ABSTRACT
Objectives
Treatment guidelines have recommended tiotropium bromide inhalation (TBI), a long-acting muscarinic antagonist, for chronic obstructive pulmonary disease (COPD); however, its efficacy in symptomatic Chinese patients with COPD remains uninvestigated.
Methods
This multicenter, prospective, observational study enrolled patients with COPD assessment test (CAT) scores exceeding 10 points from 19 hospitals spread across China. All patients received TBI and underwent follow-up for 3 months. The demographic and clinical information were assessed.
Results
The final analysis included 378 patients. The forced expiratory volume in 1 s (FEV1) and FEV1/forced vital capacity (FVC) of all participants improved markedly after 3 months of treatment (FEV1: mean 1.33 L versus 1.61 L, P < 0.001; FEV1/FVC: mean 0.53 versus 0.62, P < 0.001). The mean CAT scores decreased from 26.56 to 16.28 (P < 0.001). Patients classified into group D based on the Global Initiative for COPD guidelines showed greater improvement in FEV1 and FEV1/FVC than that in patients in group B. The proportion of patients with acute exacerbations also declined from 28.6% in the first month to 4.2% in the third month.
Conclusion
TBI for 3 months could effectively and safely attenuate symptoms and airflow obstruction in symptomatic Chinese patients with COPD.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory airway disease characterized by limited airflow reversibility, persistent respiratory symptoms, and frequent exacerbations [Citation1,Citation2]. COPD plagues more than 330 million individuals worldwide, including 8.6% adults aged above than 40 years in China [Citation3–5]. COPD has been identified as one of the top three leading causes of death by the World Health Organization based on epidemiological reports, and 90% of these deaths occur in low- and middle-income countries [Citation6–8]. The high prevalence, mortality, and healthcare burden associated with advanced COPD have garnered considerable attention, leading to the formulation of several pharmacotherapeutic modalities, chief among which are bronchodilators [Citation9].
Patients with COPD commonly present with a series of symptoms caused by obstructive airway limitations and chronic mixed inflammation, such as dyspnea, cough, and sputum, which substantially negatively effects the quality of life [Citation10,Citation11]. Smoking cessation, bronchodilators and in some cases topical steroids are considered essential approaches toward COPD rehabilitation [Citation1,Citation12]. Inhaled bronchodilators, including antimuscarinic drugs and β2-agonists, have showed great efficacy in the prevention and amelioration of symptoms during the past decade, along with an increase in the forced expiratory volume in 1 s (FEV1) and/or other spirometric variables [Citation13,Citation14]. Tiotropium bromide inhalation (TBI), a long-acting muscarinic antagonist (LAMA), is a widely used therapeutic modality for COPD that improves expiratory flow by virtue of prolonged binding to M3 muscarinic receptors and faster dissociation from M2 muscarinic receptors [Citation15,Citation16]. A few clinical trials have demonstrated that long-term (> 1 year) utilization of TBI could effectively relieve dyspnea, reduce the frequency of hospitalization, and retard the decline in lung function in patients with COPD, especially those with early-stage COPD [Citation17–19]. However, the short-term (< 6 months) efficacy and safety of TBI in symptomatic patients with COPD have little been determined.
Tian Qing Su Le® (TQSL; Chia Tai Tian Qing Pharmaceutical Group Co., Ltd, China, national drug approval number: H20060454) administered by dry powder inhaler (DPI) is one of the most commonly used TBI preparations in China; however, its post-marketing effect on the symptoms and spirometric parameters in Chinese patients with COPD remains unexplored.
Therefore, we conducted this multicenter, prospective, and observational investigation into the efficacy and safety of TBI (TQSL) in symptomatic Chinese patients with COPD who had never been treated with inhaled LAMA before this trial.
2. Patients and methods
2.1. Study design and patients
This study incorporated a multicenter, prospective, open-label and observational design. Patients diagnosed with COPD by respiratory physicians based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [Citation20,Citation21] (https://goldcopd.org) were enrolled from 19 Chinese hospitals spread over a wide region of the country and were followed up for 3 months. All participants had received TQSL at a dose of 18 mg per inhalation by DPI (manufactured by Chia Tai Tian Qing Pharmaceutical Group Co., Ltd.), and inhalation frequency was once per day. Every subject who agreed to join this study had to sign the informed consent forms at the beginning of the observation. Participants were provided instructions to take the medication for 3 months, coupled with stringent supervision from clinical research coordinators (CRCs). Participants were examined 3 times (visit 0, visit 1, and visit 2) throughout the whole observation period (), and corresponding information and data were collected for further analysis. Study enrollment commenced in June 2013 and completed in August 2014. This study was conducted in accordance with the principles of the Declaration of Helsinki (18th World Medical Assembly, 1964). The study protocol was reviewed and approved by the Ethics Committee of West China Hospital, Sichuan University (approval number: 2016.263).
2.1.1. Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) age was not less than 40 years; 2) diagnosis of COPD by respiratory specialists based on the GOLD guidelines; 3) LAMA treatment-naive patients (prior to enrollment); and 4) COPD Assessment Test (CAT) score ≥ 10 points.
The exclusion criteria were as follows: 1) history of asthma or pulmonary segmentectomy; 2) history of acute exacerbations (AEs) of COPD, respiratory infection, or oxygen therapy over 12 hours per day in the past 4 weeks before enrollment; 3) having diagnosed with active nontuberculous mycobacterium (NTM) infection or active tuberculosis; 4) inability to complete the lung function test or CAT questionnaire; and 5) prior to enrollment in another interventional clinical trial project.
2.2. Outcome measures and definitions
2.2.1. Baseline characteristics
Patients’ baseline characteristics, such as demographics, etiology, symptomatology, and radiography information, were recorded upon enrollment (visit 0). Briefly, histories of cigarette smoking, drug use, comorbidities, AEs frequency, and results of chest computed tomography (CT) were employed to evaluate the patients’ baseline health conditions and subsequently perform stratification for analysis. The evaluation of AEs followed the GOLD guidelines: an acute worsening of respiratory symptoms including increased dyspnea, sputum purulence and volume, increased cough and wheeze that results in additional therapy [Citation20]. Pulmonary spirometry and CAT scores {an 8-item unidimensional symptom-related measure applicable worldwide to evaluate the health status impairment in COPD [Citation22,Citation23] were measured to confirm the diagnosis and identify the phenotypes of patients according to the American Thoracic Society [Citation24] and European Respiratory Society guidelines [Citation25].
2.2.2. Primary endpoints and other measurements
FEV1 and FEV1/forced vital capacity (FEV1/FVC) were designated as the primary endpoints since spirometry is considered to be the most available and reproducible test of airway limitation. Other measurements including CAT scores, frequency of AE during the follow-up, and all-cause mortality were also monitored to reflect the impact on symptoms and exacerbation risk, to ensure comprehensive evaluation of multiple facets of the efficacy of TBI. Treatment with other COPD-related bronchodilators, inhaled corticosteroids (ICS), and methylxanthines in combination with TBI therapy as prescribed by experienced pulmonologists per the GOLD treatment guidelines were also implemented and recorded in the case report forms (CRFs) for further analysis.
2.2.3. Adverse events
The toxicity of inhaled anticholinergic drugs during the entire follow-up period was also assessed. Participants were provided information containing clear details on the personnel to contact in case of adverse events. Two respiratory specialists judged the relevance between the occurrence of adverse events and TBI, and recorded the date, management, ending, and conclusion of the adverse events in the CRFs.
2.3. Data collection
Two professional CRCs and 1 respiratory doctor from West China Hospital of Sichuan University collected the raw data. A monitoring team comprising 2 respiratory specialists who are independent from competing interests verified and revised the results. Experts at West China Hospital of Sichuan University performed spirometry for all participants. The CRFs, once recorded, was locked to prevent changes. Access to the identified information sets was limited to the study authors.
2.4. Statistical analysis
The sample size was calculated by statistical experts. Considering the primary endpoint of this study is FEV1, we regarded its mean difference (MD) of 0.02 L as the smallest clinically important difference between the baseline and post-treatment with a standard deviation (SD) of 0.1 L used in the calculation, which were according to the previous report of FEV1 on patients with COPD [Citation26–28]. Based on 90% power with a two-sided significance level of 5%, a smallest sample size of 214 was needed as calculated by the software PASS 15. The results were presented as the mean (95% confidence interval), rate, or percentage. Overall differences in the lung function parameters and CAT scores between visits 0 and 2 were compared using the paired Student’s t test (t test), analysis of variance, chi-squared test, or Wilcoxon rank-sum test, depending on the nature of data distribution and between-column variance. Linear regression was performed to determine the potential parameters correlated to the improvement in the CAT scores and FEV1/FVC. Two-sided P-values less than 5% were considered statistically significant. SPSS software package version 21.0 (SPSS Inc., Chicago, Illinois, USA) was used for data processing, while the figures were drawn using GraphPad Prism 9.0 (GraphPad Software Inc, La Jolla, CA, USA).
2.4.1. Subgroup analysis
2.4.1.1. Subgroup analysis 1
All participants were classified into the GOLD B or GOLD D group based on GOLD ABCD assessment which is a grade system defined by symptoms, and moderate or severe exacerbation history [Citation21] (https://goldcopd.org). Patients with at least 2 moderate or severe exacerbations, or at least 1 exacerbation history leading to hospital admission were classified into GOLD D group, while patients with 0 or 1 moderate or severe exacerbations (not leading to hospital admission) belonged to GOLD B group. The between-group differences in the baseline health-related features, spirometric parameters, CAT scores, and AEs were compared statistically via the t test, chi-squared test, or rank-sum test.
2.4.1.2. Subgroup analysis 2
Patients were classified into different subgroups for analysis on the basis of other concomitant pharmacotherapy besides TBI, including long-acting beta (2)-agonist (LABA), inhaled glucocorticoid (ICS), and theophylline. LAMA, LABA, and theophylline are bronchodilators recommended for stable COPD based on GOLD treatment guidelines [Citation21]. ICS focuses on anti-inflammation.
3. Results
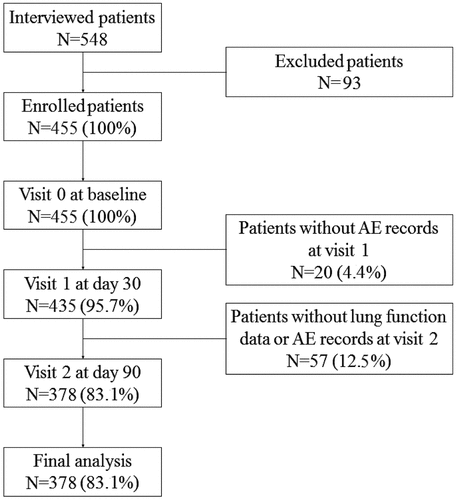
A total of 548 patients with COPD who met the inclusion criteria were interviewed, from which 93 patients were eliminated based on the exclusion criteria, and 455 patients were enrolled in this observational study. After the 3-month follow-up period, 77 patients (16.9%) dropped out due to various reasons. Eventually, 378 participants (83.1%) completed this study (), of which 242 (64.0%) were classified into the GOLD B group and 136 (36.0%) into the GOLD D group, in accordance with the GOLD ABCD grading criteria. During the observation period, 110 patients (29.1%) used inhaled TQSL alone (TBI group), 72 participants (19.0%) used TQSL in combination with theophylline (Dual group), 137 patients (36.2%) received triple therapy (TQSL + LABA + ICS), and the remaining patients (15.7%) received quadruple treatment (TQSL + LABA + ICS + theophylline) ().
Table 1. Baseline demographic and clinical characteristics.
3.1. Baseline clinical characteristics of the participants
The mean age of the 378 patients was 64.24 years, of which 72.2% were men; 34.7% were never-smokers. () The proportion of smokers and ex-smokers in the GOLD B group was almost equivalent to that in the GOLD D group. Chronic comorbidities were observed on 126 (33.3%) participants, of which cardiovascular diseases were the mostly seen. In total of 31.6% of patients in the GOLD D group had concomitant cardiovascular diseases while 23.6% of patients in the GOLD B group also were diagnosed with cardiovascular diseases (P = 0.088). All patients treated with TQSL and theophylline (dual group) had cardiovascular disease. Moreover, more than half of patients (67.5%) had a history of bronchodilator use, including salmeterol/fluticasone (LABA + ICS), budesonide/formoterol (LABA + ICS), and theophylline, in the last year prior to enrollment; theophylline was the most commonly used drug (41.0%). Emphysema, or pathological hallmarks of lung parenchymal destruction were observed on the chest CT scans of most patients with COPD (64.4%). Nearly 80% of participants in the triple and quadruple groups had emphysema, which was significantly greater than that in patients receiving single and dual therapies.()
3.2. Lung function
The mean pre-bronchodilator FEV1/FVC of 378 patients was 0.53, which increased to 0.62 after receiving corresponding therapies (P < 0.001). The FEV1/FVC rose at the final visit in 343 patients (90.7%) but not in 35 patients (9.2%). The mean FEV1 of all patients after treatment was greater than that before treatment (1.33 L versus 1.61 L, respectively, P < 0.001, ).
Table 2. Main outcomes in patients with COPD.
In subgroup analysis 1, the mean FEV1 and FEV1/FVC in the GOLD B group improved after continued inhalation compared to that at baseline (FEV1: 1.53 L versus 1.77 L, P < 0.001; FEV1/FVC: 0.60 versus 0.67, P < 0.001). In the GOLD D group, the mean of the post-bronchodilator treatment FEV1 and FEV1/FVC were 1.32 L and 0.52, respectively, which were higher than the corresponding pre-bronchodilator values (both P < 0.001, ). The changes in FEV1 and FEV1/FVC in the GOLD D group were greater than those in the GOLD B group (FEV1: P = 0.001; FEV1/FVC: P = 0.003; ).
Figure 2. This figure presents the changes of main lung function parameters between pre-treatment and post-treatment in different groups.
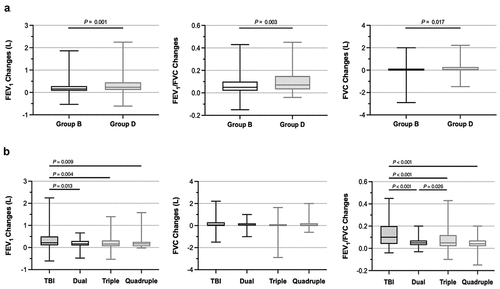
In subgroup analysis 2, the mean values for FEV1 and FEV1/FVC after bronchodilation showed significant improvement compared to those before treatment in the TBI, Dual, and Triple groups (All P < 0.05, ). However, there was no significant difference between pre-treatment and post-treatment FEV1 and FVC of patients who received quadruple therapy (TBI + LABA + ICS + theophylline) (FEV1: P = 0.076, FEV1/FVC: P = 0.356; ). The degree of increase in the mean post-bronchodilator FEV1 and FEV1/FVC was greater in patients who persisted in using inhalational TBI alone compared to those who used other medications concomitantly during the study period (All P < 0.05, ).
In addition, changes in the FEV1/FVC were correlated with the comorbidities, the concomitant intake of other medications, and the pre-treatment CAT scores (All P < 0.05, ).
Table 3. AECOPD reported during 3-month follow-up.
Table 4. Multiple linear regression analysis for FEV1/FVC changes and CAT changes.
3.3. CAT scores
At visit 1, the average CAT scores declined markedly from the baseline (mean 21.62 versus 26.56, P< 0.001), which continued to plummet to 16.28 at visit 2 (P < 0.001). Specifically, the CAT scores decreased at the end of 3-month TBI treatment in almost all patients (365, 96.6%; ).
The post-treatment CAT scores of patients in the GOLD B and GOLD D groups were lower than the corresponding pre-treatment CAT scores of each group (All P < 0.001, ). The change between the post-treatment and baseline CAT scores in the GOLD B group was greater than that in the GOLD D group (P = 0.017, ). Similarly, the CAT scores showed a distinct reduction at the end of the observation (visit 2) in the TBI, dual, triple, and quadruple groups, respectively (All P < 0.001, ). The degree of decline was the greatest in the TBI group ().
Figure 3. This figure presents the changes of CAT scores between pre-treatment and post-treatment in different groups.
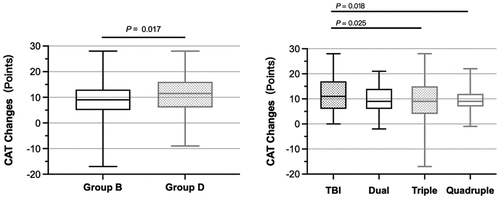
Multiple regression analysis revealed that the changes in the CAT score were related to the comorbidities, the concomitant intake of other medications, and the pre-treatment CAT scores (All P < 0.05, ).
3.4. Acute exacerbations (AEs) and mortality
During the 3-month follow-up, a total of 202 AEs were recorded, in which the highest number of AEs in a single patient was 3. The numbers of AEs was the highest in the first month (108, 28.6%), followed by the second month (78, 20.6%) and third month (16, 4.2%), respectively. details the AE events in each group. No instance of patient mortality was reported during the 3-month follow-up.
3.5. Safety
Only 9 patients (2.4%) reported adverse effects. The majority (6, 66.7%) of patients complained of dry mouth, while the other 3 presented with constipation, insomnia, and allergy. Eventually 2 experienced pulmonologists judged that 5 xerostomia cases, 1 constipation case, and 1 allergy case (6 cases in all) were related to TBI. All reported adverse effects were resolved after appropriate treatment.
3.6. Ethics approval
This study was conducted in accordance with the principles of the Declaration of Helsinki (18th World Medical Assembly, 1964). The study protocol was reviewed and approved by the Ethics Committee of West China Hospital, Sichuan University (approval number: 2016.263).
4. Discussion
This study demonstrated the short-term effectiveness and safety of TBI for COPD treatment, as evidenced by improvements in the patients’ symptoms and lung function.
A 4-year double-blind trial conducted by Donald et al. suggested that the addition of tiotropium to other standard therapies had no effect on the rate of lung function decline [Citation18]. However, a 2-year clinical trial of patients with early-stage COPD, defined as a low symptom burden and mild to moderate airflow obstruction, reported that treatment with tiotropium resulted in an increase in FEV1 in moderate but not severe exacerbation [Citation17]. Therefore, considering the discrepancy in the results of the studies, and the burden of regular long-term treatments in low-income or low-education patients, we just investigated the impact of TBI in symptomatic COPD patients whose pre-bronchodilator CAT scores were above 10 points for 3 months, and found significant improvements in FEV1, FEV1/FVC, and CAT scores in most patients. A 3-month use of TBI could effectively reduce symptoms and the degree of airflow obstruction in symptomatic patients with COPD.
The ABCD assessment tool updated by the GOLD in 2022 was a major step forward from the simple spirometric grading system because it highlighted the importance of exacerbation prevention in the management of COPD [Citation21,Citation29,Citation30]. However, clinical studies on the method of application of this new measurement guideline for COPD therapy are rare. Our study classified patients into GOLD B and GOLD D groups to compare their responses to TBI. The changes in FEV1, FEV1/FVC and CAT scores between baseline and post-treatment in the GOLD D group were greater than those in the GOLD B group after 3 months of treatment. Patients with higher AEs risks may suffer severer lung impairment and airway inflammation. The use of an inhaled LAMA may results in anti-inflammatory impact suggested by increasing evidence [Citation31]. This may be the reason why the use of TBI produced a greater improvement in FEV1, FEV1/FVC and COPD-related symptoms in patients who suffered more frequent AEs burden. The second subgroup analysis revealed that patients who used TBI alone during the observation period showed a marked amelioration in symptoms and lung function impairment, which further determined the efficacy of TBI. A combination of theophylline with salmeterol (LABA) produces a greater improvement in FEV1 and breathlessness than salmeterol alone [Citation32,Citation33], but in this study, the improvement of FEV1 and FEV1/FVC in patients in the Dual group was lower than patients who treated with TBI alone, which suggested that theophylline may not increase the effectiveness of TBI in improving lung function. Triple therapy (LAMA + LABA + ICS) also resulted in a substantial improvement in the FEV1 and FEV1/FVC, but the FEV1 of patients who received quadruple therapy (LAMA + LABA + ICS + theophylline) did not increase significantly (P> 0.05), which indicated that theophylline, to a great extent, did not enhance the efficacy of triple therapy in symptomatic patients with COPD.
Multiple regression analysis revealed that both the changes in the CAT score and FEV1/FVC were related to comorbidity, concomitant medication and CAT scores at the baseline, suggesting that the characteristics of patients himself are associated with symptom identification and lung function, suggesting that the characteristics of patients himself are associated with symptom identification and lung function. The presence of emphysema was also not related to the improvement in the CAT score and FEV1/FVC, indicating that the degree of pulmonary destruction may not be totally reflected by symptoms or decline of lung function.
This observational study first investigated the post-marketing efficacy of TQSL in Chinese patients with COPD whose CAT scores were above 10 and innovatively compared the efficacy of TBI in patients classified into GOLD B and GOLD D, finding that TBI had a greater impact on attenuating symptoms and improving lung function in patients in GOLD D. Besides, it also explores the discrepancy of TBI efficacy among different concomitant therapy. The 3-month triple therapy and the use of TBI alone could be the most effective treatment approaches for improving COPD-related symptoms, FEV1 and FEV1/FVC ratio. If confirmed, this could provide a new reference for future COPD treatment strategies.
4.1. Limitation
This study was observational in design rather than a randomized controlled trial. In the current study, we observed that the numbers of patients with exacerbations (AEs) were the highest in the first month, which decreased in the second month, and declined to the lowest levels in the third month; however, we could not determine whether the frequency of AEs decreased since the number of AEs in the last year was considered as baseline as the study entailed a follow-up of only 3 months. The standardization of mean values in different periods may result in a large bias. As for baseline information, the significant differences of comorbidity and emphysema among TBI, Dual, Triple, and Quadruple groups () may be bias to final results. Patients receiving triple and quadruple therapies had a higher proportion of comorbidity and emphysema than patients in TBI and Dual groups, which may lead to less improvement in lung function and symptoms.
5. Conclusions
A 3-month course of TBI appears to effectively and safely alleviate the symptoms and pulmonary airflow limitation in symptomatic Chinese patients with COPD.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Review of Respiratory Medicine for their review work, but have no other relevant financial relationships to disclose
Author contributions
FW designed this study; YL, HW, KW, KZ, YS, LC, TW, and JC reviewed patients and collected data; YL, TW, and HW analyzed the data; YL, HW, TW, and FW drafted the manuscript.
Additional information
Funding
References
- Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940.
- Labaki WW, Rosenberg SR. Chronic obstructive pulmonary disease. Ann Intern Med. 2020;173(3):Itc17–itc32.
- Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899–909.
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717.
- Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430.
- Halpin DMG, Celli BR, Criner GJ, et al. The GOLD Summit on chronic obstructive pulmonary disease in low- and middle-income countries. Int J Tuberc Lung Dis. 2019;23(11):1131–1141.
- Vestbo J, Halpin DMG, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365.
- Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18(114):213–221.
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351.
- Ritchie AI, Wedzicha JA, Definition C. Pathogenesis, and consequences of chronic obstructive pulmonary disease exacerbations. Clin Chest Med. 2020;41(3):421–438.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654.
- Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. Jama. 2019;321(8):786–797.
- Park SY, Kim S, Kim J-H, et al. A randomized, noninferiority trial comparing ICS + LABA with ICS + LABA + LAMA in asthma-COPD overlap (ACO) treatment: the ACO Treatment with Optimal Medications (ATOMIC) study. J Allergy Clin Immunol Pract. 2021;9(3):1304–1311.e2.
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234.
- Blair HA. Tiotropium/Olodaterol: a Review in COPD. Drugs. 2019;79(9):997–1008.
- Barnes PJ, Belvisi MG, Mak JCW, et al. Tiotropium bromide (Ba 679 BR), a novel long-acting muscarinic antagonist for the treatment of obstructive airways disease. Life Sci. 1995;56(11–12):853–859.
- Zhou Y, Zhong N-S, Li X, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377(10):923–935.
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554.
- Yu S, Zhang C, Yan Z, et al. Tiotropium bromide attenuates mucus hypersecretion in patients with stable chronic obstructive pulmonary disease. Comput Math Methods Med. 2021;2021:1341644.
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365.
- Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):12.
- Zhou Z, Zhou A, Zhao Y, et al. Evaluating the clinical COPD questionnaire: a systematic review. Respirology. 2017;22(2):251–262.
- Cheng SL, Lin CH, Wang CC, et al. Comparison between COPD Assessment Test (CAT) and modified Medical Research Council (mMRC) dyspnea scores for evaluation of clinical symptoms, comorbidities and medical resources utilization in COPD patients. J Formos Med Assoc. 2019;118(1 Pt 3):429–435.
- STANDARDIZATION OF SPIROMETRY - 1994. UPDATE. Am J Respir Crit Care Med. 1995;152(3):1107–1136.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338.
- van Noord JA, Buhl R, LaForce C, et al. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax. 2010;65(12):1086–1091.
- Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43(1):72–81.
- Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446.
- Vogelmeier CF, Román-Rodríguez M, Singh D, et al. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938.
- Mirza S, Clay RD, Koslow MA, et al. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488–1502.
- Calzetta L, Coppola A, Ritondo BL, et al. The impact of muscarinic receptor antagonists on airway inflammation: a systematic review. Int J Chron Obstruct Pulmon Dis. 2021;16:257–279.
- ZuWallack RL, Mahler DA, Reilly D, et al. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest. 2001;119(6):1661–1670.
- Zacarias EC, Castro AA, Cendon S. Effect of theophylline associated with short-acting or long-acting inhaled beta2-agonists in patients with stable chronic obstructive pulmonary disease: a systematic review. J Bras Pneumol. 2007;33(2):152–160.