ABSTRACT
Background
It is unclear the efficacy and safety of glucocorticoids compared with placebo or usual care for treatment of COVID-19.
Research design and methods
Randomized controlled trials (RCTs) of corticosteroids in COVID-19 patients from 1 December 2019, to 30 June 2022, were assessed using Cochrane bias risk assessment method and improved Jadad score scale. GRADEpro was used to rate the quality of evidence for outcomes.
Results
Fifteen RCTs were included, including 10,620 patients. Glucocorticoid treatment for severe and critical COVID-19 showed lesser all-cause mortality (OR = 0.85, 95% CI [0.76, 0.94], P = 0.002) than conventional treatment. However, for mildly ill patients, neither inhaled drugs nor intravenous drugs reduced mortality (OR = 0.64, 95% CI [0.24, 1.76], P = 0.39). Glucocorticoids had no significant effect on the adverse reactions of patients (OR = 1.18, 95% CI [0.77, 1.80], P = 0.44) compared with usual care/placebo. Subgroup analysis demonstrated that dexamethasone significantly reduced the mortality of COVID-19 patients. Low-dose glucocorticoids were also associated with lower all-cause mortality.
Conclusion
Glucocorticoids (especially dexamethasone) reduce mortality of patients with severe and critical COVID-19 with no significant effect on the incidence of adverse reactions (moderate quality). In contrast, glucocorticoids do not benefit patients with mild symptoms (low quality).
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by a new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [Citation1] has triggered a global health crisis [Citation2]. The risk of infection is high, the strain mutates quickly, and the prognosis is poor for severely ill patients. People aged ≥ 60 years and those with underlying conditions such as hypertension, heart and lung problems, diabetes, obesity, or cancer have a higher risk of developing severe COVID-19. However, individuals of any age can develop the disease, become seriously ill, or die from COVID-19 [Citation1]. In the latest update in September 2022, more than 600 million COVID-19 infections and more than 6 million COVID-19-related deaths have been recorded worldwide [Citation3].
SARS-CoV-2 enters host cells by binding to angiotensin-converting enzyme 2 (ACE2) after cleavage by transmembrane protease serine 2 (TMPRSS2) [Citation4]. Its pathogenesis is closely related to an immune-mediated cytokine storm [Citation5]. Glucocorticoids, as the standard therapy for inflammatory and autoimmune disorders, have a good inhibitory effect on inflammatory factors and are often used as adjuvant therapy for viral pneumonia [Citation6]. Its main anti-inflammatory effect is to inhibit the inflammatory process and restore balance in vivo by the inhibition of a large number of pro-inflammatory genes encoding cytokines, chemokines, cell adhesion molecules, inflammatory enzymes, and receptors [Citation7]. Hence, glucocorticoids can play a central role in immunosuppression during an acute cytokine storm for the symptomatic treatment of COVID-19.
Studies on the efficacy and safety of glucocorticoids in the treatment of COVID-19 emerge endlessly. WHO guidelines [Citation8] strongly recommend systemic glucocorticoid treatment for severe and critical COVID-19 patients. Wagner C et al. [Citation9] reached a similar conclusion that systemic glucocorticoid treatment is beneficial to reduce all-cause mortality in severe and critical diseases. With the development of time and the variation of the virus, more and more patients with mild symptoms are diagnosed. However, there is a lack of efficacy evaluation for asymptomatic patients or patients with mild symptoms, and the therapeutic effect of inhaled glucocorticoid on COVID-19 is still unclear. Therefore, it is necessary to conduct a systematic analysis again. This study summarizes RCTs carried out globally with both systemic and inhaled glucocorticoids. In addition to basic indicators such as mortality and adverse reactions, subgroup analysis was also conducted in terms of disease degree, drug type and drug dose. The goal of this study is to comprehensively assess the efficacy and safety of glucocorticoids versus usual care or placebo for COVID-19.
2. Methods
2.1. Registration and protocol
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [Citation10] and registered with PROSPERO (CRD42020193268). The PRISMA checklist and the review protocol can be accessed in Appendix 1.
2.2. Search strategy
We performed a systematic literature search in electronic databases, including PubMed, Embase, the American clinical trial database, and the Chinese database CNKI for eligible studies published until 30 June 2022. There was no language restriction. The search strategy was based on the following keywords and MeSH terms: ‘glucocorticoid, prednisolone, dexamethasone, budesonide, hydrocortisone, methylprednisolone, prednisone, COVID-19, SARS-CoV-2, and randomized controlled trials’. Detailed search strategy can be found in Appendix 2. Two investigators (Ye Zhang, Dian Li) independently performed literature screening to identify eligible studies. Disagreements were resolved by consensus. The reference lists of the relevant articles were also reviewed to identify potential studies.
2.3. Protocol and guidance
The included studies met the following criteria: (1) The included patients were clinically suspected or laboratory diagnosed as having COVID-19. (2) They were randomized controlled trials. (3) Intervention: The experimental group was administered glucocorticoids of any type in any form, dose, or duration based on standard treatment, while the control group was given standard treatment or placebo. (4) After the completion of the experiment, accurate data were obtained.
2.4. Exclusion criteria
(1) Non-randomized controlled trials (cohort studies, case-control studies, etc.) (2) Trials with unavailable data and trials with missing data without explanation or treatment. (3) Low-quality studies (including less-than-rigorous design and small enrollment) (4) Studies that repeatedly reported the same cohort of patients.
2.5. Assessment of risk of bias
The Cochrane Risk of Bias Assessment Method and the Modified Jadad Rating Scale were used to assess the risk of bias for the included studies. Funnel chart were used to evaluate publication bias. Disagreements were settled through negotiation or according to the opinions of a third party.
2.6. Outcomes
The main outcome was all-cause mortality, and subgroup analyses were conducted according to the type of glucocorticoid drug administered, doses of glucocorticoids and the severity of the patient’s condition. According to Guidelines for clinical application of glucocorticoids [Citation11], we defined > 60 mg prednisone equivalent a day as high dose of systemic glucocorticoid, and < 60 mg/d as low dose; According to the Guidelines for the Prevention and Treatment of Bronchial Asthma (2020 Edition) [Citation12], >800ug/d is defined as high dose of budesonide, and < 800ug/d is low dose; > 320ug/d is defined as the high dose of ciclesonide, and < 320ug/d is low dose. The severity of COVID-19 is determined according to the WHO guidelines [Citation13]. We analyzed the included studies. Patients with mild and moderate disease exhibited only symptoms related to COVID-19 (low fever, mild fatigue, dysosmia, and/or pneumonia) and severe and critical patients were characterized by dyspnea and/or hypoxemia, which are consistent with the definition of the guidelines.
Secondary outcomes included adverse effects (defined as the number of patients with any event), length of hospital stay, and the time to conversion/conversion rate of pharyngeal swab nucleic acid testing.
2.7. Assessment of the quality of evidence
We followed the GRADE guidelines [Citation14] and used GRADEpro 3.6 to rate the quality of evidence for the outcomes included in this study. The evaluation of evidence grade is divided into high (no degradation), moderate (reduced by 1 grade), low (reduced by 2 grades) and very low (reduced by 3 grades) based on considerations of five factors – risk of bias, consistency, indirectness, immediacy, and publication bias. Wenxiao Qiao and Dian Li rated each outcome and resolved discrepancies by consensus.
2.8. Data synthesis
Using the software ReviewManage 5.4.1, statistical analysis of the gathered data was performed. The odds ratio (OR) was used as the effect size for the count data and the confidence interval (CI) was set at 95%. The heterogeneity of the included studies was examined using x2 test, and if I2 < 50% and Paired (P) > 0.1, the heterogeneity of the included studies was considered not statistically significant, and the fixed-effects model could be used to combine the effect sizes. If I2 ≥ 50% and/or P < 0.1, this suggested actual heterogeneity among the included studies. A random-effects model was chosen to combine the effect sizes, or a sensitivity analysis was chosen for the analysis and investigation of the causes of heterogeneity and to exclude literature that caused greater heterogeneity. If a meta-analysis of data from the literature was not possible, descriptive analysis was used instead.
3. Results
3.1. Eligible studies and study characteristics
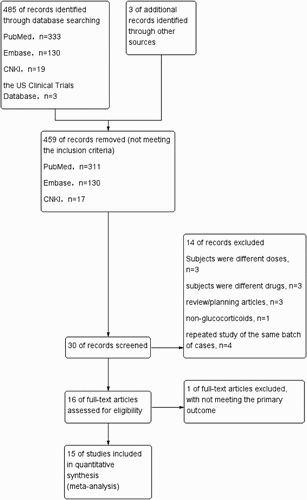
We initially identified 488 studies and included 15 eligible trials [Citation15–29] in the final meta-analysis. The trials comprised 10,620 participants, including 4090 patients in the glucocorticoid group and 6530 patients in the conventional treatment group. The literature screening process and results are demonstrated in . The details of the included trials are summarized in , including the number of participants, sex, average age, drug use, dose, main outcome of the experimental group, and control group.
Table 1. Included trails and characteristics.
3.2. Assessment of risk of bias
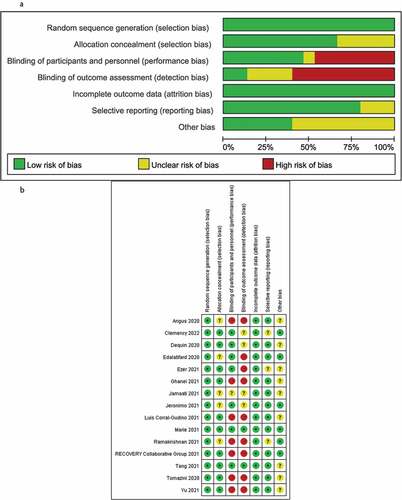
The Cochrane risk-of-bias assessment was conducted from six perspectives: random, allocation, blinding, measurement, reporting, and other sources of bias. The results demonstrated that the overall quality of the included studies was high and the risk of bias was low. The bias was mainly focused on the implementation of the blind method as some RCTs chose open research due to ethical reasons (). The Jadad modified rating scale evaluation demonstrated that 13 articles were of high quality and two articles were of low quality ().
Table 2. The Jadad modified rating scale.
3.3. Primary outcome: all-cause mortality
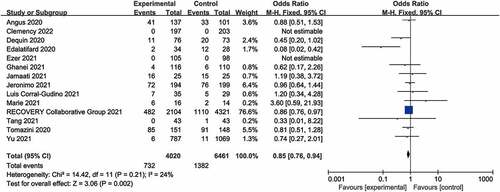
Fourteen trials [Citation15,Citation17–29] reported all-cause mortality. Among the 4020 patients who received glucocorticoid treatment, 732 died (18.2%), while 1382 of 6461 patients in the control group died (21.4%). There was a statistically significant difference between the glucocorticoid group and the control group (OR = 0.85, 95% CI [0.76, 0.94], I2 = 24%, P = 0.002; ), indicating that glucocorticoids can effectively reduce the mortality of COVID-19 patients, compared with usual care or placebo treatment. The sensitivity analysis was robust, and the conclusion was stable.
3.4. Subgroup analyses
3.4.1. Type of glucocorticoids
Six types of glucocorticoids were included in this study: dexamethasone, methylprednisolone, prednisolone, hydrocortisone, budesonide, and ciclesonide; to investigate the efficacy of these different types of glucocorticoids in adjuvant therapy of novel coronavirus, the following analyses were performed.
Three trials used dexamethasone [Citation15,Citation20,Citation21], with a total of 6774 patients, and the heterogeneity among the included studies was low (I2 = 0%, P = 0.83). The fixed effects model was selected to combine the effect size for meta-analysis, and the results demonstrated that OR = 0.86, 95% CI (0.76–0.97), P = 0.01 (Supplemental Figure 1A). The difference was statistically significant, indicating that dexamethasone adjuvant therapy can significantly reduce the mortality of patients with COVID-19.
No mortality was reported in two RCTs using ciclesonide [Citation18,Citation19]. Hence, the disease remission rate (the resolution of all COVID-19-related symptoms by day 30) was used for evaluation. Heterogeneity test yielded a result of I2 = 0, indicating that the number is completely homogeneous. The use of a fixed effect model combined with the effect of meta-analysis suggested that the effect of ciclesonide adjuvant therapy on the improvement of symptoms was not statistically significant (OR = 1.40, 95% CI [1.01, 1.96], P = 0.05, Supplemental Figure 1B).
Two trials used budesonide [Citation16,Citation17], but only one reported all-cause mortality; therefore, the disease deterioration rate (COVID-19-related urgent care visits, including emergency department assessment or hospitalization) was selected for evaluation. Heterogeneity between the studies was high (I2 = 64%). When the random effects model was used to analyze the effect, we obtained OR = 0.38, 95% CI (0.08, 1.75), and P = 0.21 (Supplemental Figure 1C), suggesting that budesonide adjuvant therapy had no significant effect on the deterioration of COVID-19 patients compared with conventional therapy or placebo.
In addition, one RCT [Citation22] used prednisolone as an adjuvant therapy, with the result P = 0.47. Four articles [Citation23–26] used methylprednisolone], and meta-analyze showed OR = 0.54, 95% CI (0.18, 1.63), and P = 0.27. Three trails [Citation27–29] used hydrocortisone [and meta-analyze showed OR = 0.80, 95% CI (0.37, 1.72), and P = 0.57. Subgroup analysis demonstrated that there was no significant difference in mortality between the three drugs and the control group (Supplemental Figure 1A)
3.4.2. Different doses of glucocorticoids
Nine RCTs [Citation15,Citation19,Citation22,Citation23,Citation25–29] used low-dose dexamethasone, the heterogeneity test I2 = 0, and the fixed effect model combined with the effect quantity was used for meta-analysis, suggesting that compared with usual care or placebo, low dose glucocorticoid is beneficial to reduce mortality (OR = 0.86, 95% CI [0.77, 0.96], P = 0.009, Supplemental Figure 2A). Five RCTs [Citation17,Citation18,Citation20,Citation21,Citation24] used high-dose glucocorticoid, and the heterogeneity between studies was high (I2 = 62%). Using a random effect model combined with the effect amount, the OR = 0.61, 95% CI (0.28, 1.34), P = 0.22 (Supplemental Figure 2B) were obtained, suggesting that high-dose glucocorticoid has no significant impact on the mortality of patients with COVID-19.
3.4.3. Different disease processes
This study analyzed the effect of glucocorticoid adjuvant therapy from the perspectives of mild, moderate, severe, and critical types of disease.
The RECOVERY study included more comprehensive cases, which were divided into mild and moderate type (1535 cases) and severe type (4890 cases). A total of 6386 severe and critically ill patients from seven RCTs [Citation15,Citation20–22,Citation25,Citation27,Citation28] were included in this study. There was no statistical difference in the heterogeneity test (I2 = 0). The meta- analysis demonstrated that OR = 0.78, 95% CI [0.69, 0.88], P < 0.0001 (Supplemental Figure 3). These results suggest that glucocorticoid adjuvant therapy has statistical significance in reducing mortality in patients with severe and critical COVID-19.
A total of 3616 patients with mild and moderate disease from four RCTs [Citation15–17,Citation23] were included in the mortality analysis; the glucocorticoid mortality rate was 6.9% (96/1401) and the control group mortality rate was 7.5% (167/2215), which was not statistically significant (OR = 0.64, 95% CI (0.24, 1.76), P = 0.39; Supplemental Figure 3).
3.5. Secondary outcome
3.5.1. Adverse reactions
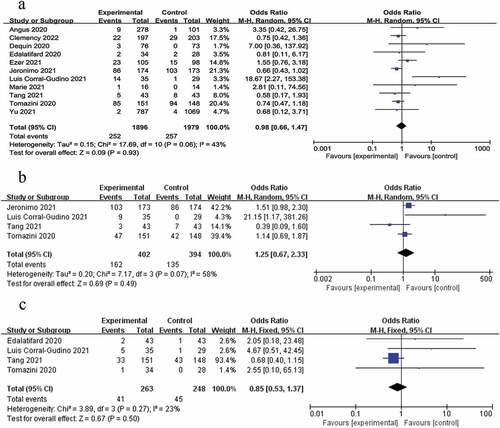
A total of 11 RCT’s [Citation17–19,Citation21,Citation23–29] were included in this study to record the adverse reactions during adjuvant therapy with glucocorticoids. Specific types of adverse reactions can be found in . Adverse effects occurred in 269 of the 1895 patients treated with glucocorticoids (14.2%) and 240 of the 1890 patients in the control group (12.7%). The random effects model combined with effect size was used for analysis, and the results demonstrated that OR = 1.88, 95% CI (0.77, 1.80), P = 0.44 (), indicating that there was no statistically significant difference in adverse reactions between glucocorticoid adjuvant therapy and conventional treatment.
Table 3. Types of adverse reactions.
Among the adverse reactions mentioned, hyperglycemia and infection are the most common. Four RCTs [Citation21,Citation23,Citation25,Citation26] mentioned hyperglycemia events, and we carried out a meta-analysis. The results showed that the heterogeneity was high (I2 = 58%). The random effect model combined with the analysis of the effect quantity obtained OR = 1.25, 95% CI (0.67, 2.33), P = 0.49 (), suggesting that glucocorticoids had no significant effect on the occurrence of hyperglycemia symptoms. Four trails [Citation21,Citation23,Citation24,Citation26] mentioned infection and the meta-analysis showed that the heterogeneity was low (I2 = 23%, P = 0.27). The fixed effect model combined with the effect quantity was analyzed, and the OR = 0.85, 95% CI (0.53, 1.37), P = 0.50 ().
3.5.2. The length of hospital stay
Three RCT’s described the effect of adjuvant glucocorticoid therapy on the length of hospital stay (). Jamaati et al. [Citation20] compared the median length of hospital stay between patients who received dexamethasone adjuvant therapy and those who received conventional therapy (11 days in the dexamethasone group versus 6 days in the conventional treatment group, P = 0.036). The length of hospital stay was prolonged for patients who received dexamethasone adjuvant therapy. Ghanei et al. [Citation22] investigated the effect of prednisolone on the average length of hospital stay and conducted modified intention-to-treat (ITT) and per-protocol (PP) analyses. There was no significant difference between prednisolone use and ITT analysis (5.5 days in the experimental group and 6.4 days in the control group, P = 0.09). PP analysis (experimental group 4.4 days, control group 5.9 days, P = 0.03) was statistically significant. Jeronimo et al. [Citation25] included 394 cases, and the median length of hospital stay was 9 days in the conventional treatment group and 10 days in the methylprednisolone group, P = 0.296; the difference was not statistically significant.
Table 4. The length of hospital stay in patients with COVID-19.
3.5.3. The time of nucleic acid test turning negative
Tang et al. [Citation23] included 86 patients with common COVID-19 and discovered that the duration of throat viral RNA detection in the methylprednisolone group was 11 days (interquartile range, 6–16 days), which was significantly longer than that in the control group (8 days, 2–12 days), P = 0.03; this was statistically significant. Hence, glucocorticoid adjuvant therapy can prolong the time of the nucleic acid test turning negative. Jeronimo et al. [Citation25] researched the negative conversion rate of nucleic acid detection on the 5th and 14th days. On the 5th day, the negative conversion rate of throat swabs in the conventional treatment group was 73/139 (52.5%), while that in the methylprednisolone group was 75/144 (52.1%), P = 0.942; the difference was not statistically significant. On the 7th day, the rate of throat swab turning negative was 45/95 (47.3%) in the conventional treatment group and 56/117 (47.9%) in the methylprednisolone group, P = 0.943, which was not statistically significant, indicating that methylprednisolone adjuvant therapy had no significant effect on the negative conversion of nucleic acid detection.
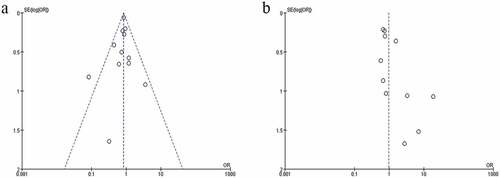
3.6. Publication bias
Only two outcomes, all-cause mortality and adverse reactions, were included in more than 10 articles. The funnel plot was selected to evaluate the risk of publication bias. The funnel plot of mortality () is almost symmetrical, indicating that there is no risk of publication bias. The funnel plot of adverse reactions () is asymmetric, indicating the high risk of publication bias.
3.7. GRADE evidence quality evaluation results
GRADE results showed that the all-cause mortality was high quality and the adverse reactions were moderate quality. Among the 12 outcomes of subgroup analysis, 4 results were moderate quality, 7 results were low quality, and 1 result was very low quality. The results are shown in .
Table 5. Types of adverse reactions.
4. Discussion
Previous studies differed in conclusions regarding all-cause mortality [Citation30,Citation31], which probably due to the inclusion of nonrandomized controlled trials. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group [Citation32] analyzed RCTs, and the research results showed that systemic glucocorticoid was associated with lower 28-day mortality. Siemieniuk et al. [Citation33] also suggested that compared with usual care or placebo, glucocorticoids may reduce the mortality of severe patients, and our research results were consistent with them. Subgroup analysis showed that it was not beneficial for ordinary and mild patients. In short, the use of glucocorticoids was more effective in patients with severe COVID-19 who had more severe inflammation and cytokine storm [Citation34].
Based on subgroup analysis, the efficacy of glucocorticoids was related to the type of drug. Only dexamethasone administration had a significant effect on the reduction of patient mortality, consistent with a previous study [Citation35]. Even though Edalatifard at al [Citation24]. reported that high-dose methylprednisolone was beneficial in reducing mortality, there was no data from other RCTs to verify it. The dose of glucocorticoid affects the curative effect. The results of meta-analysis showed that low dose of glucocorticoid may be related to the reduction of all-cause mortality, but high dose of glucocorticoid has nothing to do with it. The efficacy of glucocorticoids may also be related to the dosage form and the severity of the disease. Both ciclesonide and budesonide are inhaled drugs, and the main purpose of treatment is to relieve the patient’s condition, such as cough, asthma, loss of sense of smell, etc. The results of this study show that the above two drugs are not beneficial to the relief of symptoms. Hence, it is not recommended to use inhaled drugs to relieve the clinical symptoms of mild and common patients. For severe patients, no RCT uses inhaled glucocorticoids, so it is regrettable that its efficacy for severe patients cannot be evaluated, and more RCT results are needed to verify.
The literatures included in this study used short-term use of glucocorticoids (less than 15 days). Studies have shown that even short-term use can cause a variety of side effects [Citation36], such as hyperglycemia, hypertension, arrhythmia, and decreased immune function. This is also a concern for patients. Our study analyzed 3874 patients in 11 RCTs, and found that compared with conventional treatment, the probability of adverse reactions in the glucocorticoid group was not statistically different. When patients have been using glucocorticoids to treat underlying diseases (such as regular use of budesonide for asthma), they can continue to use them after being infected with COVID-19 without causing a greater burden on the body. However, even without COVID-19, the adverse reactions such as hypertension, hyperglycemia, and gastrointestinal reactions are common after long-term use of glucocorticoids [Citation37]. Hence, if a patient suffers from these preexisting conditions, regular monitoring and timely adjustment of medication is needed.
In addition, we found that patients who used inhaled glucocorticoids generally had mild adverse reactions such as headache, which may be related to their mild illness. However, severe patients who used systemic glucocorticoids (such as dexamethasone and methylprednisolone) were more prone to hyperglycemia and infection. Meta-analysis results showed that there was no statistical difference in hyperglycemia and infection between glucocorticoid group and usual care or placebo group. It is important to note that this study lacks long-term follow-up data of patients, and it is impossible to know the long-term adverse reactions, so further research is needed.
According to the results of the three included RCTs, glucocorticoids did not shorten the time for negative nucleic acid conversion in throat swabs, which may be related to the suppression of immune responses with the early use of glucocorticoids [Citation23].
This study had some limitations. First, there was no uniform standard for the time and dose of hormones used in various studies. Because of the limited RCTs and heterogeneity between trials, it was not possible to perform meta-analysis on other outcome indexes, such as length of hospitalization, nucleic acid detection, negative rate, negative duration, computed tomography (CT) findings, etc. Because of the release of RECOVERY’s study data [Citation15], the use of glucocorticoids in critically ill patients is highly recommended by the WHO, and many trials have been forced to stop recruiting patients without collecting the expected sample size. Due to the rapid mutation of SARS-COV-2, some studies have not been published, which will lead to publication bias. Finally, in search process, in order to avoid duplicate documents, we did not include Cochrane database in the search scope, which may lead to data loss.
In addition, as the SARS-CoV-2 virus continues to mutate over time, the likely role of glucocorticoids on different virus mutations can be used as further research in the future.
5. Conclusions
In summary, glucocorticoids, especially dexamethasone, can reduce the mortality of critically ill patients with severe COVID-19 (moderate quality). Low-dose glucocorticoids are associated with lower all-cause mortality (moderate quality). No matter inhaled or systemic glucocorticoid, it has no significant effect on the disease remission rate and mortality in patients with mild and common COVID-19 (low quality). Compared with the control group, there was no significant difference in adverse reactions caused by glucocorticoids, indicating that there was no obvious increase in adverse reactions to some extent (moderate quality). Other outcomes were low quality and shall be carefully explained. Limited by the number and quality of the included studies, hospitalization time, nucleic acid detection, and negative time could not be meta-analyzed, and more high-quality RCTs need to be carried out to verify this.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (680.5 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17476348.2023.2177155
Additional information
Funding
References
- Coronavirus disease (COVID-19) [Internet]. Geneva: World Health Organization; [cited 2023 Feb 01]. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_1
- Chifu I, Detomas M, Dischinger U, et al. Management of patients with glucocorticoid-related diseases and COVID-19. Front Endocrinol (Lausanne). 2021;12:705214.
- Home [Internet]. America: Johns Hopkins Coronavirus Resource Center; [cited 2022 Sep 30]. Available from: https://coronavirus.jhu.edu/
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8.
- Triggle CR, Bansal D, Ding H, et al. A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic. Front Immunol. 2021;12:631139.
- Shimba A, Ikuta K. Control of immunity by glucocorticoids in health and disease. Semin Immunopathol. 2020 Dec;42(6):669–680.
- Cruz-Topete D, Cidlowski JA. One hormone two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22:20–32.
- Therapeutics and COVID-19: living guideline [Internet]. Geneva: World Health Organization; [2022 Jul 14; cited 2023 Jun 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK582436/
- Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID‐19. Cochrane Database Syst Rev. 2021;2021(8):CD014963.
- Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- 宁光,马志中,王卫庆, et al 糖皮质激素类药物临床应用指导原则 [Guidelines for clinical application of glucocorticoids]. 中华内分泌代谢杂志. 2012;28(2):171–202. Chinese
- Clinical management of COVID-19: living guideline [Internet]. Geneva: World Health Organization; [2022 Jun 23; cited 2023 Jan 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK582435/
- 林苏杰,王芳,郝月琴,等. 《支气管哮喘防治指南(2020年版)》解读[Interpretation of the Guidelines for the Prevention and Treatment of Bronchial Asthma (2020 Edition)]. 中国临床医生杂志. 2022;50(12):1406–1408. Chinese
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394.
- Recovery collaborative group; Horby P, LIM WS. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704 .
- Ramakrishnan S, Nicolau DV, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9(7):763–772.
- YU LM, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398(10303):843–855.
- Ezer N, Belga S, Daneman N, et al. Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ. 2021;375:e068060.
- Clemency BM, Varughese R. Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: a Randomized Clinical Trial. JAMA Intern Med. 2022;182(1):42–49.
- Jamaati H, Hashemian SM, Farzanegan B, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947.
- Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316.
- Ghanei M, Solaymani-Dodaran M, Qazvini A, et al. The efficacy of corticosteroids therapy in patients with moderate to severe SARS-CoV-2 infection: a multicenter, randomized, open-label trial. Respir Res. 2021;22(1):245.
- Tang X, Feng YM, NI JX, et al. Early Use of Corticosteroid May Prolong SARS-CoV-2 Shedding in Non-Intensive Care Unit Patients with COVID-19 Pneumonia: a Multicenter, Single-Blind, Randomized Control Trial. Respiration. 2021;100(2):116–126.
- Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808.
- Jeronimo CMP, Farias MEL, VAL FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, Placebo-controlled trial. Clin Infect Dis. 2021;72(9):e373–e381.
- Corral-GUdino L, Bahamonde A, Arnaiz-Revillas F, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia. Wien Klin Wochenschr. 2021;133(7–8):303–311.
- Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically Ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–1306.
- Angus D, Litton E, Lorenzi E, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19. JAMA. 2020;324(13):1317–1329.
- MARIE M, Meyhoff TS, Helleberg M, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol Scand. 2021;65(10):1421–1430.
- Cheng W, LI Y, Cui L, et al. Efficacy and safety of corticosteroid treatment in patients with COVID-19: a systematic review and meta-analysis. Front Pharmacol. 2020;11:571156.
- Pei L, Zhang S, Huang L, et al. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: a systematic review and meta-analysis. Pol Arch Intern Med. 2020;130(9):726–733.
- WHO RAPID EVIDENCE APPRAISAL FOR COVID-19 THERAPIES (REACT) working group; Sterne JAC, Murthy S. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341.
- Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980.
- Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021;97(1147):312–320.
- Mohanty RR, Biswa Mohan P, Meher BR. Effectiveness of pulse dose methyl prednisolone in management of COVID 19: a systematic review and meta-analysis of observational studies. J Pharm Pharm Sci. 2022;25:110–123.
- Noetzlin S, Breville G, Seebach JD, et al. Short-term glucocorticoid-related side effects and adverse reactions: a narrative review and practical approach. Swiss Med Wkly. 2022;152:w30088.
- Bruscoli S, Puzzovio PG, Zaimi M, et al. Glucocorticoids and COVID-19. Pharmacol Res. 2022;185:106511.
Appendix 1
Search strategies
Pubmed
Search: ((((‘COVID-19’[Mesh]) AND ‘Glucocorticoids’[Mesh] AND (‘2019/12/01’[Date – Publication]: ‘2022/06/30’[Date – Publication])) NOT (meta analysis[Title])) NOT (review[Title])) NOT (systematic review[Title])
Embase:
(‘glucocorticoids’/exp OR glucocorticoids) AND (‘covid 19’/exp OR ‘covid 19’) AND ‘randomized controlled trial (topic)’ AND [01-Citation12-2019]/sd NOT [01–07-2022]/sd AND [2019–2022]/py NOT ‘systematic review’ NOT ‘review’ NOT ‘meta analysis’
CNKI:
(Subject = (glucocorticoid + prednisolone + dexamethasone + budesonide + hydrocortisone + methylprednisolone + prednisolone)) AND (Subject = (novel coronavirus pneumonia + COVID-19)) AND (Title, Keyword and Abstract = randomization + trial)
ClinicalTrials.gov
Glucocorticoids AND Completed Studies AND Studies With Results AND Interventional Studies AND COVID-19