics before and after the first wave of the pandemic, which demonstrates that salbutamol, aminophylline, ipratropium, and theophylline have not returned to their pre-pandemic levels. However, for all agents, there is great variability demonstrated by the wide standard deviations and confidence intervals (95%).
Table 1. Descriptive statistics of the total quantity (in millions) of medicines in dosage units from January 2019 to February 2020 (14 months before the pandemic) and March to October 2020 (7 months after pandemic’s onset); Standard Deviation (SD)
It is important to note that aminophylline costs £2.40-£8.50/28-tablet-pack, montelukast costs £2.04-£5.28/28-tablet-pack and theophylline costs £2.96-£5.65/56-tablet-pack, are predominantly used orally and are cheap. Inhalers like beclometasone costs £3.70-£56.56, ipratropium costs £3.21- £6.54, salbutamol costs £1.50 – £107.52 and tiotropium costs £23.00- £34.87, with inhalers traditionally costing more than tablets. These prices to some extent explain the affordability/popularity of these medicines, while their convenience and clinical benefit is well known. This price analysis is presented here, in case of future price-inflations, discontinuations, and shortages.
3.1. Interrupted time series analysis
Firstly, switching from one medicine to another is clinically unusual between these categories of medicines, due to their varied indications, standard doses, side-effects, cautions, contraindications, and interactions. As a result, the statistical gap identified in this study is likely to be an ‘unmet’ medical need. Second, no seasonal effects are expected (or detected) because follow-up prescriptions should be anticipated and are normally pre-scheduled by a month (or two). Since prescription data are not random, a one-month autocorrelation better reflects routine clinical practice (ARIMA(1,0,0) Model), which was used, see and (see supplemental Syntax).
Figure 1. ARIMA (1,0,0) montelukast, tiotropium bromide, salbutamol, aminophylline hydrate, ipratropium bromide, theophylline, beclometasone dipropionate. The x-axis presents sequential months (one representing January 2019 and 22, representing October 2020). The y-axis represents total quantities of doses reimbursed
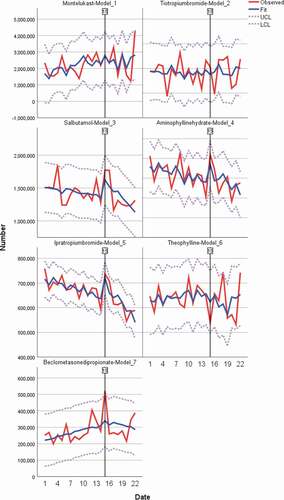
Table 2. ARIMA (1,0,0) models
Before the pandemic, montelukast showed a statistically significant monthly steady growth of 67 thousand doses (Est. 67,151 doses, P = 0.05, 95% CI: 1011, 133,291). At the intercept point (March 2020) a non-significant step change is seen with a jump of 1.6 million doses, which is clinically significant. Model estimates after the intercept shows a monthly decline of 112 thousand doses (Est. −112,098 doses, P = 0.216, 95% CI: -293,499, 69,303), negating the pre-pandemic trend. Before the pandemic, tiotropium, salbutamol, aminophylline hydrate, and ipratropium (P = 0.003) all demonstrated a monthly decline in volumes ranging from 10 to 5 thousand doses per month. Theophylline and beclometasone dipropionate demonstrate a monthly increase in volumes from 5 to 7 thousand doses per month. At the interrupt point, salbutamol (P = 0.033) and ipratropium (P = 0.001) shows a significant step-change and a sustained visible spike (). shows an initial increase in dispensing followed by a sharp decline, which is more pronounced than before the lockdown
After March 2020’s onset, ipratropium continues with the significant downward trajectory (P = 0.001), with a generalized negative trend for all other agents. While not significant, salbutamol has confidence bounds that become negative after March 2020.
With respect to drug shortages/availabilities: On November 28, 2019, the manufacturer of a theophylline branded product called Slo-phyllin® (60 mg/125 mg/250 mg capsules) went into administration with anticipated shortages by the end of November – which may explain the observed dip in November 2019 (see 2a. Supplemental Total Quantities). After the study period, medicine shortages were noted for aminophylline (Phyllocontin® 225 mg, 350 mg sustained-release tabs) which are being discontinued after Feb 2021 in the UK.
4. Discussion
4.1. Lessons learned
These findings are concerning and suggest that a significant number of patients may not have used their asthma or COPD medicines as expected. These are chronic long-term conditions that do not naturally resolve, the demand for medication should not disappear and this does not reflect ‘a better care provision’ during the pandemic, or resolution of patient’s symptoms, nor is fully explained by drug shortages. In fact, these data provide an early warning signal-detection indicating a growing unmet medical need that is accelerating and actually represents an urgent need to recall and follow-up these patients, given they may not have presented for care voluntarily. These findings may pertain to the inflexibility of the health care system to serve these patients, where access to medicines may be varied and not the ‘patient’s fault’ i.e. not a lack of willingness to adhere.
Not all analyses presented here have significant P-values; however, these extremely large variations in absolute quantities show massive fluctuations, which, while not statistically significant, are absolutely clinically important because they demonstrate that large numbers of patients were not issued their normal medications or have foregone these prescriptions. It raises questions around ‘access to basic essential medicines’ which the World Health Organization recommends every country to have.
The pandemic was associated with a change in trend, with a flattening or negation of previous (positive) trends for respiratory medicines. There was a clear change in the pattern of prescribing for all medicines in March 2020. While the generalized upward step change in March 2020 can be explained down to medicines management decisions being made in the face of the pandemic, it is difficult to explain the rapidly declining trend of the medicines after March 2020, other than linking it with pandemic-related disruptions. Prescription volumes have declined and even if this is not linked to significant morbidity now, it is a proxy for medicines-supply or patient-access and an important avenue for further enquiry.
Anecdotally, this could be for a variety of reasons including higher mortality in elderly patients (who tend to suffer from COPD) who would fall within these patient group(s), patient’s reluctance to leave their homes especially if they are shielding and reduced rates of air-pollution. General practitioner (GP) factors may also play a role and although doctors surgery’s normally have processes in place to enable patient recall and offer treatment at home where appropriate in normal times, this may be less than adequate during a pandemic. While certainty cannot be assured, the results suggest the possibility of a causal link between the pandemic and changes to prescription volumes. This analysis cannot rule out other possible causal explanatory factors but are consistent with the possibility that pandemic-related disruptions may have directly contributed to the changes observed. This also provides an early signal-detection for potentially deteriorating medium to longer term health in this group of patients with subsequent lockdowns, virus variants, and future pandemics.
This analysis reflects approximately 203 million prescription doses over the study period, probably reflecting all asthma and COPD patients. Patients may not be getting the medication they need to keep their conditions in check, with the risk that these will likely progress in disease severity with poorer health outcomes, quality of disease-free life and patients may have greater resource utilization in the medium to longer term. Their life expectancy may also be negatively affected. The individual patient-level indications for the medicines analyzed are unknown but clear correlation with adverse outcomes are beginning to appear in the literature, and the authors believe this is not just a UK trend, but a global one as discussed in the introduction [Citation8,Citation14–18]. Greater visibility is afforded in the UK because of universal health coverage, which is not afforded in many other nations, where health inequalities may accelerate faster.
First, claims data become available as a terminal step after claims have been processed and completed, which can show a delayed picture due to the lagged nature of data. In the UK, it is routine for this to occur at monthly intervals. There are a variety of inclusion and exclusion criteria that apply to this dataset, which could also introduce small biases. Analysis from the first lockdown would suggest that further declines are expected in subsequent periods.
Secondly, it must be made clear that dispensing of prescriptions does not necessarily mean that patients take or use their medications as intended. During the first wave of the COVID-19 pandemic (first lockdown March 23, 2020), all prescriptions were predominantly electronic (mass switching from paper to digital), and most consultations were digital (and not face-to-face, as most patients are accustomed to). It is unclear how many new diagnoses were made, but for this analysis, an underlying assumption is that most prescriptions relate to already diagnosed patients with a chronic long-standing history with their condition(s), which is a conservative assumption, that makes concessions for new diagnosis. To understand if there were changes to dispensing, that might have interrupted the supply of medication to patients and if there were effects on medicines shortage due to Brexit (no such evidence found), were important considerations. There has also been significant disruption to the supply chain before and during Covid-19, coupled with pharmacy reimbursement-renegotiations and manufacturing issues affecting medicine supply across Europe, but which do not affect these medicines [Citation30–32] other than those already described.
While the pandemic has provided an opportunity for digital consultations and remote supervision, they have come with added uncertainty and anxiety for patients, especially for the elderly and those who are digitally disconnected. Changes to routine have the potential for negative consequences during normal times and can be more severe during natural disasters such as pandemics. Digital consultations have the potential to create digital barriers to care. Adherence concerns and access to timely prescription refills may occur for a variety of reasons detailed above. Telephone triage may have substituted for the standard practice of a physical examination e.g. spirometry, blood-tests, or annual review. Of key concern are new patients, who may have had a delayed diagnoses or having been newly initiated on these medications, may have failed to return as a consequence of the side-effects or the pandemic.
Another consideration is the ‘prescribing’ versus ‘dispensing’ practice: it is known that varied prescribing practices occurred, deviating from routine issuance of a ‘28-day’ prescription (in some cases, people were issued up to 6 months’ worth of medication). From the analysis presented here, it is clear that this is modest. It is also known that the medicine’s supply chain can only fulfill an excess demand by two-week national average. In practice, this often means that prescriptions are partly fulfilled with an ‘owing’ or balance outstanding to the patient, which is settled when stocks become available. However, since pharmacies are contractors (to the NHS), they are highly reliant on monthly reimbursements. Hence, long-prescriptions (e.g. six-months) are sent for reimbursement and will appear in reimbursement claims data. In reality, patients may not have the medicines that appear to be fully dispensed to them, and the unmet medical needs described here may be more severe. Collectively, there may be instances across the country where patients have suboptimal disease control, where underlying complications may escalate.
Although underuse can result in morbidity and health care cost, the cost of the medication is believed to be one of the most important determinants of underuse [Citation19]. For those under 16 and over 60 years of age, dispensed-medication are free while most other patients have to pay a standard subsidised levy of approximately £10. This medication cost disproportionately negatively affects younger, working-age patients who are not exempt from prescription levies, who may fail to adhere due to financial pressures.
While this analysis provides important insight, it can only be descriptive and further work is needed to explore the underlying reasons for the trends observed and the implications for patients. The numbers presented are a fraction of the directly attributable costs of disease-management. They do not cover the costs of complications; hospital stay and onward care including the health-burden borne by family or carers.
4.2. Implications for clinical practice
For the first time, data is presented on prescription variations during this pandemic, which has implications for clinical practice – prescribers are encouraged to maintain clear documentation of offered follow-up and alternative care provided to guard against negligence suits and to actively think about patient-lists. Primary care practitioners must seriously consider recalling their patients for an urgent review. Patients also need to be trained to actively monitor their condition, in case of future lockdowns with an important reinforcement toward medicines adherence. Surgeries should also consider simplifying their prescription issuance and request processes.
Annual direct healthcare costs of COPD in England were estimated to increase from £1.50 billon (1.18–2.50) in 2011 to £2.32 (1.85–3.08) a billion in 2030, with increasing rates of prevalence [Citation33]. As a result of this pandemic, projected rates will likely increase faster and potentially further. To remove the barriers of prescription levies to poorer or younger patients adversely affected by the pandemic, policy changes waiving the prescription levy for patients prescribed medications for the management of asthma or COPD is recommended.
For researchers, improvements in the documentation and data-structure of the dataset are encouraged. The need for error-free data, its completeness, and the importance of documenting indications for medications is vital in facilitating better research that allows granular targeting of patient groups, as done here. Data collection, duration, and completeness requires that the data should be representative of practice(s) across the UK and should incorporate datasets from Scotland, Wales, and Northern Ireland. This would allow early detection of regional variations to care and prevent postcode lotteries and identify safeguarding issues faster.
4.3. Strengths and weaknesses
There are several strengths and limitations to this study. Strengths of this study include being evidence-based using real-world data. ITS studies are generally unaffected by typical confounding variables that remain fairly constant, such as population age distribution or socioeconomic status, as these only change relatively slowly over time. Nevertheless, ITS can be affected by time-varying confounders e.g. excess mortality that change more rapidly. This analysis shows that reduced prescription rates may correlate indirectly with increased mortality due to Covid-19 indirectly because of the second-degree impact that high mortality rates have e.g. lockdowns, social restrictions, etc. However, primarily the impact of reduced prescriptions may be more strongly correlate with limited GP contact or significant behavioral changes to the way in which prescription medicines are requested on a repeat basis.
Limitations pertain to the timeframe, completeness, and quality of the data. The data extracted from this study, however, have not been independently verified as complete, accurate, and are subject to potential revision. The analysis is descriptive with no adjustments, for changes in population structure (age, disease prevalence, social deprivation scores) which could impact prescriptions between periods and within regions. Hospital statistics are not represented in this analysis. Confirmed diagnosis or prescription indications as well as linked data were unavailable, making it difficult to quantify proportions. Limitations of the dataset itself is that it excludes prescriptions issued outside England (Wales, Scotland, Guernsey, Alderney, Jersey, and the Isle of Man); items not dispensed, disallowed, and those returned for further clarification; prescriptions prescribed and dispensed in prisons, hospitals, and private prescriptions; items prescribed but not presented for dispensing or not submitted to NHSBSA. This dataset included small (487 out of 2,555,396 rows) operational irregularities (e.g. 17 rows in Jan 2019 of ‘unidentified practice data’, 470 rows of ‘NULL’ chemical substance codes). This bias is acknowledged and not controlled for.
4.4. Future studies
This study generates an early warning signal from real-world data on patients’ lives and provides a model for future pandemic preparedness. Future studies must consider the impact on patients’ lives with respect to disease progression, including over the life course of this pandemic. It is important to consider subsequent periods and intervals between lockdowns to fully assess the potential impact on patients. Future studies should examine whether routine blood tests were conducted and what that missing data may imply for clinical practice.
5. Conclusion
There has been a change to asthma and COPD prescription medicines dispensed and this may have occurred because of the pandemic. Not using these medicines has the potential to result in increased morbidity and mortality. Extra effort may be needed to help these patients.
Author contributions
Ravina Barrett conceived, designed, analzyed and interpreted the data; Robert Barrett extracted the data and prepared it for analysis. All authors drafted substantially revised, and critically reviewed the article, agreed on the journal to which the article will be submitted, reviewed, and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes. All authors agreed to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Ethics approval
Ethical approval was not required for this database study, which is a secondary analysis of government data, provided freely under the Open Government Licence (OGL), incorporating anonymized participatory consent from all patients. The study was conducted according to the principles of the World Medical Association Declaration of Helsinki. [22]
Supplemental Material
Download Zip (1.1 MB)Acknowledgments
The authors would like to thank NHS Business Services Authority staff who helped with several information requests.
Data availability
Data are available from https://opendata.nhsbsa.net/dataset/english-prescribing-data-epd
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Bloom CI, Saglani S, Feary J, et al. Changing prevalence of current asthma and inhaled corticosteroid treatment in the UK: population-based cohort 2006–2016. Eur Respir J. Internet]. 2019 [cited 2021 Mar 8];53. Available from: http://erj.ersjournals.com/lookup/doi/https://doi.org/10.1183/13993003.02130-2018
- Mukherjee M, Stoddart A, Gupta RP, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med. 2016;14:113.
- Haughney J, Gruffydd-Jones K, Roberts J, et al. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43:993–1002.
- Snell N, Strachan D, Hubbard R, et al. S32 Epidemiology of chronic obstructive pulmonary disease (COPD) in the UK: findings from the British lung foundation’s ‘respiratory health of the nation’ project. Thorax. 2016;71:A20.1–A20.
- Nissen F, Morales DR, Mullerova H, et al. Concomitant diagnosis of asthma and COPD: a quantitative study in UK primary care. Br J Gen Pract. 2018;68:e775–e782.
- Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma Off J Assoc Care Asthma. 2006;43:75–80.
- Su N, Lin J, Chen P, et al. Evaluation of asthma control and patient’s perception of asthma: findings and analysis of a nationwide questionnaire-based survey in China. J Asthma. 2013;50:861–870.
- Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120.
- Centers for Disease Control and Prevention. COVID-19 and Your Health [Internet]. Cent. Dis. Control Prev. 2020 [cited 2021 Mar 8]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management Guidance [Internet]. NICE; 2018 [cited 2021 Mar 8]. Available from: https://www.nice.org.uk/guidance/ng115.
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365.
- Boulet L-P, Reddel HK, Bateman E, et al. The Global Initiative for Asthma (GINA): 25 years later. Eur Respir J. 2019;54(2):1900598
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. Eur Respir J. 2018;51.
- Oreskovic NM, Kinane TB, Aryee E, et al. The Unexpected Risks of COVID-19 on Asthma Control in Children. J Allergy Clin Immunol Pract. 2020;8:2489–2491.
- Baptist AP, Lowe D, Sarsour N, et al. Asthma disparities during the COVID-19 pandemic: a survey of patients and physicians. J Allergy Clin Immunol Pract. 2020;8(3371–3377.e1):3371–3377.e1.
- Kaye L, Theye B, Smeenk I, et al. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8:2384–2385.
- Underner M, Taillé C, Peiffer G, et al. [COVID-19 and asthma control]. Rev Mal Respir. 2021;38:111–113.
- Chang C, Zhang L, Dong F, et al. Asthma control, self-management, and healthcare access during the COVID-19 epidemic in Beijing. Allergy. 2021;76:586–588.
- Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:371–384.
- Jepson G, Butler T, Gregory D, et al. Prescribing patterns for asthma by general practitioners in six European countries. Respir Med. 2000;94:578–583.
- English Prescribing Dataset (EPD) - open data portal BETA [Internet]. [cited 2020 Apr 25]. Available from https://opendata.nhsbsa.net/dataset/english-prescribing-data-epd.
- Office for national statistics. vital statistics in the UK: births, deaths and marriages - office for national statistics [Internet]. 2021 [cited 2021 Mar 19]. Available from https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/vitalstatisticspopulationandhealthreferencetables.
- Office for National Statistics. Deaths registered monthly in England and Wales - Office for National Statistics [Internet]. [cited 2021 Mar 19]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/monthlyfiguresondeathsregisteredbyareaofusualresidence.
- Office for national statistics. provisional births in England and Wales [Internet]. [cited 2021 Mar 19]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/provisionalbirthsinenglandandwales.
- Singh D, Halpin DMG. Inhaled corticosteroids and COVID-19-related mortality: confounding or clarifying?. Lancet Respir Med. 2020;8:1065–1066.
- Benchimol EI, Smeeth L, Guttmann A, et al. The Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885.
- Cochrane Effective Practice and Organisation of Care (EPOC). Interrupted time series (ITS) analyses. EPOC Resources for review authors [Internet]. 2017. [cited 2020 Nov 27]. Available from: epoc.cochrane.org/resources/epoc-specific-resources-review-authors.
- Lopez Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2016;46(1):348–355.
- Kontopantelis E, Doran T, Springate DA, et al. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350:h2750–h2750.
- European Medicines Agency. Shortages catalogue. Internet]. Eur Med Agency. 2018 [cited 2020 Aug 6]. Available from https://www.ema.europa.eu/en/human-regulatory/post-authorisation/availability-medicines/shortages-catalogue
- European Medicines Agency. Availability of medicines. Internet]. Eur Med Agency. 2018 [cited 2020 Aug 6]. Available from https://www.ema.europa.eu/en/human-regulatory/post-authorisation/availability-medicines
- Clews G, Manufacturing problems cause 60% of medicines shortages, say European wholesalers. Pharm J. 2019;Internet] [cited 2020 Aug 10]. Available from https://www.pharmaceutical-journal.com/news-and-analysis/news/manufacturing-problems-cause-60-of-medicines-shortages-say-european-wholesalers/20207343.article?firstPass=false
- McLean S, Hoogendoorn M, Hoogenveen RT, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep. 2016;6:31893.
