ABSTRACT
Background
Interstitial lung disease (ILD) is a common complication of connective tissue diseases (CTD), but there are few clinical trials to guide disease management. We aimed to develop expert consensus statements and an algorithm for CTD-ILD management.
Research design and methods
Based on a targeted literature review, we developed 109 statements on managing CTD-ILD across six domains. We used a modified Delphi process to survey 22 physicians in Japan involved in managing CTD-ILD (specialists in pulmonology, rheumatology, pathology, and radiology). These panelists participated in two rounds of web-based survey to establish consensus statements, which were used to define an algorithm. Consensus was defined as a mean value ≥70 on a scale of 0 (strong disagreement) to 100 (strong agreement).
Results
Between May–August 2022, consensus was reached on 93 statements on CTD-ILD management. The most important consensus statements included screening CTD patients for ILD (typically with high-resolution computed tomography), using imaging, pulmonary function testing and serum biomarkers for diagnosis and severity assessment, regularly following up patients, and multidisciplinary management of CTD-ILD. Consensus statements were interpreted into an algorithm for clinical guidance.
Conclusions
Using the Delphi process, we have developed consensus statements and an algorithm to guide clinical decision-making for CTD-ILD.
1. Introduction
Connective tissue diseases (CTDs) comprise a large group of systemic disorders characterized by dysfunction of multiple organ systems due to inflammation and fibrosis. In patients with CTDs, respiratory complications are frequent, with interstitial lung disease (ILD) being the most common [Citation1]. ILD occurs in patients with various CTDs, including rheumatoid arthritis (RA), polymyositis/dermatomyositis (PM/DM), Sjögren’s syndrome, systemic sclerosis (SSc), systemic lupus erythematosus, and mixed connective tissue disease (MCTD) [Citation1,Citation2]. ILD is the leading cause of premature mortality in RA [Citation3,Citation4], PM/DM [Citation5,Citation6], Sjögren’s syndrome, SSc [Citation7,Citation8], and MCTD [Citation9]. The onset, progression speed, treatment response, and prognosis of CTD-ILDs are highly variable among patients; some develop rapidly or slowly progressive course, leading to mortality, and others have stable ILD with no clinically meaningful progression during the course of the disease. Furthermore, respiratory complications may also arise from infection and drug-induced lung injury, which often make diagnosis, evaluation, and treatment of ILD difficult. Thus, for both pulmonologists and rheumatologists, personalized management is essential for improving outcomes in patients with CTD-ILD.
Management of CTD-ILD to improve patient outcomes relies on appropriate diagnosis, evaluation, and treatment. However, there is a lack of clinical guidance on best practice, partly due to the limited evidence from randomized clinical trials. In the absence of high-quality data for evidence-based recommendations, expert consensus-based methods such as the Delphi technique [Citation10–12] can be used to develop clinical guidance. Consensus statements for identification and management of SSc-associated ILD were developed in 2020 by a panel of European experts using a modified Delphi process [Citation13]. Furthermore, the Japanese Respiratory Society and Japan College of Rheumatology collaborated in 2020 to develop a guideline on the diagnosis and treatment of CTD-ILD based on expert consensus from pulmonologists and rheumatologists [Citation14]. However, these statements were generated based on literature published before 2018, and a comprehensive guideline for CTD-ILD covering all aspects of disease management is lacking.
Consequently, we aimed to develop comprehensive consensus statements and algorithms for management of CTD-ILD across the following eight key domains: risk factors, screening tools, diagnosis, severity assessment, treatment initiation, drivers of treatment recommendations, disease progression, and follow-up and monitoring. For this purpose, we aimed to conduct a targeted literature review to develop an initial set of statements on management of CTD-ILD, and then refine them into a final set of expert consensus statements and algorithm using a modified Delphi process involving a panel of pulmonologists, rheumatologists, pathologists, and radiologists in Japan.
2. Methods
2.1. Study design and steering committee
This was a prospective healthcare provider survey conducted to define consensus statements and a disease management algorithm for CTD-ILD in Japan. The steering committee comprised two physicians: a pulmonologist (Y Kondoh) and a rheumatologist (M Kuwana). The steering committee was responsible for the planning and conduct of the study, including development of clinical questions (domains) for the targeted literature review, creating the statement-development committee, selecting Delphi panelists, reviewing survey results, and leading scientific discussions.
The study was conducted in accordance with the Japanese Ethical Guidelines for Medical and Biological Research Involving Human Subjects [Citation15], and was approved by an institutional review board from NPO-MINS.
2.2. Targeted literature review and development of draft statements
A targeted literature review was conducted to collect existing evidence in the eight key domains of management of CTD-ILD: (1) risk factors, (2) screening tools, (3) diagnosis, (4) severity assessment, (5) treatment initiation, (6) drivers of treatment recommendations, (7) disease progression, and (8) follow-up and monitoring. Any study reporting the relevant outcomes and including CTD-ILD patients was eligible for inclusion. The targeted literature review is described in detail in the Supplemental Information. Based on the targeted literature review, 309 articles published between 1 July 2018 and 5 October 2021 were selected for content extraction and analysis. A statement-development committee used the extracted information and their expert opinion to construct draft statements for the eight domains of CTD-ILD management. The statement-development committee comprised six specialists (three pulmonologists, three rheumatologists) including the two steering committee members and four others (M Bando, Y Kawahito, S Sato, T Suda). The draft statements were discussed and refined into a set of final statements for distribution to the panelists.
2.3. Expert panel
Participants in the Delphi panel were selected from the physicians involved in developing the 2020 Japanese guide for the diagnosis and treatment of CTD-ILD [Citation14]. Participants had to meet all the following eligibility criteria set by the steering committee: specialists in rheumatology, pulmonology, pathology, or radiology; more than 10 years of experience in clinical practice; experienced in treating patients with CTD-ILD; and previous experience as an advisory member of clinical guidelines. Selected experts were recruited by the study sponsor; those who agreed to participate were included in the panel.
2.4. Delphi process
Expert consensus statements for management of CTD-ILD were developed using a Delphi process involving two rounds of online survey of the panelists. In each round, panelists were asked to anonymously indicate their level of agreement with each statement on a scale of 0 (strong disagreement) to 100 (strong agreement). For each statement, consensus was regarded as achieved if the mean value of the level of agreement was ≥70 when rounded to two decimal places. Statements that did not reach consensus in round 1 were permitted to be modified by the two steering committee members for round 2 to find common ground for consensus. In round 2, a summary of the aggregate results of round 1 accompanied the statements. Statements that reached consensus after two rounds of the Delphi panel survey were used to create a draft management algorithm for CTD-ILD, which was then finalized by the statement-development committee.
2.5. Language and translation
Development of the draft statements, final statements, and the Delphi process was conducted in Japanese. The final statements were translated into English.
2.6. Statistical analysis
In each round of the Delphi panel survey, measures of central tendency (mean values) and variability (10th and 90th percentiles) were determined for each statement.
3. Results
3.1. Statements and panelists
During the discussion of the draft statements, the domains of diagnosis and severity assessment were merged, as were the domains of treatment initiation and drivers of treatment recommendations, resulting in a final set of six domains of CTD-ILD management. A total of 503 draft statements were developed, which were refined into 109 final statements across the 6 domains for the panelist survey (Supplemental Table S1). A total of 23 physicians were invited to participate in the Delphi panel and 22 (95.7%) were recruited (i.e. agreed to participate): 10 pulmonologists, 10 rheumatologists, one pathologist, and one radiologist. Most (72%) of the panelists were aged between 40 and 59 years (36% were 40–49 years old, 36% were 50–59 years old).
3.2. Delphi process and consensus statements
The Delphi process was conducted between May 2022 and August 2022. After the second round of the panel survey, consensus (mean agreement level of ≥70%) was reached on 93 of the 109 statements on CTD-ILD management () and not reached on 16 statements (). Across the six domains, consensus was reached for 34 of 36 statements on risk factors, nine of 10 on screening tools, 18 of 20 on diagnosis and severity assessment, all four statements on follow-up, 18 of 19 on initiation and/or treatment drivers, and all 10 statements on disease progression.
Table 1. Expert consensus agreement statements on CTD-ILD.
Table 2. Expert consensus disagreement statements on CTD-ILD.
3.2.1. Risk factors
For risk factors, consensus was reached on several issues, including that CTD is a risk factor for ILD, and that certain autoantibodies signified elevated risk for ILD in specific types of CTD. The following statements achieved over 95% consensus: CTD is a risk factor for developing ILD (96.5% consensus); anti-aminoacyl tRNA synthetase (ARS) antibody is a risk factor for developing ILD in patients with PM/DM (97.1%); anti-melanoma differentiation-associated gene 5 (MDA5) antibody is a risk factor for developing ILD in patients with DM (96.7%).
Statements that notably did not reach consensus included the following: male patients with SSc have a higher risk of developing ILD compared to female patients (68.2%); obesity (body mass index [BMI] ≥30 kg/m2) may be a risk factor for developing ILD in patients with RA (46.6%); poor functional status (Health Assessment Questionnaire-Disability Index score ≥1.0) may be a risk factor for developing ILD in patients with RA (53.6%).
3.2.2. Screening tools
A strong consensus was reached for the use of chest high-resolution computed tomography (HRCT) as a screening tool. Statements that achieved over 95% consensus were: screening for ILD using HRCT is informative in patients with CTD (96.5%); screening for ILD using chest HRCT is informative in patients with SSc with risk factors for developing ILD (98.2%); screening for ILD using chest HRCT is informative in patients with PM/DM with risk factors for ILD (98.6%); screening for ILD using chest HRCT is informative in patients with RA with risk factors for developing ILD (97.9%).
The only statement that did not reach consensus was the following: peripheral capillary oxygen saturation (SpO2) is considered to be a screening tool for ILD in patients with CTD (70.0%).
3.2.3. Diagnosis and severity assessment
There was also a strong consensus for the use of HRCT for diagnosis and assessment of severity of ILD, with the following statement reaching over 95% consensus: HRCT is effective for diagnosis and assessment of ILD severity in patients with CTD (97.8%). The only statements that did not achieve consensus were the following: transbronchial biopsy should be considered for diagnosis of ILD in patients with CTD (29.0%); transbronchial lung cryobiopsy should be considered for diagnosis of ILD in patients with CTD (53.5%).
3.2.4. Follow-up
HRCT was also recommended for follow-up of CTD-ILD patients (either annually or more often if patients have acute or subacute disease), as were annual pulmonary function tests. The following two statements achieved over 95% consensus: chest X-ray and/or HRCT are recommended every few days to every month for patients with CTD-ILD with progression during acute/subacute course (especially for those with anti-MDA5 antibody-positive DM) (97.3%); measurements of forced vital capacity (FVC) (and diffusing capacity of the lungs for carbon monoxide [DLco] if possible) are recommended at least once a year for patients with CTD-ILD (95.6%). No statements failed to reach consensus.
3.2.5. Initiation of treatment and/or treatment drivers
Consensus was reached that several factors should influence the decision to initiate treatment of CTD-ILD, including the presence of respiratory symptoms, the severity and progression of the ILD, the findings from chest HRCT, and pulmonary function tests. Furthermore, there was also strong agreement for multidisciplinary discussion involving pulmonologists, rheumatologists, radiologists and pathologists, and the need for individualized risk-benefit assessment. The following statements achieved over 95% consensus: evaluations of clinical course (disease progression) are informative when deciding whether to initiate treatment for patients with CTD-ILD (95.9%); evaluations of chest HRCT should be considered when deciding whether to initiate treatment for patients with CTD-ILD (96.2%); comprehensive evaluations of respiratory symptoms, quality of life (QOL), chest HRCT, and FVC, DLco, and serum Krebs von den Lungen-6 (KL-6) levels are informative when deciding whether to initiate treatment for patients with CTD-ILD (99.1%); immediate treatment of acute/subacute onset or acute exacerbation in patients with CTD-ILD is effective (98.1%); individualized risk-benefit analysis is informative when deciding whether to initiate treatment for patients with CTD-ILD (95.7%); presence of anti-MDA5 antibodies is a consideration when deciding whether to initiate treatment in patients with PM/DM-ILD (95.4%). The only statement that did not achieve consensus was the following: presence of arthritis should be considered when deciding whether to initiate treatment in patients with SSc-ILD (54.5%).
3.2.6. Disease progression
Overall, there was strong consensus for the use of HRCT, pulmonary function tests, and clinical symptoms to monitor disease progression. The following statements achieved over 95% consensus: assessment of change (decline) in FVC is informative when evaluating disease progression of CTD-ILD (95.7%); combined assessment of change (worsening) in the extent of fibrosis on chest HRCT and change (decline) in FVC and DLco is informative when evaluating disease progression of CTD-ILD (97.6%); combined assessment of worsening respiratory symptoms, change (worsening) in the extent of fibrosis on chest HRCT, and change (decline) in FVC and DLco is informative when evaluating disease progression of CTD-ILD (98.8%). No statements failed to reach consensus.
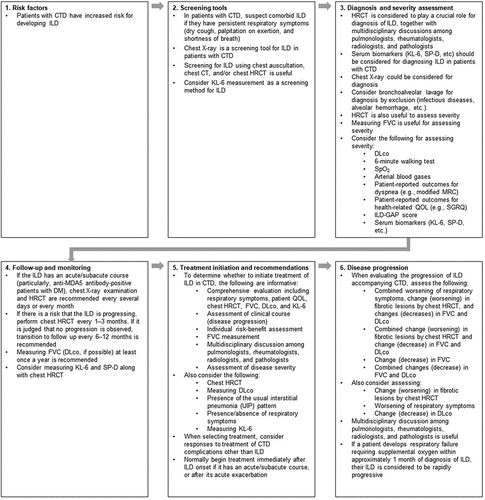
3.3. Algorithm for managing CTD-ILD
In order to assist the translation of this research into clinical practice, the consensus agreement statements were interpreted and incorporated into an algorithm for management of CTD-ILD ().
Figure 1. Management algorithm for interstitial lung disease in patients with connective tissue diseases. CT: computed tomography; CTD: connective tissue disease; DLco: diffusing capacity of the lungs for carbon monoxide; DM: dermatomyositis; FVC: forced vital capacity; GAP: gender, age, physiology; HRCT: high-resolution computed tomography; ILD: interstitial lung disease; KL-6: Krebs von den Lungen 6; MDA5: melanoma differentiation-associated gene 5; MRC: Medical Research Council dyspnea scale; QOL: quality of life; SGRQ: St George's Respiratory Questionnaire; SP-D: surfactant protein D; SpO2: peripheral oxygen saturation.
4. Discussion
In this study, we used a modified Delphi process to develop a set of consensus statements on the management of CTD-ILD, and proposed an algorithm to provide clinical guidance for the identification and management of these conditions.
The Delphi technique is increasingly used as a robust method for generating consensus-based guidance in areas of healthcare where clinical evidence might be insufficient or contradictory [Citation10–12]. In this context, use of the Delphi methodology aims to provide answers to clinical questions based on the consensus of an expert group. Prior to our study, clinical guidance on management of CTD-ILD was mostly limited to the Delphi-derived European guideline on SSc-ILD [Citation13] and Japanese guideline on diagnosis and treatment of CTD-ILD, which were generated based on narrative review of literature and experts’ opinion [Citation14].
Overall, the large majority of statements proposed to the panelists achieved consensus agreement (i.e. mean score of ≥70%). Regarding risk factors, it was strongly agreed that CTD is a risk factor for ILD. Furthermore, the presence of certain autoantibodies was agreed to be a risk factor for ILD in specific CTDs: e.g. anti-ARS antibody in patients with PM/DM and anti-MDA5 antibody in patients with DM [Citation17]; and anti-topoisomerase I (anti-Scl-70) antibody in patients with SSc [Citation18]. Regarding demographic and clinical factors, it was agreed that old age (in RA and Sjögren’s syndrome) and male sex (in RA) are risk factors for ILD, but being male was not felt to be a risk factor for ILD in SSc, nor were obesity (BMI ≥30 kg/m2) and impaired physical function felt to be risk factors for ILD in RA.
For screening, there was consensus that persistent respiratory symptoms in patients with CTD should raise suspicion of ILD. Furthermore, radiological imaging with chest X-ray, CT or HRCT were agreed to be options for screening, the latter especially in individual CTDs where the patient has a risk factor (SSc, PM/DM, RA). The screening statements did not include screening intervals; therefore, frequency of screening should be guided by clinical judgment of an individual’s risk of developing ILD, as was also recommended by the 2020 Delphi study on management of SSc-ILD [Citation13]. Although the use of HRCT for regular screening may raise some concern regarding the risk of exposure to ionizing radiation, modern scanners typically use lower doses of radiation than older models.
Chest HRCT was also agreed to be an option for both diagnosing and assessing the severity of ILD in CTD patients, while chest X-ray can be employed for diagnosis. Given this consensus and other evidence in this area [Citation19], HRCT is considered to play a crucial role for diagnosis. The panelists agreed that serum biomarkers such as KL-6 and surfactant protein D (SP-D) [Citation20–22] may also be useful for diagnosis as well as assessment of severity. Other tests agreed to be useful for assessing severity included the 6-minute walk test, FVC, DLco, and SpO2. It was also felt that patient-reported outcomes such as dyspnea and health-related QOL (assessed with validated instruments such as the Medical Research Council dyspnea scale and the St George’s Respiratory Questionnaire [Citation23], respectively) should be considered for assessing severity.
For follow-up of CTD patients with ILD, the consensus was that chest X-ray or HRCT should be performed frequently if the ILD has an acute or subacute course; otherwise, HRCT and pulmonary function tests (FVC and, if possible, DLco) should be considered at least annually.
Several findings from the present study can inform the decision to initiate treatment or not in patients with CTD-ILD, including considering the clinical course of the ILD and making individual risk-benefit assessments, with tests such as chest HRCT, FVC, DLco, and serum biomarkers (e.g. KL-6) useful in this regard. Furthermore, treatment should be initiated immediately if the ILD has an acute or subacute course, and consideration should be given to disease severity and the presence or absence of respiratory symptoms. In this present study, the panelists were not asked about specific treatment options for CTD-ILD; however, the recent Delphi-derived European consensus statements for SSc-ILD [Citation13] and Japanese guideline on CTD-ILD [Citation14] provided some suggestions in this regard. Therapeutic options include medications, non-drug treatments such as apheresis, ventilation for acute exacerbations, and lung transplant for end-stage disease [Citation14]. The choice of treatment modality depends on factors such as the specific CTD, severity of ILD, disease behavior, and the presence or absence of risk factors for mortality or progression [Citation14].
Medications, alone or in combination, include corticosteroids (e.g. prednisolone), immunosuppressants (e.g. cyclophosphamide, mycophenolate mofetil, methotrexate, cyclosporin), antifibrotic drugs such as nintedanib and pirfenidone, and other agents including molecular-targeting biologics such as tocilizumab and rituximab [Citation14,Citation24]. In many cases, there is little robust evidence to support their use in specific CTD-ILD indications [Citation14,Citation24]; however, data from randomized clinical trials exist for some medications. In the SENSCIS trial, the anti-fibrotic agent nintedanib reduced the rate of decline in FVC in patients with SSc-ILD [Citation25]. Nintedanib also reduced FVC decline in patients with chronic fibrosing ILDs with a progressive phenotype in the INBUILD trial, which included patients with RA-ILD, SSc-ILD and MCTD-ILD [Citation26]. Based on these trials, nintedanib is now approved for SSc-ILD and progressive chronic fibrosing ILD globally, i.e. the US, EU, and Japan. Another antifibrotic agent, pirfenidone, is approved for use in patients with idiopathic pulmonary fibrosis (IPF), but not in other progressive fibrotic ILDs. The RELIEF trial of pirfenidone in patients with non-IPF fibrotic ILDs (including CTD-ILD) was terminated due to slow recruitment, although there was a signal of treatment benefit in terms of slowing FVC decline [Citation27]. Tocilizumab is approved in the US to slow the rate of declining pulmonary function in adults with SSc-ILD, based on the focuSSced trial in which it significantly reduced FVC decline in patients with early, active diffuse cutaneous SSc and ILD [Citation28].
For determining if ILD is progressing in patients with CTD, there was general agreement to consider worsening of respiratory symptoms, worsening of ILD lesions using imaging, especially chest HRCT, and decreases in FVC and/or DLco – either separately or in combination. The exact levels of FVC decline and worsening in other parameters that indicate significant progression have not been delineated and may differ between specific CTD-ILDs; hence, these must be assessed by clinical judgment until further evidence is obtained.
A recurring theme across the domains of disease management was the consensus that multidisciplinary discussions involving pulmonologists, rheumatologists, radiologists, and pathologists should be considered – specifically when diagnosing ILD, initiating treatment, and evaluating progression of ILD in CTD patients. In this regard, it is noteworthy that pulmonologists and rheumatologists have complementary expertise in the use of medications such as corticosteroids and immunosuppressants, respectively.
In order to make the consensus statements more useful for clinical practice, we interpreted them into an algorithm for management of CTD-ILD. Among other recommendations, the algorithm highlights that patients with CTD should undergo screening for ILD – particularly with HRCT – and have regular follow-up, and that multidisciplinary management of the condition is important.
Our study has some noteworthy strengths and limitations. The main limitation is due to the inherent nature of the Delphi method, which is opinion-based and potentially biased by panel selection. Regarding the latter, panelists were invited based on their involvement in previous guidelines, rather than randomly selected, to ensure a certain level of clinical expertise in managing CTD-ILD. Furthermore, including specialists from four disciplines increased the diversity. Notably, patient perspectives were not included as the intention was to develop a consensus based on expert clinical opinion. Additionally, the panelists surveyed were all based in Japan, so not all findings may necessarily be generalizable to other countries.
5. Conclusions
The consensus statements and associated clinical algorithm reported here were derived from a Delphi process involving 22 Japanese specialists in pulmonology, rheumatology, pathology, and radiology. They provide clinical guidance for the identification and management of ILD in various CTDs. Many unmet needs and important questions remain, including disease pathogenesis and pathophysiology, optimal screening strategies, frequency of follow-up, and the comparative efficacy and safety of the various treatment options. Clarification of such issues will hopefully enable the development of further tailored guidance in the form of individual algorithms for specific CTD-ILDs. Until such further evidence emerges from randomized clinical trials and other studies in this area, we hope that our efforts will provide a useful framework for clinical decision-making.
Declaration of interest
The authors received no direct compensation related to the development of the manuscript. All authors received honoraria for their participation in the study. All panelists were offered honoraria for their participation in the study. Masataka Kuwana has received consulting fees, speaking fees, and research grants from AbbVie, argenx, Asahi Kasei, AstraZeneca, Astellas, Boehringer Ingelheim, Chugai, Corbus, Eisai, GlaxoSmithKline, Horizon, Janssen, Kissei, MBL, Mitsubishi Tanabe, Mochida, Nippon Shinyaku, Ono Pharmaceuticals, Pfizer, and Taisho.
Y Kondoh has received consulting fees from Asahi Kasei Pharma Corp, Boehringer Ingelheim, Chugai Pharmaceutical Co., Ltd., Healios K.K., Janssen Pharmaceutical KK, Shionogi Co, Ltd., and Taiho Pharmaceutical Co., Ltd; Speaker bureaus for Asahi Kasei Pharma Corp, Boehringer Ingelheim, Eisai Co, Ltd, Bristol Myers Squibb, Janssen Pharmaceutical K.K., KYORIN Pharmaceutical Co, Ltd, Mitsubishi Tanabe Pharma, NIPPON SHINYAKU CO., LTD, Novartis Pharma KK, Shionogi Co, Ltd., and Teijin Pharma Ltd.
M Bando has received honoraria from Nippon Boehringer Ingelheim Co., Ltd. and Shionogi & Co., Ltd.
Y Kawahito has received research grants from AbbVie GK, Asahi Kasei Pharma Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Corp., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Chugai Pharmaceutical Co. Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., and Teijin Pharma Ltd; and speaker’s fee from AbbVie GK, Ayumi Pharmaceutical Corp., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., and Teijin Pharma Ltd.
S Sato has received subsidies or donations from Asahi Kasei Pharma Corporation, Ono Pharmaceutical Co., Ltd., and Chugai Pharmaceutical Co., Ltd.
T Suda has received honoraria from AstraZeneca K.K. and Nippon Boehringer Ingelheim Co., Ltd.; grant support and/or research funding from AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd.; subsidies or donations from Astellas Pharma Inc., MSD K.K., Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., and Pfizer Japan Inc.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosure
Peer reviewers on this manuscript have received an honorarium from Expert Review of Respiratory Medicine for their review work, but have no other relevant financial relationships to disclose
Author contributions
All authors participated in the interpretation of study results and in the drafting, critical revision, and approval of the final version of the manuscript. All authors agree to be accountable for all aspects of this work.
The CTD-ILD Delphi Collaborators
Hirofumi Amano (Department of Internal Medicine and Rheumatology, Juntendo University School of Medicine, Bunkyo, Tokyo, Japan); Takao Fujii (Department of Rheumatology and Clinical Immunology, Wakayama Medical University, Wakayama, Japan); Junya Fukuoka (Department of Pathology Informatics, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan); Takahisa Gono (Department of Allergy and Rheumatology, Nippon Medical School Graduate School of Medicine, Bunkyo, Tokyo, Japan); Yoshikazu Inoue (Clinical Research Center, National Hospital Organization Kinki-Chuo Chest Medical Center, Sakai, Osaka, Japan); Takeshi Johkoh (Department of Radiology, Kansai Rosai Hospital, Amagasaki, Hyogo, Japan); Yasushi Kawaguchi (Division of Rheumatology, Department of Internal Medicine, Tokyo Women’s Medical University, Shinjuku, Tokyo, Japan); Tomohiro Koga (Department of Immunology and Rheumatology, Division of Advanced Preventive Medical Sciences, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan); Kazuhiro Kurasawa (Department of Rheumatology, Dokkyo Medical University, Mibu, Tochigi, Japan); Yutaro Nakamura (Department of Respiratory Medicine, National Hospital Organization, Tenryu Hospital, Hamamatsu, Shizuoka, Japan); Ran Nakashima (Department of Rheumatology and Clinical Immunology, Kyoto University Graduate School of Medicine, Kyoto, Japan); Yasuhiko Nishioka (Department of Respiratory Medicine and Rheumatology, Graduate School of Biomedical Sciences, Tokushima University, Tokushima, Japan); Osamu Nishiyama (Department of Respiratory Medicine and Allergology, Kindai University Faculty of Medicine, Osakasayama, Osaka, Japan); Takashi Ogura (Department of Respiratory Medicine, Kanagawa Cardiovascular and Respiratory Center, Yokohama, Kanagawa, Japan); Masaki Okamoto (Department of Respirology, National Hospital Organization Kyushu Medical Center, and Division of Respirology, Neurology, and Rheumatology, Kurume University, Fukuoka, Japan); Ken-ei Sada (Department of Clinical Epidemiology, Kochi Medical School, Nankoku, Kochi, Japan); Susumu Sakamoto (Department of Respiratory Medicine, Toho University Omori Medical Center, Tokyo, Japan); Tohru Takeuchi (Department of Internal Medicine (IV), Osaka Medical and Pharmaceutical University, Takatsuki, Osaka, Japan); Hiromi Tomioka (Department of Respiratory Medicine, Kobe City Medical Center West Hospital, Kobe, Hyogo, Japan); Yuko Waseda (Third Department of Internal Medicine, Faculty of Medical Sciences, University of Fukui, Fukui, Japan); Hidehiro Yamada (Center for Rheumatic Diseases, Seirei Yokohama Hospital, Yokohama, Kanagawa, Japan); and Yasuhiko Yamano (Department of Respiratory Medicine and Allergy, Tosei General Hospital, Seto, Aichi, Japan).
Supplemental Material
Download MS Word (1 MB)Acknowledgments
The Delphi process was managed by a contract research organization (IQVIA Solutions Japan K.K.; Tokyo, Japan). Medical writing support was provided by Giles Brooke, PhD, on behalf of Elevate Scientific Solutions (Horsham, UK) under the authors’ conceptual direction and based on feedback from the authors, and was contracted and compensated by Nippon Boehringer Ingelheim. Nippon Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The authors thank all the CTD-ILD Delphi Collaborators who generously participated in this study.
Data availability statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17476348.2023.2176303
Additional information
Funding
References
- Spagnolo P, Cordier JF, Cottin V. Connective tissue diseases, multimorbidity and the ageing lung. Eur Respir J. 2016 May;47(5):1535–1558.
- Kim EA, Lee KS, Johkoh T, et al. Interstitial lung diseases associated with collagen vascular diseases: radiologic and histopathologic findings. Radiographics. 2002 Oct;22(suppl_1):S151–65.
- Nakajima A, Inoue E, Tanaka E, et al. Mortality and cause of death in Japanese patients with rheumatoid arthritis based on a large observational cohort, IORRA. Scand J Rheumatol. 2010;39(5):360–367.
- Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011 Feb 1;183(3):372–378.
- Yamasaki Y, Yamada H, Ohkubo M, et al. Longterm survival and associated risk factors in patients with adult-onset idiopathic inflammatory myopathies and amyopathic dermatomyositis: experience in a single institute in Japan. J Rheumatol. 2011 Aug;38(8):1636–1643.
- Ishizuka M, Watanabe R, Ishii T, et al. Long-term follow-up of 124 patients with polymyositis and dermatomyositis: statistical analysis of prognostic factors. Mod Rheumatol. 2016;26(1):115–120.
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007 Jul;66(7):940–944.
- Castelino FV, Dellaripa PF. Recent progress in systemic sclerosis-interstitial lung disease. Curr Opin Rheumatol. 2018 Nov;30(6):570–575.
- Gunnarsson R, Hetlevik SO, Lilleby V, et al. Mixed connective tissue disease. Best Pract Res Clin Rheumatol. 2016 Feb;30(1):95–111.
- Milholland AV, Wheeler SG, Heieck JJ. Medical assessment by a Delphi group opinion technic. N Engl J Med. 1973 Jun 14;288(24):1272–1275.
- Page A, Potter K, Clifford R, et al. Prescribing for Australians living with dementia: study protocol using the Delphi technique. BMJ Open. 2015 Aug 11;5(8):e008048.
- Taylor E. We agree, don’t we? The Delphi method for health environments research. Herd. 2020 Jan;13(1):11–23.
- Hoffmann-Vold A-M, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol. 2020;2(2):e71–e83.
- Kondoh Y, Makino S, Ogura T, et al. 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease. Respir Investig. 2021 Nov;59(6):709–740.
- Eba J, Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol. 2022 May 31;52(6):539–544.
- Morisset J, Vittinghoff E, Elicker BM, et al. Mortality risk prediction in scleroderma-related interstitial lung disease: the SADL model. Chest. 2017 Nov;152(5):999–1007.
- Hozumi H, Fujisawa T, Nakashima R, et al. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med. 2016 Dec;121:91–99.
- Jandali B, Salazar GA, Hudson M, et al. The effect of anti-Scl-70 antibody determination method on its predictive significance for interstitial lung disease progression in systemic sclerosis. ACR Open Rheumatol. 2022 4;Apr(4):345–351.
- Cereser L, Passarotti E, De Pellegrin A, et al. Chest high-resolution computed tomography in patients with connective tissue disease: pulmonary conditions beyond “the usual suspects”. Curr Probl Diagn Radiol. 2022 Sep-Oct;51(5):759–767.
- d’Alessandro M, Bergantini L, Cameli P, et al. Krebs von den Lungen-6 as a biomarker for disease severity assessment in interstitial lung disease: a comprehensive review. Biomark Med. 2020 Jun;14(8):665–674.
- Kaieda S, Gono T, Masui K, et al. Evaluation of usefulness in surfactant protein D as a predictor of mortality in myositis-associated interstitial lung disease. PLoS One. 2020;15(6):e0234523.
- Zhong D, Wu C, Bai J, et al. Comparative diagnostic efficacy of serum Krebs von den Lungen-6 and surfactant D for connective tissue disease-associated interstitial lung diseases: a meta-analysis. Medicine (Baltimore). 2020 Apr;99(16):e19695.
- Suzuki A, Kondoh Y, Swigris JJ, et al. Performance of the St George’s Respiratory Questionnaire in patients with connective tissue disease-associated interstitial lung disease. Respirology. 2018 Mar 25;23(9):851–859.
- Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet. 2022 Sep 3;400(10354):769–786.
- Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019 Jun 27;380(26):2518–2528.
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019 Oct 31;381(18):1718–1727.
- Behr J, Prasse A, Kreuter M, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021 May;9(5):476–486.
- Khanna D, Lin CJF, Furst DE, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020 Oct;8(10):963–974.
