Despite the emergence of highly effective vaccines, the combination of a significant proportion of the world population being unvaccinated and the emergence of progressively more infectious variants of the SARS-CoV-2 virus has allowed the continuation of the Coronavirus disease-2019 (COVID-19) pandemic well into its third year. Nearly 15% of patients infected with Alpha (B.1.1.7) or Delta (B.1.617.2) variants develop severe illness requiring admission to a hospital [Citation1], among which some patients develop acute respiratory distress syndrome (ARDS) [Citation2,Citation3]. Among these, a small but significant proportion of patients, given the sheer number of infections, develop treatment-refractory and progressive ARDS [Citation3], necessitating initiation of extracorporeal membrane oxygenation (ECMO) and may culminate in significant and irreversible damage to the pulmonary parenchyma.
The extent and severity of the pulmonary physiologic impairment may leave patients dependent on significant pulmonary support without an obvious chance of meaningful recovery. All such patients merit consideration of lung transplantation (LT) as a therapeutic option. However, forming a consensus on the broad principles of a management strategy is far more easily accomplished than its bedside application toward an individual patient. There is a lack of robust evidence to guide management decisions regarding consideration of LT among patients with treatment-refractory, advanced lung disease due to COVID-19. The current review aims to discuss some of the relevant aspects regarding the management of such patients, including the timing and approach toward consideration of LT. Specifically, we will discuss the rationale for pursuing a customized strategy of permitting a longer time for pulmonary recovery among these patients. We also propose certain best practices as a framework for decision-making, especially when transitioning the goals of ECMO from recovery to LT. Finally, we highlight and briefly address a few of the unique challenges seen with prolonged periods of ECMO support.
Thus far, the general approach of the lung transplant community has been to extrapolate the learnings from other viral infections to aid decision-making among patients with COVID-19 associated ARDS [Citation4–6]. These aspects include the definition of the recovery from the acute infection, radiologic characteristics for determining reversibility of the parenchymal injury, as well as the timeline for expected recovery from ARDS, among other things. However, the last 2 years of the pandemic have repeatedly demonstrated one profound aspect of the SARS-CoV-2 virus that it is unlike any other virus seen during the bridging era of LT. Some unique aspects of COVID-19 include the development of the profound cytokine storm as the etiologic basis for the organ dysfunctions, a procoagulant state with the development of pulmonary microthrombi contributing to the acute lung injury (ALI), an unusual propensity for pleural complications, phenotypic diversity in the type of ARDS [Citation7], along with a high burden of secondary infections. Therefore, it is pertinent to approach COVID-19 as a unique illness where the temptation of extrapolating the learnings from previously studied viral infections, including consideration of LT, may be avoided at best and closely scrutinized at the very least.
The most critical aspect related to the consideration of LT among these patients pertains to its timing. The key driver of this decision is the exclusion of the possibility of pulmonary recovery. It would be helpful to base such decisions on validated clinical variables, laboratory markers, radiological patterns, or some combination of them. However, no such predictors are available for clinical use at this time. Therefore, the decision must be individualized for each patient while factoring in the evolving literature.
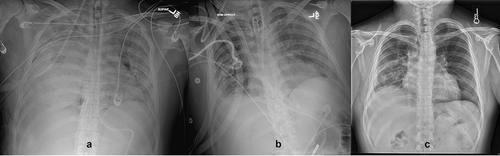
A significant body of evidence has already demonstrated that COVID-19 patients with ARDS take much longer to recover [Citation8–10]. The duration of ECMO support among COVID-19 patients is much longer than other viral pneumonia [Citation9]. Despite the longer ECMO runs, the survival among COVID-19 ARDS patients needing ECMO support is not inferior to that of other viral infections [Citation10]. In combination, these findings support a more conservative timeline for transitioning the ECMO goals of care from recovery to LT. In other words, the eventual survivors of COVID-19 ARDS without an LT should be expected to spend much longer time on ECMO than similar patients with ARDS due to other viruses. In a study conducted at our center [Citation11], where we describe the ‘ECMO long hauler’ phenotype (COVID-19 ARDS patients on ECMO for >30 days), we found pulmonary physiologic improvements among survivors well beyond the 6-week period on ECMO and as late as into the third month ().
Figure 1. A 27-year-old overweight patient who developed severe COVID-19 needing initiation of venovenous ECMO 18 days after the onset of symptoms. He was initially supported via fem-fem cannulation for 35 days that was transitioned to oxy-RVAD cannula via the internal jugular approach due to impending right ventricular failure. He was evaluated for lung transplantation but declined due to lack of social support. The pulmonary physiology started to improve >60 days on ECMO and he was decannulated after 75 days on ECMO. His serial chest radiographs show progressive improvement as compared to baseline (1A). At 1 year clinic visit after COVID-19, he had a room air 6-min walk although spirometry showed restrictive ventilatory defect. (A) Chest radiograph at 1 month after COVID-19. (B) Chest radiograph at 3 months. (C) Chest radiograph at 6 months.
In fact, the survivors in this cohort had a median length of ECMO support of more than 2 months [Citation11]. The ECMO long-haulers tended to be younger patients with few preexistent comorbidities but, physiologically, a more profound ARDS. It appeared that the baseline healthier status of the long-haulers allowed them to tolerate the complications integral to prolonged ECMO runs while permitting additional time for their lungs to recover. While such patients may also be suitable for the consideration of LT if their pulmonary status does not show any signs of recovery, their life expectancy is likely to be far longer if they can avoid, or even delay, the need for LT.
We recommend that the consideration of LT should be delayed for at least 8 weeks after the development of respiratory failure, including 6 weeks of ECMO support, and a consensus on this timeline may be emerging in the transplant community [Citation12]. It is certainly reasonable to individualize these timelines for specific patients. Still, the guiding principle should be to follow expectantly for signs of pulmonary recovery well past the conventional four-week period from the acute illness. The ECMO support during this period should be structured as a bridge to recovery while making all efforts to ‘rest the lungs’. Such patients should be followed longitudinally by a multidisciplinary team experienced in the management of COVID-19 ARDS. Early signs of pulmonary recovery may be limited to minor improvements in pulmonary compliance and minute ventilation on the ventilator, often without signs of radiological recovery [Citation11]. A ‘white-out’ on the chest radiograph is not uncommon and rather unhelpful in predicting reversibility. Despite the challenges in transporting these patients, obtaining a CT chest is worthwhile. A radiological pattern predominated by parenchymal consolidations and air bronchograms in the background of ground-glass opacities merits expectant management given the potential for recovery. In contrast, the signs of irreversibility may include an extended period of static pulmonary support parameters on ventilator or ECMO in the presence of radiological findings of parenchymal architectural distortion, traction bronchiectasis, and extensive cystic changes.
While not the driving factors, there are additional benefits of allowing an extended period for the ARDS to recover. There is little possibility of viable SARS-CoV-2 virus that far out, and recrudescence of COVID-19 after LT is not a concern. Patients are also likely to be stabilized enough to be awakened to enable their participation in the decision-making. This is a critical step given the life-changing nature of the post-transplant course, with a need for strict adherence to various medical recommendations, including several lifestyle modifications and restrictions. Last but not least, the additional time can permit the institution of strategies to address the critical illness myo-neuropathy, improve nutritional status, manage frailty and improve the physical conditioning of these critically ill patients.
While patients must meet the usual criteria of transplant candidacy, certain modifications to their assessment may be appropriate. We limit the consideration of ECMO as a bridge to transplant only among patients <65 years of age. Patients should be awakened and encouraged to participate in physical therapy. We typically require patients to be able to at least sit out of bed and stand with assistance for them to remain viable transplant candidates, although we have made exceptions among younger patients (<40 years) with profound hypoxemia despite maximal support. We have, however, avoided transplanting patients who are obtunded and unable to awaken.
Early involvement of the lung transplant team is desirable as it permits adequate time to work on potential barriers. The lung transplant teams should follow these patients longitudinally as cross-sectional assessments tend to be less instructive. Our protocols have incorporated weekly updates among all such patients during the transplant committee discussions. We have found the multidisciplinary discussions around the clinical progress, or lack thereof, including consideration of proactive strategies to preempt and manage complications among these patients, to be helpful. The ECMO long-haulers seem particularly prone to developing three significant complications: secondary infections, pleural complications, and right ventricular dysfunction (RVD). These complications are highly consequential not only from the standpoint of pulmonary recovery but also significantly impact the surgical candidacy of these patients if and when LT becomes a consideration.
While data are sparse, the risk of infections among these patients seems to be higher than non-COVID ARDS patients needing ECMO support [Citation13]. This is a result of the inherent immune dysfunction among patients with COVID-19, which is compounded by the use of immunosuppressive therapeutics. The common source of these infections tends to be the diseased lungs themselves, with the etiological agents being a mix of typical hospital-acquired infections and atypical organisms such as fungal infections. Given that these patients almost always have profoundly abnormal chest radiographs, we find radiologic assessments for pneumonia as having poor sensitivity. Furthermore, their airways tend to be colonized, thereby limiting the diagnostic utility of tracheal aspirates. We do not perform quantitative cultures. Instead, we prefer to proactively use bronchoscopy for direct visualization of the airways and collect broncho-alveolar lavage specimens to decide regarding the use of antimicrobials. Our approach among these patients tends to mimic that for assessment of pulmonary infections in an immunocompromised host.
Pleural complications often tend to be recalcitrant among these patients [Citation14]. They pose a significant challenge due to the severity of underlying lung disease and a high risk of bleeding complications with a pneumothorax often getting complicated by hemothorax. Unless the situation is emergent, the standard approach of bedside placement of large-bore chest tubes may not be suitable due to the loculated nature of pleural complications. We have found the image-guided placement of smaller-bore chest tubes to be less likely to cause complications. The development of hemothorax may necessitate surgical interventions, sometimes multiple times, to permit the underlying lung to expand. While difficult to manage pleural complications can significantly limit the chances of pulmonary recovery, a hostile pleural space also has a bearing on their surgical candidacy for LT. Indeed, dense pleural adhesions pose one of the most significant challenges in explanting the native lungs during transplant surgery.
Finally, a significant proportion of patients supported on venovenous ECMO tend to exhibit signs of RVD, which can progress to frank RV failure if not managed proactively [Citation15]. While the acute or sub-acute nature of the RVD does not preclude patients from being viable LT candidates, it does impact their clinical course, including the risk of complications and survival. Studies have found RVD to be an independent predictor of failure to bridge to LT [Citation16] and increased mortality [Citation15,Citation17], making it pertinent to implement proactive strategies to address this complication. Our practice is to obtain a pre-ECMO echocardiogram to assess the RV function. Thereafter, we monitor RV function regularly, especially among the long-haulers. Among patients with early RV failure, we proactively transition them to an oxy-RVAD cannulation strategy. In our experience, a proactive transition to oxy-RVAD cannulation along with an astute bedside management strategy consisting of low dose inotropic support, avoidance of high airway pressures, and volume overload can significantly off-load the RV and permit the prolonged ECMO run. An ‘RV-centric’ strategy during the ECMO run may also allow the RV to recover more swiftly after LT should that become necessary.
Given the dynamics of the pandemic, LT professionals will continue to be called upon to opine regarding LT among patients with COVID-19 ARDS. It is pertinent to emphasize that despite needing much longer time for pulmonary recovery, these patients experience equivalent hospital survival. The recovery from ARDS can extend well into the second or even the third month of ECMO support, and consideration for LT should be deferred for at least 6–8 weeks. Complications are not uncommon but can be managed with a proactive and customized strategy. Finally, the cornerstone of decision-making among these patients should be based upon serial assessments by a multidisciplinary group.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–765.
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area [published correction appears in JAMA. 2020 May 26;323:2098]. JAMA. 2020;323(20):2052–2059.
- Tan E, Song J, Deane AM, et al. Global impact of coronavirus disease 2019 infection requiring admission to the ICU: a systematic review and meta-analysis. Chest. 2021;159(2):524–536.
- Cypel M, Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir Med. 2020;8(10):944–946.
- Bharat A, Machuca TN, Querrey M, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9(5):487–497.
- Guidance from the international society of heart and lung transplantation regarding the SARS CoV-2 pandemic. cited 2022 Jan 17. ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-center.pdf
- Attaway AH, Scheraga RG, Bhimraj A, et al. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436.
- Dreier E, Malfertheiner MV, Dienemann T, et al. ECMO in COVID-19-prolonged therapy needed? A retrospective analysis of outcome and prognostic factors. Perfusion. 2021;36(6):582–591.
- Schmidt M, Hajage D, Lebreton G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121–1131.
- Kurihara C, Manerikar A, Gao CA, et al. Outcomes after extracorporeal membrane oxygenation support in COVID-19 and non-COVID-19 patients [published online ahead of print, 2021 Oct 25]. Artif Organs. 2022;46(4):688–696.
- Mohanka MR, Joerns J, Lawrence A, et al. ECMO Long-haulers: a distinct phenotype of COVID-19 associated ARDS with implications for lung transplant candidacy. [published online ahead of print, 2022 Feb 7]. Transplantation. 2022;106(4):E202–E211.
- King CS, Mannem H, Kukreja J, et al. Lung Transplantation for Patients With COVID-19. Chest. 2022;161(1):169–178.
- Shafran N, Shafran I, Ben-Zvi H, et al. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep. 2021;11(1):12703.
- Türk MS, Akarsu I, Tombul I, et al. The analysis of pleural complications of COVID-19 pneumonia. Turk J Med Sci. 2021;51(6):2822–2826.
- Lazzeri C, Bonizzoli M, Batacchi S, et al. Persistent right ventricle dilatation in SARS-CoV-2-related acute respiratory distress syndrome on extracorporeal membrane oxygenation support [published online ahead of print, 2021 Aug 21]. J Cardiothorac Vasc Anesth. 2021;36(7):1956–1961.
- Maharaj V, Alexy T, Agdamag AC, et al. Right ventricular dysfunction is associated with increased mortality in patients requiring venovenous extracorporeal membrane oxygenation for coronavirus disease 2019. ASAIO. 2022;Publish Ahead of Print: https://doi.org/10.1097/MAT.0000000000001666.
- Banga A, Batchelor E, Mohanka M, et al. Predictors of outcome among patients on extracorporeal membrane oxygenation as a bridge to lung transplantation. Clin Transplant. 2017;31(7):https://doi.org/10.1111/ctr.12990.
