ABSTRACT
Introduction
Quitting is the only proven method to attenuate the progression of chronic obstructive pulmonary disease (COPD). However, most COPD smokers do not seem to respond to smoking cessation interventions and may benefit by lessening the negative health effects of long-term cigarette smoke exposure by switching to non-combustible nicotine delivery alternatives, such as heated tobacco products (HTPs) and e-cigarettes (ECs).
Areas covered
Compared with conventional cigarettes, HTPs and ECs offer substantial reduction in exposure to toxic chemicals and have the potential to reduce harm from cigarette smoke when used as tobacco cigarette substitutes. In this review, we examine the available clinical studies and population surveys on the respiratory health effects of ECs and HTPs in COPD patients.
Expert opinion
The current research on the impact of ECs and HTPs on COPD patients’ health is limited, and more high-quality studies are needed to draw definitive conclusions. However, this review provides a comprehensive overview of the available literature for health professionals looking to advise COPD patients on the use of these products. While ECs and HTPs may offer some benefits in reducing harm from cigarette smoke, their long-term effects on COPD patients’ health are still unclear.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of global death, causing more than 3.2 million deaths in 2019 [Citation1]. As a result of an inherent unremitting inflammation and remodeling of the airways, COPD may result in respiratory symptoms, progressive deterioration in lung function, respiratory failure, and death [Citation2,Citation3]. Protracted exposure to smoke toxicants is assumed to be the cause of the distinct airway inflammatory response of COPD [Citation4,Citation5]. Notably, the inflammatory processes in smokers with COPD do not respond well to topical corticosteroids [Citation6,Citation7]. Additionally, current and ex-smokers with COPD have a higher risk of lung cancer, cardiovascular disease, and diabetes [Citation8–10].
Abstinence from conventional tobacco use is the only reported evidence-based strategy known to prognostically improve COPD [Citation11,Citation12], to significantly attenuate the decline in lung function and to enhance overall health status [Citation13–15]. Besides, stopping smoking reduces the risk of lung cancer, cardiovascular disease, and other tobacco-related diseases [Citation16].
Although stopping smoking should be a priority for any smokers with COPD, most of them are unable to experience high success rates during their quit attempts [Citation17,Citation18]. Licensed quitting therapies (nicotine replacement therapy – NRT, bupropion, and varenicline) have only limited success in patients with COPD who smoke and many COPD patients continue smoking despite their symptoms [Citation19,Citation20]. Studies have also documented much higher relapse rates in patients with COPD [Citation21]. This may be attributed to their higher pack-year history, enhanced degree of nicotine dependence, greater risk for depressive symptoms, and poor motivation to quit [Citation17,Citation22].
These patients struggle to completely stop nicotine use and may require prolonged treatment and/or sustained nicotine use to achieve longstanding abstinence and to prevent relapse. Obviously, cessation interventions and outcomes may be improved by taking into consideration specific predictors of quitting attempts and quitting success [Citation23,Citation24] or by promptly addressing relapse [Citation25]. However, this specific knowledge is lacking for COPD patients who smoke.
Hence, more effective strategies that enhance successful quit rates are warranted in a population that usually responds poorly to smoking cessation and that it is vulnerable to relapse. For patients with COPD who are having difficulty stopping smoking, the alternative harm reductionist approach of substituting conventional cigarettes with consumer products that do not require combustion to deliver nicotine (i.e. e-cigarettes – ECs, and heated tobacco products – HTPs) should be considered.
ECs are battery powered devices that operate by heating a metal coil that vaporizes a solution (e-liquid) mainly consisting of glycerol, propylene glycol (PG), distilled water, and flavorings, and which may or may not contain nicotine. The user inhales the aerosol generated by vaporizing the e-liquid in a process commonly referred to as ‘vaping.’ ECs do not contain tobacco, do not create smoke, and do not rely on combustion to operate. Their design and efficiency in nicotine delivery have improved substantially since their market introduction in 2006.
Another class of combustion-free products that has been introduced for cigarette substitution is that of heated tobacco products (HTP, also known as heat-not-burn). They consist of a holder that electronically transfers controlled heat at temperatures that are below 350°C (instead of burning tobacco) to tobacco sticks, plugs, or capsules that generate nicotine-containing aerosols [Citation26,Citation27]. The user places the tobacco product in a holder and draws on it in the same fashion as cigarettes or cigars.
ECs and HTPs have evolved as a popular, yet controversial, tobacco cigarettes substitute method among smokers worldwide [Citation28–33]. Compared with conventional cigarettes, they offer substantial reduction in exposure to toxic chemical emissions [Citation26,Citation27,Citation34–36] and, for this reason, they are proposed for harm reduction from cigarette smoke [Citation36–39] and for smoking cessation [Citation40].
2. Objective and methods of the review
Considering that only limited information is available about the health impact of nicotine delivery technologies in COPD, we authored this narrative review to carefully identify and critically appraise the existing evidence from human studies on the respiratory health effects of ECs/HTPs substitution for COPD patients who smoke. The primary goal is to provide clinicians with evidence on the health effects of ECs/HTPs substitution to inform their recommendations and plans. The twin goal of this narrative review is to promote health literacy in COPD patients who are using or intending to use ECs/HTPs with a specific focus on existing evidence on their respiratory health. Emphasis will be on clinical studies and surveys only. Findings from in vitro and animal studies cannot be directly applied to humans, and they tend to overemphasize negative effects of ECs/HTPs because of abnormal exposure protocols that do not replicate normal condition of use and lack of appropriate experimental controls as discussed extensively in a state-of the-art review [Citation39].
We thoroughly searched published English literature on the impact of EC and/or HTP use on COPD. The literature search was conducted in September 2021 using the following databases: PubMed, Embase, ScienceDirect library, and Scopus. Search terms included ‘COPD’ OR ‘Chronic Obstructive Pulmonary Disease’ OR ‘Chronic Obstructive Airway Disease’ OR ‘COAD’ OR ‘Chronic Obstructive Lung Disease’ OR ‘Chronic Airflow Obstructions’ OR ‘Bronchitis’ OR ‘Emphysema’ AND ‘E-Cigarette’ OR ‘Vaping’ OR ‘Electronic Nicotine Delivery Systems’ OR ‘e-cig*’ OR ‘Electronic Cigarette’ OR ‘vape*’. A separate search string was developed for HTPs and included the following terms: Alternative Nicotine Delivery Systems, Heat-not-burn, Reduced Risk Product, Modified Risk Tobacco Product, MRTP, Tobacco heating systems/products/device, THS/THP, Tobacco heated systems/products/device, Heated tobacco systems/products/device, HTS/HTP, IQOS, glo, PLOOM. Search results were filtered to include only human studies published from 1 January 2000. Titles, abstracts, and full texts of the search results were sequentially screened by JM and GC independently for inclusion, with disagreements resolved via blind review by a third reviewer (RP).
In view of the plethora of discordant findings and interpretations from surveys and clinical studies of patients with COPD, a positive health impact of nicotine delivery technologies cannot be proven or disproven and a clear conclusion cannot be reached. This review article emphasizes the poor quality of the existing scientific literature and highlights the need for high-quality research to assess health effects in COPD patients.
3. Harm reduction and cessation potential of combustion-free nicotine delivery devices
Harm reduction is a public health strategy to reduce or eliminate harms that are caused by unhealthy behaviors, one of which is illicit drugs use/abuse [Citation41]. Harm reduction acknowledges that, while the preferred goal is abstinence, this is not always achievable, and that helping people change to less harmful alternatives may be a more effective approach [Citation41].
In the case of tobacco, it refers to preventing or reducing morbidity and mortality from tobacco use among smokers. While eliminating exposure to toxic chemicals and carcinogens generated by tobacco combustion would result in the greatest reduction of harm, tobacco harm reduction (THR) acknowledges that this is not always achievable, and users may not always be able or willing to quit. So THR advocates that smokers switch to less harmful forms of nicotine consumption [Citation42].
While, in itself, nicotine cannot be ruled out completely as a cause for harm, the current scientific consensus is clear that there is no clinical proof that nicotine is linked to cancer in humans [Citation16,Citation43]. Moreover, the Lung Health Study (a multicenter clinical trial of nicotine gum in a large cohort of smokers with mild-to-moderate COPD with a long-term follow-up of about 15 years) indicated no evidence for an effect of NRT use on overall cancer [Citation44]. Likewise, studies evaluating long-term use of oral smokeless tobacco products (e.g. snus – an oral form of nitrosamine-free smokeless tobacco that has been consumed in Scandinavian countries for decades) have shown that the cancer risks from nicotine intake are minimal [Citation45,Citation46]. The most compelling evidence comes from large population studies of snus. For example, Ramstrom and Wikmans [Citation47], using data from the WHO Global Report on Mortality Attributable to Tobacco to compare rates of smoking-related lung cancer mortality between male snus consumers in Sweden and men in European nations (where snus is banned), found that Swedish men had approximately three times lower rates of lung cancer deaths than men in the European Union. Nonetheless, several experimental studies have found that nicotine can act as a tumor promoter [Citation48–51]. Tumor-promoting activity of nicotine is an area of ongoing research, and prospective clinical and epidemiological studies will be required to obtain conclusive evidence.
There is also a scientific consensus that nicotine consumption does not contribute to respiratory morbidity either [Citation16]. In general, nicotine is relatively safe for human consumption [Citation52]. Smokers die from inhaling toxicants and carcinogens present in cigarette smoke, not nicotine.
A plethora of studies supply evidence for the harm reduction potential of ECs and HTPs.
In a large cross-sectional trial of 181 participants biochemically tested for biomarkers of exposure (BoEs) in five groups: (1) cigarette-only-users, (2) ECs-only-users (>6 months smoking cessation), (3) NRT-only-users (>6 months smoking cessation), (4) dual-users of cigarettes and ECs, and (5) dual-users of cigarettes and NRT, exclusive EC users had substantially lower 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL; a proxy for the cancerogenic nicotine-derived nitrosamine ketone – NNK) levels than all other groups – equivalent to a 97% overall reduction compared to combustible cigarette smokers. In exclusive EC users, 1,3-butadiene and acrylonitrile (among two of the greatest source of cancer risk in tobacco cigarettes) levels were markedly reduced at 11.0% (CI 7.5, 16.1) and 2.9% (CI 1.7, 4.7) of cigarette smokers [Citation53]. Strong evidence of vaping-associated reduction in carcinogens (i.e. NNAL) has also been confirmed in 17.830 participants with urine samples from the Population Assessment of Tobacco and Health (PATH) study, a nationally representative longitudinal study of US adults [Citation54].
Similar evidence has been reported with HTPs in a Cochrane review showing moderate-certainty evidence for lower BoE levels at follow-up in HTP than cigarette smoking groups [Citation55]. Gale et al. enrolled 506 participants in a 12-month randomized controlled switching trial, which were tested to selected cigarette smoke toxicants (including NNAL, 3-HPMA, HMPMA, MHBMA, HEMA, 4-ABP, 2-AN, 1-OHP, eCO, S-PMA, and CEMA) [Citation56]. In continuing smokers, BoE remained stable between baseline and day 180, while huge decreases in most toxicant levels were reported in HTP users, becoming similar to those of controls abstaining from cigarette smoking. Moreover, it was found that switching to HTPs (glo) not only reduced exposure to cigarette smoke toxicants but also improved several health effect indicators when compared to continuing to smoke [Citation56]. Another large switching trial of 984 smokers allocated to continue smoking cigarettes or to use an HTP device (IQOS) for 6 months confirmed similar markedly reduced levels of BoE at follow-up [Citation57]. Another important reduction in exposure provided by EC and HTP use compared to smoking is the elimination of elevated levels of carbon monoxide, a major risk factor for cardiovascular disease, in the exhaled breath. Switching from conventional cigarettes to ECs quickly and universally leads to normalization in exhaled carbon monoxide levels (eCO) [Citation58–60]. Similar findings have been reported with HTPs [Citation55–57,Citation61].
In spite of substantial evidence from analytical chemistry and exposure studies demonstrating that chemical production in EC emission aerosols does not pose a major health concern according to quantitative risk assessment [Citation62,Citation63], opposing viewpoints have been presented [Citation64]. While BoEs are not indicators of disease rates, the significant reduction in exposures experienced by exclusive ECs/HTP users is a positive marker for tobacco harm reduction. With the growing popularity of combustion-free nicotine delivery technologies, such as ECs and HTPs, product substitution is now an important aspect of THR, with the aim of reducing health damage associated with combustible tobacco cigarettes.
There is also growing evidence that ECs and HTPs use may help smokers to achieve sustained abstinence from cigarette smoking. The most recent Cochrane review concludes that ‘There was high certainty that quit rates were higher in people randomized to nicotine EC than in those randomized to NRT (RR 1.63, 95% CI 1.30 to 2.04),’ indicating in absolute terms an additional four quitters per every 100 using ECs [Citation40]. An elegant randomized controlled trial of 886 motivated quitters at the UK National Heath Stop Smoking Service compared ECs and NRTs for successful cessation at one year with biochemical verification [Citation65]. In this study, quit rates were 18.0% for ECs compared to an atypically low 9.9% with NRT (RR 1.83; CI 1.30, 2.58; p < 0.001). Of note, among those achieving one-year abstinence with ECs, 80% were still using ECs at the one-year follow-up, a possible indication of the effectiveness of ECs for relapse prevention. As discussed earlier, relapse is a widespread problem for smoking cessation. A study drawn from survey data shows the effectiveness of EC use in preventing relapse. Based on combined data from the 2014 and 2015 US National Health Interview Surveys, the prevalence of being quit during the prior 6 years was significantly higher among daily EC users compared to those who had never used ECs (52.2% vs. 28.2%). After adjustment for co-variates, daily EC use was consistently the strongest independent correlate of smoking cessation and did not vary by gender [Citation66]. How could EC use act to prevent relapse? It is possible that for some, ECs can substitute the physical, psychological, social, cultural, and identity-related dimensions that were previously enjoyed when smoking tobacco cigarettes, thus may uniquely support long-term smoking relapse prevention [Citation67]. However, conflicting findings on the topic of relapse prevention among EC users that have stopped smoking have been shown in large cross-sectional surveys in the US [Citation68,Citation69]. There is no information about relapse prevention among EC users with COPD. Formal demonstration of the efficacy of HTPs for smoking cessation is just emerging from analyses of large surveys showing that the current use of HTPs is common among former smokers [Citation70] and from randomized controlled trials showing that HTPs are as effective as refillable ECs for stopping smoking [Citation71].
Many users do not switch exclusively to ECs and do use ECs and combustible cigarettes (dual use) for a variable period of time thus potentially prolonging exposure to harmful toxic chemicals in the tobacco smoke. Dual use is known for being a common transitory state, with transitions to exclusive use taking a variable time to occur [Citation72,Citation73]. Recent trends show a fall in dual-use prevalence; in the UK and US, dual-use prevalence among adult EC users is about 30% [Citation74–76]. Greater declines in dual usage rate have been reported for youth in the US [Citation77–79].
Complete cigarette substitution with combustion-free nicotine delivery products (either ECs or HTPs) may reduce health damage associated with tobacco smoke. A large inter-laboratory replication study conducted in parallel in Italy, Greece, Serbia, Oman, Indonesia, and USA has confirmed that combustion-free nicotine delivery technologies can substantially reduce cytotoxicity and inflammatory burden compared to conventional cigarettes by >80% [Citation80]. Experiments were conducted and validated using the International Organization for Standardization (ISO) standard and protocols resembling normal condition of use.
Therefore, it is expected that switching away from combustible tobacco cigarettes would produce significant improvements. This is consistent with what we have learnt over the last 50 years about tobacco smoke chemical composition and respiratory disease pathogenesis. Nonetheless, only limited information is available regarding the health impact of these nicotine delivery technologies in COPD.
4. Health impact of combustion-free nicotine delivery devices: surveys
A number of surveys have looked into the question of whether the use of combustion-free nicotine delivery technologies by patients at risk of, or with, COPD may impact respiratory health outcomes. We identified a total of 16 relevant papers investigating the association between EC use and COPD or COPD/asthma-like symptoms: 3 longitudinal studies [Citation81–83] and 13 cross-sectional surveys [Citation84–96] (). The search did not find any surveys on HTP use. Their quality assessment indicated poor quality ratings for most studies.
Table 1. Summary list and description of included surveys.
In summary, most of these cross-sectional studies have reported significant association between EC use and self-reported diagnosis of COPD or COPD-like symptoms. The association is expected considering that the vast majority of EC users are former (single users) or current smokers (dual users). Nonetheless, cross-sectional studies cannot establish a causal relationship. They also fail to provide a consistent and meaningful characterization of exposure; definitions of exposure for vaping studies (e.g. daily, one puff in last 30 days, one puff ever, average duration of EC use) should be tailored to the hypothesis and consistent with metrics in related research. Moreover, these studies should be adjusted for previous smoking history, a clear confounder in COPD.
For example, a paper analyzing data pooled from two large observational US cohorts – which was rated of high quality [Citation97] – concluded that EC users had more rapid decline in lung function [Citation81], but this trend did not persist after adjustment for conventional cigarette smoking (which is an obvious key factor driving the accelerated decline in lung function). Also, selection bias was an important potential confounder, with EC users having heavier cigarette smoking history thus explaining why they were more likely to report chronic bronchitis and poorer respiratory health and to have more rapid decline in lung function [Citation81]. As for most studies in this category, the authors relied on poorly constructed measures of EC use (i.e. mixing together ever and current use to define EC exposure), which makes it hard to determine if the reported association between EC use and self-reported diagnosis of COPD is real or merely the result of misclassification error. A more detailed appraisal of this study has been published [Citation98].
Another study that segmented never and current smokers was the cross-sectional random-dial phone survey by Wills et al. [Citation95]. The authors examined respiratory health among e-cigarette users in Hawaii, USA, aged 55 years and older and found no significant association between EC use and self-reported chronic respiratory conditions (asthma and COPD) in the entire sample, which included smokers (AOR 1.27, CI 0.96–1.67; p = 0.10); however, when the analysis was limited to nonsmokers, there was a barely significant association (AOR 1.33, CI 1.00–1.77; p < 0.05). The study did not appear to account for relevant confounders (e.g. information that could have an impact, such as a family history of allergy disorders, passive smoking, or occupational exposures, was not obtained). As for the other surveys in this review article, this study shares the obvious weaknesses of inferring causality from cross-sectional design that does not have information about temporal sequence between cause (i.e. vaping) and effect (i.e. disease outcomes), and the absence of exposure data that does not allow examination of potential dose–response relationships. Notably, vaping behavior was defined as ‘one puff ever’ (indicating insignificant EC exposure, probably casual experimentation with the product), whereas smoking behavior in a group with a mean age of 55 years suggests that people have smoked cigarettes more regularly and over a longer period of time (probably for decades) [Citation95]. Attributing respiratory injury to such a modest degree of exposure would imply that vaping has a significant unfavorable acute and chronic impact; this is very unlikely, as biologically implausible. The link is clearly due to residual confounding from smoking. The authors may have reported how associations could change when different strata of former smokers (recency of quitting, intensity/frequency of use) are considered. Or should have looked at the associations for regular daily vaping rather than just presenting results for all ever-triers. Or could have assessed whether the subjects had symptoms before they started vaping.
Perez et al. [Citation89] examined the association between EC use and COPD and found an association. Unfortunately, EC use is poorly characterized, and the definition of COPD is ambiguous. Exposure in this study was a vague mix of daily and occasional use of unknown duration. Even if we accept that the exposure to vape aerosols was as intense as daily and as prolonged as 5 years among never smokers, and that EC aerosol emissions are as harmful as tobacco smoke, it is implausible to expect development of COPD in such a short period of time. Given that >80% of the study population was not in the age-risk category for COPD, a major flaw of the study is that the dependent variable appraised by the authors is unlikely to be COPD (especially in younger ages). Transient dry cough is frequently (approx. 20–30%) reported in first-time users, due to the irritant effect of PG/VG inhalation. If doctors in this study have deemed this transient cough to be a symptom for the diagnosis of COPD, then we have a serious problem of misclassification.
In their cross-sectional study, using a large representative sample of 18–24 years old from the Behavioral Risk Factor Surveillance System (BRFSS), Bircan et al. [Citation84] reported significant associations between EC use and self-reported diagnosis of asthma, COPD, and asthma-COPD overlap syndrome. These authors elegantly excluded people with previous and current smoking history from their study sample, thus limiting the impact of this major confounder on chronic respiratory diseases. Once again, diagnostic mislabeling (a problem not unique to BRFSS) cannot be underestimated. Also, the reported association rests on a small number of cases and their propensity score only matches demographic conditions, but does not measure the propensity of developing COPD. Last but not least, if the causal claim is that a substantial frequency of vaping causes chronic respiratory problems to develop, and then the study design should not have been based on 18–24-year-old whose historical exposure is perforce minimal. Nonetheless, the authors found an association between vaping and COPD among never cigarette smokers in the 18–24-year-old age bracket, with over a quarter of the vapers and over a fifth of the non-vapers reporting having COPD alone or COPD plus asthma. This is an unexpected finding since COPD is very unlikely to be diagnosed prior to the age of 40 and is much less prevalent among never cigarette smokers than current or former smokers. There is no obvious explanation then that these respondents were misclassified as COPD. Most importantly, BRFSS – but same limitation also applies to another federal survey, the National Health Interview Survey (NHIS) – does not introduce questions that would generate information about the age of disease diagnosis and the correct timing of EC use initiation.
A prospective evaluation of EC users would be the most useful approach to prove or disprove the negative health impact of combustion-free nicotine delivery technologies. In a longitudinal survey of the Population Assessment of Tobacco and Health (PATH) study, Xie et al. [Citation99] investigated the respiratory health effect of EC use in a nationally representative cohort of US young adults. The authors showed that both former and current EC use was associated with higher odds of developing any respiratory symptom (aOR = 1.20 and 1.32 for former and current EC use, respectively). However, cigarette smoking history was insufficiently adjusted for in the analysis. Using cigarette smoking as a binary covariate (i.e. yes/no) in an adjusted model is insufficient. A much stronger analytical approach is to adjust for cumulative amount of cigarette smoked (i.e. pack-years) as used in the recent study by Sargent et al. [Citation100]. These authors also used dataset from the PATH study, but when they adjusted for pack-years of smoking history the significant association became non-significant – illustrating how much residual confounding there is when using ‘crude’ binary measures. While there is a tendency for EC use at baseline to be associated with the onset of new symptoms, the ORs have quite wide confidence intervals and are not always significant. Furthermore, where associations with cigarette smoking are seen, the odds ratios are typically somewhat larger. Thus (as seen in Table 3 of Xie et al.), all the ORs for EC only relative to cigarette only are below 1, and even where they are significant – e.g. wheezing in the chest where we have a fully adjusted OR of 0.62 (95% CI 0.39–0.99) – one cannot really tell whether EC is only slightly better or quite a lot better than cigarettes. Last but not least, Xie et al. note that a limitation of their study is that ‘exposure and outcome measures were self-reported and may be subject to misclassification.’
That efficient adjustment of cigarette smoking history is paramount to correctly evaluate association between EC use and COPD incidence has been confirmed by a recent analysis of PATH Study data [Citation101]. An association between 30-day EC use and increased risk of COPD was found in the unadjusted models, but this relationship disappeared after adjusting for cigarette pack-years. In addition, exclusive use of ECs was not associated with higher COPD incidence (RR = 1.36, CI [0.55, 3.39]).
In addition to the lack of adequate adjustment for previous smoking history as a clear confounder, cross-sectional population-based data that fail to include data on the age of initiation of EC and combustible cigarette use cannot be relied on for drawing conclusions regarding potentially causal associations with COPD. Rodu and Plurphanswat have used data from the first wave of the PATH Study (which has information about the age of disease diagnosis and EC and combustible cigarette use initiation) to examine the reliability of associations found in cross-sectional studies [Citation102]. The authors’ analysis concluded that COPD was only rarely diagnosed in respondents who had initiated EC use prior to the age of COPD diagnosis. On the contrary, as expected, COPD was nearly always diagnosed following many years after the age of initiation of smoking and represented 97% of all COPD diagnoses. This important article has been examined in a recent commentary [Citation103].
5. Health impact of combustion-free nicotine delivery devices: clinical studies
The negative evidence emerging from surveys is in stark contrast with longitudinal clinical studies of real patients with COPD. A 5-year multicenter study monitored the health parameters in COPD patients who substantially attenuated or ceased their cigarette consumption after switching to ECs [Citation104]. Changes in daily cigarette smoking, annualized disease exacerbations, lung function indices, patients reported outcomes (COPD assessment test – CAT scores) and 6-minute walk distance (6MWD) from baseline were measured in smokers with COPD prior to and over a 5-year follow-up period after switching to ECs in comparison with an age-sex-matched control group of smokers with COPD who did not use ECs and continued smoking. Complete data sets were available for 39 patients (20 in the EC group). The study findings indicated a significant and sustained improvement in lung function, symptoms, and functional ability in the EC users compared with the reference group, likely as a result of reduction in the harmful effects of continuing smoking. Daily solos ECs use elicited a substantial (52%) decrease in annualized COPD exacerbations by the end of the study. That respiratory exacerbations were halved in patients with COPD who ceased or markedly reduced their tobacco consumption after switching to ECs was a key finding. Smoking increases susceptibility to respiratory infections, and quitting smoking is known to reduce this risk [Citation105]. In addition, substantial and clinically significant improvements in CAT scores and 6MWD were observed in the EC cohort. No significant changes were noted in COPD patients who continued smoking. Small improvements in post-bronchodilator forced expiratory flow in one second (FEV1) were also noted over the 5-year observation period in COPD EC users. As expected, amelioration in exacerbation rates, overall health status, and lung function also caused progressive improvement in Global initiative for chronic Obstructive Lung Disease (GOLD) COPD Stages in many patients in the EC group. Another important finding of the study is that only 8.3% patients from the COPD EC user group relapsed to cigarette smoking over the 5-year duration of the study, thus suggesting that relapse prevention may be an important mechanism by which vaping contributes to long-term smoking abstinence. ECs were well tolerated. However, these findings must be interpreted cautiously because they are based on a small, self-selected sample of patients with mild-moderate COPD, and larger studies will be necessary to assess the long-term effects and safety of these products. It must also be emphasized that the impact on the severe form of the disease has not been investigated.
Health impact and tolerability of ECs on COPD have also been investigated in a small acute study by Palamidas et al. [Citation106]. The authors asked 16 smokers with COPD to puff an EC under controlled conditions (ad libitum for 10 min) before measuring their respiratory symptoms and airways physiology. No difference in cough frequency was observed between COPD patients and healthy controls. A small decrease in forced exhaled nitric oxide (NO) was shown in all participants, but changes were not statistically significant between study groups. Small changes in airway mechanics were within the variability of the test, with healthy non-smokers having the greater change.
These findings are in agreement with several acute clinical studies consistently showing no changes in respiratory symptoms, lung function, or signs of airway inflammation in response to EC use (both subjects with preexisting respiratory condition and matched healthy controls) [reviewed in ref. 39] and also consistent with results from a large internet survey reporting transient throat irritation, dry cough, and other symptoms of respiratory irritation in some smokers when switching to ECs [Citation107]. The same survey also showed improvements in respiratory symptoms in a sub-sample of 1190 regular EC users with COPD, with 75.7% of the respondents reporting symptoms improvement after switching [Citation107].
COPD patients using EC report mild transient coughing and modest changes in respiratory physiology that are no different from healthy controls. The acute changes described here are simply suggestive of non-specific irritant effects in response to EC aerosol emissions, indicating an efficient physiologic defensive reflex response. This airway response to inhaled respiratory irritants does not indicate EC vapor emission-specific effects [reviewed in ref. 39].
Nonetheless, the possibility of a negative impact on patients with COPD cannot be ruled out, particularly in patients with severe form of the disease, and the long-term health effects of combustion-free nicotine products require investigation. The potential health impact of ECs has been addressed in several review articles, with conflicting conclusions [Citation39,Citation108,Citation109].
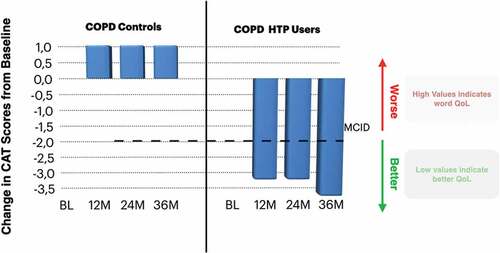
An alternative approach for patients with COPD who are having difficulty at stopping smoking or not intending to quit is that of substituting conventional cigarettes with HTPs. To date, the evidence about health outcomes in COPD patients who have switched to HTP use is limited. In the first study to investigate the long-term health effects of HTP use in COPD patients, investigators from four Italian hospitals monitored health parameters for 3-years in COPD patients who substantially attenuated or ceased cigarette consumption after switching to HTPs and findings were compared to age-sex-matched COPD patients who continued to smoke [Citation110]. On average, the HTP group achieved either a sustained smoking cessation (~60% of the group) or marked reduction in the number of cigarettes smoked per day, while no changes in smoking consumption were noted in the control group. Thus, the switch to HTPs successfully achieved the goal of smoking cessation/reduction. In spite of the small number of patients in each study group who completed the 3-year follow-up (n= 19), daily solos HTPs use elicited significant reductions in acute exacerbations of COPD (>40%) and caused improvements in symptoms, clinically relevant amelioration in health-related quality of life (), and enhanced exercise capacity (assessed by 6-minute walk distance). No improvements were observed in COPD patients who continued smoking. HTPs were well tolerated and no deterioration in post-bronchodilator forced expiratory volume in 1 s [FEV1], forced vital capacity [FVC], and %FEV/FVC was observed in patients with COPD who stopped or considerably reduced their tobacco consumption by switching to HTP use. As for the 5-year multicenter study with EC discussed earlier, the same careful interpretation of the findings applies here. Analyses of these studies have been presented in recent editorials [Citation111,Citation112].
Figure 1. Change in median COPD assessment tool (CAT) scores from baseline in COPD-heated tobacco product users and COPD controls. The bold dashed line on the bar chart illustrates the MCID for CAT score.
It is not surprising to observe harm reversal with combustion-free nicotine delivery technologies. For example, restoration of lung defense has been shown in daily solos EC and HTP users [Citation113]. A study has found that smokers who had switched to combustion-free nicotine delivery technologies (i.e. ECs and HTPs) exhibited mucociliary clearance efficiency similar to that of never and former smokers [Citation113]. This means that smokers who have switched away from combustible cigarettes regain an important protection against lung infections and inflammation. In turn, this decreases the risk of infection and provides an additional explanation for the significant reduction in respiratory exacerbations observed in patients with COPD who ceased or markedly reduced their tobacco consumption after switching to ECs or HTPs [Citation104,Citation110]. The fact that stopping smoking (including by substituting tobacco cigarettes with non-combustible sources of nicotine) would produce substantial improvement in mucociliary clearance is a significant finding, but it is not unexpected. Under normal conditions of use, the level of cilia-toxic chemicals in EC and HTP aerosol emissions are 80–99% lower compared to cigarette smoke [Citation26,Citation27,Citation34,Citation35,Citation80]. Accordingly, exposure to aerosols generated from combustion-free nicotine delivery technologies is expected to have a much less disruptive impact on the functional elements and self-repair characteristics of mucociliary defense system. This conclusion is consistent with what we have learnt about tobacco smoke chemical composition and COPD pathogenesis over the last 40–50 years.
Given the current status quo in tobacco control and the rising global burden of COPD [Citation114], it is sensible to review any data that may show real progress. Recent work using real-world data from Japan has gathered evidence that widespread substitution of cigarettes with combustion-free products can have a substantial positive impact. In particular, a time-trend analysis on hospitalizations for COPD exacerbations among the Japanese population showed a statistically significant decrease in the number of hospitalizations after the introduction of HTPs in Japan [Citation115].
6. Conclusion
Slowing down disease progression, reducing respiratory exacerbations, and improving quality of life are unmet needs in the management of patients with COPD. The only proven method for improving prognosis is quitting smoking, but it is discouraging that most smoking cessation schemes do not seem to work for the vast majority of COPD smokers, and many continue smoking despite their symptoms. Moreover, serious implementation of this approach requires time and dedication from the clinician, as well as awareness and appreciation of its effectiveness, requirements that – despite their importance – may be sorely lacking in the medical community [Citation116–118].
Although combustion-free nicotine delivery alternatives (e-cigarettes, heated tobacco, etc.) carry a much-reduced risk compared to conventional cigarettes, it is imperative to emphasize that they should only be used by patients with COPD unable to quit smoking despite having seriously attempted to quit using conventional measures, including approved pharmacotherapy and counseling. However, considerable controversy continues to surround the use of these products, mainly with regard to their potential use (misuse) by nonsmoking youths, a controversy illustrated by several commentaries and editorials [Citation119–122].
Nevertheless, any measures that can improve respiratory symptoms and quality of life should not be dismissed light-heartedly. As many COPD smokers prefer to smoke, conventional cigarette substitution should be considered as a valuable solution to the persistent problem of smoking, and combustion-free nicotine delivery technologies should be weighted as a component of this strategy.
Our analysis of existing human studies on the respiratory health impact of ECs/HTPs substitution for COPD patients who smoke fails to reach a clear conclusion because of the discordant findings and unreliable interpretations driven from surveys and clinical studies of modest quality.
This review article highlights the need for large, carefully designed, adequately controlled, long-term follow-up clinical trials to assess the true potential of combustion-free nicotine delivery technologies for sustained smoking cessation and reducing the risk of harm from smoking, particularly among smokers with chronic obstructive pulmonary disease (COPD). Appropriate outcomes of these studies should include, among others, changes in lung function, respiratory symptoms, health-related quality of life, exacerbations of COPD, physical functional ability, thoracic imaging (e.g. high-resolution computed tomography), as well as readily accessible biomarkers associated with the severity and progression of this disease. These recommendations have been discussed in a recent commentary [Citation123].
When evaluating the non-acute effects of combustion-free nicotine delivery technologies, epidemiologists must consider the health consequences of previous smoking history, including the duration of smoking, the time since quitting, and the frequency of cigarette consumption, as well as ensure the temporal relationships are consistent with the association being tested. Propagation of common mistakes in epidemiological surveys of respiratory health in COPD must be fixed. Common flaws in the methodology of epidemiology research have been identified and analyzed systematically [Citation102,Citation124,Citation125].
Implementation of shared methodologies within the scientific community that will set common standards on tobacco harm reduction science is necessary. Formulating and establishing standardization of methodologies for scientific research on ECs and HTPs is a crucial step.
7. Expert opinion
Evidence-based recommendations urge systematic identification of any cigarette users, which includes COPD patients [Citation19,Citation126]. All smokers with COPD should be advised to quit with clear, personalized instructions. Health professionals should assist COPD patients intending to quit by presenting a cessation plan with a commitment to a quit date and frequent follow-up visits. Physicians should also consider prescribing medications (e.g. NRT, bupropion, and varenicline) to ameliorate nicotine withdrawal symptoms in combination with cessation counseling for best results [Citation126,Citation127]. In general, these medications are also effective in COPD and have no specific contraindications in patients with mild-moderate COPD [Citation128,Citation129].
Establish a strong alliance with your COPD patient by explaining what to expect. For example, mention the possibility that withdrawal symptoms might still occur even with medications, but point out that some people do not experience any of these problems and a few might experience all of them. COPD patients should also be advised that their respiratory symptom (primarily cough) might worsen within the first couple of weeks after smoking cessation soon after smoking cessation [Citation130]. Although medical management of respiratory patients who smoke is no different from those who do not smoke, patients experiencing worsening respiratory symptoms when stopping smoking can benefit from a temporary increase in bronchodilators [Citation131].
Recognizing risk factors that predict cessation failure might require more intensive treatment and referral to specialized centers. For example, COPD patients with major depression, mental illnesses, and/or substance abuse are very vulnerable to relapse [Citation17,Citation22].
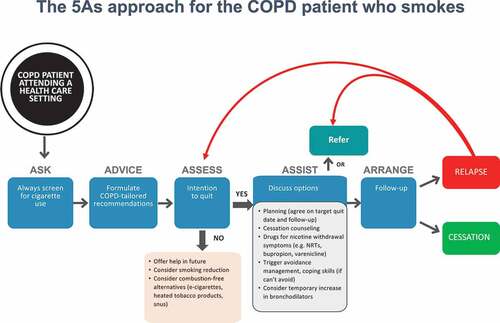
Patients who are having difficulty stopping smoking should receive a brief intervention designed to increase future quit attempts. For these smokers, it is better to wait until they feel ready and confident enough to stop. Smoking reduction might be a viable treatment approach to promote future smoking abstinence among smokers who are not ready to repeat a quit attempt. Alternatively, substitution of conventional cigarettes with a less harmful source of nicotine (i.e. ECs or HTPs) should be considered. A decisional algorithm for assisting smokers with COPD is shown in .
Figure 2. An algorithm for the management of the COPD patient who smoke. The approach has been adapted from that recommended by US Agency for Health Care Policy and Research (AHCPR) JAMA 2000; 283:3244–54.
Electronic cigarettes (ECs) and heated tobacco products (HTPs) are continuing to gain popularity among consumers worldwide [Citation28–33]. However, many health professionals are uncertain about the potential benefits or adverse effects of these reduced risk products. In spite of the existing controversy surrounding the use of combustion-free nicotine products [Citation119–122], a number of significant positive actions have been adopted by regulatory bodies and scientific authorities. For instance, in the United States, the Food and Drug Administration (FDA) has recently authorized the legal commercialization of tobacco heated products and e-cigarettes, affirming ‘that marketing of these products is appropriate for the protection of public health’ based on the FDA’s robust, scientific evaluation of reduced consumers’ exposure to harmful chemicals when completely switching away from conventional cigarettes [Citation132,Citation133]. However, some ECs have been denied approval. Although not designed as smoking cessation products, the latest Cochrane review confirmed that e-cigarettes may help smokers quit, recognizing the effectiveness of these devices in cessation programs [Citation40]. Along similar lines, the National Institute for Health and Care Excellence (NICE) committee ‘agreed that, because many of the harmful components of cigarettes are not present in e-cigarettes, switching to nicotine-containing e-cigarettes was likely to be significantly less harmful than continuing smoking’ and ‘ … that people should be able to access them as part of the range of [cessation] interventions they can choose to use’ [Citation134].
Fast innovation in combustion-free technologies is likely not only to further reduce residual health risks but also to increase health benefits in exclusive users. With the development of improved product designs, we are now beginning to see that products’ adoption rates (and consequently the extent of reduction in cigarette consumption) are strongly associated with efficient smoking cessation, smoking cessation being the main ‘collateral benefit’ for many smokers switching to their regular use [Citation135–137]. In addition, people concerned about the toxicology profile of vaping products should consider that modern devices are continuing to improve in quality and safety. The risk of harm from modern regulated devices from reputable manufacturers who fully comply with standards of quality and safety is extremely small.
Research on these emerging products is intense and scientific publications are growing at an exponential rate. More high-quality work is necessary to quantify the relative risk of using these emerging technologies compared to cigarette smoking, to accurately establish product quality and safety in absolute terms, and to assess health effects in vulnerable populations, including COPD patients.
Article highlights
Patients with chronic obstructive pulmonary disease (COPD) who smoke find it very hard to quit smoking and many do not intend to quit smoking
Most COPD patients who smoke rarely have success with standard cessation interventions, with many continuing to smoke despite their symptoms
Compared with conventional cigarettes, e-cigarettes (ECs) and heated tobacco products (HTPs) offer substantial reductions in exposures to toxic chemicals
For patients with COPD who are having difficulty stopping smoking, the alternative of lessening the negative health effects of smoking by switching from conventional cigarettes to EC or HTPs should be considered
Relapse prevention may be an important mechanism by which EC or HTP use contributes to long-term smoking abstinence
Clear conclusions on the impact of EC and HTP on COPD patients’ health long term cannot be reached due to limitations in the available studies
Well-controlled clinical trials and large-scale prospective cohort studies with long-term follow-up are needed to obtain conclusive evidence for or against EC and HTPs use in patients with COPD.
Declaration of interest
J Morjaria has received honoraria for speaking and financial support to attend meetings/advisory boards from Wyeth, Chiesi, Pfizer, MSD, Boehringer Ingelheim, Teva, GSK/Allen & Hanburys, Napp, Almirall, AstraZeneca, Trudell, Cook Medical, Medela AG, and Novartis. J Morjaria has been an expert witness in a court case relating to the impact of smoking on illness severity, ITU admissions, and mortality from Covid-19 in South Africa in 2020. The entire proceeds of the work were donated to various charities. R Polosa is a full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same university. In relation to his recent work in the area of respiratory diseases, clinical immunology, and tobacco control, R Polosa has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics, and Forest Laboratories. Lecture fees from a number of European EC industries and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vaper advocacy no-profit organizations. R Polosa has also received grants from European Commission initiatives (U-BIOPRED and AIRPROM) and from the Integral Rheumatology & Immunology Specialists Network (IRIS) initiative. R Polosa has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, Boehringer Ingelheim, Novartis, Duska Therapeutics, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl., Health Diplomats, and Sermo Inc. R Polosa has served on the Medical and Scientific Advisory Board
of Cordex Pharma, Inc., CV Therapeutics, Duska Therapeutics Inc., Pfizer, and PharmaCielo. R Polosa is also founder of the Center for Tobacco Prevention and Treatment (CPCT) at the University of Catania and of the Center of Excellence for the acceleration of HArm Reduction (CoEHAR) at the same university, which has received support from the Foundation for a Smoke Free World to conduct eight independent investigator-initiated research projects on harm reduction. R Polosa is currently involved in a patent application concerning an app tracker for smoking behavior developed for ECLAT Srl. R Polosa is also currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti-Smoking League), the Consumer Advocates for Smoke-free Alternatives (CASAA), and the International Network of Nicotine Consumers Organizations (INNCO); Chair of the European Technical Committee for standardization on ‘Requirements and test methods for emissions of electronic cigarettes’ (CEN/TC 437; WG4). R O’Leary is supported with a contract from ECLAT Srl. ECLAT receives funding from the Foundation for a Smoke-Free World. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The contents, selection, and presentation of facts, as well as any opinions expressed in this paper, are the sole responsibility of the authors and under no circumstances shall be regarded as reflecting the positions of industry and traders promoting combustion-free nicotine products. We wish to thank the five anonymous reviewers for their insightful comments and careful consideration of the manuscript.
Additional information
Funding
References
- World Health Organization (WHO). The top 10 causes of death. 2020. [cited 2022 Mar 03] Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(4):258–266. discussion 290-251.
- Morjaria JB, Malerba M, Polosa R. Biologic and pharmacologic therapies in clinical development for the inflammatory response in COPD. Drug Discov Today. 2010;15(9–10):396–405.
- How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. cited Sept 2017. 2010. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
- Stratton K, Shetty P, Wallace R, et al. Clearing the smoke: the science base for tobacco harm reduction–executive summary. Tob Control. 2001;10(2):189–195.
- Pauwels RA, Löfdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European respiratory society study on chronic obstructive pulmonary disease. N Engl J Med. 1999 Jun 24;340(25):1948–1953.
- Culpitt SV, Maziak W, Loukidis S, et al. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999 Nov;160(5 Pt 1):1635–1639.
- Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2(4682):739–748.
- Chen W, Thomas J, Sadatsafavi M, et al. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639.
- Peng Y, Zhong GC, Wang L, et al. Chronic obstructive pulmonary disease, lung function and risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. BMC Pulm Med. 2020 May 11;20(1):137.
- U.S. Department of Health and Human Services. Smoking Cessation. A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2020.
- Hersh CP, DeMeo DL, Al-Ansari E, et al. Predictors of survival in severe, early onset COPD. Chest. 2004;126(5):1443–1451.
- Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The lung health study. JAMA. 1994;272:1497–1505.
- Burchfiel CM, Marcus EB, Curb JD, et al. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. Am J Respir Crit Care Med. 1995;151(6):1778–1785.
- Kanner RE, Connett JE, Williams DE, et al. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med. 1999;106(4):410–416.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: a Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014.
- Jimenez-Ruiz CA, Masa F, Miravitlles M, et al. Smoking characteristics: differences in attitudes and dependence between healthy smokers and smokers with COPD. Chest. 2001;119(5):1365–1370.
- Hoogendoorn M, Feenstra TL, Hoogenveen RT, et al. Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax. 2010;65(8):711–718.
- Tashkin DP. Smoking cessation in chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2015 Aug;36(4):491–507.
- van der Meer RM, Wagena EJ, Ostelo RW, et al. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003;2003(2). DOI:10.1002/14651858.cd002999.
- Wagena EJ, Knipschild PG, Huibers MJH, et al. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Arch Intern Med. 2005;165(19):2286–2292.
- Zhang MW, Ho RC, Cheung MW, et al. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33(3):217–223.
- Klemperer EM, Mermelstein R, Baker TB, et al. Predictors of smoking cessation attempts and success following motivation-phase interventions among people initially unwilling to quit smoking. Nicotine Tob Res. 2020 Aug 24;22(9):1446–1452.
- Caponnetto P, Polosa R. Common predictors of smoking cessation in clinical practice. Respir Med. 2008;102(8):1182–1192.
- Caponnetto P, Keller E, Bruno CM, et al. Handling relapse in smoking cessation: strategies and recommendations. Intern Emerg Med. 2013 Feb; 8(1):7–12.
- Haziza C, de La Bourdonnaye G, Merlet S, et al. Assessment of the reduction in levels of exposure to harmful and potentially harmful constituents in Japanese subjects using a novel tobacco heating system compared with conventional cigarettes and smoking abstinence: a randomized controlled study in confinement. Regul Toxicol Pharmacol. 2016Nov;81:489–499. DOI:10.1016/j.yrtph.2016.09.014
- Gale N, McEwan M, Camacho OM, et al. Changes in biomarkers of exposure on switching from a conventional cigarette to the glo tobacco heating product: a randomized, controlled ambulatory study. Nicotine Tob Res. 2021 Feb 16;23(3):584–591.
- European Commission. Special Eurobarometer 506: attitudes of Europeans towards tobacco and electronic cigarettes. European Commission. 2021 [cited 2022 Sep 05] https://data.europa.eu/data/datasets/s2240_506_eng?locale=en
- GOV.UK. Vaping in England: evidence update February 2021. A report commissioned by Public Health England. Public Health England. 2021 [cited 2022 Mar 03]. Available from: https://www.gov.uk/government/publications/vaping-in-england-evidence-update-february-2021.
- Jerzynski T, Stimson GV, Shapiro H, et al. Estimation of the global number of e-cigarette users in 2020. Harm Reduct J. 2021Oct23;18(1):109. PMID: 34688284; PMCID: PMC8541798.
- Hori A, Tabuchi T, Kunugita N. Rapid increase in heated tobacco product (HTP) use from 2015 to 2019: from the Japan ‘Society and New Tobacco’ Internet Survey (JASTIS). Tob Control. 2020 Jun 5;30(4):474–475.
- Ratajczak A, Jankowski P, Strus P, et al. Heat not burn tobacco product-a new global trend: impact of heat-not-burn tobacco products on public health, a systematic review. Int J Environ Res Public Health. 2020 Jan 8;17(2):409.
- Miller CR, Sutanto E, Smith DM, et al. Characterizing heated tobacco product use among adult cigarette smokers and nicotine vaping product users in the 2018 ITC four country smoking & vaping survey. Nicotine Tob Res. 2022 Mar 1;24(4):493–502.
- Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67–86.
- Daynard R. Public health consequences of e-cigarettes: a consensus study report of the National Academies of Sciences, Engineering, and Medicine.J Public Health Pol.2018;39(3):379–381.
- Polosa R, Rodu B, Caponnetto P, et al. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J. 2013;4(10):19.
- Morjaria JB, Mondati E, Polosa R. E-cigarettes in patients with COPD: current perspectives. Int J Chron Obstruct Pulmon Dis. 2017;1(12):3203–3210.
- O’Leary R, Polosa R. Tobacco harm reduction in the 21st century. Drugs Alcohol Today. 2020;20(3):219–234.
- Polosa R, O’Leary R, Tashkin D, et al. The effect of e-cigarette aerosol emissions on respiratory health: a narrative review. Expert Rev Respir Med. 2019 Sep;13(9):899–915.
- Hartmann-Boyce J, Lindson N, Butler AR, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216.
- Lenton S, Single E. The definition of harm reduction. Drug Alcohol Rev. 1998 Jun;17(2):213–219.
- Zeller M, Hatsukami D, Strategic Dialogue on Tobacco Harm Reduction Group. The strategic dialogue on tobacco harm reduction: a vision and blueprint for action in the US. Tob Control. 2009 Aug;18(4):324–332.
- World Health Organization International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. In: Cancer I, editor. Tobacco smoke and involuntary smoking 2004 . Geneva: World Health Organization International Agency for Research on Cancer; 2004. Vol. 83, p. 9–1413.
- Murray RP, Connett JE, Zapawa LM, et al. Does nicotine replacement therapy cause cancer? Evidence from the lung health study. Nicotine Tob Res. 2009;11(9):1076–1082.
- Lee PN, Hamling J. Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Med. 2009;7(1):36.
- Lee PN. Epidemiological evidence relating snus to health–an updated review based on recent publications. Harm Reduct J. 2013;10(1):36.
- Ramstrom L, Wikmans T. Mortality attributable to tobacco among men in Sweden and other European countries: an analysis of data in a WHO report. Tob Induc Dis. 2014;24(14):1–4.
- Young RP, Scott RJ. Inhaled nicotine and lung cancer: potential role of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 2020;117(9):4460–4461.
- Tang MS, Wu XR, Lee HW, et al. Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice. Proc Natl Acad Sci U S A. 2019 Oct 22;116(43):21727–21731.
- Sanner T, Grimsrud TK. Nicotine: carcinogenicity and effects on response to cancer treatment - A review. Front Oncol. 2015 Aug 31;5:196. DOI:10.3389/fonc.2015.00196.
- Kyte SL, Gewirtz DA. The influence of nicotine on lung tumor growth, cancer chemotherapy, and chemotherapy-induced peripheral neuropathy. J Pharmacol Exp Ther. 2018 Aug;366(2):303–313.
- Greenland S, Satterfield MH, Lanes SF. A meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Saf. 1998;18(4):297–308.
- Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med. 2017 Mar 21;166(6):390–400.
- Dai HD, Leventhal AM, Khan AS. Trends in urinary biomarkers of exposure to nicotine and carcinogens among adult e-cigarette vapers vs cigarette smokers in the US, 2013-2019. JAMA. 2022 Nov 8;328(18):1864–1866.
- Tattan-Birch H, Hartmann-Boyce J, Kock L, et al. Heated tobacco products for smoking cessation and reducing smoking prevalence. Cochrane Database Syst Rev. 2022 Jan 6;1(1):CD013790.
- Gale N, McEwan M, Camacho OM, et al. Changes in biomarkers after 180 days of tobacco heating product use: a randomised trial. Epub 2021 Jul 1, Intern Emerg Med. 2021 Nov;16(8):2201–2212.
- Lüdicke F, Ansari SM, Lama N, et al. Effects of switching to a heat-not-burn tobacco product on biologically relevant biomarkers to assess a candidate modified risk tobacco product: a randomized trial. Cancer Epidemiol Biomarkers Prev. 2019 Nov;28(11):1934–1943.
- Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-cigarette) on smoking cessation and reduction: a prospective pilot study. BMC Public Health. 2011;11(1):786.
- Caponnetto P, Campagna D, Cibella F, et al., EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317.
- Adriaens K, Gucht DV, Baeyens F. IQOSTMvs. e-cigarette vs. tobacco cigarette: a direct comparison of short-term effects after overnight-abstinence. Int J Environ Res Public Health. 2018 Dec 18;15(12):2902.
- Caponnetto P, Maglia M, Prosperini G, et al. Carbon monoxide levels after inhalation from new generation heated tobacco products. Respir Res. 2018 Aug 31;19(1):164.
- Chen J, Bullen C, Dirks K, et al. Risk assessment of electronic cigarettes and conventional cigarettes. Int J Environ Res Public Health. 2017 Apr 5;14(4):382.
- Rodrigo G, Jaccard G, Tafin Djoko D, et al. Cancer potencies and margin of exposure used for comparative risk assessment of heated tobacco products and electronic cigarettes aerosols with cigarette smoke. Arch Toxicol. 2021 Jan;95(1):283–298.
- Gordon T, Karey E, Rebuli ME, et al. E-Cigarette Toxicology. Annu Rev Pharmacol Toxicol. 2022 Jan;6(62):301–322.
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–637.
- Giovenco DP, Delnevo CD. Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers. Addict Behav. 2018;76:129–134.
- Notley C, Ward E, Dawkins L, et al. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduct J. 2018;15(1):1–12.
- Pierce JP, Chen R, Kealey S, et al. Incidence of cigarette smoking relapse among individuals who switched to e-cigarettes or other tobacco products. JAMA Network Open. 2021 Oct 1;4(10):e2128810.
- Dai H, Leventhal AM. Association of electronic cigarette vaping and subsequent smoking relapse among former smokers. Drug Alcohol Depend. 2019 Jun 1;199:10–17. DOI:10.1016/j.drugalcdep.2019.01.043.
- Laverty AA, Vardavas CI, Filippidis FT. Prevalence and reasons for use of Heated Tobacco Products (HTP) in Europe: an analysis of Eurobarometer data in 28 countries. Lancet Reg Health Eur. 2021 Jul 14;8:100159. doi:10.1016/j.lanepe.2021.100159.
- Caponnetto P, Campagna D, Maglia M, et al., Comparing effectiveness, tolerability, and acceptability of heated tobacco productS vs. refillable electronic cigarettes for cIgaREttes substitution: CEASEFIRE randomized controlled trial, JMIR Public Health Surveill, 2022;in press
- Brouwer AF, Jeon J, Hirschtick JL, et al. Transitions between cigarette, ENDS and dual use in adults in the PATH study (waves 1-4): multistate transition modelling accounting for complex survey design. Tob Control. 2020 Nov 16. DOI:10.1136/tobaccocontrol-2020-055967.
- Conner TS, Zeng J, Blank ML, et al. Analysis of transitions from smoking to Electronic Nicotine Delivery System (ENDS) use: a daily diary investigation. Int J Environ Res Public Health. 2021 Jun 10;18(12):6301.
- Ash. Action on smoking and health. Use of e-cigarettes (vapes) among adults in Great Britain. Ask.org. 2021 [cited 2022 Sep 5]. Available from: https://ash.org.uk/uploads/Use-of-e-cigarettes-vapes-among-adults-in-Great-Britain-2021.pdf
- Selya AS, Shiffman S, Greenberg M, et al. Dual Use of Cigarettes and JUUL: trajectory and Cigarette Consumption. Am J Health Behav. 2021 May 1;45(3):464–485.
- Mattingly DT, Zavala-Arciniega L, Hirschtick JL, et al. Trends in exclusive, dual and polytobacco use among U.S. adults, 2014–2019: results from two nationally representative surveys. Int J Environ Res Public Health. 2021;18(24):13092.
- Polosa R, Casale TB, Tashkin DP, et al. Look at Vaping in adolescents and young adults in the USA. J Allergy Clin Immunol Pract. 2022 Jun 16;10(11):2831–2842.
- Centers for Disease Control and Prevention. National Youth Tobacco Survey (NYTS). Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. 2022 [cited 2022 Sep 5]. Available from: https://www.cdc.gov/tobacco/data_statistics/surveys/nyts/index.htm
- Centers for Disease Control and Prevention. National health interview survey. About the National Health Interview Survey. 2022 [cited 2022 Mar 03]. Available from: https://www.cdc.gov/nchs/nhis/about_nhis.htm
- Caruso M, Emma R, Distefano A, Rust S, Poulas K, Zadjali F, Giordano A, Volarevic V, Mesiakaris K, Al Tobi M, Boffo S, Arsenijevic A, Zuccarello P, Giallongo C, Ferrante M, Polosa R, Li Volti G; Replica Project Group. Electronic nicotine delivery systems exhibit reduced bronchial epithelial cells toxicity compared to cigarette: the Replica Project. Sci Rep. 2021 Dec 17;11(1):24182.
- Bowler RP, Hansel NN, Jacobson S, et al. Electronic cigarette use in us adults at risk for or with COPD: analysis from two observational cohorts. J Gen Intern Med. 2017;32(12):1315–1322.
- Xie W, Kathuria H, Galiatsatos P, et al. Association of electronic cigarette use with incident respiratory conditions among US adults from 2013 to 2018. JAMA Network Open. 2020 Nov 2;3(11):e2020816.
- Bhatta DN, Glantz SA. Association of E-cigarette use with respiratory disease among adults: a longitudinal analysis. Am J Prev Med. 2020 Feb;58(2):182–190.
- Bircan E, Bezirhan U, Porter A, et al. Electronic cigarette use and its association with asthma, chronic obstructive pulmonary disease (COPD) and asthma- COPD overlap syndrome among never cigarette smokers. Tob Induc Dis. 2021 Apr 7;19(September):23.
- Kang HS, Jung JW, Park HJ, et al., Korean Smoking Cessation Study Group. A pilot investigation of e-cigarette use and smoking behaviour among patients with chronic airway disease or respiratory symptoms. Clin Respir J. 2022 Jan;16(1):17–26.
- Antwi GO, Rhodes DL. Association between E-cigarette use and chronic obstructive pulmonary disease in non-asthmatic adults in the USA. J Public Health (Oxf). 2020 Dec 22; fdaa229. DOI:10.1093/pubmed/fdaa229.
- Parekh T, Owens C, Fay K, et al. Use of e-Cigarettes and Development of Respiratory Conditions in Women of Childbearing Age. South Med J. 2020 Oct;113(10):488–494.
- Xie Z, Ossip DJ, Rahman I, et al. Use of electronic cigarettes and self-reported chronic obstructive pulmonary disease diagnosis in adults. Nicotine Tob Res. 2020 Jun 12;22(7):1155–1161.
- Perez MF, Atuegwu NC, Mead EL. Adult E-cigarettes use associated with a self-reported diagnosis of COPD. Int J Environ Res Public Health. 2019 Oct 16;16(20):3938.
- Kioi Y, Tabuchi T. Electronic, heat-not-burn, and combustible cigarette use among chronic disease patients in Japan: a cross-sectional study. Tob Induc Dis. 2018 Sep 10;16(September):41.
- Wang JB, Olgin JE, Nah G, et al. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS One. 2018 Jul 25;13(7):e0198681.
- Lewis NM, Friedrichs M, Wagstaff SS, et al. Characteristics of adults who use both marijuana and E-cigarette, or vaping, products: a cross-sectional study, Utah, 2018. 433021. 2021May;26:333549211018679. DOI:10.1177/00333549211018679
- Soriano JB, Alfageme I, Miravitlles M, et al. Determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol (Engl Ed). 2021 Jan;57(1):61–69.
- Osei AD, Mirbolouk M, Orimoloye OA. Association between E-cigarette use and chronic obstructive pulmonary disease by smoking status: behavioral risk factor surveillance system 2016 and 2017. Am J Prev Med. 2020 Mar;58(3):336–342.
- Wills TA, Pagano I, Williams RJ, et al. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend. 2019 Jan 1;194:363–370. DOI:10.1016/j.drugalcdep.2018.10.004.
- Kruse GR, Kalkhoran S, Rigotti NA. Use of electronic cigarettes among U.S. adults with medical comorbidities. Am J Prev Med. 2017 Jun;52(6):798–804.
- Hajat C, Stein E, Shantikumar S, et al. A scoping review of studies on the health impact of electronic nicotine delivery systems. Intern Emerg Med. 2021 Oct 12;17(1):241–268.
- Cummings KM, Polosa R. E-cigarette and COPD: unreliable conclusion about health risks. J Gen Intern Med. 2018 Jun;33(6):784–785.
- Xie W, Tackett AP, Berlowitz JB, et al. Association of electronic cigarette use with respiratory symptom development among U.S. young adults. Am J Respir Crit Care Med. 2022 Jun 1;205(11):1320–1329.
- Sargent JD, Halenar MJ, Edwards KC, et al. Tobacco use and respiratory symptoms among adults: findings from the longitudinal Population Assessment of Tobacco and Health (PATH) study 2014-16. Nicotine Tob Res. 2022 Apr 2;ntac080. DOI:10.1093/ntr/ntac080.
- Paulin LM, Halenar MJ, Edwards KC, et al. Association of tobacco product use with chronic obstructive pulmonary disease (COPD) prevalence and incidence in waves 1 through 5 (2013-2019) of the Population Assessment of Tobacco and Health (PATH) Study. Respir Res. 2022 Oct 1;23(1):273.
- Rodu B, Plurphanswat N. Cross-sectional e-cigarette studies are unreliable without timing of exposure and disease diagnosis. Intern Emerg Med. 2022. DOI:10.1007/s11739-022-03141-3.
- Polosa R, Farsalinos K. A tale of flawed e-cigarette research undetected by defective peer review process. Intern Emerg Med. 2022 Dec 8. DOI:10.1007/s11739-022-03163-x.
- Polosa R, Morjaria JB, Prosperini U, et al. COPD smokers who switched to e-cigarettes: health outcomes at 5-year follow up. Ther Adv Chronic Dis. 2020 Oct 10;11:2040622320961617. doi: 10.1177/2040622320961617.
- Huttunen R, Heikkinen T, Syrjanen J. Smoking and the outcome of infection. J Intern Med. 2011;269(3):258–269.
- Palamidas A, Tsikrika S, Katsaounou PA, et al. Acute effects of short term use of ecigarettes on airways physiology and respiratory symptoms in smokers with and without airway obstructive diseases and in healthy non smokers. Tob Prev Cessat. 2017 Mar 1;3:5. doi:10.18332/tpc/67799.
- Farsalinos KE, Romagna G, Tsiapras D, et al., Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health. 2014;11(4):4356–4373.
- Gotts JE, Jordt S-E, McConnell R, et al. What are the respiratory effects of e-cigarettes? BMJ. 2019;l5275. DOI:10.1136/bmj
- Wills TA, Soneji SS, Choi K, et al. E-cigarette use and respiratory disorders: an integrative review of converging evidence from epidemiological and laboratory studies. Eur Respir J. 2021 Jan 21;57(1):1901815.
- Polosa R, Morjaria JB, Prosperini U, et al. Health outcomes in COPD smokers using heated tobacco products: a 3-year follow-up. Intern Emerg Med. 2021 Apr; 16(3):687–696.
- Tashkin DP. Smoking cessation in COPD: confronting the challenge. Intern Emerg Med. 2021Mar25;16(3):545–547. Epub ahead of print. PMID: 33765300.
- Polosa R. Cessation of smoking in COPD: a reality check. Intern Emerg Med. 2021Oct;16(7):2029–2030. Epub 2021 May 10. PMID: 33970427.
- Polosa R, Emma R, Cibella F, et al. Impact of exclusive e-cigarettes and heated tobacco products use on muco-ciliary clearance. Ther Adv Chronic Dis. 2021 Aug 12;12: 20406223211035267. DOI:10.1177/20406223211035267.
- Reitsma MB, Kendrick PJ, Ababneh E, GBD 2019. Tobacco collaborators. spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the global burden of disease study 2019. Lancet. 2021;397(10292):2337–2360.
- van der Plas A, Antunes M, Romero-Kauss A, et al. Ischemic heart disease and chronic obstructive pulmonary disease hospitalizations in Japan before and after the introduction of a heated tobacco product. Front Public Health. 2022 Jun 28;10:909459. doi: 10.3389/fpubh.2022.909459.
- Stead M, Angus K, Holme I, et al. Factors influencing European GPs’ engagement in smoking cessation: a multi-country literature review. Br J Gen Pract. 2009;59(566):682–690.
- Vogt F, Hall S, Marteau TM. General practitioners’ and family physicians’ negative beliefs and attitudes towards discussing smoking cessation with patients: a systematic review. Addiction. 2005;100(10):1423–1431.
- van Eerd EAM, Bech Risør M, Spigt M, et al. Why do physicians lack engagement with smoking cessation treatment in their COPD patients? A multinational qualitative study. NPJ Prim Care Respir Med. 2017 Jun 23;27(1):41.
- Sahu KK, Mishra SK, Lal A, et al. A double-edged sword: e-cigarettes, and other electronic nicotine delivery systems (ENDS). Intern Emerg Med. 2020;15(6):1117–1118.
- Polosa R, Farsalinos K, Prisco D. E-cigarettes, and other electronic nicotine delivery systems (ENDS: reply). Intern Emerg Med. 2020;15(6):1119–1121.
- Cohen JE, Krishnan-Sarin S, Eissenberg T, et al. Balancing risks and benefits of E-cigarettes in the real world. Am J Public Health. 2022 Feb;112(2):e1–e2.
- Balfour DJK, Benowitz NL, Colby SM, et al. Balancing consideration of the risks and benefits of E-Cigarettes. Am J Public Health. 2021 Sep;111(9):1661–1672.
- Polosa R. Examining the evidence for the health impact of combustion-free products: progress and prospects for tobacco harm reversal and reduction. Intern Emerg Med. 2021 Nov;16(8):2043–2046.
- Hajat C, Stein E, Selya A, et al., the CoEHAR study group. Analysis of common methodological flaws in the highest cited e-cigarette epidemiology research. Intern Emerg Med. 2022;17(3):887–909.
- Chan GCK, Stjepanovic D, Lim C, et al. Gateway or common liability? A systematic review and meta-analysis of studies of adolescent e-cigarette use and future smoking initiation. Addiction. 2021;116(4):743–756.
- Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Washington (DC): US Department of Health and Human Services, Public Health Service; 2008.
- Polosa R, Benowitz NL. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol Sci. 2011;32(5):281–289.
- Tashkin D, Kanner R, Bailey W, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet. 2001 May 19;357(9268):1571–1575.
- Tashkin DP, Rennard S, Hays JT, et al. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011Mar;139(3):591–599. Epub 2010 Sep 23.
- Cummings KM, Giovino G, Jaen CR, et al. Reports of smoking withdrawal symptoms over a 21-day period of abstinence. Addict Behav. 1985;10(4):373–381.
- Thomson NC, Polosa R, Sin DD. Cigarette Smoking and Asthma. J Allergy Clin Immunol Pract. 2022 May 6;10(11):2783–2797.
- FDA U.S. FOOD & DRUG ADMINISTRATION. FDA authorizes marketing of IQOS tobacco heating system with ‘reduced exposure’ information. agency will closely monitor real-world data to assess if marketing continues to be appropriate. FDA NEWS RELEAS. 2020. [cited 2022 Mar 03] Available from: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-iqos-tobacco-heating-system-reduced-exposure-information
- FDA U.S. FOOD & DRUG ADMINISTRATION. FDA PERMITS MARKETing of E-cigarette products, marking first authorization of its kind by the agency. FDA NEWS RELEAS. 2021. [cited 2022 Mar 03] Available from: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-e-cigarette-products-marking-first-authorization-its-kind-agency
- NICE. National Institute for Health and Care Excellence. Tobacco: preventing uptake, promoting quitting and treating dependence. NICE guideline. 2021. [cited 2022 Sep 05] Available from: https://www.nice.org.uk/guidance/ng209/resources/tobacco-preventing-uptake-promoting-quitting-and-treating-dependence-pdf-66143723132869
- Polosa R, Caponnetto P, Cibella F, et al. Quit and smoking reduction rates in vape shop consumers: a prospective 12-month survey. Int J Environ Res Public Health. 2015;12(4):3428–3438.
- Caponnetto P, Maglia M, Polosa R, et al. Commentary on Dawkins et al. (2015): electronic cigarettes - from smoking cessation to smoking sensation and back. Addiction. 2015;110(4):678–679.
- Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220–11248. PMID:25358095.
