ABSTRACT
Introduction
Small-cell lung cancer (SCLC) accounts for 15% of lung cancers and has a dismal prognosis due to early dissemination and acquired chemoresistance. The initial good response to chemotherapy is followed by refractory relapses within 1-2 years. Mechanisms leading to chemoresistance are not clear and progress is poor.
Areas covered
This article reviews the current evidence of the resistance of SCLCs at the cellular level including alteration of key proteins and the possible presence of cancer stem cells (CSCS). Without compelling evidence for cellular mechanisms and clinical failures of novel approaches, the study of SCLC has advanced to the role of 3D tumor cell aggregates in chemoresistance.
Expert opinion
The scarcity of viable tumor specimen from relapsed SCLC patients has hampered the investigations of acquired chemoresistance but a panel of nine SCLC circulating tumor cell (CTC) cell lines have revealed characteristics of SCLC in the advanced refractory states. The chemoresistance of relapsed SCLC seems to be linked to the spontaneous formation of large spheroids, termed tumorospheres, which contain resistant quiescent and hypoxic cells shielded by a physical barrier. So far, drugs to tackle large tumor spheroids are in preclinical and early clinical development.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.1. Introduction
SCLC is a highly proliferative neuroendocrine lung tumor which accounts for approximately 15% of all lung cancers. Worldwide, the number of SCLC deaths is estimated to be around 200,000 patients worldwide and smoking accounts for more than 95% of SCLC cases [Citation1–4]. Median overall survival (OS) is approximately 10 - 12 months in patients with extensive stage disease (ES) and approximately 17 months for those with limited-stage SCLC [Citation5,Citation6]. The great majority of the patients have disseminated disease at first presentation and the tumor develops rapidly since the Ki-67 proliferation index of SCLC cells is consistently high (50–100%) [Citation7]. Despite a high initial response rate of 60 - 70% for first-line cytotoxic SCLC therapy, resistance to chemotherapy and radiotherapy develops invariably and leads to early recurrent disease. The 70% of patients diagnosed at an ES have a 5-year OS rate below 7% [Citation8]. So far, the mechanisms by which this global resistance occurs are poorly understood. Recently, SCLC has been subdivided into four subtypes according to their expression of the transcription factors ASCL1, NEUROD1, YAP1 and POU2F3 but no clear relationship between these SCLC subtype and chemoresistance has been found to date [Citation2,Citation9]. Little improvement has been achieved in SCLC treatment and survival during the last decades, with minor improvements due to novel agents and addition of immunotherapy. The majority of SCLC patients in screening trials were not detected by low-dose CT (LDCT), indicating the aggressive biology and rapid growth of SCLC [Citation10]. Given the ineffectiveness of LDCT in improving survival for SCLC, novel approaches for early detection of this malignancy are urgently needed.
Patients with SCLC are assorted into limited versus extensive disease (LD vs ED) according to the Veteran’s Administration Lung Study Group (VALSG) staging system [Citation11]. The majority of patients (approximately 85%) present with ES-SCLC that exhibit malignant pleural or pericardial effusion alone or other metastatic lesions [Citation3]. The patients with ED-SCLC are treated with a chemotherapy regimen combining a cisplatin agent with etoposide (EP), that has been introduced several decades ago [Citation12]. Although chemotherapy and (prophylactic) radiotherapy yield high initial responses they are short-lived and relapses are obvious within approximately 1 year [Citation13,Citation14]. Thus, despite an initial overall response rate (ORR) of approximately 66.0%, based on a COCIS meta-analysis, the median OS is 9.4 months [Citation6]. Moreover, almost all EP-treated ED-SCLC patients reveal primary or secondary resistance for a host of chemotherapeutic drugs and resistance modulators resulting in a dismal prognosis [Citation15, Citation16]. EP is the favored regimen for three decades, but carboplatin and etoposide has shown equivalent efficacy with better tolerability in a randomized trial. Second-line chemotherapy consists of either topotecan or a cyclophosphamide-epirubicin-vincristine (CEV) regimen with both modalities showing high toxicity and minor responses of short duration [Citation17,Citation18]. In essence, all agents used in the chemotherapy of SCLC target rapidly proliferating tumor cells and seem not to reach more quiescent cells in protected regions that eventually account for these relapses. Immunotherapy or combinations of checkpoint inhibitors with chemotherapeutics have a limited efficacy in the treatment of SCLC [Citation19]. Nevertheless, in absence of detailed knowledge of the mechanisms responsible for the observed chemoresistance, more of classical and novel cytotoxic compounds are evaluated in hope of a superior outcome.
2. Immunotherapy of SCLC
It is reassuring that the relatively poor clinical activity of checkpoint inhibitors (ICIs) in SCLC have shown a good correlation with the cell biological factors that indicate a limited immunogenicity of SCLC cells, such as poor expression of HLA antigens, scarce infiltration of immune effector cells, presence of immunosuppressive cell populations, inaccessible avascular tumor regions and others [Citation20]. SCLC is strongly associated with smoking and possesses a high mutational burden favoring a good response to immune checkpoint blockade. Nevertheless, chemotherapy combined with the PD-L1 blocking antibodies, durvalumab and atezolizumab, only revealed a modest survival benefit by approximately 3 months in SCLC [Citation5,Citation21]. Only a minor fraction of non-descript SCLC patients respond to immunotherapy, and resistance against ICI treatment alone can occur [Citation20]. Incorporation of ICIs into platinum-based chemotherapy resulted in modest yet notable improvements in patient outcomes, which become more evident with longer follow-up [Citation19]. Thus, immunotherapy trials in SCLC yielded only modest improvements in survival compared to the positive results in NSCLC [Citation22]. In detail, three phase III randomized clinical SCLC trials (IMpower133, CASPIAN, and KEYNOTE-604) have proved a small but significant effect of adding ICIs to first-line chemotherapy [Citation23]. The results of the IMpower133 trial, combining atezolizumab with EP, reporting a prolongation of median OS of 2 months obviously presented a major breakthrough in the treatment of SCLC. For KEYNOTE-604, the median OS was 10.8 months versus 9.7 months of controls. A small subset of patients, that cannot be identified so far, may have durable responses to ICI. Expression of PD-L1 and measurement of the tumor mutational burden (TMB) did not predict responses. In addition, the positive clinical trial patients are generally highly selected with better performance status and the long-term survival benefit in patients from the general population is largely unknown. [Citation24].
3. Genomics of SCLC
Genomics of SCLC, including comprehensive whole exome and whole genome analyses were published in 2012 and 2015 [Citation25, Citation26]. SCLC genomes revealed an extremely high mutation rate of 8.6 nonsynonymous mutations per million base pairs. This high mutational rate is attributed to the smoking history of SCLC patients as supported by the tobacco exposure signature (C:G > A:T transversion) in a significant portion of all mutations. Most of the mutations obtained in SCLC are passenger mutations, indicating that they do not significantly contribute to the growth and progression of the disease. Analyses showed universal functional loss of two key tumor suppressor genes, namely TP53 and RB1, but potential targetable mutations in known oncogenes, including BRAF, PTEN, PIK3CA and SLIT2, were only found in rare cases [Citation26,Citation27]. In contrast, known epigenetic regulators including histone-modifying genes and inactivating mutations in Notch family members were present at high frequencies. Target genes of epigenetic controllers may play key roles in cell homeostasis, neuroendocrine differentiation and cell adhesion. Despite the recurrent inactivation of TP53 and RB1 genes, SCLC is a heterogeneous disease with a range of distinct potential drivers in individual patients [Citation26]. Loss of p53 and RB1 seems to play a synergistic role in combination with mutations for carcinogenesis [Citation28]. The MYC transcription factor is amplified in approximately 20% of the cases [Citation29,Citation30]. The amplification of MYC family members includes MYC, MYCL, and MYCN and is mutually exclusive for the different subtypes [Citation27,Citation31,Citation32]. However, no consistent mechanisms explaining the universal chemoresistance of SCLC have been derived from genomic data.
4. Tumor specimen availability for the study of SCLC
There has been some progress in the understanding of the mechanisms of chemoresistance using cell lines and preclinical models in SCLC. Nevertheless, the molecular and cellular alterations that lead to chemoresistance in SCLC are poorly understood. Since relapsed patients are referred to chemotherapy without further biopsies there is a scarcity of human samples procured at this time during the progress of the disease [Citation3]. In primary cases, core tumor biopsies would provide higher numbers of cells but in most cases, only small fine-needle biopsies or CTCs may be available. In contrast to genomic tests that may be done on small tumor cell samples, valid chemosensitivity tests need higher cell numbers to provide reliable results and to overcome the problem of tumor heterogeneity [Citation33,Citation34]. Comprehensive exploration of genomic, transcriptomic, and proteomic alterations of samples at the time of relapse would help to clear the causes of the resistance of SCLC [Citation2].
Diagnosis of SCLC is established by tissue biopsies, often in form of fine-needle aspirations, leaving none or only small samples for research and, therefore, it was not possible to include SCLC in The Cancer Genome Atlas (TCGA). A lot of experimental studies is performed using permanent SCLC cell lines, some of which has been cultivated for decades and are suspected to have lost some of their differentiated features in extended in vitro cultures. It is well-established that cultured cancer cells may bear little or no resemblance phenotypically and in terms of gene expression patterns to cells derived directly from cancers [Citation35,Citation36]. In principle, genomes of the permanent SCLC cell lines should be validated by comparison to the genomic landscapes of real tumor tissues [Citation37,Citation38]. Of course, basic characteristics such as chemosensitivity are preserved but important properties such as growth characteristics, interaction with benign cell types and other features may have been altered in vitro. Therefore, for a range of experimental studies, the effects observed in vitro has poorly translated into clinical practice.
Frequently, patient-derived xenografts (PDX) of SCLC tumors are established in immunocompromised mice and used for basic characterization and in vivo drug assessment [Citation38,Citation39]. PDX models are created by extracting tissue or cells from a patient tumor directly into an immunodeficient mouse. PDXs are complex tissues and include fibroblast and endothelial cells of the parent tumors [Citation40]. Instead of tumor biopsies, blood samples can be employed to establish CTC-derived xenografts (CDXs) [Citation41]. PDXs are genetically similar to the parent tumors but need serial passaging that is associated increasingly with altered genetics and partial changes of human characteristics under the control of the murine microenvironemt [Citation42]. PDX may require a mean period of 3 months to develop in vivo and because actual SCLC tumor-initiating cells are very rare nonrelevant tumor cells may have been expanded. PDX/CDX models have been used to investigate novel therapies in SCLC but few new findings have been reported. For example, SCLC PDX models examined the synergistic effect of a Bcl-2 inhibitor (ABT-737) with etoposide, which significantly reduced the tumor size in these models but failed in clinics [Citation43]. In PDX models, the topoisomerase I inhibitor, irinotecan, when conjugated with a HSP90 targeting moiety showed increased drug delivery and elevated DNA damage [Citation44]. However, these positive reports from the PDX model did not translate into clinical success as a general observation with PDX models. Specific novel mechanisms of the chemoresistance of SCLC have not been revealed by PDX/CDX models.
SCLC is distinguished by rapid dissemination and at the extended stage by an extreme number of circulating tumor cells (CTCs). Whereas in other tumors, for example NSCLC, a few tumor cells are detectable in 7.5 ml blood, thousands of CTCs may be present in the same volume of blood in SCLC and offer the possibility to obtain truly metastasis-initiating tumor cells [Citation41]. Only a very small faction of the CTCs has the potential to initiate metastases and the remaining CTCs perish in the circulation [Citation45]. Other tumors with high CTC counts are inflammatory breast cancer and patients with a very high metastatic load, shedding CTCs from primary tumors and the other lesions. We have established a panel of 9 SCLC CTC lines from blood samples of advanced patients before second-line chemotherapy. Neither biopsies nor pleural effusions have been available for these patients, except for one case for which a CTC line has been set up from a blood sample (BHGc59) the same day a pleural effusion has been obtained for the cultivation of a corresponding cell line [Citation46]. These proprietary SCLC CTC lines may have been ascribed to the limited and scarce human samples procured at the time at relapse [Citation3]. A series of studies on their characteristics and molecular features have been published and furthermore, STR profiles, RNA sequences and whole genome Nanopore sequences have been obtained [Citation47]. The most distinguishing feature of all these SCLC CTC lines is the spontaneous formation of large spheroids, termed tumorospheres, under regular tissue culture conditions in vitro. These CTC cell lines were tumorigenic in NOD mice upon transfer of 0.05 – 0.5 million tumor cells at four different ventral sites suspended in extracellular matrix (ECM) within 1.5 months. The BHGc10 SCLC CTC line is shown after the recovery of the tumor tissue of the first passage in NOD mice and cultivation in tissue culture that resulted in the development of numerous tumorospheres and with dark necrotic cores in the largest spheroids ().
Figure 1. Light microscopic picture of BHG10 SCLC tumorospheres after passaging the cell line in NOD mice and subsequent cultivation in regular tissue culture. The attached cells form spheroids of different sizes, with the largest one beginning to show a darker necrotic core as found previously for 5 other BHGcX lines [Citation48].
![Figure 1. Light microscopic picture of BHG10 SCLC tumorospheres after passaging the cell line in NOD mice and subsequent cultivation in regular tissue culture. The attached cells form spheroids of different sizes, with the largest one beginning to show a darker necrotic core as found previously for 5 other BHGcX lines [Citation48].](/cms/asset/ca97c29b-5555-4657-bc18-dbbb1b8a38b2/ierx_a_2388288_f0001_oc.jpg)
5. Cellular chemoresistance: SCLC cells have no sweet spot
Despite high initial response to first line platinum-etoposide with or without immunotherapy, all patients eventually relapse within approximately 1 year. Second-line treatment options are limited for patients with SCLC [Citation49]. Topotecan has an unfavorable toxicity profile but is mostly used for second-line chemotherapy of SCLC, with CEV (cyclophosphamide, epirubicin, vincristine), lurbinectedin or diverse combinations of other drugs as alternatives [Citation50]. In a trial of 105 patients (B-005), lurbinectedin elicited an ORR of 35% and duration of response of 5.3 months with acceptable safety and, thus, was approved by the FDA [Citation51]. In a meta-analysis including 77 publications covering 6349 patients,70% of the publications reported low-/very-low-quality evidence due to the lack of randomization and small sample sizes [Citation49].
For the SCLC subtypes SCLC-A (ASCL1-high), SCLC-N (NEUROD1-high), SCLC-Y (YAP1-high), and SCLC-P (POU2F3-high) no clear association to chemosensitivity could be detected [Citation9,Citation13]. In vitro experiments testing over 500 drugs suggested SCLC-P cells to be sensitive and SCLC-N and SCLC-I resistant to cisplatin with variable responses for SCLC-A [Citation13]. Studies suggesting the involvement of increased drug efflux by MRP1 or P-gp with chemoresistance has not been substantiated in clinics [Citation52].
Comparison of the gene expression of SCLC lines before and after exposure to chemotherapeutics have identified DNA repair proteins such as PARP1, CHEK1, and others as therapeutic targets. This has been expected as consequence of the DNA Damage Response (DDR) triggered by the DNA-directed drugs. MYC-controlled genes correlated with resistance to EP and, in good correspondence, downregulation of MYC by PROTACS directed to MYC-controlling BRD4 bromodomain proteins suppressed the proliferation of SCLC cell lines [Citation53,Citation54]. Chemoresistance was induced in SCLC PDX tissues by passaging and repeated cycles of treatment with chemotherapeutics but no recurrent mutations were observed between for the resistant samples, although new mutations were detectable [Citation16] This finding may point to chemoresistance at the tumor physiological level in contrast to molecular alterations at the cellular level. SLFN11 (Schlafen Family Member 11) was one of the most down-regulated genes and reexpression of SLFN11 by inhibition of EZH2 increased sensitivity to topotecan [Citation16]. In a phase 1/2 trial involving 50 patients with relapsed SCLC, the combination of temozolomide with the PARP inhibitor olaparib revealed an ORR of 41.7%, a median PFS and OS of 4.2 and 8.5 months, respectively [Citation55]. Thomas et al. [Citation56] determined agents that enhance responses to topoisomerase I inhibitors and evaluated ATR inhibition with M6620 in combination with topotecan in patients resulting in some partial responses (NCT02487095). Analysis of chemoresistant SCLC cell lines and their parental controls demonstrated altered amino acid metabolism of arginine in the chemoresistant variants [Citation57]. Platinum compounds are inactivated by conjugation with glutathione (GSH) but depletion of GSH by of D,L-buthionine-S, R-sulphoximine (BSO) exhibited no clear effects on the cytotoxic activity of chemotherapeutic agents in SCLC [Citation58,Citation59]. The antiapoptotic Bcl was found overexpressed in 77% of SCLC specimens but clinical use of Bcl-2 inhibitors was largely ineffective [Citation60]. Novel targeted drugs, such as PARP inhibitors, delta-like canonical Notch ligand 3 (DLL3) monoclonal antibodies and toxic conjugates, as well as EZH2 inhibitors showed low activity in clinical trials [Citation46].
6. Cancer Stem Cell (CSC) hypothesis and SCLC
CSCs are regarded as a rare population of tumor stem cells, capable of self-renewal and multilinear differentiation that survive the killing of the bulk tumor cells by chemotherapy. SCLC CSCs have been hypothesized to be involved in tumorigenesis, treatment resistance, and dissemination via epithelial-mesenchymal transition (EMT) [Citation61]. Treatments such as chemoradiotherapy kill most SCLC cells resulting in rapid tumor shrinkage; however, residual resistant CD133+ SCLC reconstitute the tumor as leading cause of tumor relapses [Citation62]. Like normal stem cells, SCLC CSCs are thought to penetrate the basement membrane after EMT transformation, intravasate into the bloodstream as CTCs and metastasize [Citation61]. Expression of Nanog, OCT4, and SOX2 in SCLC are believed to be associated with CSC phenotype, although SOX2 is also found in NSCLC [Citation63]. Further common CSC markers comprise aldehyde-dehydrogenase(ALDH), CD133, CD44, CD24, and EpCAM [Citation64]. Identification of tumor cells as stem cells solely by expression of these molecules is no sufficient proof. Xenografts in immunodeficient mice are the gold standard to demonstrate the tumorigenic properties of enriched CSC population. However, the critical proof of a distinct subpopulation of SCLC cells which exhibit increased tumorgenicity in immunocompromised mice is lacking so far. Nevertheless, SCLC is recognized as a prototype for CSC research due to its multilinear neuroendocrine nature and high dissemination capacity [Citation65]. For SCLC, an attempt to increase the CSC capabilities of SCLC cells by transplanting cell line spheroids into mice has been reported by [Citation66]. The transplantation of 3D SCLC H69 cells into mice led to a significant increase in tumor-formation capacity along with elevated expression of CSC markers. The H69 cell line has been established in 1980 [Citation67]. Although the tumor volume of H69 spheroids was larger in the 3D environment, necrosis inside the aggregates has not been observed, indicating that using this H69 cells no dense 3D structures with internal gradients truly mimicking tumor tissue have formed.
While similar evidence was found for many cancer types, the CSC model has since been
challenged as well. All characteristics of CSCs, such as a rarity, specific surface antigens, tumorigenicity in immunocompromised mice, differentiation, self-renewal and chemoresistance, constitute no definite proof of CSCs [Citation68]. Additionally, xenotransplantation, the gold standard for the in vivo testing of enriched CSCs has revealed markedly varying percentages among tumor entities [Citation69]. A meta-analysis evaluated the empirical support for the CSC hypothesis and found that it amounted only to 49.0% in favor of CSCs [Citation70]. Furthermore, the conception that putative CSCs are a rare subset of tumor cells could not be confirmed by most studies (13.5% support). The empirical support of CSCs varied also between types of cancer, animal models and cell isolation method used.
7. SCLC and Tumorospheres
Since blood samples are in most cases the single clinical specimen available from patients with advanced and relapsed SCLC, we tried to cultivate CTCs and succeeded for the first two cell lines, termed BHGc7 and BHGc10 [Citation71]. Cells first grow as attached cell patches but then large spheroids began to form under regular tissue culture conditions without any sphere-forming manipulations. Additional patients and blood samples led to the permanent cultivation of three new independent CTC cell lines, termed BHGc16, BHGc26 and BHGc27 exhibiting similar growth characteristics with generation of unenforced SCLC spheroids, termed tumorospheres [Citation48,Citation49]. These 3D structures grow up to 1-2 mm in size. The CTC cell lines exhibit the typical SCLC markers (NCAM, CHGA and Enolase-2). Comparison of CTC lines single cell suspensions and tumorospheres demonstrated significantly higher chemoresistance of the 3D spheroids against cisplatin, etoposide, doxorubicin and topotecan [Citation48]. Tumorospheres of the five CTC lines established so far were fixated, embedded and tissue sections used to study the interior structure. Immunohistochemical staining revealed positivity for the proliferation marker Ki67 for the outer cell layers of the spheroids, with positivity for the hypoxia marker carbonic anhydrase 9 (CAIX) for the inner layers, followed by necrotic core regions. Thus, staining of tumorosphere sections revealed proliferating cells at the rim, followed by quiescent and hypoxic cells, respectively. Therefore, universal chemoresistance of relapsing SCLC seems to rely on formation of large tumorospheres that limit drug penetration and contain cells with a lower growth fractions and a hypoxic phenotype. SCLC seems to represent a peculiar model to study the association of CTCs, metastasis and drug resistance. The SCLC CTCs established from relapsed patients were shown to lack a typical CSC phenotype in respect to surface markers, expression of pluripotent stem cell and transcription factors as well as lack of chemoresistance to CSC-selective drugs [Citation72]. Successively we have obtained 9 permanent SCLC CTC cell lines (BHGc7, BHGc10, BHGc16, BHGc26, BHGc27, BHGc50, BHGc59, BHGc71, and UHGc5) of blood samples of distinct patients exhibiting similar characteristics and spontaneous formation of tumorospheres [Citation73]. Still, most of the 3D models in vitro lack characteristics of complex tumor tissues and have not been validated for their adequacy to select clinically useful drugs [Citation74]. Patient-derived spheroids from blood, namely tumorospheres of CTCs are 3D spheroid models most similar to patient’s tumors [Citation74].
In one case of a ROVA-T-treated patient, two pleural-derived cell lines have been established and the BHGc59 CTC cell line were set up at the same time point at which the first pleural effusion was obtained, thus establishing the first M1 metastasis – CTC cell line pair [Citation46]. The chemosensitivity assays demonstrate that most SCLC lines show IC50 values exceeding the ROVA-T in vivo concentrations and that slow-growing cells and lines showing spheroidal growth as CTCs exhibit high resistance. Chemosensitivity of the cell lines is not correlated with the DLL3 protein expression most likely due to the toxic effect of the released payload in tissue culture. The CTC cell line BHGc50 was established from an NSCLC patient, obviously representing an NSCLC-SCLC transformation [Citation76]. CTCs are competent to specifically manipulate TAMs to increase invasiveness, angiogenesis, immunosuppression and possibly lipid catabolism [Citation76,Citation77]. SCLC CTCs express MMP-9 and a range of cathepsins for proteolysis [Citation78]. CTC lines express EpCAM and lack a full-blown vimentin-positive epithelial-mesenchymal transition (EMT) phenotype [Citation47]. Upon formation of CTC tumorospheres the expression of EpCAM is upregulated and seems to have a key role in cell-cell adhesion. Proteins such as E-Cadherin, p27 KIP1, Progranulin, BXclx, Galectin-3, and Survivin showed variable alterations during formation of spheroids for the distinct SCLC CTC cell lines. In conclusion, EpCAM presents the most critical marker for the formation of SCLC CTCs chemoresistant tumorospheres. The shedding of CTCs is most likely via irregular and leaky tumor vessels or in case of SCLC, by vessels formed by vasculogenic mimicry. Therefore, the lower microvessel densities (MVD) in NSCLC can be held responsible for the relative rarity of CTCs in NSCLC and the extremely high counts in SCLC [Citation45].
8. 3D Spheroids and solid tumors
2D cell culture models are not able to mimic truly the characteristics of in vivo solid tumors and their chemoresistance [Citation79]. The 3D architecture better resembles the cellular organization, proliferation and differentiation of the cancer cells. Therefore, 3D models have been established in tissue culture to assess chemosensitivity and biomarkers of resistance [Citation74]. There are different techniques to enforce a 3D growth of cancer cells in vitro. In scaffold‐based approaches, 3D cell cultures are obtained by cultivating the cells on artificial 3D supports, where the cells attach and fill the interstices within the matrix [Citation80]. For these approaches, acellular artificial matrices are used or the cells are embedded in a hydrogel often followed by crosslinking of the matrix [Citation80,Citation81]. ECM is produced by the cells or can be supplied in form of Matrigel® [Citation82]. In scaffold-free approaches, 3D cellular aggregates are generated as spheroids, in which ECM is produced by the cells themselves [Citation80]. There are four major techniques for promoting the spheroid formation that rely either on the inhibition of cells adhesion to surfaces by agitation, in hanging drop or liquid overlay cultures or on growth on microfluidic chips. Spheroids reproduce features of solid tumors including growth kinetics, gradients of oxygen, nutrients and waste, as well as the presence of proliferative, senescent and necrotic cells [Citation83,Citation84]. In particular, large spheroids (>500 µm in diameter) are also capable to emulate tumor evasion mechanisms in response to chemotherapeutics as found in solid tumors [Citation85].
In tumors tissue, cell–ECM and cell–cell interactions form a physical barrier that limits the penetration of the drugs inside the malignancy [Citation86,Citation87]. Increased interstitial fluid pressure (IFP) in tumors further hinders the penetration of pharmaceuticals. Drug penetration is often heterogeneous within a tumor lesion and at the cellular level, sanctuary sites may result in insufficient drug concentrations in relation to the required therapeutic dose [Citation88]. Assessment of systemic exposure can be used to select a dose with the highest probability to achieve maximal receptor occupancy in the tumor [Citation89]. Biomarkers that reflect downstream drug effects can also be assessed through target binding at the effector site [Citation90]. The limited drug penetration by the physical barrier has been shown in spheroids [Citation91,Citation92]. For example, in neuroblastoma spheroids, penetration of doxorubicin is limited to the layers within approximately 70 µm distance of the surface of the spheroids. Doxorubicin showed increased penetration in spheroids of smaller sizes compared to spheroids with diameters between 450 - 500 μm [Citation93]. In spheroids, cells in the external layer have a high access to oxygen whereas those in the interior (100 μm apart from the surface) are hypoxic and upregulate the expression of hypoxia‐inducible factor HIF1α [Citation87]. In MCF‐7 breast spheroids this factor caused upregulation P-gp and reduced the accumulation of doxorubicin [Citation94]. Additionally, HIF‐1 promotes the expression of antiapoptotic factors, such as Bak, Bax, Bcl‐xL, Bcl‐2, Bid, Mcl‐1 and NF‐κB [Citation95].
Drugs, such as 5‐fluorouracil, cisplatin, doxorubicin, and irinotecan need oxygen for their anticancer effect that relies on reactive oxygen species (ROS) that damage DNA [Citation96]. Also, hypoxic cancer cells from the core of spheroids are typically more radioresistant and the increased lactate production also promotes the acidification of its core [Citation97]. The cellular uptake of weak basic drugs such as doxorubicin, mitoxantrone, vincristine, vinblastine, anthraquinones, and vinca alkaloids is reduced through protonation that impairs the transport of charged drugs through tissues [Citation98]. In combination with hypoxia, acidic pH and lack of nutrients a cellular dormant state is induced in spheroids a dormant state is induced on cells present in spheroids [Citation99]. This quiescent state of cells within spheroids causes a resistance to drugs such as carboplatin, cisplatin, doxorubicin, oxaliplatin, methotrexate, and paclitaxel that require proliferating cells [Citation100].
9. Spheroid-directed therapies
The factors contributing to the chemoresistance of spheroids have been summarized in the chapter above. These mechanisms hold true for the tumorospheres that present the real circulating metastasis-inducing cancer cells in patients with relapsed SCLC. All 5 CTC lines in form of tumorospheres exhibited significantly higher chemoresistance to all 4 chemotherapeutics in use for the treatment of SCLC compared to the same cells employed as single cell suspensions. The mean increase in chemoresistance was approximately 5-fold for cisplatin, 4.5-fold for etoposide, 9-fold for topotecan for all CTCs, and approximately 7-50-fold for epirubicin, respectively. This increased resistance to the chemotherapeutics is sufficient to leave the tumorospheres intact. Furthermore, The SCLC CTC lines revealed layers of cellular fragments with a range of decreasing sizes compared to intact cells (approximately 12 µm) down to small debris (approximately 2 µm) which are not detectable in permanent SCLC lines [Citation101]. Chemosensitivity tests employing SCLC and SCLC CTC lines with chemotherapeutics used in therapy of SCLC demonstrated an inhibitory activity of the released cell fragments on the resulting cytotoxicity and possible immunotherapy.
SCLC CTCs are functional in the dissemination of the tumor cells and possibly in form of tumorospheres for the chemoresistant survival as assembly of quiescent and hypoxic tumor cells. How to attack tumor spheroids and tumorospheres in special is unclear but a range of possible approaches has been reported in the literature. The nine SCLC CTC lines were compared for their expression of 84 proteins associated with cancer either as single cells or in the form of tumorospheres [Citation42]. With exception of one line, all other CTC lines expressed EpCAM and lacked an EpCAM-negative, vimentin-positive epithelial-mesenchymal transition (EMT) phenotype. Upon formation of tumorospheres, the expression of EpCAM, that mediates cell-cell adhesion, was found to be markedly upregulated, most likely presenting a suitable target.
EpCAM (or CD326A) is a differentiation antigen expressed universally by epithelial tissues that regulates cell signaling, proliferation, differentiation, and migration [Citation102]. Additionally, EpCAM represents a tumor antigen of various human carcinomas and suppresses antitumor immunity [Citation103,Citation104]. Overexpression of EpCAM is linke to invasion, metastasis, and poor prognosis in many cancers, including breast cancer [Citation105–107]. EpCAM affects the Wnt pathway and activates genes involved in cell cycle regulation like c-MYC, cyclines A and E [Citation106]. Gao et al. found that knockdown of EpCAM repressed the malignant behavior of cancer cells and application of humanized anti-EpCAM antibodies has resulted in successful targeting of positive cell lines and prevention of relapse [Citation108,Citation109]. Catumaxomab (Removab) is a trifunctional bispecific antibody targeting human EpCAM and CD3 with further binding to Fcγ receptor type I, IIa and III [Citation110]. This antibody effected an antitumor response against tumor spheroids of EpCAM-positive FaDu tumor cells and exhibited synergism with cisplatin. Thus, EpCAM-directed agents may help to eliminate tumorospheres in SCLC patients and this therapy may be applied as neoadjuvant approach instead waiting for the invariable relapse after first-line therapy.
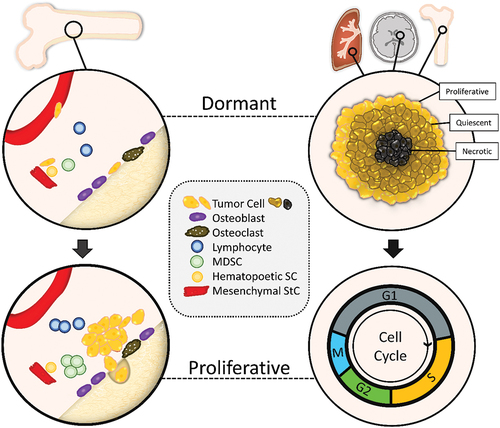
Breast cancer cells (left) and SCLC tumor cells (right) proliferate and generate secondary lesions in distinct organs (). The disseminated breast cancer cells may reside dormant in hypoxic regions of the bone and SCLC CTCs form tumorospheres with hypoxic regions in the periphery and as metastatic lesions in lung, brain, and bone, respectively. Due to lack of oxygen and nutrients in the interior of these spheroids, cells are quiescent with low proliferation and survive exposure to chemotherapeutics. After chemotherapy, the dormant or protected tumor cells are activated, enter the proliferative cell cycle and cause relapses. In tumorospheres this kind of dormancy is phenotypically like putative CSCs and dormant disseminated tumor cells. The tumorospheres may eventually disintegrate and release proliferative tumor cells that cause relapses.
The cytostatic drugs applied in SCLC target proliferating cells and leave dormant/quiescent cancer cell of tumorospheres intact as a key mechanism of resistance. Compounds detected in a screen for spheroid-damaging agents were inhibitors of the respiratory chain thatexhibited induction of cell death in inner spheroid regions by impairing extracellular glucose concentrations, such as metformin [Citation98]. Sodium-potassium exchangers have been proposed for the disintegration of CTC clusters but agents such as ouabain have no specific mode of action but are highly toxic for the tumor cells (unpublished observation) [Citation111].
Novel BET-directed Proteolysis Targeting Chimeras (PROTACs) show high antiproliferative activity in SCLC [Citation112]. Particularly compounds such as ARV825, targeting specifically BRD4, exhibits superior cytotoxic effects on SCLC cell lines and may become a valuable adjunct to SCLC combination chemotherapy. For the therapy of 3D aggregates, activated allogeneic Natural Killer (NK) cells were demonstrated to enter tumor spheroids and to show cytotoxicity against pediatric cancer cells [Citation113]. These effects of NK cells were elevated in combination with apoptosis-inducing BH3 mimetics that act as antagonists of the anti-apoptotic BCL-2 family members. Furthermore, oxygen-generating manganese dioxide nanoparticles were employed to reverse the immunosuppressive hypoxic conditions in spheroids [Citation114]. For the increased cell–cell contacts in spheroids, E‐cadherins play a key role in tumor cells to provide strong cohesiveness and to act as barrier for the penetration of cytotoxic drugs [Citation115,Citation116]. A competitive inhibitor of E‐cadherins, ADH‐1 (Exherin™), has been tested in clinical trials to treat solid tumors achieving disease control [Citation117]. The universal chemoresistance of SCLC seem to be related to physiological resistance at the tumor tissue level and presence of large spheroids that enclose quiescent and hypoxic tumor cells in regions with poor access of chemotherapeutics. This type of chemoresistance is difficult to overcome and impairs therapeutic interventions directed to individual tumor cells [Citation118].
10. Expert opinion
SCLC has been termed the “graveyard of pharmacology” due to the inability to find drugs that could significantly improve survival for more than a few weeks or months. Therapy has not changed much for the last decades, with the exception of the addition of immunotherapy in form of checkpoint inhibitors that achieve a marginal extension of OS in selected patients. Despite an extreme smoking-associated mutation rate, SCLC is distinguished by producing a range of factors detrimental to immunotherapy. The mechanisms of chemoresistance in relapsed SCLC patients are not clear and, thus, progress is still thought to capitalize on the essential similar cytotoxic drugs that have failed in the past. However, one can still administer DNA-damaging compounds and hope for a better outcome. Experimentation using SCLC cell lines and PDX/CTX models failed to identify recurrent and targetable mutations at the cellular level. Although a lot of investigations have been invested to characterize the genomics of SCLC, few actionable targets were described and agents directed to these unique characteristics fared poor in clinical trials. Simply, the molecular biology of the SCLC cells and the complex interactions of proteins that confer metastatic and resistant properties are difficult to be deduced from DNA/RNA data. Lack of suitable tumor specimen is held responsible for the lack of progress. Since an extremely wide range of chemical unrelated drugs exhibited low activity in SCLC, the assumption that SCLC cells possess manifold different mechanisms of chemoresistance is not realistic. Comparison of tumor specimen from primary and relapsed patients did not reveal reproducible and recurrent alterations, such pointing either to complex changes or lack of significant changes at the cellular level. Most SCLC cell lines available are derived from bulk tumors and are likely to have lost specific differentiated features of native SCLCs. In the 1990ties groups led by Tannock, Sutherland, Kerbel and others have pioneered the chemoresistance derived from cell association and spheroids caused by cell-cell adhesion and multicellular effects. With the advent of DNA/RNA sequencing, the underlying mechanisms of chemoresistance were searched at the gene expression level and the level of cellular organization in tumor resistance became somewhat neglected. Many research groups reported adverse intratumor conditions, such as increased interstitial pressure, hypoxic and acidic conditions resulting in lower drug efficacy and penetration. A rapidly proliferation tumor such as SCLC seem to present a prototype of a cancer containing hypoxic and acidic regions poorly amenable to chemotherapy. A panel of SCLC CTC cell lines established in our lab seem to represent the currently best available live samples from relapsed patients. Surprisingly SCLC is competent to provide a protected region for the highly active dissemination process. All of these lines exhibit unsolicited generation of tumorospheres, large spheroids, conferring high chemoresistance that may have been present early in tumor development and metastasis. Tumor cells in the interior of these spheroids are protected as quiescent and hypoxic cells against chemotherapy and irradiation as well. Weeding out rapidly proliferating SCLC cells by DNA-damaging agents leaves the tumor cells protected by tumorospheres intact. Several approaches have been developed to kill cells in spheroids but few have reached the clinical trial stage. However, after decades of unfruitful attempts to really prolong the life of SCLC patients, there seems time to try novel ways of treating patients, not at least to spare patients from highly toxic and ineffective trials. Tackling tumorospheres seem to direct a novel way for overcoming chemoresistance in SCLC victims.
Article highlights
| - | Small-cell Lung Cancer has a dismal prognosis due to acquired chemoresistance | ||||
| - | Resistance at the cellular level cannot account for the marked refractoriness | ||||
| - | Proof of Cancer Stem Cells in SCLC is largely lacking | ||||
| - | Scarcity of tumor specimen from relapsed tumor hampers research | ||||
| - | A panel of nine SCLC circulating tumor cell lines (CTCs) represent actual metastasis-inducing cells of the metastatic state | ||||
| - | These SCLC CTC lines form large spheroids spontaneously (tumorospheres) | ||||
| - | The tumorospheres provide a physical barrier und contain resistant quiescent and hypoxic cancer cells | ||||
| - | Chemoresistance of relapsed SCLC patients seems to be related to EpCAM-positive 3D aggregations of the cancer cells | ||||
| - | Approaches to tackle tumor spheroids are in an early preclinical/clinical state | ||||
Abbreviations
| 2D | = | Two-dimensional |
| 3D | = | Three-dimensional |
| ABCC1 | = | ATP Binding Cassette Subfamily C Member 1 |
| ABT-737 | = | Bcl-2 inhibitior |
| ALDH | = | aldehyde-dehydrogenase |
| ASCL1 | = | achaete-scute homolog 1 |
| ATR | = | Ataxia Telangiectasia And Rad3-Related Protein |
| Bak1 | = | BCL2 Antagonist/Killer 1 |
| BAMBI | = | BMP And Activin Membrane Bound Inhibitor |
| Bax | = | BCL2 Associated X, Apoptosis Regulator |
| BC | = | breast cancer |
| Bcl-2 | = | B-cell lymphoma 2 |
| BET | = | Bromodomain and Extra-Terminal Domain |
| Bid | = | Apoptic Death Agonist |
| BMP | = | Bone Morphogenetic Protein |
| BRAF | = | V-Raf Murine Sarcoma Viral Oncogene Homolog B |
| BRD4 | = | Bromodomain-containing protein 4 |
| BRD4 | = | Bromodomain Containing 4 |
| BSO | = | D,L-buthionine-S, R-sulphoximine |
| CAIX | = | carbonic anhydrase 9 |
| CDX | = | cancer-derived xenograft |
| CEV | = | cyclophosphamide-epirubicin-vincristine |
| CHEK1 | = | Checkpoint Kinase 1 |
| CHGA | = | Chromogranin A |
| CSC | = | Cancer Stem Cell |
| CTC | = | circulating tumor cell |
| DKK | = | Dickkopf WNT Signaling Pathway Inhibitor |
| DLL3 | = | delta-like canonical Notch ligand 3 |
| DLL3 | = | Delta-like-Protein 3 |
| DNA | = | Desoxyribonuclei acid |
| ECM | = | Extracellular Matrix |
| EMT | = | epithelial-mesenchymal transition |
| EP | = | etopside platinum |
| EpCAM | = | epithelial cell adhesion molecule |
| ES | = | extensive stage |
| ES | = | Ewing sarcoma |
| EZH2 | = | Enhancer Of Zeste 2 Polycomb Repressive Complex 2 Subunit |
| FDA | = | Food and Drug Administration |
| FHL2 | = | Four And A Half LIM Domains Protein 2 |
| GSH | = | Glutathione |
| HIF1α | = | hypoxia‐inducible factor 1α |
| HLA | = | human leukocyte antigen |
| HSP90 | = | heat shock protein 90 |
| ICI | = | checkpoint inhibitor |
| IFP | = | interstitial fluid pressure |
| LDCT | = | low-dose computed tomography |
| Lef-1 | = | Lymphoid enhancer-binding factor-1 |
| LS | = | limited stage |
| Mcl‐1 | = | Induced Myeloid Leukemia Cell Differentiation Protein |
| MCTS | = | multicellular tumor spheroids |
| MMP-9 | = | Matrix metalloproteinase-9 |
| MRP1 | = | Multidrug resistance protein 1 |
| mTOR | = | Mechanistic Target Of Rapamycin Kinase |
| MVD | = | microvessel densities |
| MYC | = | Avian Myelocytomatosis Viral Oncogene Homolog |
| MYCL | = | Myc-Related Gene From Lung Cancer |
| MYCN | = | V-Myc Avian Myelocytomatosis Viral Oncogene Neuroblastoma Derived Homolog |
| Nanog | = | Homeobox Transcription Factor Nanog |
| NCAM | = | Neural Cell Adhesion Molecule |
| NEUROD1 | = | neurogenic differentiation 1 |
| NF‐κB | = | Nuclear Factor Of Kappa Light Polypeptide Gene Enhancer In B-Cells |
| NK | = | Natural Killer |
| NOD mice | = | Non-Obese Diabetic mouse |
| NSCLC | = | non small-cell lung cancer |
| OCT4 | = | octamer binding trascription factor 4 |
| ORR | = | overall response rate |
| OS | = | overall survival |
| PARP | = | Poly(ADP-Ribose) Polymerase |
| PARP1 | = | Poly(ADP-Ribose) Polymerase 1 |
| PDL1 | = | programmed cell death ligand-1 |
| PDX | = | patient-derived xenograft |
| PFS | = | Progression Free survival |
| P-gp | = | P-Glykoprotein |
| PIK3CA | = | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase |
| POU2F3 | = | POU domain, class 2, transcription factor 3 |
| PROTAC | = | Proteolysis targeting chimera |
| PTEN | = | Phosphatase And Tensin Homolog |
| RB1 | = | Protein Phosphatase 1 |
| RNA | = | ribonucleic acid |
| ROS | = | reactive oxygen species |
| ROVA-T | = | Rovalpituzumab tesirine |
| SCLC | = | small-cell lung cancer |
| SLFN11 | = | Schlafen Family Member 11 |
| SLIT2 | = | Slit Guidance Ligand 2 |
| SOX2 | = | Sex Determining Region Y-Box 2 |
| STR | = | short tandem repeat |
| TAM | = | Tumor-Associated Macrophage |
| TCGA | = | The Cancer Genome Atlas |
| TMB | = | tumor mutational burden |
| TP53 | = | Tumor Protein P53 |
| VALSG | = | Veteran’s administation lung study group |
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
REFERENCES
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660
- Herzog BH, Devarakonda S, Govindan R Overcoming Chemotherapy Resistance in SCLC. J Thorac Oncol. 2021 Dec;16(12):2002–2015. doi: 10.1016/j.jtho.2021.07.018
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006 Oct 1;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859
- Gazdar AF. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019 May;19(5):289–297. doi: 10.1038/s41568-019-0133-9.
- Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012 May 10;30(14):1692–1698. doi: 10.1200/JCO.2011.40.4905
- McNamee N, da Silva IP, Nagrial A, et al. Small-Cell Lung Cancer-An Update on Targeted and Immunotherapies. Int J Mol Sci. 2023 May 1;24(9):8129. doi: 10.3390/ijms24098129
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7–33. doi: 10.3322/caac.21708
- Rudin CM, Poirier JT, Byers LA, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019 May;19(5):289–297. doi: 10.1038/s41568-019-0133-9
- Silva M, Galeone C, Sverzellati N, et al. Screening with Low-Dose Computed Tomography Does Not Improve Survival of Small Cell Lung Cancer. J Thorac Oncol. 2016 Feb;11(2):187–193. doi: 10.1016/j.jtho.2015.10.014
- Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7(1):69–79. doi: 10.21037/tlcr.2018.01.16
- Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol. 1985;3(11):1471–1477. doi: 10.1200/JCO.1985.3.11.1471
- Gay CM, Stewart CA, Park EM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021 Mar 8;39(3):346–360.e7. doi: 10.1016/j.ccell.2020.12.014
- Chen P, Kuang P, Wang L, et al. Mechanisms of drugs-resistance in small cell lung cancer: DNA-related, RNA-related, apoptosis-related, drug accumulation and metabolism procedure. Transl Lung Cancer Res. 2020 Jun;9(3):768–786. doi: 10.21037/tlcr-19-547
- Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11(1):78–98. doi: 10.6004/jnccn.2013.0011
- Gardner EE, Lok BH, Schneeberger VE, et al. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 axis. Cancer Cell. 2017;31(2):286–299. doi: 10.1016/j.ccell.2017.01.006
- Chouaïd C, Baize N, Monnet I Second-line therapy for disseminated small-cell lung cancer: optimal management remains to be defined. Transl Lung Cancer Res. 2020 Oct;9(5):1732–1735. doi: 10.21037/tlcr-20-362.
- Zugazagoitia J, Paz-Ares L Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J Clin Oncol. 2022 Feb 20;40(6):671–680. doi: 10.1200/JCO.21.01881.
- Gomez-Randulfe I, Leporati R, Gupta B, et al. Recent advances and future strategies in first-line treatment of ES-SCLC. Eur J Cancer. 2024 Jan 29;200:113581. doi: 10.1016/j.ejca.2024.113581
- Hamilton G, Rath B Immunotherapy for small cell lung cancer: mechanisms of resistance. Expert Opin Biol Ther. 2019 May;19(5):423–432. doi: 10.1080/14712598.2019.1592155
- Barrows ED, Blackburn MJ, Liu SV Evolving role of immunotherapy in small cell lung cancer. Semin Cancer Biol. 2022 Nov;86(Pt 3):868–874. doi: 10.1016/j.semcancer.2022.02.021
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018 May 31;378(22):2078–2092. doi: 10.1056/NEJMoa1801005
- Giunta EF, Addeo A, Rizzo A, et al. First-Line Treatment for Advanced SCLC: What Is Left Behind and Beyond Chemoimmunotherapy. Front Med (Lausanne). 2022;9:924853. doi: 10.3389/fmed.2022.924853.
- Spigel DR, Cheng Y, Cho BC, et al: ADRIATIC: Durvalumab as consolidation treatment for patients with limited-stage small-cell lung cancer. 2024 ASCO Annual Meeting. Abstract LBA5. Presented June 2, 2024
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012 Oct;44(10):1111–1116. doi: 10.1038/ng.2405
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015 Aug 6;524(7563):47–53. doi: 10.1038/nature14664
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012 Oct;44(10):1104–1110. doi: 10.1038/ng.2396
- Lázaro S, Pérez-Crespo M, Enguita AB, et al. Ablating all three retinoblastoma family members in mouse lung leads to neuroendocrine tumor formation. Oncotarget. 2017 Jan 17;8(3):4373–4386. doi: 10.18632/oncotarget.13875
- Mollaoglu G, Guthrie MR, Böhm S, et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell. 2017;31(2):270–285. doi: 10.1016/j.ccell.2016.12.005
- Sos ML, Dietlein F, Peifer M, et al. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):17034–17039. doi: 10.1073/pnas.1207310109
- Borromeo MD, Savage TK, Kollipara RK, et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep. 2016 Aug 2;16(5):1259–1272. doi: 10.1016/j.celrep.2016.06.081
- Fiorentino FP, Tokgün E, Solé-Sánchez S, et al. Growth suppression by MYC inhibition in small cell lung cancer cells with TP53 and RB1 inactivation. Oncotarget. 2016 May 24;7(21):31014–31028. doi: 10.18632/oncotarget.8826.
- Stickler S, Eggerstorfer M-T, Hochmair M, et al. Implementation of functional precision medicine for Non-Small Lung Cancer. Explor Med. In press.
- Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017 May 2;7(1):1339. doi: 10.1038/s41598-017-01571-0
- Gillet JP, Calcagno AM, Varma S, et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18708–18713. doi: 10.1073/pnas.1111840108
- Liu X, Sun Q, Wang Q, et al. Epithelial Cells in 2D and 3D Cultures Exhibit Large Differences in Higher-order Genomic Interactions. Genomics, Proteomics & Bioinformatics. 2022 Feb;20(1):101–109. doi: 10.1016/j.gpb.2020.06.017
- Lee JK, Sivakumar S, Schrock AB, et al. Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-world outcomes in solid tumors. NPJ Precis Oncol. 2022 Dec 9;6(1):91. doi: 10.1038/s41698-022-00334-z.
- Dentro SC, Leshchiner I, Haase K, et al. PCAWG Evolution and Heterogeneity Working Group and the PCAWG Consortium. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell. 2021 Apr 15;184(8):2239–2254.e39. doi: 10.1016/j.cell.2021.03.009.
- Vidhyasagar V, Haq S UI, Lok BH. Patient-derived Xenograft Models of Small Cell Lung Cancer for Therapeutic Development. Clinic Oncol. 2020;32(10):619– 625. Doi: 1 0.1016/j.clon.2020.05.017
- Hernandez MC, Leiting JL, Joshi VB, et al. Patient-derived xenografts in surgical oncology: A short research review. Surgery. 2020 Dec;168(6):1021–1025. doi: 10.1016/j.surg.2020.07.031.
- Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014 Aug;20(8):897–903. doi: 10.1038/nm.3600.
- Ben-David U, Ha G, Tseng YY, et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat Genet. 2017 Nov;49(11):1567–1575. doi: 10.1038/ng.3967.
- Hann CL, Daniel VC, Sugar EA, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008 Apr 1;68(7):2321–2328. doi: 10.1158/0008-5472.CAN-07-5031.
- Gaponova AV, Nikonova AS, Deneka A, Kopp MC, et al. A Novel HSP90 Inhibitor-Drug Conjugate to SN38 Is Highly Effective in Small Cell Lung Cancer. Clin Cancer Res. 2016 Oct 15;22(20):5120–5129. doi: 10.1158/1078-0432.CCR-15-3068.
- Hamilton G, Rath B, Stickler S Significance of circulating tumor cells in lung cancer: a narrative review. Transl Lung Cancer Res. 2023 Apr 28;12(4):877–894. doi: 10.21037/tlcr-22-712
- Rath B, Plangger A, Krenbek D, et al. Rovalpituzumab tesirine resistance: analysis of a corresponding small cell lung cancer and circulating tumor cell line pair. Anticancer Drugs. 2022 Mar 1;33(3):300–307. doi: 10.1097/CAD.0000000000001267
- Stickler S, Rath B, Hochmair M, et al. Changes of protein expression during tumorosphere formation of small cell lung cancer circulating tumor cells. Oncol Res. 2023 Mar;31(1):13–22. doi: 10.32604/or.2022.027281
- Klameth L, Rath B, Hochmaier M, et al. Small cell lung cancer: model of circulating tumor cell tumorospheres in chemoresistance. Sci Rep. 2017 Jul 13;7(1):5337. doi: 10.1038/s41598-017-05562-z
- Bernabé-Caro R, Chen Y, Dowlati A, et al. Current and Emerging Treatment Options for Patients With Relapsed Small-cell Lung Carcinoma: A Systematic Literature Review. Clin Lung Cancer. 2023 May;24(3):185–208. doi: 10.1016/j.cllc.2023.01.012
- Aix SP, Ciuleanu TE, Navarro A, et al. Combination lurbinectedin and doxorubicin versus physician’s choice of chemotherapy in patients with relapsed small-cell lung cancer (ATLANTIS): a multicentre, randomised, open-label, phase 3 trial. Lancet Respir Med. 2023 Jan;11(1):74–86. doi: 10.1016/S2213-2600(22)00309-5
- Singh S, Jaigirdar AA, Mulkey F, et al. FDA Approval Summary: Lurbinectedin for the Treatment of Metastatic Small Cell Lung Cancer. Clin Cancer Res. 2021 May 1;27(9):2378–2382. doi: 10.1158/1078-0432.CCR-20-3901
- Triller N, Korosec P, Kern I, et al. Multidrug resistance in small cell lung cancer: expression of P-glycoprotein, multidrug resistance protein 1 and lung resistance protein in chemo-naive patients and in relapsed disease. Lung Cancer. 2006 Nov;54(2):235–240. doi: 10.1016/j.lungcan.2006.06.019.
- Drapkin BJ, George J, Christensen CL, et al. Genomic and Functional Fidelity of Small Cell Lung Cancer Patient-Derived Xenografts. Cancer Discov. 2018 May;8(5):600–615. doi: 10.1158/2159-8290.CD-17-0935
- Hamilton G, Stickler S, Rath B. Bromodomain protein-directed agents and MYC in Small Cell Lung Cancer. Curr Cancer Drug Targets. 2024; in press.
- Farago AF, Yeap BY, Stanzione M, et al. Combination Olaparib and Temozolomide in Relapsed Small-Cell Lung Cancer. Cancer Discov. 2019 Oct;9(10):1372–1387. doi: 10.1158/2159-8290.CD-19-0582
- Thomas A, Takahashi N, Rajapakse VN, et al. Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress. Cancer Cell. 2021;39(4):566–579.e7. doi: 10.1016/j.ccell.2021.02.014
- Chalishazar MD, Wait SJ, Huang F, et al. MYC-driven small-cell lung cancer is metabolically distinct and vulnerable to arginine depletion. Clin Cancer Res. 2019;25(16):5107–5121. doi: 10.1158/1078-0432.CCR-18-4140
- Meijer C, Mulder NH, Hospers GA, et al. The role of glutathione in resistance to cisplatin in a human small cell lung cancer cell line. Br J Cancer. 1990;62(1):72–77. doi: 10.1038/bjc.1990.232
- Campling BG, Baer K, Baker HM, et al. Do glutathione and related enzymes play a role in drug resistance in small cell lung cancer cell lines? Br J Cancer. 1993;68(2):327–335. doi: 10.1038/bjc.1993.336
- Stefanaki K, Rontogiannis D, Vamvouka C, et al. Immunohistochemical detection of bcl2, p53, mdm2 and p21/waf1 proteins in small-cell lung carcinomas. Anticancer Res. 1998 May-Jun;18(3A):1689–1695.
- Pantazaka E, Vardas V, Roumeliotou A, et al. Clinical Relevance of Mesenchymal- and Stem-Associated Phenotypes in Circulating Tumor Cells Isolated from Lung Cancer Patients. Cancers (Basel). 2021 Apr 29;13(9):2158. doi: 10.3390/cancers13092158
- Sarvi A, Mackinnon A, Avlonitis N, et al. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist, Cancer Res. 2014;74(5):1554–1565.doi: 10.1158/0008-5472.CAN-13-1541
- Sodja E, Rijavec M, Koren A, et al. The prognostic value of whole blood SOX2, NANOG and OCT4 mRNA expression in advanced small-cell lung cancer. Radiol Oncol. 2016 Apr 23;50(2):188–196. doi: 10.1515/raon-2015-0027
- Gires O Lessons from common markers of tumor-initiating cells in solid cancers. Cell Mol Life Sci. 2011 Dec;68(24):4009–4022. doi: 10.1007/s00018-011-0772-9
- Obermayr E, Koppensteiner N, Heinzl N, et al. Cancer Stem Cell-Like Circulating Tumor Cells Are Prognostic in Non-Small Cell Lung Cancer. J Pers Med. 2021 Nov 18;11(11):1225. doi: 10.3390/jpm11111225.
- Jung YH, Kim IK, Eom SY, et al. Establishment of a Small-cell Lung Cancer (SCLC) Mouse Model Using Enhanced Cancer Stem Cell–functioning 3D SCLC Spheroids. Research Square. 2023. doi: 10.21203/rs.3.rs-3700346/v1
- Gazdar AF, Carney DN, Russell EK, et al. Establishment of continuous, clonable cultures of small-cell carcinoma of lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res. 1980 Oct;40(10):3502–3507
- Vinogradova TV, Chernov IP, Monastyrskaya GS, et al. Cancer stem cells: plasticity works against therapy. Acta Naturae. 2015 Oct-Dec; 7(4): 46–55.10.32607/20758251-2015-7-4-46-55
- Aguirre-Ghiso JA Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007 Nov;7(11):834–846. doi: 10.1038/nrc2256
- Bartram I, Jeschke JM, Nie D Do cancer stem cells exist? A pilot study combining a systematic review with the hierarchy-of-hypotheses approach. PLoS One. 2019 Dec 13;14(12):e0225898. doi: 10.1371/journal.pone.0225898
- Hamilton G, Burghuber O, Zeillinger R Circulating tumor cells in small cell lung cancer: ex vivo expansion. Lung. 2015 Jun;193(3):451–452. doi: 10.1007/s00408-015-9725-7
- Hochmair M, Rath B, Klameth L, et al. Effects of salinomycin and niclosamide on small cell lung cancer and small cell lung cancer circulating tumor cell lines. Invest New Drugs. 2020 Aug;38(4):946–955. doi: 10.1007/s10637-019-00847-8
- Hamilton G, Rath B. Role of circulating tumor cell spheroids in drug resistance. Cancer Drug Resist. 2019 Sep;2(3):762–772. doi: 10.20517/cdr.2019.47
- Hamilton G, Rath B. Applicability of tumor spheroids for in vitro chemosensitivity assays. Expert Opin Drug Metab Toxicol. 2019;15(1):15–23. doi: 10.1080/17425255.2019.1554055
- Rath B, Plangger A, Hamilton G. Non-small cell lung cancer-small cell lung cancer transformation as mechanism of resistance to tyrosine kinase inhibitors in lung cancer. Cancer Drug Resist. 2020 Feb 28;3(2):171–178. doi: 10.20517/cdr.2019.85.
- Hamilton G, Rath B, Klameth L, Hochmair MJ. Small cell lung cancer: Recruitment of macrophages by circulating tumor cells. Oncoimmunology. 2015 Oct 29;5(3):e1093277. doi: 10.1080/2162402X.2015.1093277
- Hamilton G, Rath B Circulating tumor cell interactions with macrophages: implications for biology and treatment. Transl Lung Cancer Res. 2017 Aug;6(4):418–430. doi: 10.21037/tlcr.2017.07.04
- Rath B, Klameth L, Plangger A, et al. Expression of Proteolytic Enzymes by Small Cell Lung Cancer Circulating Tumor Cell Lines. Cancers (Basel). 2019 Jan 19;11(1):114. doi: 10.3390/cancers11010114.
- Nunes AS, Barros AS, Costa EC, et al. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol Bioeng. 2019 Jan;116(1):206–226. doi: 10.1002/bit.26845.
- Knight E, Przyborski S. Advances in 3D cell culture technologies enabling tissue‐like structures to be created in vitro. J Anatomy. 2015;227(6):746–756. doi:10.1111/joa.12257.
- Fang Y, Eglen RM. Three‐dimensional cell cultures in drug discovery and development. SLAS Discov. 2017;22(5):456–472. doi: 10.1177/1087057117696795
- Badea MA, Balas M, Hermenean A, et al. Influence of Matrigel on single- and multiple-spheroid cultures in breast cancer research. SLAS Discov. 2019;24(5):563–578. doi: 10.1177/2472555219834698
- Mehta G, Hsiao AY, Ingram M, et al. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 2012;164(2):192–204. doi: 10.1016/j.jconrel.2012.04.045.
- Lee KH, Kim TH Recent Advances in Multicellular Tumor Spheroid Generation for Drug Screening. Biosensors (Basel). 2021 Nov 11;11(11):445. doi: 10.3390/bios11110445.
- Nath S, Devi GR. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol Ther. 2016;163:194–108. doi: 10.1016/j.pharmthera.2016.03.013.
- Costa EC, Moreira AF, de Melo-Diogo D, et al. 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol Adv. 2016;34(8):1427–1441. doi: 10.1016/j.biotechadv.2016.11.002
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nature Rev Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893.
- Morgan P, Van Der Graaf PH, Arrowsmith J, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today. 2012;17(9–10):419–424. doi: 10.1016/j.drudis.2011.12.020
- Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20–33. doi: 10.7497/j.issn.2095-3941.2014.01.002
- Bartelink IH, Jones EF, Shahidi-Latham SK, et al. Tumor Drug Penetration Measurements Could Be the Neglected Piece of the Personalized Cancer Treatment Puzzle. Clin Pharmacol Ther. 2019 Jul;106(1):148–163. doi: 10.1002/cpt.1211
- Zanoni M, Piccinini F, Arienti C, et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep. 2016 Jan 11;6:19103. doi: 10.1038/srep19103.
- Wang X, Zhen X, Wang J, et al. Doxorubicin delivery to 3D multicellular spheroids and tumors based on boronic acid-rich chitosan nanoparticles. Biomaterials. 2013;34(19):4667–4679. doi: 10.1016/j.biomaterials.2013.03.008.
- Gong X, Lin C, Cheng J, et al. Generation of multicellular tumor spheroids with Microwell-based agarose scaffolds for drug testing. PLoS One. 2015;10(6):e0130348. doi: 10.1371/journal.pone.0130348
- Doublier S, Belisario DC, Polimeni M, et al. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer. 2012;12:4. doi: 10.1186/1471-2407-12-4.
- Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–389. doi: 10.1016/j.apsb.2015.05.007
- Wigerup C, Påhlman S, Bexell D Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009.
- Hirschhaeuser F, Menne H, Dittfeld C, et al. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148(1):3–15. doi: 10.1016/j.jbiotec.2010.01.012
- Saggar JK, Yu M, Tan Q, et al. The tumor microenvironment and strategies to improve drug distribution. Front Oncol. 2013;3:154. doi: 10.3389/fonc.2013.00154.
- Wenzel C, Riefke B, Gründemann S, et al. 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp Cell Res. 2014;323(1):131–143. doi: 10.1016/j.yexcr.2014.01.017
- Cheung-Ong K, Giaever G, Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. 2013;20(5):648–59. doi: 10.1016/j.chembiol.2013.04.007.
- Rath B, Plangger A, Moser D, et al. Protection of small-cell lung cancer circulating tumor cells by cellular fragmentation. J Cancer Metastasis Treat 2020;6:30. Doi: 10.20517/2394-4722.2020.51
- Barzaman K, Vafaei R, Samadi M, et al. Anti-cancer therapeutic strategies based on HGF/MET, EpCAM, and tumor-stromal cross talk. Cancer Cell Int. 2022 Aug 19;22(1):259. doi: 10.1186/s12935-022-02658-z.
- Kapka-Skrzypczak L, Popek S, Sawicki K, et al. IL-6 prevents CXCL8-induced stimulation of EpCAM expression in ovarian cancer cells. Mol Med Rep. 2019 Mar;19(3):2317–2322. doi: 10.3892/mmr.2019.9890
- Chaudry MA, Sales K, Ruf P, et al. EpCAM an immunotherapeutic target for gastrointestinal malignancy: current experience and future challenges. Br J Cancer. 2007 Apr;96(7):1013–1019. doi: 10.1038/sj.bjc.6603505
- van der Gun BT, Melchers LJ, Ruiters MH, et al. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010 Nov;31(11):1913–21. doi: 10.1093/carcin/bgq187
- Keller L, Werner S, Pantel K. Biology and clinical relevance of EpCAM. Cell Stress. 2019 May;3(6):165–180. doi: 10.15698/cst2019.06.188
- Armeanu-Ebinger S, Hoh A, Wenz J, et al. Targeting EpCAM (CD326) for immunotherapy in hepatoblastoma. Oncoimmunology. 2013 Jan;2(1):e22620. doi: 10.4161/onci.22620
- Wellbrock C, Arozarena I The Complexity of the ERK/MAP-Kinase Pathway and the Treatment of Melanoma Skin Cancer. Front Cell Dev Biol. 2016 Apr;4:33. doi: 10.3389/fcell.2016.00033
- Münz M, Murr A, Kvesic M, et al. Side-by-side analysis of five clinically tested anti-EpCAM monoclonal antibodies. Cancer Cell Int. 2010 Nov;10:44. doi: 10.1186/1475-2867-10-44 1
- Hirschhaeuser F, Walenta S, Mueller-Klieser W Efficacy of catumaxomab in tumor spheroid killing is mediated by its trifunctional mode of action. Cancer Immunol Immunother. 2010 Nov;59(11):1675–1684. doi: 10.1007/s00262-010-0894-1.
- Ring A, Nguyen-Sträuli BD, Wicki A, Aceto N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat Rev Cancer. 2023 Feb;23:95–111. doi: 10.1038/s41568-022-00536-4.
- Hamilton G, Stickler S, Rath B Bromodomain Protein-directed Agents and MYC in Small Cell Lung Cancer. Curr Cancer Drug Targets. 2024 Jan. doi: 10.2174/0115680096272757231211113206 24 9 930–940
- Särchen V, Shanmugalingam S, Kehr S, et al. Pediatric multicellular tumor spheroid models illustrate a therapeutic potential by combining BH3 mimetics with Natural Killer (NK) cell-based immunotherapy. Cell Death Discov. 2022 Jan 10;8(1):11. doi: 10.1038/s41420-021-00812-6.
- Murphy DA, Cheng H, Yang T, et al. Reversing Hypoxia with PLGA-Encapsulated Manganese Dioxide Nanoparticles Improves Natural Killer Cell Response to Tumor Spheroids. Mol Pharm. 2021 Aug 2;18(8):2935–2946. doi: 10.1021/acs.molpharmaceut.1c00085.
- Jeanes A, Gottardi CJ, Yap AS Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008 Nov 24;27(55):6920–6929. doi: 10.1038/onc.2008.343.
- Alpaugh ML, Tomlinson JS, Ye Y, et al. Relationship of sialyl-Lewis(x/a) underexpression and E-cadherin overexpression in the lymphovascular embolus of inflammatory breast carcinoma. Am J Pathol. 2002 Aug;161(2):619–628. doi: 10.1016/S0002-9440(10)64217-4.
- Yarom N, Stewart D, Malik R, et al. Phase I clinical trial of Exherin (ADH-1) in patients with advanced solid tumors. Curr Clin Pharmacol. 2013 Feb 1;8(1):81–88. 10.2174/1574884711308010011
- Hamilton G Comparative characteristics of small cell lung cancer and Ewing’s sarcoma: a narrative review. Transl Lung Cancer Res. 2022 Jun;11(6):1185–1198. doi: 10.21037/tlcr-22-58.