ABSTRACT
Introduction
Complementing recognition of biomedical phenotypes, a primary care approach to asthma care recognizes diversity of disease, health beliefs, and lifestyle at a population and individual level.
Areas covered
We review six aspects of personalized care particularly pertinent to primary care management of asthma: personalizing support for individuals living with asthma; targeting asthma care within populations; managing phenotypes of wheezy pre-school children; personalizing management to the individual; meeting individual preferences for provision of asthma care; optimizing digital approaches to enhance personalized care.
Expert opinion
In a primary care setting, personalized management and supporting individuals to live with asthma extend beyond the contemporary concepts of biological phenotypes and pharmacological ‘treatable traits’ to encompass evidence-based tailored support for self-management, and delivery of patient-centered care including motivational interviewing. It extends to how we organize clinical practiceand the choices provided in mode of consultation. Diagnostic uncertainty due to recognition of phenotypes of pre-school wheeze remains a challenge for primary care. Digital health can support personalized management, but there are concerns about increasing inequities. This broad approach reflects the traditionally holistic ethos of primary care (‘knowing their patients and understanding their communities’), but the core concepts resonate with all healthcare.
1. Introduction
1.1. ‘Asthma’ – an umbrella term
In 2017, the Lancet Commission on asthma, crystallized the concept that asthma was a ‘collection of symptoms’ that could be caused by a range of pathophysiologies [Citation1]. The implication was that a diagnosis of asthma was imprecise and patients should be investigated to identify ‘treatable traits’ to inform ‘precision medicine’ rather than a one-size-fits-all approach’ [Citation1]. Distinguishing phenotypes of asthma is now the norm in severe asthma clinics to tailor a therapeutic approach potentially including biologics [Citation2]. This approach is uncommon in primary care where there may be limited access to diagnostic testing (for example, spirometry especially in children, FeNO), though guidelines now identify clusters of clinical features associated with different phenotypes (See ) [Citation2]. Many of these phenotypes could be identified in primary care as they can be determined clinically with a knowledge of the patient’s demographic characteristics and observation of response (or not) to treatment. Although phenotype-guided treatment is effective in severe asthma, it is not clear that adopting this approach in patients with mild/moderate asthma in primary care improves outcomes’ [Citation2].
Table 1. Phenotypes of asthma [Citation2].
Although ‘precision medicine’ with formal phenotyping of patients with asthma is not yet the norm in primary care, recognizing diversity of disease, health beliefs and lifestyle at a population and individual level is embodied in the holistic approach to care of family practice. The UK Royal College of General Partitioners defines ‘delivering high quality, person-centered care’ and ‘knowing their patients and understanding their communities’ as the core values of general practice’ [Citation3]. We have adopted broad terminology (for example: ‘management’ as opposed to ‘medicine’; ‘care’ to describe the personalized services provided) that aligns with these primary care values and reflects conceptual thinking about ‘treatable traits’ [Citation4]. See for definitions [Citation1–7].
Table 2. Clarifying the terminology used in this review.
In this review we highlight six aspects of ‘personalized care’ particularly pertinent to primary care:
Personalising support for individuals living with asthma. Personalising self-management support for people with asthma, guided by an understanding of different attitudes to asthma and the burden of disease.
Personalising asthma care within targeted populations. Identifying and personalizing the organization of care for people at risk of severe asthma events.
Personalising management of phenotypes of wheezy pre-school children. Optimising treatment of pre-school wheeze despite the diagnostic and management uncertainties.
Personalising patient-centered care for individuals. Implementing patient-centered care in a routine asthma service.
Meeting personal preferences for provision of asthma care. Understanding the role of face-to-face or remote care and responding appropriately to personal preferences.
Optimising digital approaches to enhance personalized care. Exploring the potential of digital technology to support self-management, target care, manage diagnosis and uncertainty, provide options for delivery of care.
We highlight research gaps and our views on future directions for development and implementation in each of these areas in the section ‘Expert Opinion’ below.
1.2. Methods used in this review
The six sections were written by professionals currently working in the different aspects of personalized support addressed in this review. They bring contemporary understanding of the literature in their specialist area, supplemented by PubMed literature searches for their specialist section updated from their most recent searches to March 2023. In addition, as recommended in the context of updating literature on complex interventions, we undertook forward citations on key papers [Citation8]. The sections were collated, synergies emphasized, and differences discussed to improve understanding, ensure coherence and add depth to the broad exploration of the topics covered. In addition to their academic expertise, all authors are working in, or with, UK primary care enabling a practical clinical interpretation.
2. Personalising support for individuals living with asthma
2.1. The central role of supported self-management
Asthma is, by definition, a variable condition and all people with asthma need to know what to do if their control deteriorates, how to abort an imminent attack as well as how safely to reduce treatment when their asthma is stable [Citation2,Citation9]. This advice needs to encompass changes in preventer medication (or use of combined maintenance and reliever therapy), emergency treatment of acute symptoms and when to seek medical help [Citation9]. In an ambulant care setting, professionals are responsible for advising on appropriate management strategies, but day-to-day treatment and lifestyle decisions are taken by the patient influenced by their ‘common-sense’ understanding of their condition often in discussion with family and friends [Citation10]. Some behaviors will be habitual – actions taken without a conscious decision [Citation11] – such as the habit of taking a reliever inhaler for respiratory symptoms [Citation12]. Personalising support for self-management (or more broadly ‘supporting people to live with asthma’) is thus central to all professional/patient interactions [Citation6].
2.2. Supported self-management improves outcomes
Guideline recommendations are based on ‘overwhelming’ evidence that supported self-management is an effective intervention [Citation13]. A metareview that synthesized the findings of 270 randomized controlled trials concluded that ‘supported self-management for asthma can reduce unscheduled care and improve asthma control, can be delivered effectively for diverse demographic and cultural groups, and is applicable in a broad range of clinical settings’ [Citation13].
Resource implications are often perceived as a barrier to delivering supported self-management in routine care, and a network meta-analysis (105 trials; 27767 participants) concluded that at least two hours of healthcare professional support over the trial period was required to optimize the benefit to patients’ quality-of-life and to reduce use of healthcare resources [Citation14]. This conclusion, however, is based on initiating self-management in the context of a trial; once skills are established, maintaining benefits may require less intensive support. Overall, the reduction in acute care offsets the investment of time in establishing and supporting self-management skills and total healthcare costs are not significantly increased [Citation13,Citation14].
2.3. Personalising components of supported self-management
The priority for healthcare professionals and the focus of most of the evidence cited in support of self-management [Citation13,Citation14], is empowering patients to adjust their treatment to improve clinical outcomes [Citation13,Citation15], but this narrow focus may not reflect the broader priorities of people living with asthma [Citation16]. The PRISMS taxonomy [Citation17], defined 14 categories of support strategies used in the interventions included in a series of meta-reviews (102 systematic reviews reporting 969 trials) of self-management of long-term conditions [Citation18]. The relevance of these strategies to individuals living with asthma, and thus personalization of the support provided, will depend on asthma status, co-morbidities, social and demographic context as well as individual preferences. lists the components and suggests how they may be personalized.
Table 3. The PRISMS taxonomy of supported self-management with suggestions for personalization and potential digital support (Adapted from [Citation17]).
Typically symptoms, biomarkers and other disease attributes are used to derive phenotypes [Citation2], but clusters based on perceptions of asthma control and attitudes toward asthma management have also been described. Using data from an on-line survey of 2,467 people with asthma from eight Asian countries [Citation19], five attitudinal – control clusters have been defined [Citation20]:
Well-adjusted and at least partly controlled: 29% of the individuals were coping well so that asthma has minimal impact on their lives. Happy to adhere to medical advice, this group are likely to follow self-management advice summarized in action plans.
In denial: Despite concern about their symptoms, 18% of the individuals were not convinced they have asthma and have not come to terms with living with the condition. ‘Asthma’ self-management will not seem relevant, so advice may be better related to symptom management.
Adrift and poorly controlled: 14% of individuals were stressed about their asthma and the impact on their daily lives but have little confidence in healthcare services. Building a trusting relationship will be a pre-requisite for supporting self-management.
Tolerating poor control: 29% of the individuals had little interest in learning to self-manage their asthma, instead ignoring their symptoms (whenever possible). Enabling timely access to emergency care will be important as this group will leave it late to present in an emergency.
Worried with multiple symptoms: Asthma was a constant worry to 11% of the individuals who live with a high level of stress and anxiety about their symptoms, some of which may not be due to their asthma. Psychological support may be helpful.
Recognising these ‘attitudinal – control phenotypes’ could inform personalization of supported self-management.
2.4. Tailoring to cultural and demographic groups
Supported self-management has been delivered effectively in a broad range of sociodemographic and cultural groups [Citation13], with an over-arching conclusion that interventions need to be tailored to the specific needs of the target group. For example, self-management education for young people with asthma often used digital approaches [Citation21,Citation22], or were peer-led [Citation23–25] to enhance the relevance for teenagers. School-based programmes, effective at reducing acute attacks [Citation26], offer a convenient opportunity to reach not only the child with asthma but also the whole school population, and the parents of younger children [Citation27]. Strategies for older people tailored interventions to meet personal goals or address the concerns identified by the participants [Citation28,Citation29]. The only age-group where traditional asthma self-management has been shown not be effective is pre-school children [Citation30], probably reflecting the diverse phenotypes of pre-school wheeze (see section 4).
Culturally tailored self-management programmes developed explicitly for a community can reduce unscheduled care and improve asthma control [Citation31]. A systematic review in 2018 (N = 17 trials; n = 2944 participants) concluded that trials based in South Asian countries, were more effective than those delivered to South Asian minority populations [Citation32], perhaps reflecting the limitations of tailoring in the context of complex sociocultural processes, such as acculturation [Citation33]. Poor literacy will need to be addressed in many populations [Citation34], and action plans featuring carefully selected illustrations have been developed in the UK [Citation35] and tailored for other communities [Citation36].
2.5. Implementation and digital support for self-management
Implementation in routine practice is challenging, but a systematic review of implementation studies (N = 18) showed that a whole systems approach incorporating strategies that explicitly addressed patient, professional and organizational factors showed consistent improvement in both process and clinical outcomes [Citation37]. Digital approaches can contribute to many of the components of self-management support [Citation38] (see ), for example providing information [Citation22,Citation39], logging clinical status [Citation40–42] or supporting self-management [Citation22,Citation43–45], facilitating remote reviews [Citation46], monitoring adherence [Citation47], and enabling peer support [Citation23,Citation48].
3. Personalising asthma care within targeted populations
3.1. The challenge of reducing asthma morbidity and mortality
Worldwide, asthma affects over 250 million people, nearly half of whom have symptoms that regularly interfere with everyday life [Citation49]. Asthma is responsible for more than 1,000 deaths every day [Citation49]. Despite improved understanding of the pathophysiology of asthma and the development of effective treatments, morbidity and mortality show no sign of significant improvement [Citation50,Citation51].
Strategies to improve asthma care include the widespread availability of global evidence-based guidelines [Citation2], professional education [Citation52], and ‘pay-for-performance’ incentives as (for example) the UK Quality and Outcomes Framework which rewards the provision of regular reviews for people on an asthma register [Citation53]. Implementation, however, is complex and guidelines, education and incentives do not ensure that high quality care reaches those most at-risk many of whom struggle to access healthcare or engage with treatment. This matters, because risk of poor outcomes (acute attacks, hospitalisation or death) is not evenly distributed across asthma populations, raising the possibility of identifying and targeting care for the 5–10% of people with asthma who have greatest risk of asthma crises [Citation54].
3.2. Identifying patients with ‘at-risk’ asthma
Many risk factors associated with poor asthma outcomes have been identified in observational, epidemiological and cross-sectional studies (). These include demographic factors (including ethnicity [Citation55] and social deprivation [Citation56]), difficulty accessing healthcare, poor adherence to preventative medication, a prior history of emergency care or hospitalization, multimorbidity and a wide range of psychological and social factors [Citation57–59].
Access to healthcare is a significant contributory factor highlighted in the UK National Review of Asthma Deaths [Citation60]. Better patient-reported access to primary care is associated with fewer admissions [Citation61], but patients at-risk of poor outcomes are more likely than those with well-controlled asthma to miss scheduled appointments or not engage in initiatives [Citation62,Citation63].
A wide range of psychosocial factors are associated with fatal and near-fatal asthma [Citation60,Citation64]. These factors often underpin risk-laden health behaviours such as poor adherence or smoking and add considerably to the complexity of the patient narratives and challenge patient engagement with health services. Such patients also tend to be excluded from, or choose not to participate in, clinical trials so evidence on how to improve their care is scarce.
Guidelines now recommend that clinicians should consider factors associated with poor outcomes in their service provision. For example, global guidelines recommend identifying poor adherence, short-acting beta-agonist (SABA) overuse, co-morbidities (including obesity and rhinosinusitis), smoking, medication side effects, anxiety and depression, and social difficulties [Citation2]. Guidelines call for risk stratification and targeting care on those with poor adherence, psychosocial problems and repeated episodes of unscheduled care [Citation9].
3.2.1. Predictive risk algorithms
The heterogeneity of patients within any at-risk grouping and the presence of multiple, often interacting, risk factors makes the development of predictive risk algorithms challenging. Some, such as Asthma UK’s Asthma Attack Risk Checker tool [Citation65], the Wheeze frequency, Admissions, Reliever use and Step on the BTS medication guidelines (WARS) score [Citation66], and the TENOR risk score [Citation67] help patients and clinicians to identify and manage individual risks. Such tools, however, require current personal characteristics such as can be obtained during a consultation. As failure to engage with healthcare is, in itself, a significant risk factor, these algorithms may miss non-attending high-risk patients.
An alternative strategy has been to derive and validate stratified risk prediction tools using routine electronic healthcare data from large databases [Citation68]. The QAdmission score [Citation69] estimates absolute risk of an emergency hospital admission due to any cause and has been embedded into primary care software systems in the UK. However, it is not linked to specific interventions and does not identify risk for specific diseases. One recent validated algorithm which stratifies asthma risk has been developed and is being tested as part of a complex intervention [Citation68]. This has the advantage of including and weighting all coded risk factors in the risk prediction model and overcomes the need to engage with patients directly before deriving a score. Not all risk factors, however, are routinely coded in electronic records, so patient review and local clinical knowledge are still needed to complete the picture. Combining algorithms derived in different ways to improve predictive values has not been formally tested.
3.3. A structured primary care approach to targeting care for poorly controlled asthma
Accurate risk stratification is only the first step in improving care and outcomes of at-risk patients. Subsequent incorporation of this information into existing healthcare delivery models has received relatively little attention. In the UK, the British Thoracic Society Difficult Asthma Registry was established in 2007 to describe the clinical and demographic characteristics of at-risk patients with refractory disease [Citation70]. Compiled from attendees at specialist clinics, the patients registered were those willing and able to engage with healthcare and often had pathophysiological phenotypes amenable to novel pharmacotherapies available via such clinics. Comparison of the International Severe Asthma Registry (ISAR [Citation71]) [Citation72]) with UK primary care data (Optimum Patient Care Research Database (OPCRD) [Citation73]), suggested that nearly three quarters of people potentially with severe asthma were ‘hidden’ in primary care [Citation74]). Further, evidence from the National Review of Asthma Deaths (NRAD) [Citation60] confirmed that most patients dying from asthma were not recorded as being under specialist supervision during the 12 months prior to death. One home-based intervention which targeted a wider at-risk group with intensive support from a specialist asthma nurse following hospital admission improved quality-of-life for the duration of the study, but this was not sustained [Citation75].
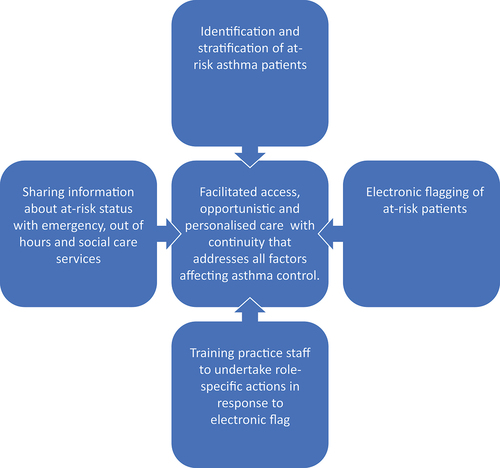
In primary care, practice-agreed development plans have shown promise in improving the management of acute asthma [Citation76]. A pilot study in a single practice in the UK developed an asthma at-risk register using factors derived from observational studies of asthma deaths and ‘flagged’ the patients’ electronic health record (EHR) to alert staff to the at-risk status [Citation77]. All practice staff were trained on role-specific responses with the aim of facilitating patient access, promoting opportunistic asthma management, monitoring medication adherence, and addressing barriers to good asthma control. After one year, compared to age, sex and treatment-matched controls, there were reductions in emergency events, missed scheduled appointments and healthcare costs. A subsequent regional cluster randomized controlled trial [Citation78], involved 29 practices in Norfolk, UK and used a definition of at-risk asthma that included severity defined by treatment step, episodes of unscheduled hospital care, and co-existing adverse psychosocial characteristics. This practice-level intervention which involved electronic flagging of at-risk patient records and staff training () did not reduce the composite primary end-point of asthma attacks, because an increase in prednisolone prescriptions masked a 50% reduction in hospital admissions and a 26% reduction in emergency department attendances. There was also an increase in the prescription of preventative treatments. These results were sufficiently encouraging for the on-going At-Risk Registers Integrated into primary care to Stop Asthma crises in the UK (ARRISA-UK [Citation79]) study to be funded scaling up to 275 practices [Citation80]. This study used on-line practice-wide training and derived the at-risk registers from the electronic health record (EHR) supplemented if participating practices were aware of uncoded risk factors.
4. Personalising management of phenotypes of wheezy pre-school children
Up to a third of children will have experienced one episode of wheeze by three years of age, and up to half by six years of age [Citation81,Citation82]. Most episodes are mild and self-limiting, but some children will experience persistent or frequent symptoms resulting in multiple emergency healthcare attendances, and poor quality of life [Citation83]. Although not every pre-school child that wheezes has asthma [Citation84], around a third are diagnosed with asthma by age eight [Citation85]. Compared to children who had infrequent or no history of wheeze during their preschool years, pre-school children with wheeze have nearly three times the odds of being diagnosed with asthma by age 14 years [Citation86].
Three-quarters of pediatric hospital admissions, and the highest rate of unscheduled primary care consultations for wheeze/asthma are in pre-school children [Citation87,Citation88]. Although there are fewer pharmacological treatments for asthma/wheeze in pre-school children, regular treatment can help to reduce attacks [Citation89]. Many healthcare professionals, however, are uncertain when, and in which children, to start these medications.
4.1. Phenotypes
Longitudinal birth cohort studies have characterized several phenotypes of wheeze in pre-school children, based on the trajectory of wheezing episodes during the first few years of life, and subsequent asthma diagnoses. The US Tucson birth cohort study reported findings from 826 children recruited in the early 1980s followed up at three and six years of age. They identified four phenotypes of wheeze based on wheezing status between 0–3 years and 4–6 years: 1) never wheezed, 2) transient early wheeze, 3) persistent wheeze, and 4) late onset wheeze [Citation81]. (See for description). The UK Avon Longitudinal Study of Parents and Children (ALSPAC), which included 6,265 children, confirmed these four phenotypes but added two additional groups: 1) intermediate onset wheeze, and 2) prolonged early wheeze [Citation82]. All pre-school wheezing phenotypes were associated with physician-diagnosed asthma by aged 7–8 years, but the association was strongest with persistent wheeze or late onset wheeze [Citation81,Citation82]. In both studies, atopy (positive skin prick test) was associated with persistent wheeze and intermediate/late onset wheeze phenotypes, but not with never wheeze or transient/prolonged early wheeze phenotypes [Citation81,Citation82], suggesting an important association between allergic inflammation and subsequent childhood asthma.
Table 4. Phenotypes of pre-school wheeze (data from ALSPAC [Citation82]).
4.2. Diagnostic challenges in clinical practice
Information from birth cohort studies predicts population risk but has limited utility to inform an asthma diagnosis in an individual pre-school child seen in the clinical setting. Wheeze can be caused by anything which obstructs airflow in the lower airways, and a key decision is to determine whether this child’s recurrent wheeze symptoms are due to asthma or not, and thus whether they are likely to benefit from asthma treatment.
Primary care clinicians describe several challenges in diagnosing and managing wheezing disorders in young children. There may be reluctance about initiating inhaled corticosteroids without a confirmed diagnosis of asthma, which is not straightforward in this age group as symptoms overlap with other common respiratory conditions such as recurrent viral lower respiratory tract infections. On a practical level, there may be concern that a preventer treatment prescribed as a ‘one-off’ trial may be automatically repeated by another clinician who reviews the child [Citation90].
Existing guidelines and strategy documents [Citation2,Citation9,Citation91,Citation92] define asthma as a condition characterized by variable symptoms of wheeze, shortness of breath, cough, and chest tightness, associated with objective evidence of obstructed and reversible airflow limitation. Diagnosis based on symptoms alone is discouraged. Tests that can be used in older children in primary care (spirometry with bronchodilation; peak flow diary; fraction of exhaled nitric oxide (FeNO) measurements) [Citation93] are not feasible in very young pre-school children. Less effort-dependent tests, such as impulse oscillometry, may be able to detect abnormal lung function in children with asthma as young as three years [Citation94], but are not available outside specialist/research settings.
4.2.1. Practical approaches to diagnosing pre-school children with wheeze
In 2008, a European Respiratory Society (ERS) taskforce [Citation95] recommended avoiding the term ‘asthma’ to describe recurrent wheeze in pre-school children, arguing that there is insufficient evidence to inform how epidemiological phenotypes of pre-school wheeze can be used to make a clinical diagnosis in an individual. Pragmatically, after seeking ‘red-flags’ (such as failure to thrive, onset from birth, symptoms triggered by feeds, chronic productive cough, heart murmur, digital clubbing) and excluding other serious causes of wheeze, they recommended characterizing pre-school wheeze into two phenotypes based on temporal patterns of symptoms that could be recognized in clinical practice:
Episodic viral wheeze (EVW) – wheezing during viral upper respiratory tract infections (URTIs) but no interval symptoms. Children with EVW were assumed to be more likely to stop wheezing by school-age and only require as-needed symptomatic treatment.
Multi-trigger wheeze (MTW) – wheezing with viral URTIs and symptoms in-between episodes – Children with MTW were assumed to be more likely to continue to wheeze and be diagnosed with asthma in later childhood and were more likely to benefit from inhaled steroids.
This approach was criticized for not considering the frequency or severity of symptoms, and that additional symptoms of multi-trigger wheeze may be a marker of severity rather than of different phenotypes [Citation96]. In addition, these phenotypes are unstable, with the pattern of wheezing changing from EVW to MTW, and vice versa, in a significant proportion of pre-school children [Citation97].
Multiple asthma prediction rules have been developed with the aim of identifying pre-school children who will be diagnosed with asthma by school age [Citation98]. Typically based on variables such as presence of parental asthma, personal history of eczema, atopy, raised eosinophils, and the temporal nature of wheeze [Citation28,Citation99], the sensitivity and specificity were low [Citation98] limiting their clinical utility. Until more accurate, widely accessible, and feasible diagnostic tests are available, healthcare professionals and families of children with pre-school wheeze have to accept the diagnostic uncertainty. The aim should be for families to receive consistent information, using terms such as ‘suspected asthma’ or ‘possible asthma’, and feel supported until there is clarity about the diagnosis.
4.3. Challenges in treatment
Awareness of this diagnostic uncertainty, combined with a general concern about over-diagnosis of asthma [Citation100], are likely reasons for an observed fall in coded asthma diagnoses in pre-school children (from 4% to 2% over 10 years [Citation88]) despite an overall increase in acute wheeze presentations [Citation85]. The concern now is that asthma is probably under-diagnosed in pre-school children, with consequent underuse of preventer medications that could reduce morbidity and over-reliance on short acting beta agonists (SABA) and courses of oral corticosteroids [Citation85]. In addition, the lack of a coded diagnosis means that regular reviews are unlikely to be prompted.
In a meta-analysis of trials comparing regular inhaled corticosteroids (ICS) versus placebo for pre-school children with recurrent wheeze (regardless of phenotype), children who received ICS had significantly fewer exacerbations of wheeze/asthma compared with children given placebo (18% vs 32%) [Citation89]. The Individualized Therapy for Asthma in Toddlers (INFANT) study [Citation101] randomized 300 children aged 12–59 months with recurrent wheeze to either daily ICS, daily leukotriene receptor antagonist (LTRA), or as-needed combined ICS/salbutamol in a double-blind, double-dummy, crossover trial. Participants demonstrated a differential response to the three treatment options. The probability for a good response was highest for daily ICS, although some children responded better to LTRAs. Eosinophilia and/or evidence of aeroallergen sensitization increased the likelihood of a response to ICS [Citation101].
In summary, in children with recurrent pre-school wheeze, preventers can reduce exacerbation rates, with ICS probably having the best efficacy for most children, especially those with evidence of eosinophilia or atopy. Asthma guidelines now recommend a trial of regular preventers in pre-school children if they have ‘frequent wheeze’ or ‘more severe wheezing episodes’ [Citation2,Citation9]. However, the definitions of ‘frequent’ differ markedly: the Global Initiative for Asthma (GINA) considers three or more episodes of wheeze per season as ‘frequent’ [Citation2], whilst UK guidelines suggest a trial of preventers if a child needs salbutamol three or more times per week [Citation9]. Neither define what they mean by ‘more severe episodes of wheeze’.
4.4. Overcoming challenges in primary care
Our evolving understanding of pre-school asthma/wheeze phenotypes has resulted in uncertainty [Citation102] compounded by inconsistent recommendations from current guidelines, a focus on acute episodes, lack of co-ordination between healthcare settings and little standardization of ongoing management or advice given to families [Citation103]. To achieve the aim of reducing attacks, and improve quality-of-life, there needs to be a shift toward a whole system approach to managing pre-school children with wheeze integrated across healthcare settings and potentially including practical decision support aids for non-asthma specialists. A probability-based approach is recommended for the diagnosis of asthma [Citation2,Citation9], using qualifiers such as ‘probable’ or ‘suspected’ until confirmed with objective tests. In the meantime, diagnostic uncertainty can be mitigated through provision of standardized information for families. The decision for a trial of regular preventer therapy should be shared with families, based on the burden of illness and not dependent on confirming an asthma diagnosis. An NIHR-funded research programme is currently underway to develop a pragmatic ‘pre-school wheeze care pathway’ which can be integrated across different care settings [Citation104].
5. Personalising patient-centered care for individuals
Personalising management to the individual and supporting them to live with their asthma is a key aim of asthma management. Evidence-based strategies for enabling this include patient-centered care, shared decision making, motivational interviewing to support lifestyle change, and promoting adherence.
5.1. Patient-centered care
Patient-centered care has been promoted in healthcare provision for several decades [Citation105]. Patient-centered care involves consideration of patient participation and involvement in their care, communication between the patient and the healthcare professional, the relationship between the patient and the healthcare professional, and the context in which care is delivered [Citation106,Citation107].
The Health Foundation has described a framework that defines four principles of person-centered care (See ) [Citation7]:
Figure 3. The four principles of person-centered care (Reproduced with permission from the Health Foundation [Citation7]).
![Figure 3. The four principles of person-centered care (Reproduced with permission from the Health Foundation [Citation7]).](/cms/asset/52557d22-ce41-4844-ba28-aeac3df63b1d/ierx_a_2241357_f0003_oc.jpg)
Affording people dignity, compassion, and respect
Offering coordinated care, support, or treatment
Offering personalized care, support, or treatment
Supporting people to recognize and develop their own strengths and abilities to enable them to live an independent and fulfilling life
A mixed methods systematic review (133 articles; 58 interventional) concluded that patient-centered care for asthma can improve patient outcomes (e.g. asthma control, symptoms) and reduce hospitalizations [Citation108]. Emphasising the importance attached to patient-centered care, a primary care overview of asthma guidelines highlighted that the management of asthma should encourage and support self-management and make treatment decisions in partnership with the individual [Citation109]. Echoing this approach, primary care staff interviewed about delivering supported self-management described the need to customize their approach to individual patients and their clinical situation (e.g. offering a range of asthma action plans, alternative modes of consultation) [Citation110]. On a practical level, a recent systematic review (N = 12 qualitative and 14 quantitative studies) concluded that electronic templates used in (asthma) reviews should be designed to promote patient-centered care, for example by asking an open question to establish the patient’s agenda, and a closing question to check the agenda has been addressed [Citation111].
5.2. Shared decision making
Shared decision making is a collaborative process in which a healthcare professional and patient work together to reach a decision about care. Patient/professional partnership has been a central recommendation of guidelines since the British Thoracic Society in 1990 recommended that ‘patients should be given adequate opportunity to express their expectations of treatment and to hear how far those expectations can be met’ [Citation112]. Thirty years later, the message has not changed. Global asthma management guidelines in 2022 explicitly recommend shared decision making: ‘Patients should be encouraged to participate in decisions about their treatment, and given the opportunity to express their expectations and concerns. This partnership needs to be individualized to each patient’ [Citation2].
A Cochrane review (N = 4 studies; 1342 participants) of shared decision making for people with asthma concluded that it may benefit quality-of-life, patient (and parent) satisfaction, adherence to medication, reduce asthma-related healthcare visits, and improve asthma control [Citation113], though the small number of studies included in the review limited the confidence in the findings.
5.3. Motivational interviewing
Motivational interviewing is a patient-centered approach that can promote behavior change [Citation114]. Motivational interviewing involves helping patients to say why and how they might change [Citation115], using three core skills – asking, listening, and informing – in a guiding style to draw out patients’ ideas and their own solutions [Citation114]:
‘Ask’ open-ended questions – invite the patient to consider how and why they might change.
‘Listen’ to understand the patient’s experience – ‘capture’ their account with brief summaries or reflective listening statements such as ‘quitting smoking feels beyond you at the moment’. This expresses empathy, encourages the patient to elaborate, and are often the best way to respond to resistance.
‘Inform’- by asking permission to provide information, and then asking what the implications might be for the patient.
A number of strategies can be used in motivational interviewing to elicit motivation to change from the patient, these include [Citation114]:
Agenda setting (what to change): Inviting the patient to select an issue or behavior that they are most ready to tackle.
Pros and cons (why change): Invite the patient to discuss the pros and cons of a situation, then ask them to clarify whether change is possible.
Assess importance (why) and confidence (how): Patients who are not convinced of the importance of change are unlikely to benefit from advice about how to change.
Exchange information: A guiding style ‘elicit-provide-elicit’ strategy can be used with a patient to share information.
Make decisions about change (setting goals): Use a guiding style to elicit practical solutions from the patient and offer suggestions.
Motivational interviewing can be used to promote asthma medication adherence [Citation116], and there is systematic review evidence (N = 11 studies) to suggest that it can improve asthma self-management behaviors by increasing engagement with primary care providers, and increasing asthma knowledge [Citation117].
5.4. Improving adherence
Poor adherence to ICS is common, with reported adherence rates in children with asthma varying between 30% and 70% of the total number of doses prescribed [Citation118] and similar concerns in adults [Citation119]. Habit is an important influence on adherence behavior, both positively promoting regular preventer medication use as well as negatively contributing to overuse of rescue medication [Citation12]. In a systematic review of 771 medication adherence intervention trials [Citation120], analyses revealed larger effect sizes for habit-based and behavioral-targeted (vs. cognitive-focused) interventions. The content of habit-based interventions included assessing an individual’s daily routines, or personal practices (e.g. pattern of eating meals, walking the dog) then linking medication administration with their existing habits. The content of behaviourally-focused interventions included cues or prompts to take medications (e.g. telephone calls, text messages, beepers), or behavioral goal setting (e.g. ‘take medication with breakfast every day this week’). A Cochrane review in 2022 (40 trials; n = 15,207 participants) showed the potential of digital support for adherence [Citation47], concluding that smart inhalers can improve adherence by 23%, and SMS interventions by 12%. This was reflected in improved asthma outcomes, especially if there was an in‐person element to the intervention.
6. Meeting personal preferences for provision of asthma care
6.1. Modes of delivery of asthma care
Face-to-face asthma reviews are the traditional and most common mode of routine asthma care delivery, although telephone reviews have also been in use for many years and have been shown to increase the proportion of people with asthma reviewed with no detriment to asthma quality of life [Citation121]. Since the outbreak of the COVID-19 pandemic, other modes of consultation for the delivery of asthma care are being used routinely in primary care practice [Citation122], and the list of options for reviewing – or contributing to reviewing – asthma has expanded. Modes of consultation are typically categorized as synchronous (face-to-face/in person consultation; video consultation; telephone consultations) or asynchronous (online questionnaires/e-consultations; text messaging; telemedicine). Each of these has advantages and disadvantages (see ) and the appropriate option for an individual patient will be determined by the clinical context as well as the preferences of the patient. Recent calls to restore face-to-face consultations for routine asthma care result from concerns about the quality of care that people with asthma received during the pandemic. For example, many patients have not had their inhaler technique checked for two or more years [Citation128].
Table 5. Modes of consultation and features that can personalize their use.
A realist review exploring self-management in remote asthma reviews (video and telephone) concluded that remote asthma care delivery is convenient for the patient and reduced barriers to routine care [Citation123]. Video consultations offer the advantage of enabling visual assessment of the patient and potentially improving rapport during the consultation [Citation123,Citation124], though require suitable technology infrastructure [Citation126,Citation127]. Asthma action plans need to be discussed and agreed with patients, and it is possible to complete an action plan by sharing the screen in a video call [Citation125].
Group consultations, offering patients the opportunity to share their healthcare appointment with other patients, may be an efficient mode of consultation for the management of long-term conditions such as asthma in primary care. Provision of education and opportunity for peer support are cited as particular benefits of this consultation mode, however barriers including lack of clinician confidence and organizational support can hinder provision in primary care [Citation129].
Telemedicine can support logging of symptoms and physiological parameters to inform any mode of consultation (see section 7), and it is anticipated that there will be much greater use of digital strategies to deliver asthma care in the future, though these will not be acceptable to all patients. The partnership between patients and their healthcare professional is a key facilitator for the development of effective self-management skills [Citation130] and the principle of being ‘partners-in-care’ applies to patient choice in the mode of their asthma review.
6.2. Practical approaches to meeting individual preferences in primary care
Consideration of preferences for asthma care encompass patient, healthcare professional and organizational factors. Understanding of the local context and patient population inform the organization of asthma care in primary care, but meeting individual patient preferences can be challenging. Access to care can be seen as a patient-centered concept [Citation107], and the mode of consultation should be aligned to patient need and preference [Citation131]. Reconciling organizational approaches with patient preference is ideal but the mode of asthma review offered to patients may be determined by other factors such as cost, availability and functionality of technology, time, and the confidence of healthcare professionals as well as local strategic or financial drivers.
6.2.1. Digital-first initiatives
A ‘digital-first’ approach, in which first contact with healthcare is made digitally (by phone or increasingly on-line) and requests triaged to appropriate professionals and modes of consultation, is advocated in many countries and the move toward implementing this has been accelerated by the pandemic [Citation132]. In primary care in England, this ‘digital-first’ policy [Citation133] is being applied to the provision of asthma care through the utilization of text messaging and online questionnaires. The rationale for digital-first is that it can address barriers of workforce capacity and patient access, though there is limited evidence to support this policy and raises concerns about exacerbating health inequalities [Citation134]. Indeed, Rodgers et al. found that digital options are more likely to be used by patients who are younger, female, and with higher income and education levels [Citation135]. In an example of the inverse care law [Citation136], those living in socio-economic deprivation are least likely to have the technology and e-health literacy to be able benefit from the digital access, but are also communities with poor asthma outcomes [Citation56].
6.2.2. Personalisation and offering a choice
On the other hand, digital-first may offer a practical approach to meeting patient preferences. A digital first contact opens a line of communication and can be used to offer choice for the mode of review, either face-to-face or remote. In addition, asynchronous contact may be used to identify those for whom a full asthma review is a priority, whilst remaining mindful that a digital-first approach may not establish contact with all patients and alternatives such as sending letters or reviewing asthma opportunistically, will be needed. In their online consultations implementation toolkit [Citation133], NHS England highlights the importance of offering a range of modes of consultation and adapting service provision to patient preferences in order to meet their needs.
Supporting patients to self-manage (‘live with’) their asthma occurs over time as learning is experiential [Citation12], and regular reviews are a core component [Citation14]. A personalized and patient-centered approach extends to providing choice in the mode of consultation [Citation110], though the choice needs to be consistent with providing the range of components including education, support for behavior change and provision of an asthma action plan. Embedded in the community, primary care understands its patient population and needs to be flexible to meet patient preferences for the mode of their asthma reviews.
7. Optimising digital approaches to enhance personalized care
7.1. Digital approaches to enhance care for people with asthma
Emerging technologies are available (and will be maturing in the next five years [Citation137]) that can support the GINA management cycle for personalized asthma care (see ) [Citation2]. Many existing technologies have evidence of technical safety/accuracy, some have proved to be clinically effective in research contexts, but implementation questions remain including the impact on socioeconomic inequities [Citation138].
Figure 4. Existing and emerging technologies to support the GINA guideline management cycle for personalized asthma care [Citation2].
![Figure 4. Existing and emerging technologies to support the GINA guideline management cycle for personalized asthma care [Citation2].](/cms/asset/78a902c2-ec00-4402-963c-ba9e5d9d9f16/ierx_a_2241357_f0004_oc.jpg)
7.1.1. Screening and diagnosis of asthma
Classic machine learning algorithms such as artificial neural network, support vector machine, decision tree and natural language processing, can be used in clinical decision support systems (CDSS: software, ideally embedded in the EHR, that aims to improve healthcare delivery by enhancing medical decisions with targeted clinical knowledge, patient information, and other health information [Citation139]) to reduce the under- or over-diagnosis rate [Citation140]. In addition, CDSS can prompt communication with the patient about the diagnostic process, link to tailored information to support understanding of asthma and relieve uncertainty by providing a personalized step-by-step guide on what to expect during the diagnostic process [Citation141]. Clinical data from the patients’ EHR along with data on symptom patterns, pulmonary function tests, digital auscultation, collected over time by wearable sensors and smartphones could confirm/refute the variability that is characteristic of asthma. The data collection required to inform a CDSS is already available, and the incorporation of machine learning algorithms is a subject of research and rapid development [Citation142]. Adoption of such systems in primary care practice, however, requires not only evidence of technical feasibility and acceptable diagnostic accuracy, but must also align with clinicians’ preferences and organizational routines for the screening, testing and management of asthma [Citation143].
7.1.2. Personalised support for self-management
Traditionally, patients are given a paper-based asthma action plan, reminding them about regular treatment, advising them on how to adjust their medication according to their asthma status, and prompting when they needed medical help. Artificial intelligence embedded in a ‘superapp’ that can collect real-time patient data from multiple devices (e.g. smart inhaler, smart peak flow meter, smart watch), collate environmental data and learn from retrospective healthcare data, will be able to provide personalized early warning of an impending asthma attack along with individualized timely self-management advice [Citation140]. Smart inhalers and app reminders can already significantly improve medication adherence [Citation47,Citation144], and AI-enabled augmented reality, video and games are being developed to check and advise on inhaler technique [Citation145–147]. Smoking cessation features can support patients wanting to quit smoking though few are explicitly designed for asthma [Citation148,Citation149].
7.1.3. Support for management tasks
Many of the personalized tasks described in previous sections of this review can be supported digitally. Regular reviews can be conducted remotely via video consultation to reduce travel and followed up by text messages/telephone (See section 6) [Citation150,Citation151]. Self-monitoring data can be transferred to GP practices enabling triage and identification of those who would benefit from priority review and potentially a referral to a severe asthma clinic (see section 3) [Citation142]. Hospital admissions can be prevented or shortened by ‘virtual wards’ with short-term intensive remote monitoring by clinicians, a strategy widely used during the COVID-19 pandemic [Citation152]. Online supervision of rehabilitation, with virtual reality technologies and virtual therapists can be conducted remotely at the patients’ home to overcome individual’s access problems [Citation153–155].
7.2. Connected systems to provide personalized choice
Most existing smart monitoring devices present data via their own branded app so that to use multiple devices patients have to download multiple apps to their phones, and clinicians receive multiple health reports in different formats. Linking multiple apps/smart devices and summarizing the monitoring data on a ‘plug and play’, device-agnostic platform is still in the pilot stages [Citation156–158] but will be an important practical advance in personalizing asthma services. Potential benefits include:
Enabling patients to build their own suite of monitoring devices from different brands that fits their needs and that they trust [Citation158,Citation159].
Supported by distributed ledger technology [Citation160] the patients will own their self-monitoring data and can decide with whom they share their medical summary and asthma monitoring data: for example, allowing clinicians in different healthcare organisations/clinical settings to access their data for clinical purposes.
Creating a new eco-culture on supporting care that enables clinicians to set-up and co-evaluate an optimized personalized monitoring plan that fits with the patients’ daily routine, and does not overload their clinician with a mass of irrelevant data [Citation161].
7.3. Artificial intelligence to increase personalization
‘Artificial intelligence’ (AI) enables a machine to learn from individual patient data in order to provide timely personalized advice to improve control or prevent exacerbations. Artificial intelligence is categorized as:
Artificial narrow intelligence (ANI) requires human intelligence to determine the rules that the AI uses to make a decision. For example, AI based on ‘rules’ derived from clinical guidelines, can identify high risk patients and provide individualized evidence-based treatment advice [Citation142,Citation146].
Artificial general intelligence (AGI) uses machine learning techniques to make decisions based on observed patterns in patients’ data. For example, AI can learn a patients’ past pattern of attacks and use real-time patient-reported outcome measures to predict the future likelihood of an asthma attack and provide personalized management advice based on an asthma action plan previously agreed with the clinician [Citation142,Citation146].
Artificial super-intelligence (ASI) uses deep learning techniques to make rules that predict future attacks based on their own observation of the patient. ASI requires multiple, diverse, large population datasets/biobanks to increase its accuracy (‘intelligence’); enabling it to provide tailor-made, dynamic treatment advice to the patient [Citation162].
The latest real-life AI applications (e.g. ‘ChatGPT’ [Citation163]) ‘talk’ with patients in natural language, enhancing a dynamic interaction with patients, and encouraging adoption of AI to support personalized care. However, there are challenges when deploying AI in real-life clinical care [Citation164,Citation165], including preventing biased predictions based on limited historical data generated in a single ethnic group [Citation166], and building trust in AI so that it is perceived to be reliable and secure by patients and clinicians.
8. Conclusion
In a primary care setting, personalized management and supporting individuals to live with asthma moves beyond the pathophysiological definition of phenotypes and pharmacological ‘treatable traits’ to encompass patient-centered care and tailored self-management support. It also extends to how we organize our practices and the choices we provide in the mode of consultation and use of digital health. This reflects the traditionally holistic ethos of primary care (‘knowing their patients and understanding their communities’), but the core concepts resonate with all healthcare.
9. Expert opinion
Knowing individual patients and their families, and embedding holistic care within the community is the essence of primary care/family medicine [Citation3]. Personalized care aims to support individuals to live with their asthma whatever their demographic, cultural, socio-economic or clinical context. Although much progress has been made, there remains research gaps related to development and evaluation of implementation strategies and digital solutions that will increasingly offer innovative approaches to delivery of personalized care.
The research evidence for supported self-management is ‘overwhelming’ [Citation13], but there are dual research challenges for the future. Firstly, there is a need to focus on how supported self-management can be implemented within routine clinical practice. Reflected in ongoing work (IMPlementing IMProved Asthma self-management as RouTine (IMP2ART) [Citation167]), this is likely to require a whole systems approach giving attention to the needs and preferences of patients, the skills of professionals and the priorities and routines of practice organizations. Secondly, most of the literature on self-management is from high-income countries and western cultures. There is a need to explore diversity and adapt the concept to a broad range of societal and cultural settings and to specific groups such as minority ethnic populations, deprived communities, or those with poor health literacy.
The large-scale trial on targeting care for high-risk populations (ARRISA-UK) will soon be reporting [Citation79]. The intervention is embedded in a healthcare system with electronic records and a strong primary tradition that aims to provide continuity of care to support people at high risk, many of whom are hard to engage with care. Ideally in the future, asthma risk-prediction algorithms will be routinely embedded in primary care software systems. Through the application of AI, there is the potential to develop an interactive template format which can capture risk factors (such as psychosocial factors) that currently are not routinely recorded, use CDSS to connect risk stratification to appropriate guidelines to support clinicians and personalized self-help resources for patients.
Multiple research questions remain about the management of pre-school wheeze including how poverty and ethnicity affect risk. Although there is general agreement that the decision to prescribe preventer treatment should be based on symptom burden rather than awaiting a confirmed diagnosis of asthma, there is a need to determine the thresholds that should trigger a trial of regular treatment. Predictive algorithms are needed both to identify those at risk of future wheezing attacks as well as predicting who will develop asthma in childhood. An on-going trial testing whether two years of omalizumab therapy can prevent asthma development will further inform care in pre-school wheezers.
Patient-centered care not only encompasses personalized management of the individual, but also embraces flexibility of organization of care to meet patient preferences for convenient access to care. Delivering personalized management can improve adherence, self-management, and asthma outcomes. Future research will need to focus on the implications of increasing use of remote consulting on professional/patient relationships, shared decision-making and the delivery of behavior change support.
Digital healthcare is poised to progress from designing and testing initiatives typically limited by disease, brand or locality, to development as connected systems integrated within routine healthcare. This offers the promise of personalized suites of digital services generating patient-owned data for sharing with healthcare advisors, but raises implementation challenges of ensuring security of data and equity of access. AI is developing rapidly, and rule-based algorithms are already being implemented. Artificial super-intelligence is being developed, but there are issues of trust with patients, professionals and organizations which will need to be resolved before implementation in routine practice.
Article highlights
Although ‘precision medicine’ with biomedical phenotyping of patients with asthma is not yet the norm in primary care, recognizing diversity of disease, health beliefs and lifestyle at a population and individual level is embodied in the holistic approach to care of family practice.
Supported self-management aims to enable individuals to live with asthma and, beyond tailoring to asthma status, this needs to reflect social context, cultural beliefs and as well as personal attitudes to management strategies.
Targeting care for people at high risk of a severe asthma attack shows promise, involving a structured approach to identifying and ‘flagging’ the electronic record so that the primary healthcare team recognize and respond appropriately to the risk.
Managing the multiple phenotypes of pre-school wheeze remains a challenge, but there is concern that diagnostic uncertainty is leading to a reluctance to prescribe preventer medication that could improve outcomes.
There is evidence that patient-centered care, including techniques such as shared-decision making and motivational interviewing can improve adherence and clinical outcomes.
Flexible use of remote consulting, tailored to patient preferences as well as clinical status can improve access and support patient-centered care.
Digital care can enhance personalized care, and existing initiatives in remote care and artificial intelligence are set to become mainstream over the next decade, though societal concerns about increasing inequities need to be addressed.
Declaration of interest
H Pinnock is the Principal Investigator of the IMP2ART programme, K McClatchey is a researcher with IMP2ART, V Marsh is employed as a nurse facilitator with IMP2ART so received funding from the NIHR Programme Grants for Applied Research (RP-DG-1016–10008).
D Lo holds an Advanced Research Fellowship award from the National Institute of Health and Care Research (NIHR302205).
CY Hui is a visitor in the University of Edinburgh and a senior consultant in net zero and sustainability (healthcare) at Turner and Townsend. Her research with the University of Edinburgh, is independent from, and not financially supported by Turner and Townsend. Her view in this publication is her own, and not those of the Turner and Townsend. Neither she, nor Turner and Townsend, stand to gain financially from work reported in this manuscript.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that their research group founded a digital system for management and diagnosis of asthma, AsthmaTuner. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. The lancet commission. Lancet 2017;391:p. 350–400.
- Global Initiative for Asthma. Global strategy for asthma management and prevention 2023. GINA. 2023. Available from: https://ginasthma.org/gina-reports/
- Royal College of General Practitioners. Fit for the future: a vision for general practice. RCGP; 2019. Available from: https://www.rcgp.org.uk/getmedia/ff0f6ea4-bce1-4d4e-befc-d8337db06d0e/RCGP-fit-for-the-future-report-may-2019.pdf
- McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: treatable traits down under international workshop report. Eur Respir J. 2019;53(5):1802058. doi: 10.1183/13993003.02058-2018
- US Food and Drug Administration. Precision medicine. FDA; 2018. Available from: https://www.fda.gov/medical-devices/in-vitro-diagnostics/precision-medicine#:~:text=Precision%20medicine%2C%20sometimes%20known%20as,genes%2C%20environments%2C%20and%20lifestyles
- Pinnock H. Supported self-management for asthma. Breathe. 2015;11(2):98–109. doi: 10.1183/20734735.015614
- The Health Foundation. Person-centred care made simple what everyone should know about person-centred care. The Health Foundation; 2016. Available from: https://www.health.org.uk/publications/person-centred-care-made-simple
- Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331(7524):1064–1065. doi: 10.1136/bmj.38636.593461.68
- British Thoracic Society/Scottish Intercollegiate Guideline Network. SIGN 158: british guideline on the management of asthma [internet]. SIGN; 2019. Available from: www.sign.ac.uk
- Leventhal H, Phillips LA, Burns E. The common-sense model of self-regulation (CSM): a dynamic framework for understanding illness self-management. J Behav Med. 2016;39(6):935–946. doi: 10.1007/s10865-016-9782-2
- Gardner B. Habit as automaticity, not frequency. Eur Health Psychol. 2012;14:32–36.
- Daines L, Morrow S, Wiener-Ogilvie S, et al. Pinnock H for the IMP2ART team. understanding how patients establish strategies for living with asthma: IMP2ART qualitative study. Br J Gen Pract. 2020;70:e303–11. doi: 10.3399/bjgp20X708869
- Pinnock H, Parke HL, Panagioti M, et al.; for the PRISMS group. Systematic meta-review of supported self-management for asthma: a healthcare service perspective. BMC Med. 2017;15:64.
- Hodkinson A, Bower P, Grigoroglou C, et al. Self-management interventions to reduce healthcare utilisation and improve quality of life among patients with asthma: a systematic review and network meta-analysis. BMJ. 2020;370:m2521.
- McKeever T, Mortimer K, Wilson A, et al. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. New Eng J Med. 2018;378:902‐910. doi: 10.1056/NEJMoa1714257
- Ring N, Booth H, Wilson C, et al. The ‘vicious cycle’ of personalised asthma action plans implementation in primary care: a qualitative study of patients and health professionals’ views. BMC Fam Pract. 2015;16:145. doi: 10.1186/s12875-015-0352-4
- Pearce G, Parke H, Pinnock H, et al. The PRISMS taxonomy of self-management support: derivation of a novel taxonomy and initial testing of its utility. J Health Serv Res Policy. 2016;21(2):73–82. doi: 10.1177/1355819615602725
- Taylor SJC, Pinnock H, Epiphaniou E, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions (PRISMS practical systematic review of self-management support for long-term conditions). Health Serv Deliv Res. 2014;2:54.
- Price D, David-Wang A, Cho SH, et al.; for the REALISE Asia Working Group. Time for a new language for asthma control: results from REALISE asia study. J Asthma Allergy. 2015;8:93–103. doi: 10.2147/JAA.S82633
- Chisholm A, Price D, Pinnock H, et al.; on behalf of the Respiratory Effectiveness Group. Personalising care of adults with asthma from asia: a modified e-Dephi consensus study to inform management tailored to attitude and control profiles. Prim Care Respir Med. 2017;27:16089. doi: 10.1038/npjpcrm.2016.89
- Joseph CL, Ownby DR, Havstad SL, et al. Evaluation of a web-based asthma management intervention program for urban teenagers: reaching the hard to reach. J Adolesc Health. 2013;52:419–426. doi: 10.1016/j.jadohealth.2012.07.009
- Rikkers‐Mutsaerts ER, Winters AE, Bakker MJ, et al.; for the Smashing Study Group. Internet-based self-management compared with usual care in adolescents with asthma: a randomized controlled trial. Pediatr Pulmonol. 2012;47(12):1170–1179.
- Kew KM, Carr R, Crossingham I. Lay-led and peer support interventions for adolescents with asthma. Cochrane Database Syst Rev. 2017;2017(4): Art. No.: CD012331. doi: 10.1002/14651858.CD012331.pub2.
- Grape A, Rhee H, Sanchez P. Evaluation of a peer-led asthma self-management group intervention for urban adolescents. J Pediat Nursing. 2019;45:1‐6. doi: 10.1016/j.pedn.2018.12.011
- Rhee H, Love T, Wicks MN, et al. Long-term effectiveness of a peer-led asthma self-management program on asthma outcomes in adolescents living in urban areas: a randomized clinical trial. JAMA Netw Open. 2021;4(12):e2137492. doi: 10.1001/jamanetworkopen.2021.37492
- Harris K, Kneale D, Lasserson TJ, et al. School-based self-management interventions for asthma in children and adolescents: a mixed methods systematic review. Cochrane Database Syst Rev. 2019;2019(1). Art. No.: CD011651. doi: 10.1002/14651858.CD011651.pub2.
- Ramdzan SK, Suhaimi J, Harris KM, et al.; RESPIRE collaborators. School-based self-management interventions for asthma among primary school children: A systematic review. npjPrim Care Respir Med. 2021;31:18.
- Baptist AP, Hao W, Song PX, et al. A behavioral intervention can decrease asthma exacerbations in older adults. Ann Allergy Asthma Immunol. 2020;124:248‐253.e3. doi: 10.1016/j.anai.2019.12.015
- Federman AD, O’Conor R, Mindlis I, et al. Effect of a self-management support intervention on asthma outcomes in older adults: the SAMBA study randomized clinical trial. JAMA Int Med. 2019;179(8):1113‐1121. doi: 10.1001/jamainternmed.2019.1201
- Stevens CA, Wesseldine LJ, Couriel JM, et al. Parental education and guided self-management of asthma and wheezing in the pre-school child: a randomised controlled trial. Thorax. 2002;57:39–44. doi: 10.1136/thorax.57.1.39
- McCallum GB, Morris PS, Brown N, et al. Culture‐specific programs for children and adults from minority groups who have asthma. Cochrane Database Syst Rev. 2017;8: CD006580. doi: 10.1002/14651858.CD006580
- Ahmed S, Harris K, Taylor SJC, et al. Enhancing the adoption of asthma self-management behaviour in the south asian and african american population: a systematic review. npjPrim Care Respir Med. 2018;28. Art no 5. doi: 10.1038/s41533-017-0070-6
- Ahmed S, Pinnock H, Dowrick A, et al. Generational perspective on asthma self-management in bangladeshi and pakistani people in the UK: A qualitative study. Health Expect. 2022;25:2534–2547. doi: 10.1111/hex.13579
- Salim H, Ramdzan SN, Ghazali SS, et al. A systematic review of interventions addressing limited health literacy to improve asthma self-management. J Glob Health. 2020;10(1):010428. doi: 10.7189/jogh.10.010428
- Roberts NJ, Mohamed Z, Wong P-S, et al. The development and comprehensibility of a pictorial asthma action plan. Pat Ed Counsel. 2009;74:12–18. doi: 10.1016/j.pec.2008.07.049
- Radzniwan MR, Chow SY, Shamsul AS, et al. Effectiveness of pictorial based self-management among adult with asthma in a suburban primary care health clinic: a randomised controlled trial. Brunei Int Med J. 2016;12:183–190.
- Pinnock H, Epiphaniou E, Pearce G, et al. Implementing supported self-management for asthma: a systematic review of implementation studies. BMC Med. 2015;13:127.
- Pinnock H. Connecting professionals and patients: how technology can support asthma self-management. Respir Drug Delivery Eur. 2017;1:43–52.
- Kohler B, Kellerer C, Schultz K, et al. An internet-based asthma self-management program increases knowledge about asthma. Dtsch Arztebl Int. 2020;117:64‐71. doi: 10.3238/arztebl.2020.0064
- Marcano Belisario JS, Huckvale K, Greenfield G, et al. Smartphone and tablet self management apps for asthma. Cochrane Database Syst Rev. 2013;(11). Art. No.: CD010013. doi: 10.1002/14651858.CD010013.pub2.
- Hanlon P, Daines L, Campbell C, et al. Telehealth interventions to support self-management of long-term conditions: a systematic meta-review of diabetes, heart failure, asthma, chronic obstructive pulmonary disease and cancer. J Med Internet Res. 2017;19:e172.
- Hui CY, Walton R, McKinstry B, et al. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc. 2017;24(3):619–632. doi: 10.1093/jamia/ocw143
- Salim H, Lee PY, Ghazali SS, et al. Developing an asthma self-management intervention through a web-based design workshop for people with limited health literacy: user-centered design approach. J Med Internet Res. 2021;23(9):e26434. doi: 10.2196/26434
- Khusial RJ, Honkoop PJ, Usmani O, et al. for myAircoach study group. effectiveness of myAircoach: a mHealth self-management system in asthma. J Allergy Clin Immunol. 2020;8:1972‐1979.e8. doi: 10.1016/j.jaip.2020.02.018
- Ljungberg H, Carleborg A, Gerber H, et al. Clinical effect on uncontrolled asthma using a novel digital automated self-management solution: a physician-blinded randomised controlled crossover trial. Eur Respir J. 2019;54(5):1900983. doi: 10.1183/13993003.00983-2019
- Kew KM, Cates CJ. Home telemonitoring and remote feedback between clinic visits for asthma. Cochrane Database Syst Rev. 2016;2016(8): Art. No.: CD011714. doi: 10.1002/14651858.CD011714.pub2.
- Chan A, De Simoni A, Wileman V, et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database Syst Rev. 2022;(6). Art. No: CD013030. doi: 10.1002/14651858.CD013030.pub2
- De Simoni A, Shah AT, Fulton O, et al. Superusers’ engagement in asthma online communities: asynchronous web-based interview study. J Med Internet Res. 2020;22(6):e18185. doi: 10.2196/18185
- Global Asthma Network. The global asthma report 2022.[Internet] GAN 2022. Available from: http://globalasthmareport.org/burden/burden.php
- Gupta RP, Mukherjee M, Sheikh A, et al. Persistent variations in national asthma mortality, hospital admissions and prevalence by socioeconomic status and region in england. Thorax. 2018;73(8):706–712. doi: 10.1136/thoraxjnl-2017-210714
- NHS Digital. Mortality from asthma: directly standardised rate, all ages, annual trend, MFP. NHS Digital. 2020. https://digital.nhs.uk/data-and-information/publications/statistical/compendium-mortality/current/mortality-from-respiratory-diseases/mortality-from-asthma-directly-standardised-rate-all-ages-annual-trend-mfp
- European Respiratory Society. Education, funding and continuing professional development. ERS; 2023. Available from: https://www.ersnet.org/education-and-professional-development/
- Roland MD. Linking physicians’ pay to the quality of care—a major experiment in the united kingdom. N Engl J Med. 2004;351:1448–1454. doi: 10.1056/NEJMhpr041294
- Fleming L, Heaney L. Severe asthma – perspectives from adult and pediatric pulmonology. Front Pediatr. 2019;7:389. doi: 10.3389/fped.2019.00389
- Sheikh A, Steiner MFC, Cezard G, et al. Ethnic variations in asthma hospital admission, readmission and death: a retrospective, national cohort study of 4.62 million people in scotland. BMC Med. 2016;14(1):3. doi: 10.1186/s12916-015-0546-6
- Al Sallakh MA, Rodgers SE, Lyons RA, et al. Association of socioeconomic deprivation with asthma care, outcomes and deaths in wales: A 5-year national linked primary and secondary care cohort study. PLOS Med. 2021;18:e1003497. doi: 10.1371/journal.pmed.1003497
- Thomas M, Bruton A, Moffat M, et al. Asthma and psychological dysfunction. Prim Care Respir J. 2011;20(3):250–256. doi: 10.4104/pcrj.2011.00058
- Scott KU, Von Korff M, Ormel J, et al. Mental disorders among adults with asthma: results from the world mental health survey. Gen Hosp Psychiatry. 2007;29:123–133. doi: 10.1016/j.genhosppsych.2006.12.006
- Buelo A, McLean S, Julious S, et al. on behalf of the ARC group. identifying the child (5-12 years) with asthma at increased risk of attacks: the At-Risk Child with asthma (ARC) systematic review of risk factors. Thorax. 2018;73:813–824.
- Royal College of Physicians. National Review Of Asthma Deaths. London: Royal College of Physicians. 2015. Available from: https://www.rcplondon.ac.uk/projects/national-review-asthma-deaths. .
- Fleetcroft R, Noble M, Martin A, et al. Emergency hospital admissions for asthma and access to primary care: a cross-sectional analysis. Br J Gen Pract. 2016;66:e640–6. doi: 10.3399/bjgp16X686089
- McGovern CM, Redmond M, Arcoleo K, et al. A missed primary care appointment correlates with a subsequent emergency department visit among children with asthma. J Asthma. 2017;54(9):977–982. doi: 10.1080/02770903.2017.1283697
- Yoon R, McKenzie DK, Miles DA, et al. Characteristics of attenders and non-attenders at an asthma education programme. Thorax. 1991;46(12):886–890. doi: 10.1136/thx.46.12.886
- Harrison BDW. Psychosocial aspects of asthma in adults. Thorax. 1998;53(6):519–525. doi: 10.1136/thx.53.6.519
- Asthma UK. Asthma attack risk checker. A&Luk; 2021. Available from: https://www.asthma.org.uk/advice/manage-your-asthma/risk/
- Blakey JD, Obediat M, Pogson Z, et al. A simple asthma severity score predicts exacerbations. Am J Respir Crit Care Med. 2011;183:A2248.
- Miller MK, Lee JH, Blanc PD, et al. TENOR risk score predicts healthcare in adults with severe or difficult-to-treat asthma. Eur Respir J. 2006;28(6):1145–1155. doi: 10.1183/09031936.06.00145105
- Noble MJ, Burden A, Stirling S, et al. Predicting asthma-related crisis events using routine electronic data: a quantitative database analysis study. Br J Gen Pract. 2021;71:e948–57. doi: 10.3399/BJGP.2020.1042
- Hippersley-Cox J, Coupland C. Predicting risk of emergency hospital admissions using primary care data: derivation and validation of qAdmissions score. BMJ Open. 2013;3:e003482. doi: 10.1136/bmjopen-2013-003482
- Jackson DJ, Busby J, Pfeffer PE, et al. Characteristics of patients with severe asthma in the UK severe asthma registry in the biologic era. Thorax. 2020;76:220–227. doi: 10.1136/thoraxjnl-2020-215168
- International Severe Asthma Registry. The registry. ISAR; 2023. Available from: https://www.isaregistries.opcglobal.org/about
- FitzGerald JM, Tran TN, Alacqua M, et al. International severe asthma registry (ISAR): protocol for a global registry. BMC Med Res Methodol. 2020;20(1):212. doi: 10.1186/s12874-020-01065-0
- Optimum patient care research database. our databases. OPCRD; 2023. Available from: https://www.opcrd.co.uk/our-database
- Ryan D, Heatley H, Heaney L, et al. Potential severe asthma hidden in UK primary care. J Allergy Clin Immunol. 2021;9:1612–1623. doi: 10.1016/j.jaip.2020.11.053
- Smith JR, Mildenhall S, Noble MJ, et al. The coping with asthma study: a randomised controlled trial of a home-based, nurse-led psychoeducational intervention for adults at risk of adverse asthma outcomes. Thorax. 2005;60:1003–1011. doi: 10.1136/thx.2005.043877
- Foster JM, Hoskins G, Smith B, et al. Practice development plan to improve the primary care management of acute asthma: randomised controlled trial. BMC Fam Pract. 2007;8:23. doi: 10.1186/1471-2296-8-23
- Noble MJ, Smith JR, Windley J. A controlled retrospective pilot study of an ‘at-risk asthma register’ in primary care. Prim Care Resp J. 2006;15(2):116–124. doi: 10.1016/j.pcrj.2006.01.002
- Smith JR, Noble MJ, Musgrave S, et al. The at-risk registers in severe asthma (ARRISA) study: a cluster-randomised controlled trial examining effectiveness and costs in primary care. Thorax. 2012;67(12):1052–1060. doi: 10.1136/thoraxjnl-2012-202093.
- At-risk registers integrated into primary care to stop asthma crises. About. ARRISA-UK 2022. https://www.uea.ac.uk/web/groups-and-centres/arrisa-uk/about
- Smith JR, Musgrave S, Payerne E, et al. At-risk registers integrated into primary care to stop asthma crises in the UK (ARRISA-UK): study protocol for a pragmatic, cluster randomised trial with nested health economic and process evaluations. Trials. 2018;19(1):466. doi: 10.1186/s13063-018-2816-z
- Martinez FD, Wright AL, Taussig LM, et al.; for Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332(3):133–138.
- Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974–980. doi: 10.1136/thx.2007.093187
- Groen E H-D, Mohangoo AD, Landgraf JM, et al. The impact of preschool wheezing patterns on health-related quality of life at age 4 years. Eur Respir J. 2013;41:952–959. doi: 10.1183/09031936.00015712
- Douros K, Everard ML. Time to say goodbye to bronchiolitis, viral wheeze, reactive airways disease, wheeze bronchitis and all that. Front Pediatr. 2020;8:218. doi: 10.3389/fped.2020.00218
- Bloom CI, Franklin C, Bush A, et al. Burden of preschool wheeze and progression to asthma in the UK: population-based cohort 2007 to 2017. J Allergy Clin Immunol. 2021;147(5):1949–1958. doi: 10.1016/j.jaci.2020.12.643
- Granell R, Henderson AJ, Sterne JA. Associations of wheezing phenotypes with late asthma outcomes in the avon longitudinal study of parents and children: A population-based birth cohort. J Allergy Clin Immunol. 2016;138(4):1060–1070.e11. doi: 10.1016/j.jaci.2016.01.046
- Paton J British thoracic society national paediatric asthma audit summary report. BTS; 2016. Available from: https://www.brit-thoracic.org.uk/quality-improvement/clinical-audit/bts-national-audit-reports/
- Bloom CI, Saglani S, Feary J, et al. Changing prevalence of current asthma and inhaled corticosteroid treatment in the UK: population-based cohort 2006-2016. Eur Respir J. 2019;53:1802130. doi: 10.1183/13993003.02130-2018
- Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics. 2009;123(3):e519–25. doi: 10.1542/peds.2008-2867
- Akindele A, Daines L, Cavers D, et al. Qualitative study of practices and challenges when making a diagnosis of asthma in primary care. npjPrim Care Respir Med. 2019;29:27. doi: 10.1038/s41533-019-0140-z
- Gaillard EA, Kuehni CE, Turner S, et al. European respiratory society clinical practice guidelines for the diagnosis of asthma in children aged 5-16 years. Eur Respir J. 2021;58:2004173. doi: 10.1183/13993003.04173-2020
- The National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management (NG80). NICE; 2017. Available from: https://www.nice.org.uk/guidance/ng80
- Lo DK, Beardsmore CS, Roland D, et al. Lung function and asthma control in school-age children managed in UK primary care: a cohort study. Thorax. 2020;75(2):101–107. doi: 10.1136/thoraxjnl-2019-213068
- Grell AV, Vera RG, Yarur AM, et al. Impulse oscillometry in preschool children with persistent asthma can predict spirometry at school age. Pediatr Pulmonol. 2023 Jan 26;58(5):1411–1416. Early view. doi: https://doi.org/10.1002/ppul.26333
- Brand PL, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008;32(4):1096–1110. doi: 10.1183/09031936.00002108
- Brand PL, Caudri D, Eber E, et al. Classification and pharmacological treatment of preschool wheezing: changes since 2008. Eur Respir J. 2014;43(4):1172–1177. doi: 10.1183/09031936.00199913
- Schultz A, Devadason SG, Savenije OE, et al. The transient value of classifying preschool wheeze into episodic viral wheeze and multiple trigger wheeze. Acta Paediatr. 2010;99:56–60. doi: 10.1111/j.1651-2227.2009.01508.x
- Savenije OE, Kerkhof M, Koppelman GH, et al. Predicting who will have asthma at school age among preschool children. J Allergy Clin Immunol. 2012;130:325–331. doi: 10.1016/j.jaci.2012.05.007
- Castro-Rodriguez JA, Cifuentes L, Martinez FD. Predicting asthma using clinical indexes. Front Pediatr. 2019;7:320. doi: 10.3389/fped.2019.00320
- Looijmans-van den Akker I, van Luijn K, Verheij T. Overdiagnosis of asthma in children in primary care: a retrospective analysis. Br J Gen Pract. 2016;66(644):e152–7. doi: 10.3399/bjgp16X683965
- Fitzpatrick AM, Jackson DJ, Mauger DT, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 2016;138(6):1608–1618.e12. doi: 10.1016/j.jaci.2016.09.028
- Bacharier LB, Guilbert TW, Jartti T, et al. Which wheezing preschoolers should be treated for asthma? J Allergy Clin Immunol Pract. 2021;9(7):2611–2618. doi: 10.1016/j.jaip.2021.02.045
- Makrinioti H, Keating E, Holden B, et al. Patient reported outcomes for preschool children with recurrent wheeze. npjPrim Care Respir Med. 2019;29:7. doi: 10.1038/s41533-019-0120-3
- National Institute for Health and Care research. Development of an integrated pathway for preschool asthma/wheeze management (IP2AM): a mixed methods study. NIHR; 2022. Available from: https://fundingawards.nihr.ac.uk/award/NIHR302205
- Balint E. The possibilities of patient-centered medicine. J R Coll Gen Pract. 1969;17(82):269–276.
- Eklund JH, Holmström IK, Kumlin T, et al. “Same same or different?” a review of reviews of person-centered and patient-centered care. Pat Ed Counsel. 2019;102:3–11. doi: 10.1016/j.pec.2018.08.029
- Kitson A, Marshall A, Bassett K, et al. What are the core elements of patient-centred care? A narrative review and synthesis of the literature from health policy, medicine and nursing. J Adv Nurs. 2013;69:4–15. doi: 10.1111/j.1365-2648.2012.06064.x
- Qamar N, Pappalardo AA, Arora VM, et al. Patient-centered care and its effect on outcomes in the treatment of asthma. Patient Relat Outcome Meas. 2011;2:81–109. doi: 10.2147/PROM.S12634
- Daines L, Baxter N, Gruffydd-Jones K, et al. Asthma guidelines in practice: a PCRS consensus. PCRS; 2019. Available from: https://www.pcrs-uk.org/resource/asthma-guidelines-practice-pcrs-consensus
- Morrow S, Daines L, Wiener-Ogilvie S, et al. Exploring the perspectives of clinical professionals and support staff on implementing supported self-management for asthma in UK general practice: an IMP2ART qualitative study. npjPrim Care Respir Med. 2017;27:45. doi: 10.1038/s41533-017-0041-y
- Morrissey M, Shepherd E, Kinley E, et al. Effectiveness and perceptions of using templates in long-term condition reviews: a systematic synthesis of quantitative and qualitative studies. Br J Gen Pract. 2021;71(710):e652–9. doi: 10.3399/BJGP.2020.0963.
- British Thoracic Society. Royal college of physicians of london, king’s fund centre, national asthma campaign. guidelines for management of asthma in adults: I-chronic persistent asthma. BMJ. 1990; 301:651–653. doi: 10.1136/bmj.301.6753.651
- Kew KM, Malik P, Aniruddhan K, et al. Shared decision‐making for people with asthma. Cochrane Database Syst Rev. 2017;2017(10). Art. No.: CD012330. doi: 10.1002/14651858.CD012330.pub2
- Rollnick S, Butler CC, Kinnersley P, et al. Motivational interviewing. BMJ. 2010;340(apr27 2):c1900. doi: 10.1136/bmj.c1900
- Rollnick S, Miller WR, Butler C. Motivational interviewing in health care: helping patients change behavior. (NY): Guilford Press; 2008.
- Borrelli B, Riekert KA, Weinstein A, et al. Brief motivational interviewing as a clinical strategy to promote asthma medication adherence. J Allergy Clin Immunol. 2007;120(5):1023–1030. doi: 10.1016/j.jaci.2007.08.017
- Gesinde B, Harry S. The use of motivational interviewing in improving medication adherence for individuals with asthma: a systematic review. Perspect Public Health. 2018;138(6):329–335. doi: 10.1177/1757913918786528
- Klok T, Kaptein AA, Brand PL. Non‐adherence in children with asthma reviewed: the need for improvement of asthma care and medical education. Pediatr Allergy Immunol. 2015;26:197–205. doi: 10.1111/pai.12362
- Engelkes M, Janssens HM, de Jongste JC, et al. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396–407. doi: 10.1183/09031936.00075614
- Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prevent Med. 2017;99:269–276. doi: 10.1016/j.ypmed.2017.03.008
- Pinnock H, Bawden R, Proctor S, et al. Accessibility, acceptability, and effectiveness in primary care of routine telephone review of asthma: pragmatic, randomised controlled trial. BMJ. 2003;326:477–479. doi: 10.1136/bmj.326.7387.477
- Car J, Koh GC, Foong PS, et al. Video consultations in primary and specialist care during the COVID-19 pandemic and beyond. BMJ. 2020;371:m3945. doi: 10.1136/bmj.m3945
- Kinley E, Skene I, Steed E, et al. Delivery of supported self‐management in remote asthma reviews: A systematic rapid realist review. Health Expect. 2022;25:1200–1214. doi: 10.1111/hex.13441
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: A qualitative study in primary care. Br J Gen Pract. 2019;69:e586–9. doi: 10.3399/bjgp19X704141
- Hamour O, Smyth E, Pinnock H. Completing asthma action plans by screen-sharing in video-consultations: practical insights from a feasibility assessment. npjPrim Care Respir Med. 2020;30:48. doi: 10.1038/s41533-020-00206-8
- Shaw SE, Hughes G, Wherton J, et al. Achieving spread, scale up and sustainability of video consulting services during the COVID-19 pandemic? findings from a comparative case study of policy implementation in england, wales, scotland and northern ireland. Front Digit Health. 2021;3:754319. doi: 10.3389/fdgth.2021.754319
- Murphy M, Scott LJ, Salisbury C, et al. Implementation of remote consulting in UK primary care following the COVID-19 pandemic: a mixed-methods longitudinal study. Br J Gen Pract. 2021;71(704):e166–e177. doi: 10.3399/BJGP.2020.0948
- Asthma UK. Transforming asthma care in the UK. London: Asthma and Lung UK. 2022. Available from: https://www.asthmaandlung.org.uk/sites/default/files/Fighting%20back_V3.pdf
- Swaithes L, Paskins Z, Duffy H, et al. Experience of implementing and delivering group consultations in UK general practice: A qualitative study. Br J Gen Pract. 2021;71(707):e413–22. doi: 10.3399/BJGP.2020.0856
- Miles C, Arden-Close E, Thomas M, et al. Barriers and facilitators of effective self-management in asthma: systematic review and thematic synthesis of patient and healthcare professional views. npjPrim Care Respir Med. 2017;27:57. doi: 10.1038/s41533-017-0056-4
- Berry LL, Seiders K, Wilder SS. Innovations in access to care: a patient-centered approach. Ann Int Med. 2003;139(7):568–574. doi: 10.7326/0003-4819-139-7-200310070-00009
- Digital Health & Care Scotland. Digital health & care strategy 2021. DHCS; 2021. Available from: https://www.gov.scot/publications/scotlands-digital-health-care-strategy/
- NHS England. Using online consultations in primary care: implementation toolkit. NHSE; 2020. Available from: https://www.england.nhs.uk/wp-content/uploads/2020/01/online-consultations-implementation-toolkit-v1.1-updated.pdf
- Latulippe K, Hamel C, Giroux D. Social health inequalities and eHealth: a literature review with qualitative synthesis of theoretical and empirical studies. J Med Internet Res. 2017;19(4):e136. doi: 10.2196/jmir.6731
- Rodgers M, Raine G, Thomas S, et al. Informing NHS policy in ‘digital-first primary care’: a rapid evidence synthesis. Health Serv Deliv Res. 2019;7(41):1–124. doi: https://doi.org/10.3310/hsdr07410
- Tudor-Hart J. The inverse care law. Lancet. 1971;297(7696):405–412. doi: 10.1016/S0140-6736(71)92410-X
- Gartner. What is new in the 2022 gartner hype cycle for emerging technologies. Gartner; 2022. Available from: https://www.gartner.co.uk/en/articles/what-s-new-in-the-2022-gartner-hype-cycle-for-emerging-technologies
- Davies B, Kenia P, Nagakumar P, et al. Paediatric and adolescent asthma: a narrative review of telemedicine and emerging technologies for the post‐COVID‐19 era. Clin Exp Allergy. 2021;51(3):393–401. doi: 10.1111/cea.13836
- Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. doi: 10.1038/s41746-020-0221-y
- Feng Y, Wang Y, Zeng C, et al. Artificial intelligence and machine learning in chronic airway diseases: focus on asthma and] chronic obstructive pulmonary disease. Int J Med Sci. 2021;18:2871. doi: 10.7150/ijms.58191
- Canny A, Donaghy E, Murray V, et al. Patient views on asthma diagnosis and how a clinical decision support system could help: A qualitative study. Health Expect. 2023;26:307–317. doi: 10.1111/hex.13657
- Exarchos KP, Beltsiou M, Votti CA, et al. Artificial intelligence techniques in asthma: a systematic review and critical appraisal of the existing literature. Eur Respir J. 2020;56(3):2000521. doi: 10.1183/13993003.00521-2020
- Kouri A, Yamada J, Lam Shin Cheung J, et al. Do providers use computerized clinical decision support systems? A systematic review and meta-regression of clinical decision support uptake. Implementation Sci. 2022;17(1):21. doi: 10.1186/s13012-022-01199-3
- Häussermann S, Andersen L, Frisch M. Adherence to inhaled drugs–more than just reminders and nudging. Eur Respir J. 2022;60:4697.
- McCrossan P, O’Donoghue D, McElnay JC, et al. The use of remote video directly observed therapy to improve both inhaler technique and adherence to asthma medications. Front Public Health. 2022;10:965629. doi: 10.3389/fpubh.2022.965629
- Tony SM, Abdelrahman MA, Abd Elsalam M, et al. Effect of using acoustic flo-tone training device and its smartphone application on enhancing inhalation technique from metered-dose inhaler with spacer in asthmatic children. Exp Lung Res. 2022;48(7–8):224–238. doi: 10.1080/01902148.2022.2113573
- O’Connor A, Tai A, Brinn M, et al. The acceptability of using augmented reality as a mechanism to engage children in asthma inhaler technique training: qualitative interview study with deductive thematic analysis. JMIR Pediatr Parent. 2023;6:e40231. doi: 10.2196/40231
- Portelli P, Bogolyubova O, Tristan Lopez B, et al. A quality review of smart-phone applications for smoking cessation. J Consumer Health. 2022;26:64–81. doi: 10.1080/15398285.2021.2021372
- Chan A, Kodali S, Lee GY, et al. Evaluating the effect and user satisfaction of an adapted and translated mobile health application aSTHMAXcel© among adults with asthma in pune, india. J Asthma. 2023;60(8):1513–1523. doi: 10.1080/02770903.2022.2155188
- Patil R, Shrivastava R, Juvekar S, et al. Specialist to non-specialist teleconsultations in chronic respiratory disease management: A systematic review. J Glob Health. 2021;11:04019. doi: 10.7189/jogh.11.04019
- Diedrich L, Dockweiler C. Video-based teleconsultations in pharmaceutical care - a systematic review. Res Social Adm Pharm. 2021;17:1523–1531. doi: 10.1016/j.sapharm.2020.12.002
- Vindrola-Padros C, Singh KE, Sidhu MS, et al. Remote home monitoring (virtual wards) for confirmed or suspected COVID-19 patients: a rapid systematic review. E Clin Med. 2021;37:100965. doi: 10.1016/j.eclinm.2021.100965
- Cox NS, Dal Corso S, Hansen H, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;2021(1). Art. No.: CD013040. doi: 10.1002/14651858.CD013040.pub2
- Rutkowski S. Management challenges in chronic obstructive pulmonary disease in the COVID-19 pandemic: telehealth and virtual reality. J Clin Med. 2021;10(6):1261. doi: 10.3390/jcm10061261
- de Las Heras J C, Tulppo M, Kiviniemi AM, et al. Augmented reality glasses as a new tele-rehabilitation tool for home use: patients’ perception and expectations. Disabil Rehabil Assist Technol. 2022;17:480–486. doi: 10.1080/17483107.2020.1800111
- Venkataramanan R, Thirunarayan K, Jaimini U, et al. Determination of personalized asthma triggers from multimodal sensing and a mobile app: observational study. JMIR Pediatr Parent. 2019;2(1):e14300. doi: 10.2196/14300
- Bui AA, Hosseini A, Rocchio R, et al. Biomedical real-time health evaluation (BREATHE): toward an mHealth informatics platform. JAMIA Open. 2020;3:190–200. doi: 10.1093/jamiaopen/ooaa011
- Hui CY, McKinstry B, Mclean S, et al. Assessing the technical feasibility of a flexible, integrated internet-of-things connected for asthma (C4A) system to support self-management: a mixed method study exploring patients’ and healthcare professionals’ perspectives. JAMIA Open. 2022;5:ooac110. doi: 10.1093/jamiaopen/ooac110
- Hui CY, McKinstry B, Fulton O, et al. Patients and clinicians perceived trust in internet-of-things (IoTs) system to support asthma self-management: a qualitative study. JMIR mHealth uHealth. 2021;9:e24127.
- Pinto F, da Silva CF, Moro S. People-centered distributed ledger technology-IoT architectures: A systematic literature review. Telemat Inform. 2022;70:101812. doi: 10.1016/j.tele.2022.101812
- Rapport DJ, Maffi L. Eco-cultural health, global health, and sustainability. Ecol Res. 2011;26(6):1039–1049. doi: 10.1007/s11284-010-0703-5
- Subramanian M, Wojtusciszyn A, Favre L, et al. Precision medicine in the era of artificial intelligence: implications in chronic disease management. J Transl Med. 2020;18(1):1–2. doi: 10.1186/s12967-020-02658-5
- OpenAI. Introducing ChatGPT. OpenAi; 2023. https://openai.com/blog/chatgpt
- Johnson KB, Wei WQ, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86–93. doi: 10.1111/cts.12884
- Khanijahani A, Iezadi S, Dudley S, et al. Organizational, professional, and patient characteristics associated with artificial intelligence adoption in healthcare: A systematic review. Health Policy Technol. 2022;13:100602. doi: 10.1016/j.hlpt.2022.100602
- O’Brien N, Van Dael J, Clarke J, et al. Addressing racial and ethnic inequities in data-driven health technologies. Imperial College 2022 . Available from https://spiral.imperial.ac.uk/bitstream/10044/1/94902/2/Imperial_IGHI_AddressingRacialandEthnicInequities%20_Report.pdf
- Implementing iMProved asthma self-management as RouTine. The IMP2ART Programme IPCRG; 2021. Available from: https://www.ed.ac.uk/usher/imp2art/the-imp2art-programme